Assessing the Effect of Cold Plasma on the Softening of Postharvest Blueberries through Reactive Oxygen Species Metabolism Using Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Blueberry Samples
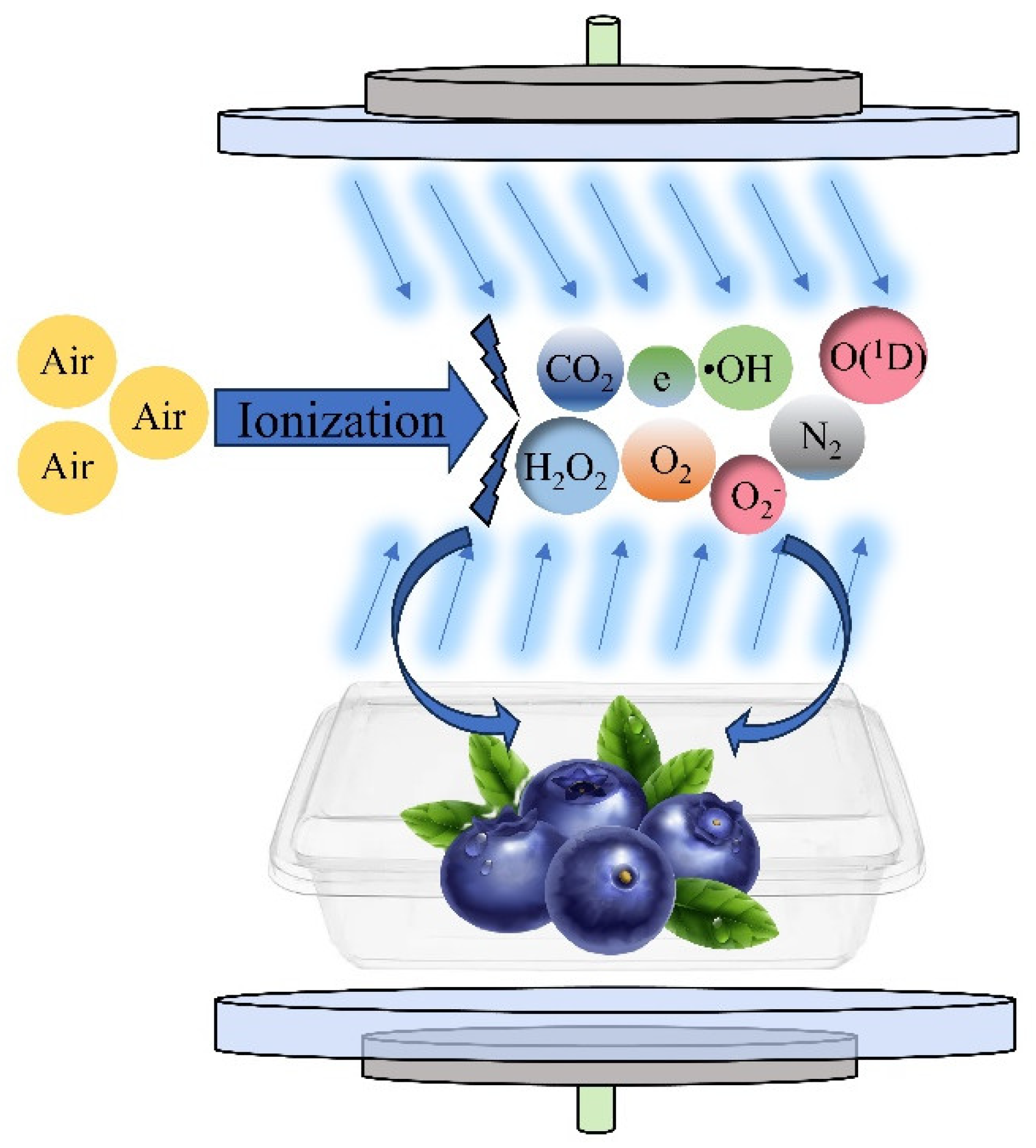
2.3. CP Treatment
2.4. Determination of Emission Spectrum
2.5. Firmness Test
2.6. H2O2 Content
2.7. Antioxidant Metabolism
2.7.1. Antioxidant Content
2.7.2. Antioxidant Enzyme Activity
2.7.3. Total Antioxidant Capacity (T-AOC) Determination
2.8. Transcriptomic Analysis
2.8.1. Transcriptome Sequencing and Gene Expression Analysis
2.8.2. DEG Enrichment Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Emission Spectroscopic Determination of Plasma
3.2. Firmness Changes
3.3. H2O2 Content Changes
3.4. The ROS-Scavenging System
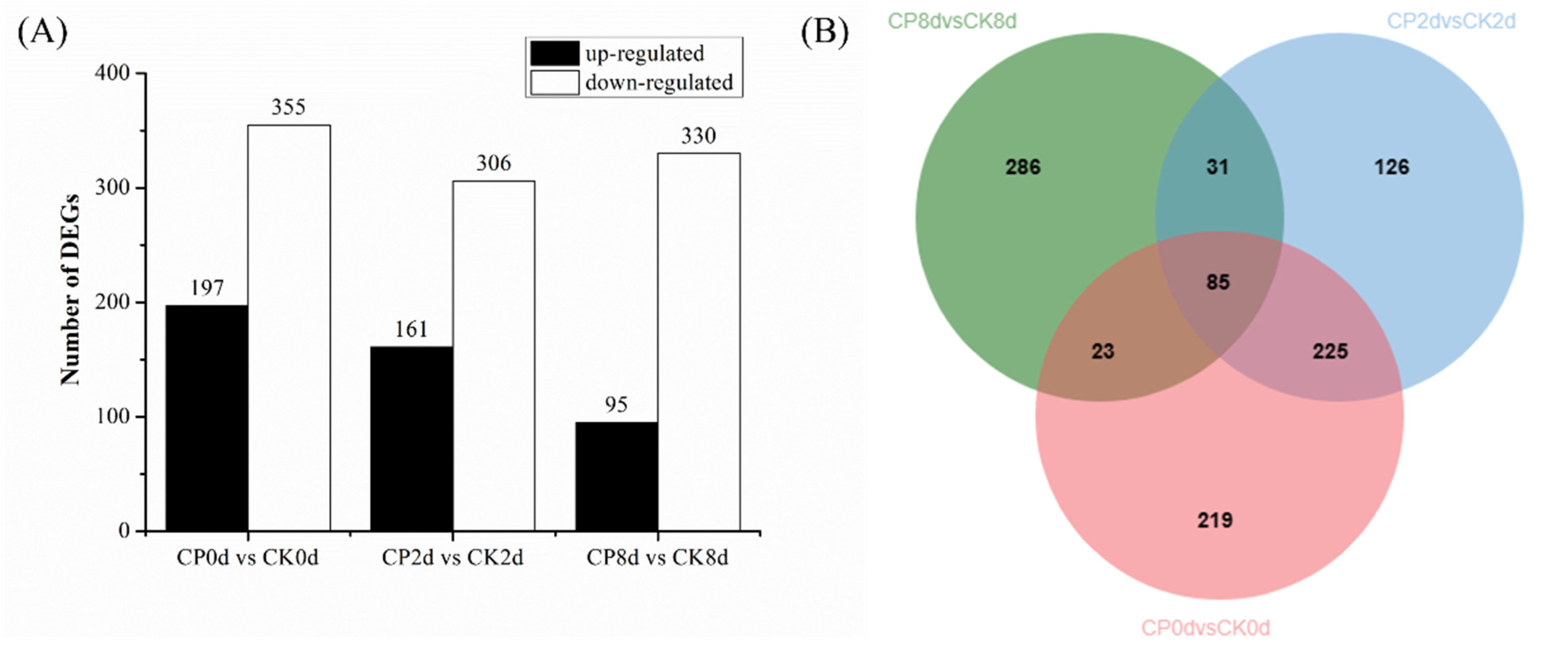
3.5. Transcriptome Data and Differential Gene Expression
3.6. Key DEG Analysis
3.6.1. Dentification and Analysis of DEGs Involved in the Cell Wall Metabolism Pathway
3.6.2. Dentification and Analysis of DEGs Involved in the ROS Metabolism Pathway
3.6.3. Dentification and Analysis of DEGs Involved in the MAPK Signaling Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folmer, F.; Basavaraju, U.; Jaspars, M.; Hold, G.; El-Omar, E.; Dicato, M.; Diederich, M. Anticancer Effects of Bioactive Berry Compounds. Phytochem. Rev. 2014, 13, 295–322. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Q.; Zhou, X.; Wei, B.; Ji, S. The Effect of Ethylene Absorbent Treatment on the Softening of Blueberry Fruit. Food Chem. 2018, 246, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Q.; Zhou, X.; Zhang, F.; Ji, S. Ethylene Plays an Important Role in the Softening and Sucrose Metabolism of Blueberries Postharvest. Food Chem. 2020, 310, 125965. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Hu, W.; Xiu, Z.; Yang, X.; Guan, Y. Integrated Transcriptomics-Proteomics Analysis Reveals the Regulatory Network of Ethanol Vapor on Softening of Postharvest Blueberry. LWT 2023, 180, 114649. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Xiu, Z.; Jiang, A.; Guan, Y.; Yang, X.; Feng, K. Ethanol Vapor Delays Softening of Postharvest Blueberry by Retarding Cell Wall Degradation during Cold Storage and Shelf Life. Postharvest Biol. Technol. 2021, 177, 111538. [Google Scholar] [CrossRef]
- Song, X.; Dai, H.; Wang, S.; Ji, S.; Zhou, X.; Li, J.; Zhou, Q. Putrescine Treatment Delayed the Softening of Postharvest Blueberry Fruit by Inhibiting the Expression of Cell Wall Metabolism Key Gene VcPG1. Plants 2022, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Li, B.; Song, Z.; Tian, J.; Wei, B.; Lang, X.; Zhou, Q. Anthocyanin Treatment Delays the Senescence of Blueberry Fruit by Regulating Abscisic Acid and Anthocyanin Synthesis Processes. Food Qual. Saf. 2024, 8, fyad053. [Google Scholar] [CrossRef]
- Sun, H.; Hao, D.; Tian, Y.; Huang, Y.; Wang, Y.; Qin, G.; Pei, J.; Abd El-Aty, A.M. Effect of Chitosan/Thyme Oil Coating and UV-C on the Softening and Ripening of Postharvest Blueberry Fruits. Foods 2022, 11, 2795. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in Antioxidative Metabolism Accompanying Pitting Development in Stored Blueberry Fruit. Postharvest Biol. Technol. 2014, 88, 88–95. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.-C.; Chen, M.; Lin, M.; Lin, H. Enhanced Storability of Blueberries by Acidic Electrolyzed Oxidizing Water Application May Be Mediated by Regulating ROS Metabolism. Food Chem. 2019, 270, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Duan, B.; Li, C.; Tang, Q.; Li, X.; Wei, M.; Chen, Y.; Li, J. γ-Aminobutyric Acid Delays Senescence of Blueberry Fruit by Regulation of Reactive Oxygen Species Metabolism and Phenylpropanoid Pathway. Sci. Hortic. 2018, 240, 303–309. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A. Cold Plasma Reduction of Salmonella and Escherichia coli O157:H7 on Almonds Using Ambient Pressure Gases. J. Food Sci. 2012, 77, M171–M175. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Mhaske, P.; Zing, X.; Kasapis, S.; Majzoobi, M.; Farahnaky, A. Cold Plasma: Microbial Inactivation and Effects on Quality Attributes of Fresh and Minimally Processed Fruits and Ready-To-Eat Vegetables. Trends Food Sci. Technol. 2021, 116, 146–175. [Google Scholar] [CrossRef]
- Asl, P.J.; Rajulapati, V.; Gavahian, M.; Kapusta, I.; Putnik, P.; Mousavi Khaneghah, A.; Marszałek, K. Non-Thermal Plasma Technique for Preservation of Fresh Foods: A Review. Food Control 2022, 134, 108560. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Liao, J.; Jiang, A.; Xiu, Z.; Gaowa, S.; Guan, Y.; Yang, X.; Feng, K.; Liu, C. Effect of Atmospheric Cold Plasma Treatment on Antioxidant Activities and Reactive Oxygen Species Production in Postharvest Blueberries during Storage. J. Sci. Food Agric. 2020, 100, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Costa, V.; Angelini, C.; De Feis, I.; Ciccodicola, A. Uncovering the Complexity of Transcriptomes with RNA-Seq. J. Biomed. Biotechnol. 2010, 2010, 853916. [Google Scholar] [CrossRef]
- Chen, F.; Hao, Y.; Yin, Z.; Wu, G.; Jiang, X. Transcriptome of Wax Apple (Syzygium samarangense) Provides Insights into Nitric Oxide-Induced Delays of Postharvest Cottony Softening. Acta Physiol. Plant. 2017, 39, 273. [Google Scholar] [CrossRef]
- Ma, L.; Sun, L.; Guo, Y.; Lin, H.; Liu, Z.; Li, K.; Guo, X. Transcriptome Analysis of Table Grapes (Vitis vinifera L.) Identified a Gene Network Module Associated with Berry Firmness. PLoS ONE 2020, 15, e0237526. [Google Scholar] [CrossRef] [PubMed]
- Application of OES Diagnostics on Plasma Etching-All Databases. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/INSPEC:10243527 (accessed on 1 April 2024).
- Ge, Y.; Zhang, J.; Li, C.; Xue, W.; Zhang, S.; Lv, J. Trisodium Phosphate Delays Softening of Jujube Fruit by Inhibiting Cell Wall-Degrading Enzyme Activities during Ambient Storage. Sci. Hortic. 2020, 262, 109059. [Google Scholar] [CrossRef]
- Liu, L. Oxidative Stress Induces Gastric Submucosal Arteriolar Dysfunction in the Elderly. World J. Gastroenterol. 2013, 19, 9439. [Google Scholar] [CrossRef]
- Wei, M.; Wu, Y.; Chen, D.; Gu, Y. Changes of Free Radicals and Digestive Enzymes in Saliva in Cases with Deficiency in Spleen-Yin Syndrome. J. Biomed. Res. 2010, 24, 250–255. [Google Scholar] [CrossRef]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Mosnier, J.-P.; Cullen, P.J. In-Package Atmospheric Pressure Cold Plasma Treatment of Cherry Tomatoes. J. Biosci. Bioeng. 2014, 118, 177–182. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Cheng, W.; Zhang, W.; Xue, G.; Ognier, S. Study on Remediation of Phenanthrene Contaminated Soil by Pulsed Dielectric Barrier Discharge Plasma: The Role of Active Species. Chem. Eng. J. 2016, 296, 132–140. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive Species in Non-Equilibrium Atmospheric-Pressure Plasmas: Generation, Transport, and Biological Effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Bonnafous, P.; Vernhes, M.-C.; Teissié, J.; Gabriel, B. The Generation of Reactive-Oxygen Species Associated with Long-Lasting Pulse-Induced Electropermeabilisation of Mammalian Cells Is Based on a Non-Destructive Alteration of the Plasma Membrane. Biochim. Biophys. Acta BBA-Biomembr. 1999, 1461, 123–134. [Google Scholar] [CrossRef]
- Ahmadnia, M.; Sadeghi, M.; Abbaszadeh, R.; Ghomi Marzdashti, H.R. Decontamination of Whole Strawberry via Dielectric Barrier Discharge Cold Plasma and Effects on Quality Attributes. J. Food Process. Preserv. 2021, 45, e15019. [Google Scholar] [CrossRef]
- Moon, A.-Y.; Noh, S.; Moon, S.Y.; You, S. Feasibility Study of Atmospheric-Pressure Plasma Treated Air Gas Package for Grape’s Shelf-Life Improvement. Curr. Appl. Phys. 2016, 16, 440–445. [Google Scholar] [CrossRef]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric Gas Plasma Treatment of Fresh-Cut Apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Brennan, T.; Frenkel, C. Involvement of Hydrogen Peroxide in the Regulation of Senescence in Pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S. Control of Postharvest Quality in Blueberry Fruit by Combined 1-Methylcyclopropene (1-MCP) and UV-C Irradiation. Food Bioprocess Technol. 2017, 10, 1695–1703. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wang, L.; Zhang, Z.; Li, J.; Zhao, C. Impact of Ozone on Quality of Strawberry during Cold Storage. Front. Agric. China 2011, 5, 356–360. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A Novel Approach to Enhance Blueberry Quality During Storage Using Cold Plasma at Atmospheric Air Pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of Plasma Activated Water on the Postharvest Quality of Button Mushrooms, Agaricus Bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Mousavi Khaneghah, A. Impacts of Cold Plasma Treatment on Physicochemical, Functional, Bioactive, Textural, and Sensory Attributes of Food: A Comprehensive Review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, Z.; Tu, S.; Chen, S.; Peng, J.; Tu, K. Effects of Cold Plasma, UV-C or Aqueous Ozone Treatment on Botrytis Cinerea and Their Potential Application in Preserving Blueberry. J. Appl. Microbiol. 2019, 127, 175–185. [Google Scholar] [CrossRef]
- Ke, Z.; Bai, Y.; Bai, Y.; Chu, Y.; Gu, S.; Xiang, X.; Ding, Y.; Zhou, X. Cold Plasma Treated Air Improves the Characteristic Flavor of Dry-Cured Black Carp through Facilitating Lipid Oxidation. Food Chem. 2022, 377, 131932. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell Wall Metabolism in Fruit Softening and Quality and Its Manipulation in Transgenic Plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A. Cell Wall Disassembly in Ripening Fruit. Funct. Plant Biol. 2006, 33, 103. [Google Scholar] [CrossRef] [PubMed]
- Vicente, A.R.; Saladié, M.; Rose, J.K.; Labavitch, J.M. The Linkage between Cell Wall Metabolism and Fruit Softening: Looking to the Future. J. Sci. Food Agric. 2007, 87, 1435–1448. [Google Scholar] [CrossRef]
- Zhai, Z.; Feng, C.; Wang, Y.; Sun, Y.; Peng, X.; Xiao, Y.; Zhang, X.; Zhou, X.; Jiao, J.; Wang, W.; et al. Genome-Wide Identification of the Xyloglucan Endotransglucosylase/Hydrolase (XTH) and Polygalacturonase (PG) Genes and Characterization of Their Role in Fruit Softening of Sweet Cherry. Int. J. Mol. Sci. 2021, 22, 12331. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. Physiological and Metabolomic Analysis of Cold Plasma Treated Fresh-Cut Strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold Plasma Treatment Induces Phenolic Accumulation and Enhances Antioxidant Activity in Fresh-Cut Pitaya (Hylocereus undatus) Fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Lethal and Sublethal Effect of a Dielectric Barrier Discharge Atmospheric Cold Plasma on Staphylococcus aureus. J. Food Prot. 2017, 80, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Singh, A.; Pratap Singh, A. Recent Developments in Cold Plasma Decontamination Technology in the Food Industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Surowsky, B.; Fischer, A.; Schlueter, O.; Knorr, D. Cold Plasma Effects on Enzyme Activity in a Model Food System. Innov. Food Sci. Emerg. Technol. 2013, 19, 146–152. [Google Scholar] [CrossRef]
- Zambon, Y.; Contaldo, N.; Laurita, R.; Várallyay, E.; Canel, A.; Gherardi, M.; Colombo, V.; Bertaccini, A. Plasma Activated Water Triggers Plant Defence Responses. Sci. Rep. 2020, 10, 19211. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.J.; Kim, M.-H.; Kwak, J.M. MAPK Cascades in Guard Cell Signal Transduction. Front. Plant Sci. 2016, 7, 177817. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.M.; Zargar, M.Y. (Eds.) Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Springer: Singapore, 2018; ISBN 978-981-10-7478-3. [Google Scholar]
- Liu, Y.; He, C. A Review of Redox Signaling and the Control of MAP Kinase Pathway in Plants. Redox Biol. 2017, 11, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Chiu, W.-L.; Tena, G.; Sheen, J. Functional Analysis of Oxidative Stress-Activated Mitogen-Activated Protein Kinase Cascade in Plants. Proc. Natl. Acad. Sci. USA 2000, 97, 2940–2945. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, B.; Choi, G.; Shin, D.; Prasad, D.T.; Lee, O.; Kwak, S.-S.; Kim, D.H.; Nam, J.; Bahk, J.; et al. NDP Kinase 2 Interacts with Two Oxidative Stress-Activated MAPKs to Regulate Cellular Redox State and Enhances Multiple Stress Tolerance in Transgenic Plants. Proc. Natl. Acad. Sci. USA 2003, 100, 358–363. [Google Scholar] [CrossRef] [PubMed]








| Gene ID | Name | Functional Annotation | log2Fold Change | ||
|---|---|---|---|---|---|
| CP0d-vs-CK0d | CP2d-vs-CK2d | CP8d-vs-CK8d | |||
| TRINITY_DN3072_c0_g1 | PG | Polygalacturonase | −2.76 | −6.84 | −7.79 |
| TRINITY_DN27616_c0_g3 | PE | Pectinesterase | - | - | −2.97 |
| TRINITY_DN32319_c1_g1 | BGAL | Beta-galactosidase | - | −1.10 | −3.89 |
| TRINITY_DN39374_c2_g2 | PL | Pectate lyase | - | −7.27 | −4.48 |
| TRINITY_DN17987_c1_g1 | XTH8 | Xyloglucan endotransglucosylase/ hydrolase protein 8 | −7.20 | - | - |
| TRINITY_DN18512_c0_g1 | XTH8 | Xyloglucan endotransglucosylase/ hydrolase protein 8 | - | −7.06 | −4.99 |
| TRINITY_DN33049_c0_g4 | XTH23 | Xyloglucan endotransglucosylase/ hydrolase protein 23 | 1.10 | - | 2.02 |
| TRINITY_DN36105_c0_g1 | GLU | Beta-glucosidase | −9.77 | −9.80 | - |
| TRINITY_DN34957_c0_g2 | At4g29360 | Beta-1,3-endoglucanase 12 | - | 1.51 | 1.11 |
| TRINITY_DN28630_c2_g1 | At1g32860 | Beta-1,3-endoglucanase 11 | - | - | 1.28 |
| TRINITY_DN11208_c0_g1 | EXPA8 | Expansin | - | - | −7.22 |
| TRINITY_DN22419_c0_g2 | BAM3 | Beta-amylase 6 | 1.11 | - | - |
| TRINITY_DN28798_c0_g2 | SUS1 | Sucrose synthase | −10.07 | - | - |
| TRINITY_DN8373_c0_g1 | WAXY | Granule-bound starch synthase 1 | −8.41 | −7.71 | −5.08 |
| TRINITY_DN34835_c0_g1 | AMY1.1 | Alpha-amylase | - | - | −1.44 |
| Gene ID | Name | Functional Annotation | Gene Expression Level Expressed by TPM | |||||
|---|---|---|---|---|---|---|---|---|
| CP0d | CK0d | CP2d | CK2d | CP8d | CK8d | |||
| TRINITY_DN3072_c0_g1 | PG | Polygalacturonase | 0.27 | 0.91 | 0.00 | 0.91 | 0.00 | 3.80 |
| TRINITY_DN27616_c0_g3 | PE | Pectinesterase | 2.54 | 1.87 | 0.94 | 1.19 | 0.26 | 2.53 |
| TRINITY_DN32319_c1_g1 | BGAL | Beta-galactosidase | 3.79 | 3.44 | 34.64 | 77.51 | 0.33 | 6.63 |
| TRINITY_DN39374_c2_g2 | PL | Pectate lyase | 618.82 | 922.6 | 0.00 | 2.24 | 0.25 | 7.84 |
| Gene ID | Name | Functional Annotation | log2Fold Change | ||
|---|---|---|---|---|---|
| CP0d-vs-CK0d | CP2d-vs-CK2d | CP8d-vs-CK8d | |||
| TRINITY_DN41456_c0_g1 | CAT1 | Catalase isozyme 1 | −1.71 | 6.18 | - |
| TRINITY_DN12758_c0_g1 | CAT2 | Catalase isozyme 2 | 1.19 | 5.95 | 1.55 |
| TRINITY_DN976_c0_g1 | SODCC | Superoxide dismutase | - | - | 2.51 |
| TRINITY_DN18708_c1_g1 | SODCC.3 | Superoxide dismutase [Cu-Zn] | 1.09 | 1.86 | - |
| TRINITY_DN18363_c0_g1 | SODCP | Superoxide dismutase [Cu-Zn] | - | - | 3.21 |
| TRINITY_DN22770_c1_g1 | POD | Peroxidase 4 | 1.08 | 2.08 | 1.25 |
| TRINITY_DN41457_c0_g1 | APX1 | L-ascorbate peroxidase 1 | 1.83 | - | 3.37 |
| TRINITY_DN17896_c0_g1 | GPX7 | Glutathione peroxidase 7 | - | - | 2.74 |
| TRINITY_DN17462_c0_g2 | GSTL3 | Glutathione S-transferase L3 | - | - | −4.83 |
| TRINITY_DN8736_c0_g1 | GSTU19 | Glutathione S-transferase U19 | - | - | 1.31 |
| TRINITY_DN18940_c0_g1 | GSTU20 | Glutathione S-transferase U20 | 1.72 | 7.39 | 2.26 |
| TRINITY_DN41198_c0_g1 | DHAR3 | Glutathione S-transferase DHAR3 | - | - | 1.53 |
| Gene ID | Name | Functional Annotation | log2Fold Change | ||
|---|---|---|---|---|---|
| CP0d-vs-CK0d | CP2d-vs-CK2d | CP8d-vs-CK8d | |||
| TRINITY_DN12758_c0_g1 | CAT2 | Catalase isozyme 2 | 1.19 | 5.95 | 1.55 |
| TRINITY_DN41456_c0_g1 | CAT1 | Catalase isozyme 2 | −1.71 | 6.18 | - |
| TRINITY_DN16006_c0_g1 | PYL | Abscisic acid receptor PYR/PYL family | - | - | −4.13 |
| TRINITY_DN22372_c0_g1 | FLS2 | Serine/threonine protein kinase | 1.35 | - | - |
| TRINITY_DN15561_c0_g2 | NDPK2 | Nucleoside diphosphate kinase-like | 4.44 | - | - |
| TRINITY_DN18128_c0_g2 | NME2 | Nucleoside diphosphate kinase | - | - | −3.42 |
| TRINITY_DN25013_c0_g1 | WRKY75 | WRKY transcription factor 33 | - | - | 1.25 |
| TRINITY_DN25539_c0_g1 | WRKY7 | WRKY transcription factor 22 | - | - | 1.16 |
| TRINITY_DN22163_c0_g1 | PR1 | Pathogenesis-related protein PR-1 type-like | - | - | 2.37 |
| TRINITY_DN30325_c0_g1 | NPL7 | Transcription factor MYC2 | 1.51 | - | - |
| TRINITY_DN15873_c0_g1 | - | Endo chitinase | - | - | −3.39 |
| TRINITY_DN17764_c0_g1 | My16 | Calmodulin | 2.02 | - | - |
| TRINITY_DN18948_c0_g2 | PCM5 | Calmodulin-5 | - | - | 4.42 |
| TRINITY_DN18948_c0_g5 | CAM1-1 | Calmodulin-1 | - | - | 7.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Cheng, J.-H. Assessing the Effect of Cold Plasma on the Softening of Postharvest Blueberries through Reactive Oxygen Species Metabolism Using Transcriptomic Analysis. Foods 2024, 13, 1132. https://doi.org/10.3390/foods13071132
Zhang C, Cheng J-H. Assessing the Effect of Cold Plasma on the Softening of Postharvest Blueberries through Reactive Oxygen Species Metabolism Using Transcriptomic Analysis. Foods. 2024; 13(7):1132. https://doi.org/10.3390/foods13071132
Chicago/Turabian StyleZhang, Can, and Jun-Hu Cheng. 2024. "Assessing the Effect of Cold Plasma on the Softening of Postharvest Blueberries through Reactive Oxygen Species Metabolism Using Transcriptomic Analysis" Foods 13, no. 7: 1132. https://doi.org/10.3390/foods13071132





