Functionality and Health-Promoting Properties of Polysaccharide and Plant-Derived Substances from Mesona chinensis
Abstract
1. Introduction
2. Proximate and Mineral Composition
3. Polysaccharides and Phytochemicals of M. chinensis Extracts
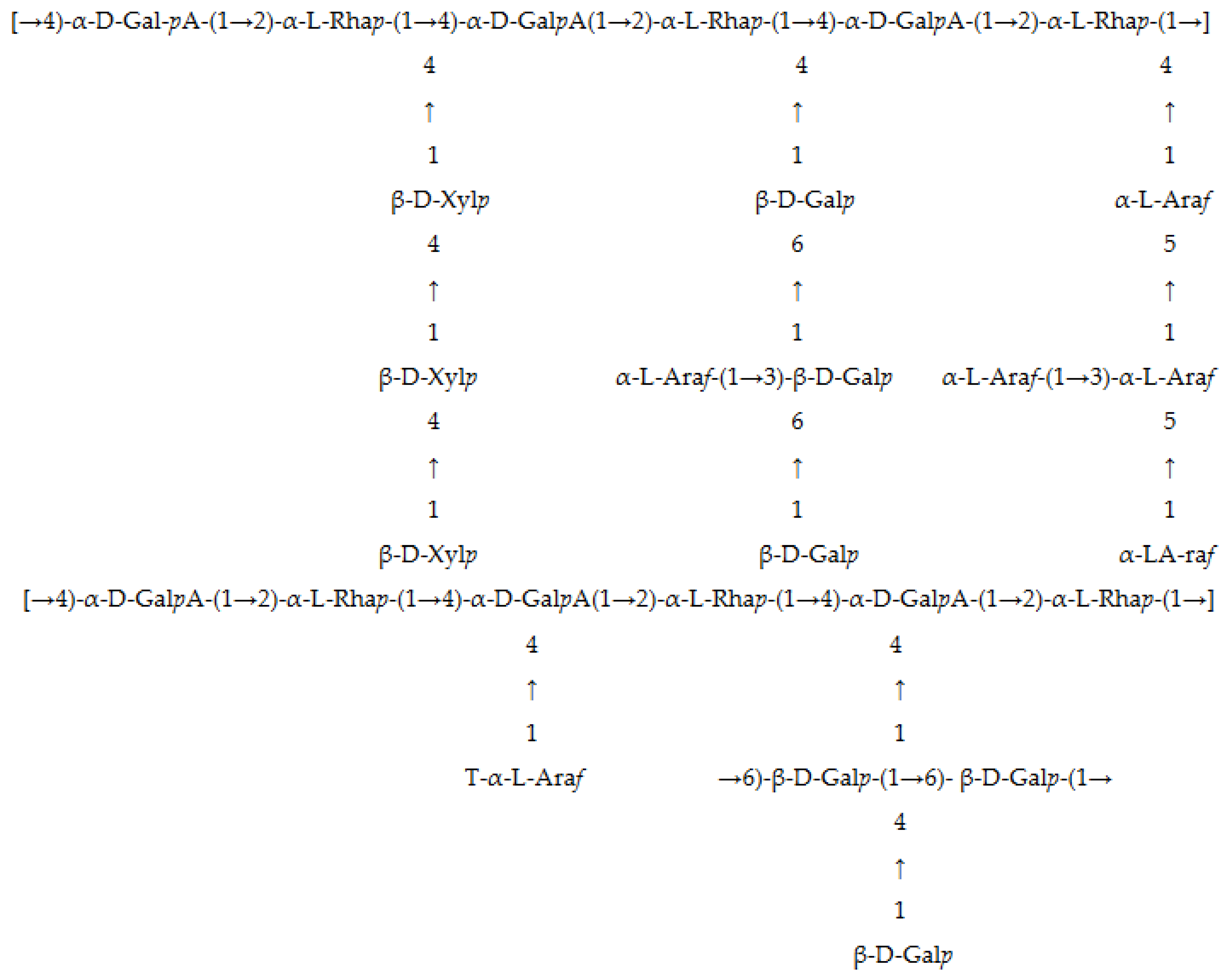
3.1. Physicochemical Properties
3.2. Phytochemicals
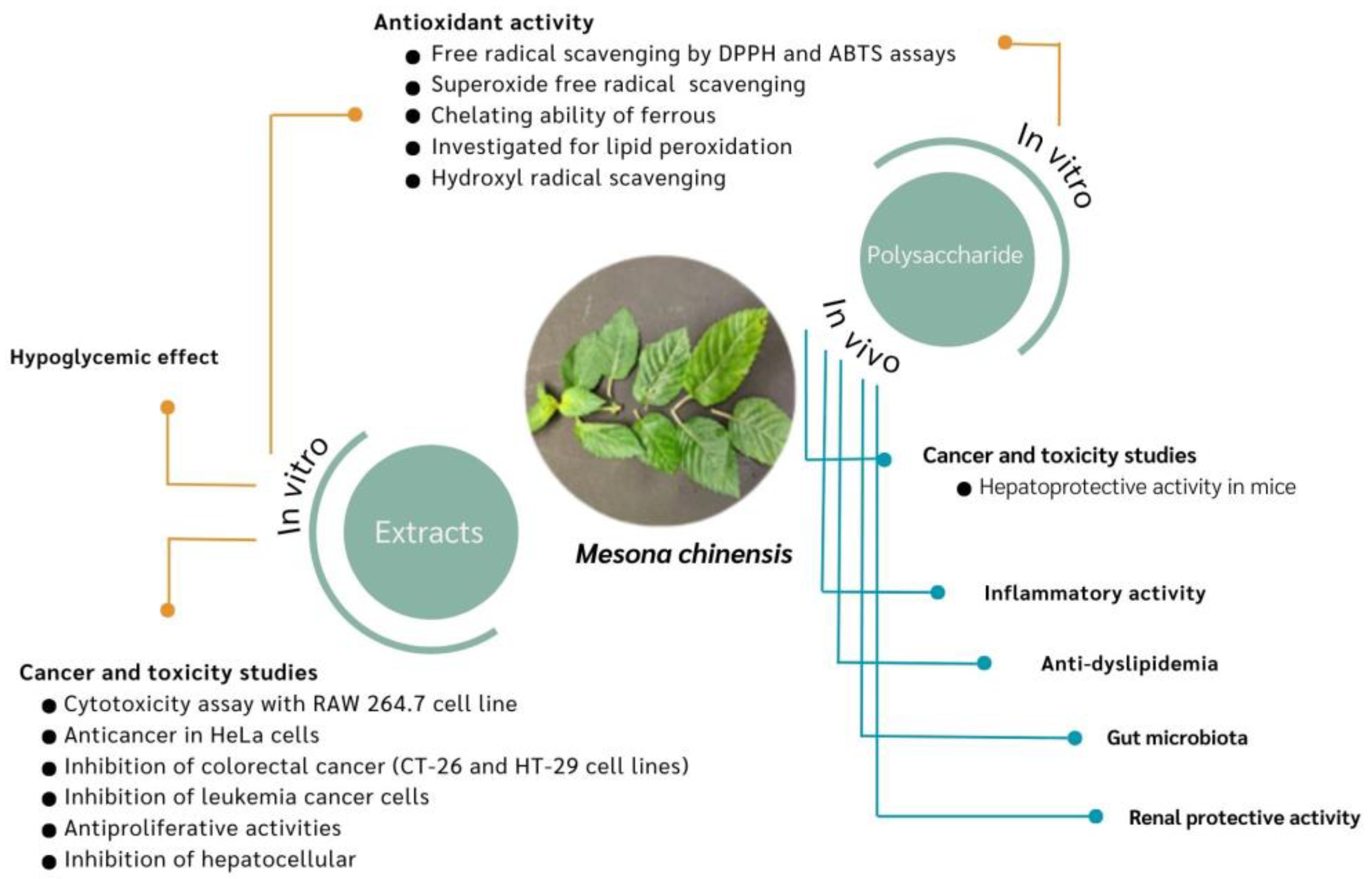
4. Pharmacological Properties
4.1. Pharmacological Properties
4.2. Antioxidant Activity
4.3. Cancer and Toxicity Studies
4.4. Hypolipidemic Effect
4.5. Hypoglycemic Effect
4.6. Renal Protective Activity
4.7. Inflammatory Activity
4.8. Gut Microbiota
5. Application of Mesona Polysaccharides as Biomaterials for Medicine
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, D.; Wei, F.; Cai, Z.; Wei, Y.; Khan, A.; Miao, J.; Wei, K. Analysis of codon usage bias and evolution in the chloroplast genome of Mesona chinensis Benth. Dev. Genes Evol. 2021, 231, 1–9. [Google Scholar] [CrossRef]
- Huang, H.C.; Chuang, S.H.; Wu, Y.C.; Chao, P.M. Hypolipidaemic function of Hsian-tsao tea (Mesona procumbens Hemsl.): Working mechanisms and active components. J. Funct. Foods 2016, 26, 217–227. [Google Scholar] [CrossRef]
- Wang, H.; Qin, L. Determination of natural benzoic acid in different Mesona Chinensis Benth. China Pharm. 2014, 12, 1493–1495. [Google Scholar]
- Huang, L.; Shen, M.; Zhang, X.; Jiang, L.; Song, Q.; Xie, J. Effect of high-pressure microfluidization treatment on the physicochemical properties and antioxidant activities of polysaccharide from Mesona chinensis Benth. Carbohydr. Polym. 2018, 200, 191–199. [Google Scholar] [CrossRef]
- Govaerts, R. World Checklist of Selected Plant Families Database in ACCESS: 1-216203; The Board of Trustees of the Royal Botanic Gardens: London, UK, 2003. [Google Scholar]
- Suddee, S.; Paton, A.J.; Parnell, J.A.N. Taxonomic Revision of the tribe Ocimeae Dumort. (Lamiaceae) in continental South East Asia III. Ociminae. Kew Bull. 2005, 60, 3–75. [Google Scholar]
- Bramley, G.L.C. Flora Malesiana; Noordhoff-Kolff N.V.: Djakarta, Indonesia, 2019; Volume 23, pp. 1–444. [Google Scholar]
- Sasmita, A.O.; Ling, A.P.K. Bioactivity of Mesona palustris (Black Cincau) as a Nutraceutical Agent. J. Eng. Sci. Res. 2017, 1, 47–53. [Google Scholar] [CrossRef]
- Widyaningsih, T.D.; Widjanarko, S.B.; Waziiroh, E.; Wijayanti, N.; Maslukhah, Y.L. Pilot plant scale extraction of black cincau (Mesona palustris BL) using historical data response surface methodology. Int. Food Res. J. 2018, 25, 712–719. [Google Scholar]
- Leelawat, B.; Permpoonchokkana, P.; Jirapornsirikun, T. Development of grass jelly processing using modified starches and higher efficient extraction method. Int. J. Agric. Technol. 2020, 16, 297–308. [Google Scholar]
- Fan, S.L.; Lin, J.A.; Chen, S.Y.; Lin, J.H.; Lin, H.T.; Chen, Y.Y.; Yen, G.C. Effects of Hsian-tsao (Mesona procumbens Hemsl.) extracts and its polysaccharides on the promotion of wound healing under diabetes-like conditions. Food Funct. 2021, 12, 119–132. [Google Scholar] [CrossRef]
- Tang, W.; Chen, X.; Liu, D.; Xie, J. Bioactive components of Mesona Blume and their potential health benefits. Food Rev. Int. 2020, 26, 70–85. [Google Scholar] [CrossRef]
- Zhao, Z.G.; Shi, Y.P.; Huang, N.Z.; Fu, C.M.; Tang, F.L.; Jiang, Q.Y. The research advances on Mesona chinensis Benth in China. J. South. Agric. 2011, 42, 657–660. [Google Scholar]
- Su, H.L.; Li, S.; Chen, J.Y. Research progress of Mesona chinensis Benth. Res. Pract. Chin. Med. 2008, 22, 79–81. [Google Scholar]
- Tang, D.; Quan, C.; Lin, Y.; Wei, K.; Qin, S.; Liang, Y.; Wei, F.; Miao, J. Physio-Morphological, Biochemical and Transcriptomic Analyses Provide Insights into Drought Stress Responses in Mesona chinensis Benth. Front. Plant. Sci. 2022, 13, 809723. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.U.; Lay, H.L.; Wu, M.C. Antioxidant activities and HepG2 cells growth inhibitory capacity of whole plant ethanol extracts (Eclipta alba Hassk and Mesona procumbens Hemsl). J. Food Biochem. 2018, 42, e12454. [Google Scholar] [CrossRef]
- Rahmah, R.; Astuti, Y.; Salimo, H.; Pamungkasari, E.P.; Wasita, B. Beneficial Effect of Mesona palustris BL: A Review on Human and Animal Intervention. J. Med. Sci. 2022, 10, 171–174. [Google Scholar] [CrossRef]
- Huang, C.Y.; Yen, G.C. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. Agric. Food Chem. 2002, 50, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.F.; Wu, S.H. The development of regulations of Chinese herbal medicines for both medicinal and food uses. Trends Food Sci. Technol. 2006, 17, 313–323. [Google Scholar] [CrossRef]
- Feng, T.; Biao, G.Z.; Jin, Z.Y.; Zhuang, H.N. Isolation and characterization of an acidic polysaccharide from Mesona Blumes gum. Carbohydr. Polym. 2008, 71, 159–169. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.P.; Xu, C.J.; Meng, J.; Ding, G.Q.; Zhang, X.M.; Weng, Y. Renal Protective Activity of Hsian-tsao Extracts in Diabetic Rats. Biomed. Environ. Sci. 2008, 21, 222–227. [Google Scholar] [CrossRef]
- Lin, L.H.; Shen, M.Y.; Liu, S.C.; Tang, W.; Wang, Z.J.; Xie, M.Y.; Xie, J.H. An acidic hetero polysaccharide from Mesona chinensis: Rheological properties, gelling behavior and texture characteristics. Int. J. Biol. Macromol. 2018, 107, 1591–1598. [Google Scholar] [CrossRef]
- Lim, J.; Adisakwattana, S.; Henry, C.J. Effects of grass jelly on glycemic control: Hydrocolloids may inhibit gut carbohydrase. Asia Pac. J. Clin. Nutr. 2018, 27, 336–340. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Xie, J. Effect of different Mesona chinensis polysaccharides on pasting, gelation, structural properties and in vitro digestibility of tapioca starch-Mesona chinensis polysaccharides gels. Food Hydrocoll. 2020, 99, 105327. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Li, Q.; Chen, Z.; Chen, J.; Geng, S. Evaluation of morphological and phytochemical characteristics of Mesona chinensis populations in southern China. J. Plant Prod. Sci. 2021, 24, 374–387. [Google Scholar] [CrossRef]
- Li, D.Y.; Lu, G.; Wang, D.D.; Wang, M. The influence of Xiancao hypolipidemic tea on the TC and TG metabolism of the experimental rabbits. Chin. Gen. Pract. 2010, 13, 9–10. [Google Scholar]
- Xiao, L.; Lu, X.; Yang, H.; Lin, C.; Li, L.; Ni, C.; Fang, Y.; Mo, S.; Zhan, R.; Yan, P. The Antioxidant and Hypolipidemic Effects of Mesona Chinensis Benth Extracts. Molecules 2022, 27, 3423. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.T.; Liaw, C.C.; Lin, Y.C.; Liao, G.Y.; Chao, C.H.; Chiou, C.T.; Kuo, Y.H.; Lee, K.T. New Diterpenoids from Mesona procumbens with Antiproliferative Activities Modulate Cell Cycle Arrest and Apoptosis in Human Leukemia Cancer Cells. Pharmaceuticals 2021, 14, 1108. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; TriDewanti, W.; Novita, W.; Mey, E.; Hanifa, H. Black Grass Jelly (Mesona Palustris Bl) Effervescent Powder has Anti-Dyslipidemia in High Cholesterol Diet-Fed Rats and Antioxidant Activity. Res. J. Life. Sci. 2017, 4, 159–167. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Z.; Zhang, R.; Zhan, P.; Wen, Y.; Shen, Y.; Zhang, X. Protection of myocardium in streptozotocin-induced diabetic rats by water extracts of Hsian-tsao (Mesona procumbens Hemsl.). Asia. Pac. J. Clin. Nutr. 2008, 17, 23–29. [Google Scholar] [PubMed]
- Yeh, C.T.; Huang, W.H.; Yen, G.C. Antihypertensive effects of Hsian-tsao and its active compound in spontaneously hypertensive rats. J. Nutr. Biochem. 2009, 20, 866–875. [Google Scholar] [CrossRef]
- Yen, G.C.; Hung, Y.L.; Hsieh, C.L. Protective effect of extracts of Mesona procumbens Hemsl. on DNA damage in human lymphocytes exposed to hydrogen peroxide and UV irradiation. Food Chem. Toxicol. 2000, 38, 747–754. [Google Scholar] [CrossRef]
- Huang, G.J.; Liao, J.C.; Chiu, C.S.; Huang, S.S.; Lin, T.H.; Deng, J.S. Anti-inflammatory activities of aqueous extract of Mesona procumbens in experimental mice. Sci. Food Agric. 2012, 92, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.; Duh, P.; Hung, Y. Contributions of Major Components to the Antimutagenic Effect of Hsian-tsao (Mesona procumbens Hemsl.). J. Agric. Food Chem. 2001, 49, 5000–5004. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Feng, C.L. In vitro antibacterial test of Hsian-tsao (Mesona chinensis Benth) against avian Escherichia coli. Guangdong. J. Anim. Vet. Sci. 2008, 33, 17–43. [Google Scholar]
- Feng, T.; Gu, Z.B.; Jin, Z.Y. Chemical Composition and Some Rheological Properties of Mesona Blumes Gum. Food Sci. Technol. Int. 2007, 13, 55–61. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.N.; Iliadis, K. Differences in chemical composition of field pea (Pisum sativum) cultivars: Effects of cultivation area and year. Food Chem. 2007, 103, 847–852. [Google Scholar] [CrossRef]
- Yuris, A.; Merino, L.M.; Hardacre, A.K.; Hindmarsh, J.; Goh, K.K.T. Molecular interactions in composite wheat starch-Mesona chinensis polysaccharide gels: Rheological, textural, microstructural and retrogradation properties. Food Hydrocoll. 2018, 79, 1–12. [Google Scholar] [CrossRef]
- Lai, L.S.; Tung, J.; Lin, P.S. Solution properties of hsian-tsao (Mesona procumbens Hemsl) leaf gum. Food Hydrocoll. 2000, 14, 287–294. [Google Scholar] [CrossRef]
- Lai, L.S.; Chou, S.T.; Chao, W.W. Studies on the Antioxidative Activities of Hsian-tsao (Mesona procumbens Hemsl) Leaf Gum. Agric. Food Chem. 2001, 49, 963–968. [Google Scholar] [CrossRef]
- Lai, L.S.; Liu, Y.L.; Lin, P.H. Rheological/textural properties of starch and crude hsian-tsao leaf gum mixed systems. J. Sci. Food Agric. 2003, 83, 1051–1058. [Google Scholar] [CrossRef]
- Delattre, C.; Fenoradosoa, T.A.; Michaud, P. Galactans: An overview of their most important sourcing and applications as natural polysaccharides. Braz. Arch. Biol. Technol. 2011, 54, 1075–1092. [Google Scholar] [CrossRef]
- Elboutachfaiti, R.; Delattre, C.; Petit, E.; Michaud, P. Polyglucuronic acids: Structures, functions and degrading enzymes. Carbohydr. Polym. 2011, 84, 1–13. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, P.; Sanghi, R. Use of microwave irradiation in the grafting modification of the polysaccharides—A review. Prog. Polym. Sci. 2012, 37, 340–364. [Google Scholar] [CrossRef]
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Gan, L.; Wang, J.; Guo, Y. Polysaccharides influence human health via microbiota-dependent and independent pathways. Front. Nutr. 2022, 9, 1030065. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiao, Y.; Shen, M.; Zhang, X.; Wang, W.; Xie, J. Effect of sodium carbonate on the gelation, rheology, texture and structural properties of maize starch-Mesona chinensis polysaccharide gel. Food Hydrocoll. 2019, 87, 943–951. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Wu, T.; Luo, Y.; Li, M.; Wen, K.; Xie, J. Role of salt ions and molecular weights on the formation of Mesona chinensis polysaccharide-chitosan polyelectrolyte complex hydrogel. Food Chem. 2020, 333, 127493. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, L.; Ren, Y.; Shen, M.; Xie, J. Characterizations and hepatoprotective effect of polysaccharides from Mesona blumes against tetrachloride-induced acute liver injury in mice. Int. J. Biol. Macromol. 2019, 124, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xie, J.; Liu, S.; Shen, M.; Tang, W.; Xie, M. Polysaccharide from Mesona chinensis: Extraction optimization, physicochemical characterizations and antioxidant activities. Int. J. Biol. Macromol. 2017, 99, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, W.; Shen, M.; Yu, Q.; Chen, Y.; Yang, J.; Xie, J. Changes in polysaccharides structure and bioactivity during Mesona chinensis Benth storage. Curr. Res. Food Sci. 2022, 5, 392–400. [Google Scholar] [CrossRef]
- Tang, W.; Shen, M.; Xie, J.; Liu, D.; Du, M.; Lin, L.; Gao, H.; Hamake, B.R.; Xie, M.Y. Physicochemical Characterization, Antioxidant Activity of Polysaccharides from Mesona Chinensis Benth and Their Protective Effect on Injured NCTC-1469 Cells Induced by H2O2. Carbohydr. Polym. 2017, 175, 538–546. [Google Scholar] [CrossRef]
- Yan, L.; Xiong, C.; Xu, P.; Zhu, J.; Yang, Z.; Ren, H.; Luo, Q. Structural characterization and in vitro antitumor activity of A polysaccharide from Artemisia annua L. (Huang Huahao). Carbohydr. Polym. 2019, 213, 361–369. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Shen, M.; Jiang, L.; Ren, Y.; Luo, Y.; Wen, H.; Xie, J. Physicochemical, rheological and thermal properties of Mesona chinensis polysaccharides obtained by sodium carbonate assisted and cellulase assisted extraction. Int. J. Biol. Macromol. 2018, 126, 30–36. [Google Scholar] [CrossRef]
- Huang, L.; Huang, M.; Shen, M.; Wen, P.; Wu, T.; Hong, Y.; Yu, Q.; Chen, Y.; Xie, J. Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress. Int. J. Biol. Macromol. 2019, 136, 1000–1006. [Google Scholar] [CrossRef]
- Huang, J.; Ding, L.; Tian, W.; Zhi, H.; Chen, J.; Wu, L.; Wang, L.; Xie, J.; Bai, J.; Fan, H.; et al. Polyphaenolic profiling, antioxidant properties, and inhibition of α-glucosidase of Mesona chinensis benth from Southern China. J. Microchem. 2021, 168, 106399. [Google Scholar] [CrossRef]
- Yen, G.C.; Hung, C.Y.; Chen, Y.J. Antioxidant Properties of Hsian-tsao (Mesona procumbens Hemsl.). Orient. Food Herb. 2003, 859, 202–214. [Google Scholar] [CrossRef]
- ElSamahy, S.K.; Abd El-Hady, E.A.; Habiba, R.A.; Moussa-Ayoub, T.E. Some Functional, Chemical, and Sensory Characteristics of Cactus Pear Rice Based Extrudates. J. Prof. Assoc. Cactus. 2007, 9, 136–147. [Google Scholar]
- Ren, Y.; Jiang, L.; Wang, W.; Xiao, Y.; Liu, S.; Luo, Y.; Shen, M.; Xie, J. Effects of Mesona chinensis Benth polysaccharide on physicochemical and rheological properties of sweet potato starch and its interactions. Food Hydrocoll. 2020, 99, 105371. [Google Scholar] [CrossRef]
- Zhuang, H.; Feng, T.; Xie, Z.; Toure, A.; Xu, X.; Jin, Z.; Su, Q. Effect of Mesona Blumes gum on physicochemical and sensory characteristics of rice extrudates. Int. J. Food Sci. Technol. 2010, 45, 2415–2424. [Google Scholar] [CrossRef]
- Wongverawattanakul, C.; Suklaew, P.; Chusak, C.; Adisakwattana, S.; Thilavech, T. Encapsulation of Mesona chinensis Benth Extract in Alginate Beads Enhances the Stability and Antioxidant Activity of Polyphenols under Simulated Gastrointestinal Digestion. Foods 2022, 11, 2378. [Google Scholar] [CrossRef]
- Iguchi, C.; Nio, Y.; Takeda, H.; Yamasawa, K.; Hirahara, N.; Toga, T.; Tamura, K. Plant polysaccharide PSK: Cytostatic effects on growth and invasion; modulating effect on the expression of HLA and adhesion molecules on human gastric and colonic tumor cell surface. Anticancer Res. 2000, 21, 1007–1013. [Google Scholar]
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Simpson, R.; Morris, G.A. The anti-diabetic potential of polysaccharides extracted from members of the cucurbit family: A review. Bioact. Carbohydr. Diet. Fibre 2014, 3, 106–114. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Chen, Z.; Gao, X.; Yuan, G.; Pan, Y.; Chen, H. Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Res. Int. 2018, 103, 280–288. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biological function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Lee, J.S.; Synytsya, A.; Kim, H.B.; Choi, D.; Lee, S.; Lee, J.; Kim, W.J.; Jang, S.; Park, Y. Purification, characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Int. Immunopharmacol. 2013, 17, 858–866. [Google Scholar] [CrossRef]
- Li, C.; Dong, Z.; Zhang, B.; Huang, Q.; Liu, G.; Fu, X. Structural characterization and immune enhancement activity of a novel polysaccharide from Moringa oleifera leaves. Carbohydr. Polym. 2020, 234, 115897. [Google Scholar] [CrossRef]
- Xu, H.S.; Wu, Y.W.; Xu, S.F.; Sun, H.X.; Chen, F.Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Zhao, Q.; Li, C.; Xie, M.Y. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydr. Polym. 2014, 101, 479–483. [Google Scholar] [CrossRef]
- Aruoma, I.O. Free radicals, oxidative stress and antioxidants in human health and disease. J. Am. Oil. Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.A.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Bon, R.S.; Waldmann, H. Bioactivity-guided navigation of chemical space. Acc. Chem. Res. 2010, 43, 1103–1114. [Google Scholar] [CrossRef]
- Widyaningsih, T.D. Cytotoxic Effect of Water, Ethanol and Ethyl Acetate Extract of Black Cincau (Mesona Palustris BL) against HeLa Cell Culture. APCBEE Procedia 2012, 2, 110–114. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.H.; Chang, Y.X.; Chen, C.C.; Chen, H.Y.; Hung, Y.C.; Chi, T.Y.; Lin, C.Y.; Chen, G.H.; Huang, P.M.; Wang, Y.P.; et al. Effects of Mesona chinensis ethanolic extracts and commercial herbal tea on the cell viability of colorectal cancer cells. GSC. Biol. Pharm. Sci. 2022, 18, 326–330. [Google Scholar] [CrossRef]
- Chandra, K.S.; Bansal, M.; Nair, T.; Iyengar, S.S.; Gupta, R.; Manchanda, S.C.; Mohanan, P.P.; Rao, V.D.; Manjunath, C.N.; Sawhney, J.P.S.; et al. Consensus statement on management of dyslipidemia in Indian subjects. Indian Heart J. 2014, 66, S1–S51. [Google Scholar] [CrossRef]
- Rauf, A.; Akram, M.; Anwar, H.; Daniyal, M.; Munir, N.; Bawazeer, S.; Bawazeer, S.; Rebezov, M.; Bouyahya, A.; Ali Shariati, M.; et al. Therapeutic potential of herbal medicine for the management of hyperlipidemia: Latest updates. Environ. Sci. Pollut. Res. Int. 2022, 29, 40281–40301. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Ivanova, E.A. Conventional, traditional and alternative therapies for cardiovascular disorders. Part 2: Traditional therapy. Curr. Pharm. Des. 2017, 23, 967–968. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Thu, N.T.; Hanh, N.T.H. Hypolipidemic effect of ethanol extract from Mesona chinensis Benth. in high fat diet-induced obesity mice. VNU J. Sci. Med. Pharm. Sci. 2019, 35, 37–43. [Google Scholar] [CrossRef]
- Lin, Y.; Sun, Z. Current views on type 2 diabetes. A review. J. Endocrinol. 2010, 204, 1–11. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2013, 36 (Suppl. 1), S67–S74. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Reinehr, T. Type 2 diabetes mellitus in children and adolescents. World J. Diabetes 2013, 4, 270. [Google Scholar] [CrossRef]
- Chusak, C.; Thilavech, T.; Adisakwattana, S. Consumption of Mesona chinensis Attenuates Postprandial Glucose and Improves Antioxidant Status Induced by a High Carbohydrate Meal in Overweight Subjects. Am. J. Chin. Med. 2014, 42, 315–336. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou1, Y.S.; Tsai, M.L.; Ho, C.T. Anti-inflammatory activity of traditional Chinese medicinal herbs. Tradit. Complement. Med. 2011, 1, 8–24. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmate) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Zeng, B.Y.; Su, M.H.; Chen, Q.X.; Chang, Q.; Wang, W.; Li, H.H. Protective effect of a polysaccharide from Anoectochilus roxburghii against carbon tetrachloride induced acute liver injury in mice. J. Ethnopharmacol. 2017, 200, 124–135. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, M.; Huang, L.; Wu, T.; Xie, J. Mesona chinensis Benth. Polysaccharides alleviates liver injury by beneficial regulation of gut microbiota in cyclophosphamide-induced mice. Food Sci. Hum. Wellness 2022, 11, 74–84. [Google Scholar] [CrossRef]
- Lu, H.; Shen, M.; Chen, T.; Yu, Y.; Chen, Y.; Yu, Q.; Chen, X.; Xie, J. Mesona chinensis Benth Polysaccharides Alleviate DSS-Induced Ulcerative Colitis via Inhibiting of TLR4/MAPK/NF-κB Signaling Pathways and Modulating Intestinal Microbiota. Mol. Nutr. Food Res. 2022, 66, e2200047. [Google Scholar] [CrossRef]
- Chen, G.; Xie, M.; Wan, P.; Chen, D.; Ye, H.; Chen, L.; Zeng, X.; Liu, Z. Digestion under saliva, simulated gastric and small intestinal conditions and fermentation in vitro by human intestinal microbiota of polysaccharides from Fuzhuan brick tea. Food Chem. 2018, 244, 331–339. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.D. Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 2015, 9, 1441–1452. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Backhed, F. Gut microbial metabolites as multikingdom intermediates. Nat. Rev. Microbiol. 2020, 19, 77–94. [Google Scholar] [CrossRef]
- Song, Q.; Wang, Y.; Huang, L.; Shen, M.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2021, 140, 109858. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, M.; Yu, Q.; Chen, Y.; Xie, J. UPLC-Q-TOF/MS-based metabolomics reveals modulatory effects of Mesona chinensis Benth polysaccharide in liver injury mice induced by cyclophosphamide. Food Sci. Hum. Wellness 2023, 12, 584–595. [Google Scholar] [CrossRef]
- Yang, J.; Lin, J.; Zhang, J.; Chen, X.; Wang, Y.; Shen, M.; Xie, J. Fabrication of Zein/Mesona chinensis Polysaccharide Nanoparticles: Physical Characteristics and Delivery of Quercetin. ACS Appl. Bio Mater. 2022, 5, 1817–1828. [Google Scholar] [CrossRef]
- Yang, J.; Lin, J.; Chen, X.; Rong, L.; Shen, M.; Wang, Y. Mesona chinensis polysaccharide/zein nanoparticles to improve the bioaccesibility and in vitro bioactivities of curcumin. Carbohydr. Polym. 2022, 295, 119875. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, X.; Wen, H.; Chen, Y.; Yu, Q.; Shen, M.; Xie, J. Curcumin-Loaded pH-Sensitive Biopolymer Hydrogels: Fabrication, Characterization, and Release Properties. ASC Food Sci. Technol. 2022, 2, 512–520. [Google Scholar] [CrossRef]

| Source | Extraction | Proximate Composition (%) | Refs. | |||
|---|---|---|---|---|---|---|
| Crude Protein | Crude Fat | Crude Fiber | Ash | |||
| Mesona chinensis leaf powder (China) | With sodium bicarbonate in heated water at 95 °C for 2 h | 9.74 | - | 2.98 | 30.9 | [36] |
| Mesona chinensis powder (China) | Water extract | 9.0 | 0.1 | - | 28.2 | [38] |
| Mesona chinensis leaves (farm market in Taiwan) | With sodium bicarbonate in heated water at 95 °C for 4 h | 4.56 | - | 1.07 | 26.97 | [39] |
| Mesona chinensis leaves (contracted farmer, Taiwan) | With sodium bicarbonate in heated water at 95 °C for 4 h | 10.04 | 0.52 | 1.47 | 26.2 | [40] |
| Mesona chinensis leaves (contracted farmer in Miao-Li, Taiwan) | With sodium bicarbonate in heated water at 95 °C for 4 h | 4.60 | 0.90 | 1.10 | 27.0 | [41] |
| Collected Region | Extraction | Yield (%) | Molecular Weight (kDa) | Chemical Composition (%) | Monosaccharide Composition (Mole Ratios) | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Sugar | Uronic Acid | Protein | Glu | Gal | Gala | Rha | Ara | Man | Xyl | |||||
| Xiaoshicheng, Ganzhou, Jiangxi, China | Boil in hot water 95 °C for 2 h with Na2CO3 | - | 158 | 29.03 | 17.06 | 22.64 | N.D. | 2.80 | 2.40 | N.D. | N.D. | N.D. | 5.50 | [4] |
| China | Boil in hot water 95 °C for 2 h with Na2HCO3 | 29.36 | 16.26 | 42.20 | 13.80 | 9.74 | 2.30 | 3.10 | 1.40 | 1.20 | 2.30 | 0.20 | 1.00 | [36] |
| Xiaoshicheng, Jiangxi, China | Boil in hot water 95 °C for 3 h with Na2CO3 | - | 141.6 | 16.88 | 36.91 | - | 1.36 | 3.76 | 17.5 | 0.87 | 0.14 | - | 2.00 | [48] |
| Ganzhou, Jiangxi, China. | Boil in hot water 95 °C for 2.5 h | - | 375 | 81.12 | - | 14.07 | 4.90 | 2.16 | 6.75 | 1.38 | 1.64 | - | 0.42 | [49] |
| Xiaoshicheng, Jiangxi, China | Boil in hot water 90 °C for 2 h with Na2CO3 | 7.05 | 1450 | - | 29.30 | 10.40 | 1.38 | 1.0 | - | - | - | - | - | [50] |
| Yichun, Jiangxi, China | Boil in hot water 100 °C | 0.84 | 44.39 | 30.69 | 20.86 | 25.30 | 1.12 | 1.97 | 1.69 | 0.42 | 0.30 | 0.50 | N.D. | [51] |
| Xiaoshicheng, Ganzhou, Jiangxi, China | Boil in hot water 95 °C for 2.5 h with Na2CO3 | 11.14 | 195 | 34.40 | 24.30 | 17.30 | 1.00 | 1.34 | 0.25 | - | N.D. | - | N.D. | [52] |
| Xiaoshicheng, Ganzhou, Jiangxi, China | Boil in hot water 95 °C for 2.5 h with Na2CO3 | - | 204 | 32.28 | 29.52 | 31.35 | 6.24 | 0.82 | - | 0.11 | 0.32 | - | 0.34 | [54] |
| Ganzhou, Jiangxi, China. | Boil in hot water 100 °C for 2 h | 1.68 | 157 | 39.01 | 29.30 | 27.52 | 1.49 | 0.68 | 6.33 | - | - | - | 2.54 | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seah, R.; Siripongvutikorn, S.; Wichienchot, S.; Usawakesmanee, W. Functionality and Health-Promoting Properties of Polysaccharide and Plant-Derived Substances from Mesona chinensis. Foods 2024, 13, 1134. https://doi.org/10.3390/foods13071134
Seah R, Siripongvutikorn S, Wichienchot S, Usawakesmanee W. Functionality and Health-Promoting Properties of Polysaccharide and Plant-Derived Substances from Mesona chinensis. Foods. 2024; 13(7):1134. https://doi.org/10.3390/foods13071134
Chicago/Turabian StyleSeah, Romson, Sunisa Siripongvutikorn, Santad Wichienchot, and Worapong Usawakesmanee. 2024. "Functionality and Health-Promoting Properties of Polysaccharide and Plant-Derived Substances from Mesona chinensis" Foods 13, no. 7: 1134. https://doi.org/10.3390/foods13071134
APA StyleSeah, R., Siripongvutikorn, S., Wichienchot, S., & Usawakesmanee, W. (2024). Functionality and Health-Promoting Properties of Polysaccharide and Plant-Derived Substances from Mesona chinensis. Foods, 13(7), 1134. https://doi.org/10.3390/foods13071134






