Golden Barrel Cactus: Unveiling Its Potential as a Functional Food and Nutraceutical Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Acetonitrile Extract of GBC
2.3. Phytochemical Profile of GBC Crude Extract
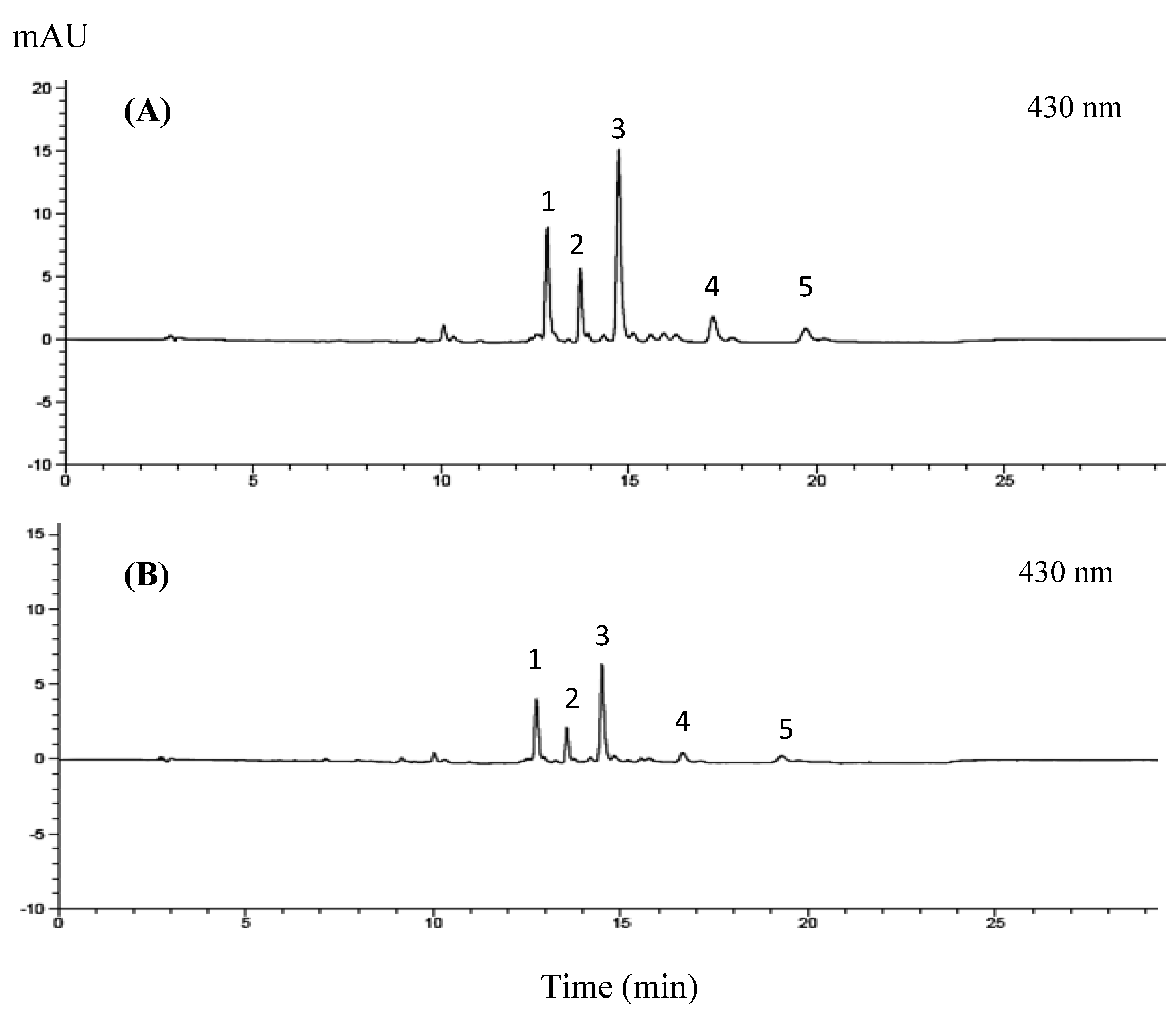
2.3.1. Chlorophyll and Lutein Analysis
2.3.2. Total Phenolic Compounds Quantification
2.4. Fiber Antioxidant Activities
2.4.1. DPPH Free Radical Scavenging Activity
2.4.2. Scavenging Activity of ABTS Radical Cation
2.4.3. Ferric Reducing Antioxidant Power Assay
2.5. Bioaccessibility and Bioavailability
2.5.1. In Vitro Digestion
Gastric Phase
Small Intestinal Phase
2.5.2. Bioaccessibility
2.5.3. Bioavailability
Cell Culture
Cellular Uptake of Aqueous Fraction
Transport of Chlorophyll Derivatives, Lutein, and Total Phenolic by Caco-2 Cells
Digesta, Aqueous Fraction, Media, and Cell Pellet Extraction
2.6. Protein Determination
2.7. Statistical Analysis
3. Results
3.1. Chemical Approximate Analysis
3.2. Phytochemical Profile of GBC Extract
3.2.1. Chlorophyll and Lutein Profile of GBC Extract
3.2.2. Total Phenolic Contents
3.3. Antioxidant Activities
3.3.1. DPPH Free Radical Scavenging Activity
3.3.2. Scavenging Activity of ABTS Radical Cation
3.3.3. Ferric Reducing Antioxidant Power Assay (FRAP)
3.4. Bioaccessibility and Bioavailability
3.4.1. Cytotoxicity
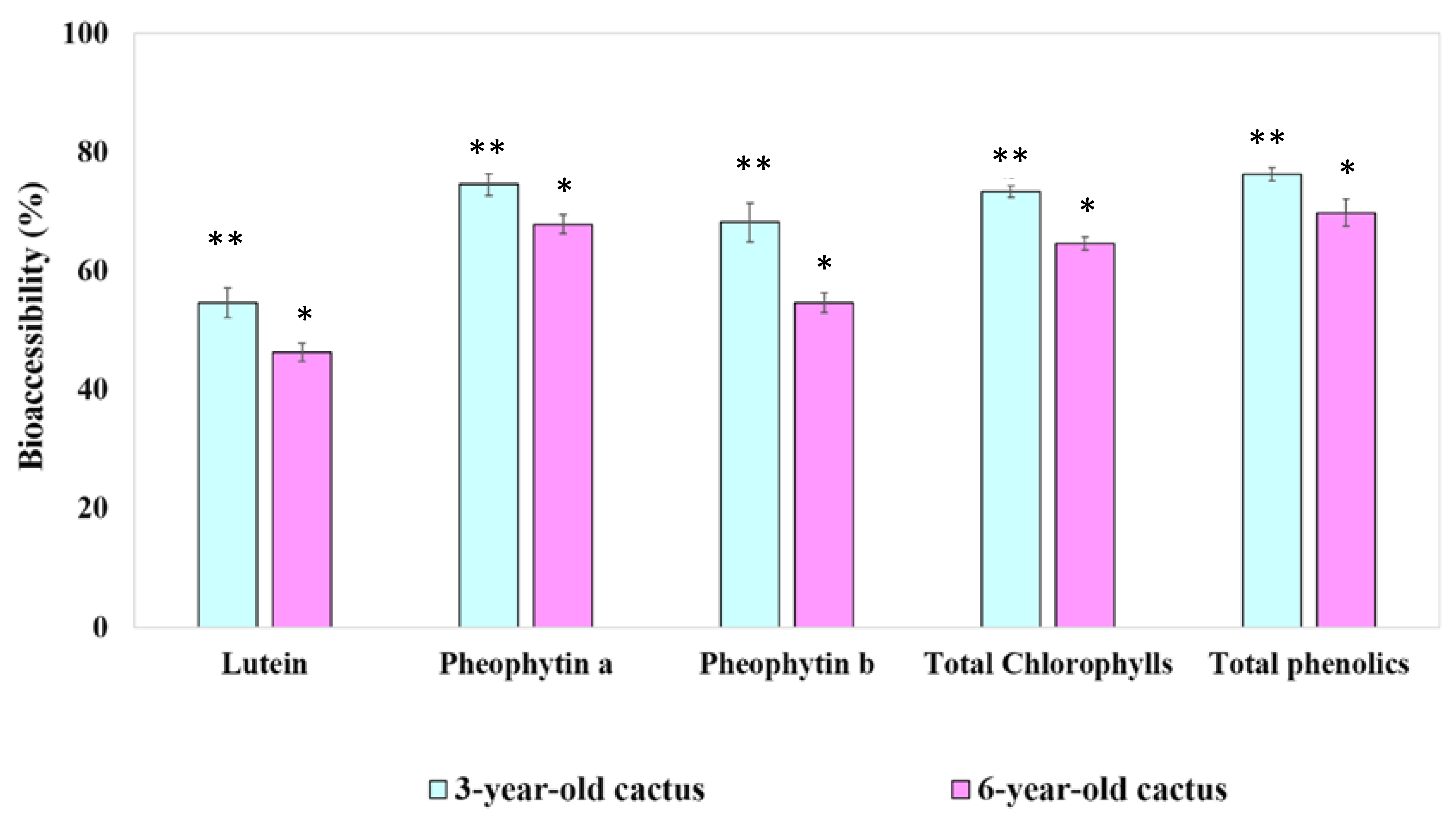
3.4.2. Digestive Stability and Percentage of Aqueous Micellar Fraction of Phytochemical Compounds
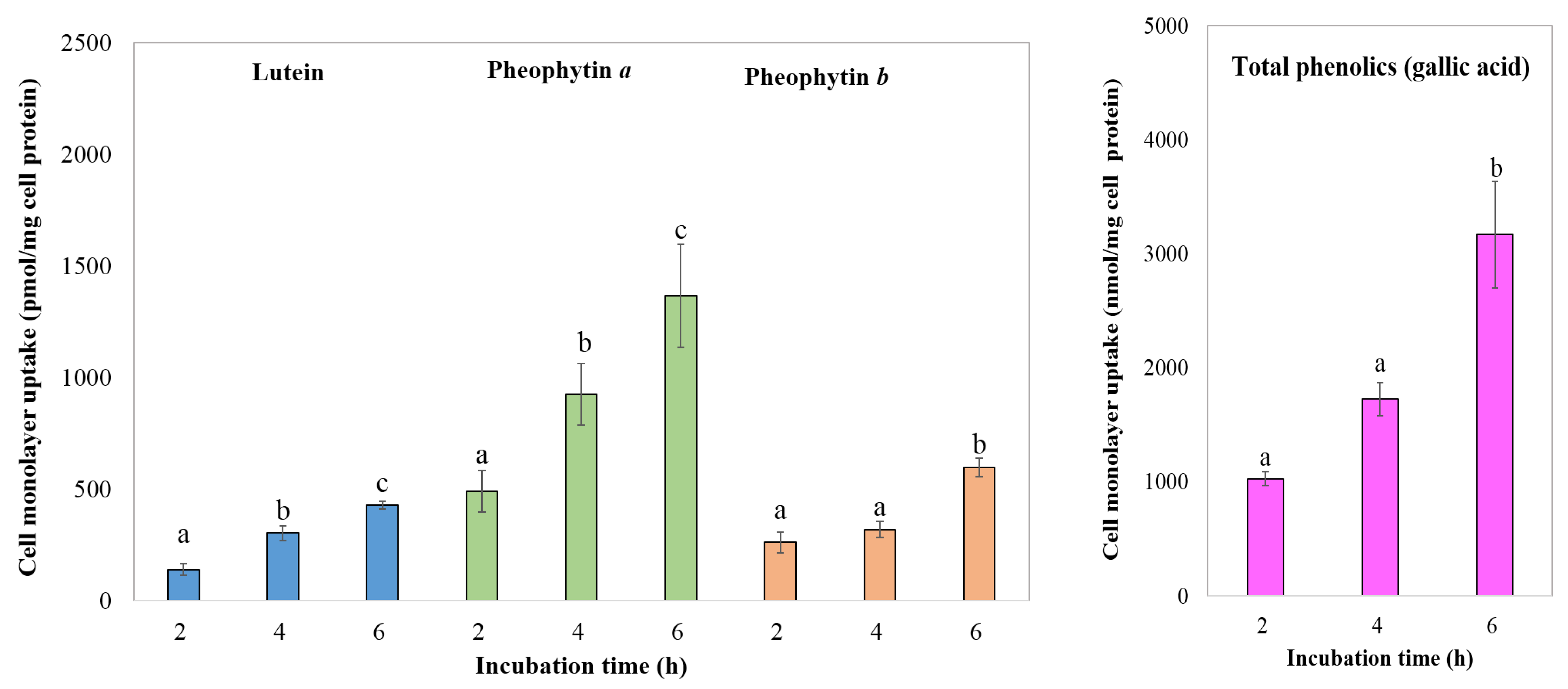
3.4.3. Bioavailability of Phytochemical Compounds
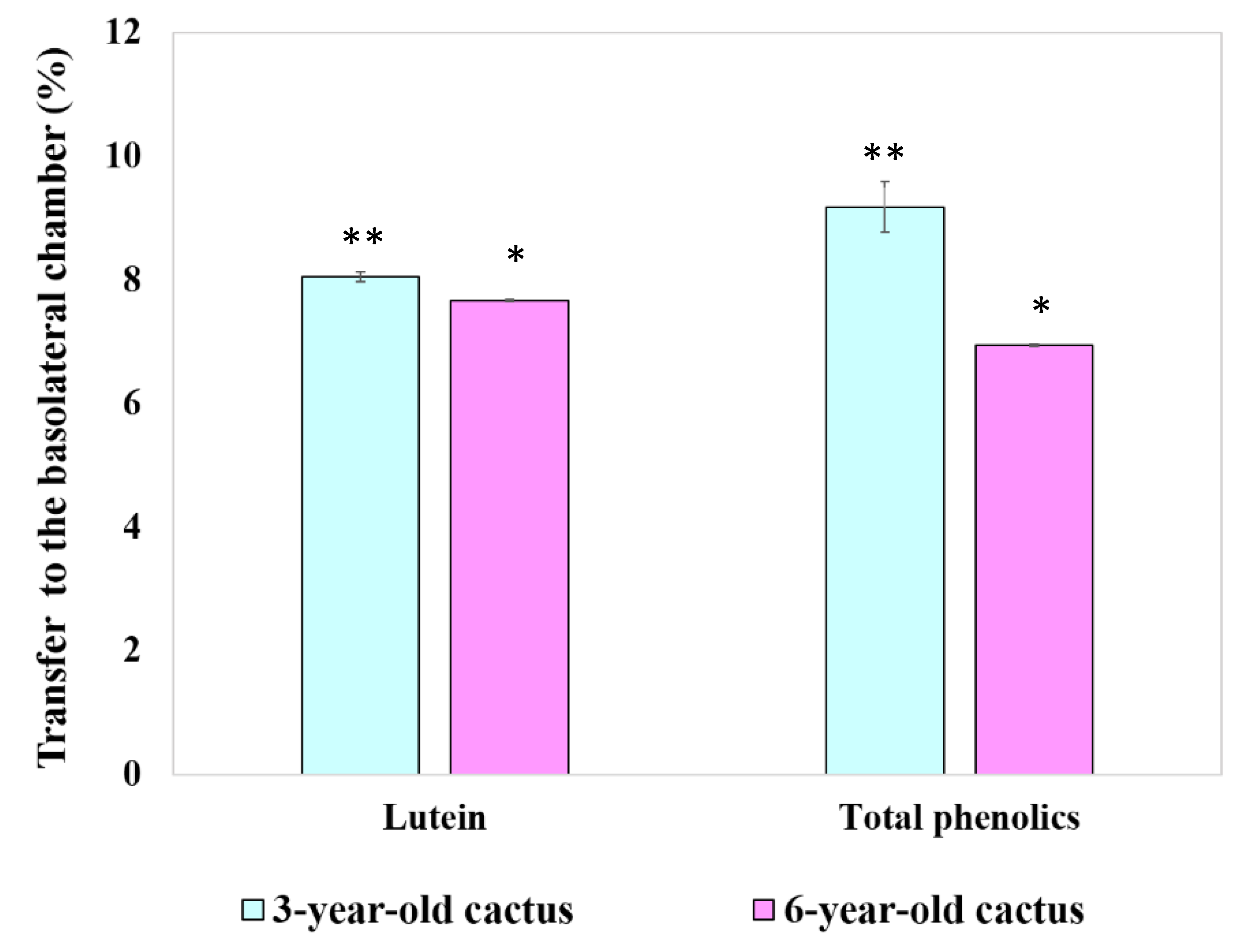
3.4.4. Transport of Chlorophyll Derivatives, Lutein, and Total Phenolics by Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuniga-Vega, M.F.; Vazquez-Flores, B.; Lopez-Cervantes, M.G.; Perez-Torres, A.; Guzman-Cruz, L.A. Chemical composition and biological activities of golden barrel cactus (Echinocactus grusonii): A review. Molecules 2023, 28, 84. [Google Scholar]
- Gonzalez-Flores, M.L.; Rodriguez-Garcia, R.; Reyes-Moreno, C.; Vargas-Torres, A.; Guzman-Cruz, L.A. Evaluation of the antioxidant activity and phenolic compounds of extracts from three species of Cactaceae using different extraction methods. Ind. Crops Prod. 2015, 77, 849–855. [Google Scholar]
- Perez-Garcia, Y.Y.; Hernandez-Muñoz, P.; Garcia-Mellado, G.; Moreno-Rojas, J.M. Antioxidant activity and bioactive compounds of eight prickly pear cactus (Opuntia spp.) cultivars. J. Sci. Food Agric. 2020, 100, 5206–5215. [Google Scholar]
- Avila-Flores, Y.M.; Rodriguez-Ramirez, M.I.; Rodriguez-Garcia, R.; Diaz-Sanchez, R.; Zazueta-Sandoval, Y.G. In vitro digestibility and Caco-2 cell uptake of bioactive compounds from golden barrel cactus (Echinocactus grusonii) extracts. Plant Foods Hum. Nutr. 2022, 77, 129–135. [Google Scholar]
- De Leo, M.; Durante, M.; Raimondi, S.; Lombardi, V.; Borrelli, R.; De Feo, V. Antioxidant properties and phytochemical profile of Opuntia ficus-indica fruit extracts. J. Agric. Food Chem. 2010, 58, 6425–6430. [Google Scholar]
- Galati, E.M.; Miceli, N.; Miceli, A.; Rozza, L.; Chisari, M.V. Opuntia ficus-indica cladodes can protect gastric mucosa from ethanol-induced lesions in rats. J. Pharm. Pharmacol. 2003, 55, 953–957. [Google Scholar]
- Hernández-Urbiola, A.; Castañeda-Sauza, C.; Torres-Tinoco, R.; Rodríguez-Ramírez, M.I.; Martínez-Flores, I.E. Nutritional composition and antioxidant capacity of nopal (Opuntia ficus-indica, cv. Redonda) at different maturity stages. J. Sci. Food Agric. 2012, 92, 1610–1616. [Google Scholar]
- Chen, H.; Wang, M.; Zhao, M.; Wang, W.; He, X. Bioaccessibility and antioxidant activity of phenolic compounds in prickly pear leaves extracted by pressurized solvent extraction. Food Nutr. Res. 2019, 63, 1069. [Google Scholar]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomas-Barberan, M.A. In vitro bioaccessibility and intestinal uptake of beta-carotene from carrot assessed by an in vitro digestion model. J. Agric. Food Chem. 2002, 50, 5205–5210. [Google Scholar]
- Mineta, T.; Okubo, S.; Arai, A.; Okigami, A. Improvement of chlorophyll uptake from spinach by β-cyclodextrin and its evaluation by Caco-2 cell model. Int. J. Mol. Sci. 2020, 21, 8905. [Google Scholar]
- Sáez-Plaza, P.; Perez-Esteban, R.; Garcia-Alonso, S.; Moreno-Rojas, J.M. Bioaccessibility and intestinal uptake of phenolic compounds from four prickly pear cactus cultivars using an in vitro digestion model. J. Agric. Food Chem. 2011, 59, 13106–13112. [Google Scholar]
- Vermaak, I.; Hamman, J.; Viljoen, A. High performance thin layer chromatography as a method to authenticate Hoodia gordonii raw material and products. S. Afr. J. Bot. 2010, 76, 119–124. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Cheng, C.; Bomser, J.; Ferruzzi, M.G.; Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 2007, 114, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Oonsivilai, R.; Ferruzzi, M.; Ningsanond, S. Antioxidant activity and cytotoxicity of Rang Chuet (Thunbergia laurifolia Lindl.) extracts. Asian J. Food Agro-Ind. 2008, 1, 116–128. Available online: https://www.researchgate.net/publication/237386904_Antioxidant_activity_and_Cytotoxicity_of_Rang_Chuet_Thunbergia_laurifolia_Lindl_Extracts (accessed on 5 January 2024).
- Ksouri, R.; Falleh, H.; Megdiche, W.; Trabelsi, N.; Mhamdi, B.; Chaieb, K.; Bakrouf, A.; Magné, C.; Abdelly, C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem. Toxicol. 2009, 47, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-C.; Li, H.-B.; Cheng, K.-W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Garrett, D.A.; Failla, M.L.; Sarama, R.J. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. J. Agric. Food Chem. 1999, 47, 4301–4309. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Failla, M.L.; Schwartz, S.J. Assessment of degradation and intestinal cell uptake of carotenoids and chlorophyll derivatives from spinach puree using an in vitro digestion and Caco-2 human cell model. J. Agric. Food Chem. 2001, 49, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Ellwood, K.C.; Chatzidakis, C.; Failla, M.L. Fructose utilization by the human intestinal epithelial cell line, Caco-2. Exp. Biol. Med. 1993, 202, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chitchumroonchokchai, C.; Schwartz, S.J.; Failla, M.L. Assessment of lutein bioavailability from meals and a supplement using simulated digestion and Caco-2 human intestinal cells. J. Nutr. 2004, 134, 2280–2289. [Google Scholar] [CrossRef] [PubMed]
- Mehran, M.; Levy, E.; Bendayan, M.; Seidman, E. Lipid, apolipoprotein, and lipoprotein synthesis and secretion during cellular differentiation in Caco-2 cells. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 118–128. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Ryan, L.; O’Brien, N. Comparison of the uptake and secretion of carotene and xanthophyll carotenoids by Caco-2 intestinal cells. Br. J. Nutr. 2007, 98, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Duangrat, C.; Anuchpreeda, S.; Tachakittirungrod, S.; Chowwanapoonpohn, S. Comparison of antioxidant capacities and cytotoxicities of certain fruit peels. Food Chem. 2007, 103, 839–846. [Google Scholar] [CrossRef]
- Liu, C.-S.; Glahn, R.P.; Liu, R.H. Assessment of carotenoid bioavailability of whole foods using a Caco-2 cell culture model coupled with an in vitro digestion. J. Agric. Food Chem. 2004, 52, 4330–4337. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Hussain, M.M.; Morel, D.W.; Harrison, E.H. Carotenoid uptake and secretion by CaCo-2 cells β-carotene isomer selectivity and carotenoid interactions. J. Lipid Res. 2002, 43, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Urbiola, M.I.; Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Quevedo, G.; Rojas-Molina, J.; Cortes, M.; Rodríguez-García, M. Study of nutritional composition of nopal (Opuntia ficus indica cv. Redonda) at different maturity stages. Nutr. J. 2010, 4, 11–16. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Stintzing, F.C.; Carle, R. Cactus stems (Opuntia spp.): A review on their chemistry, technology, and uses. Mol. Nutr. Food Res. 2005, 49, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Holasova, K.; Remsey, S.; Turova, B.; Kokoska, L.; Busta, M. Content of photosynthetic pigments and carotenoids in green and etiolated tissues of cactus Pereskia aculeata Miller. J. Sci. Food Agric. 2009, 89, 2444–2448. [Google Scholar] [CrossRef]
- Shetty, S.; Prakash, J.; Shantaram, S. Age-dependent variations in carotenoid content and antioxidant activity of cactus pear (Opuntia ficus indica) fruit. Food Chem. Toxicol. 2012, 50, 4314–4319. [Google Scholar]
- Preetham, T.; Deviprasad, J.; Raju, V.S.; Krishnamoorthy, R. Effect of age on antioxidant and antimicrobial activity of Opuntia ficus indica Mill. fruit extracts. J. Food Sci. Technol. 2018, 55, 227–234. [Google Scholar]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.; De León-Rodríguez, A.; Fomsgaard, I.S.; de la Rosa, A.P.B. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Walsh, R.B.; Zhang, Y.; Vodovotz, Y.; Schwartz, S.J.; Failla, M.L. Bioavailability of apigenin from single and multiple doses of green tea in humans. Am. J. Clin. Nutr. 2003, 78, 826–830. [Google Scholar]
- Scheer, H. The interfacial factor in the heat-induced conversion of chlorophyll to pheophytin in green leaves. J. Sci. Agric. 1991, 56, 137–142. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Perez-Sacalu, L.; Garcia-Alonso, F.J.; Granado, F.; Gil-Izquierdo, A. In vitro bioaccessibility of carotenoids from raw and cooked spinach cultivars using different digestion models. J. Food Compos. Anal. 2020, 91, 103600. [Google Scholar]
- Yi, J.; Sun, M.; Li, B. In vitro digestion and bioaccessibility of carotenoids from orange vegetables. J. Food Compos. Anal. 2011, 24, 611–618. [Google Scholar] [CrossRef]



| Composition | Content (% Dry Weight) | |
|---|---|---|
| 3-Year-Old Cactus Powder | 6-Year-Old Cactus Powder | |
| Moisture | 0.86 ± 0.06 | 0.53 ± 0.25 |
| Ash | 22.83 ± 0.03 * | 25.33 ± 0.02 ** |
| Crude protein | 14.48 ± 0.12 | 14.82 ± 1.33 |
| Crude fat | 1.39 ± 0.36 | 1.02 ± 0.09 |
| Crude fiber | 11.18 ± 0.20 * | 15.99 ± 0.80 ** |
| Available carbohydrates | 49.26 ± 0.54 * | 43.64 ± 1.11 ** |
| Phytochemical Content | GBC Extract | |
|---|---|---|
| 3-Year-Old | 6-Year-Old | |
| Lutein (µg/g RM) | 36.14 ± 0.39 ** | 30.44 ± 0.45 * |
| Chlorophyll a (µg/g RM) | 179.41 ± 2.89 ** | 115.15 ± 5.83 * |
| Chlorophyll b (µg/g RM) | 97.26 ± 0.31 ** | 91.28 ± 0.80 * |
| Pheophytin a (µg/g RM) | 243.46 ± 10.59 ** | 154.08 ± 5.23 * |
| Pheophytin b (µg/g RM) | 6.16 ± 1.45 * | 5.87 ± 1.48 * |
| Total Chlorophylls (µg/g RM) | 526.29 ± 10.45 ** | 366.37 ± 1.22 * |
| Samples | IC50 of DPPH Radical Scavenging Activity (mg RM/mL) |
|---|---|
| 3-year-old cactus | 112.60 ± 1.42 b |
| 6-year-old cactus | 191.90 ± 0.52 c |
| BHT | 0.35 ± 0.04 a |
| Ascorbic acid | 0.08 ± 0.01 a |
| Samples | IC50 of ABTS+ Radical Scavenging Activity (mg RM/mL) |
|---|---|
| 3-year-old cactus | 44.62 ± 2.43 b |
| 6-year-old cactus | 81.84 ± 0.42 c |
| BHT | 0.09 ± 0.003 a |
| Ascorbic acid | 0.04 ± 0.002 a |
| Samples | FRAP Values (mmol Fe2+/g RM) |
|---|---|
| 3-year-old cactus | 0.014 ± 0.001 a |
| 6-year-old cactus | 0.010 ± 0.002 a |
| BHT | 2.50 ± 0.21 b |
| Ascorbic acid | 8.61 ± 0.14 d |
| Samples | LC50 (µg RM/mL) | |
|---|---|---|
| Caco-2 Cells | HepG2 Cells | |
| 3-year-old cactus | >200 | >200 |
| 6-year-old cactus | >200 | >200 |
| 3-year-old cactus (digesta) | >200 | >200 |
| 6-year-old cactus (digesta) | >200 | >200 |
| Phytochemical | 3-Year-Old Cactus Extract | 6-Year-Old Cactus Extract | ||||
|---|---|---|---|---|---|---|
| Pre-Digested | Digesta | Aqueous Micellar Fraction | Pre-Digested | Digesta | Aqueous Micellar Fraction | |
| Lutein (µg/g RM) * | 36.14 ± 0.39 | 24.95 ± 1.19 | 22.35 ± 0.08 | 30.44 ± 0.45 | 17.75 ± 0.17 | 12.12 ± 0.22 |
| Chlorophyll a (µg/g RM) * | 179.41 ± 2.89 | n.d. | n.d. | 115.155.83 | n.d. | n.d. |
| Chlorophyll b (µg/g RM) * | 97.26 ± 0.31 | n.d. | n.d. | 91.28 ± 0.80 | n.d. | n.d. |
| Pheophytin a (µg/g RM) * | 243.46 ± 10.59 | 159.50 ±2.04 | 118.97 ± 1.97 | 154.08 ± 5.23 | 92.17 ± 7.32 | 62.59 ± 4.91 |
| Pheophytin b (µg/g RM) * | 6.16 ± 1.45 | 38.54 ± 1.04 | 26.34 ± 1.83 | 5.87 ± 1.48 | 29.98 ± 1.19 | 16.40 ± 0.39 |
| Total chlorophylls (µg/g RM) | 526.29 ± 10.45 | 198.04 ± 2.76 | 145.31 ± 1.12 | 366.37 ± 1.22 | 122.15 ± 6.18 | 78.98 ± 4.67 |
| Total phenolics (mg GAE/g of RM) ** | 35.45 ± 1.43 | 21.43 ± 0.45 | 16.37 ± 0.19 | 25.58 ± 0.26 | 14.55 ± 0.16 | 10.17 ± 0.34 |
| Phytochemical | % Digestive Stability or Recovery | |
|---|---|---|
| 3-Year-Old Cactus | 6-Year-Old Cactus | |
| Lutein | 69.03 ± 2.65 ** | 58.33 ± 1.38 * |
| Pheophytin a | 65.62 ± 3.63 * | 59.83 ± 4.70 * |
| Pheophytin b | 655.24 ± 187.59 * | 534.84 ± 146.47 * |
| Total chlorophylls | 37.64 ± 1.09 ** | 33.34 ± 1.73 * |
| Total phenolics | 60.52 ± 2.73 * | 56.89 ± 1.20 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaicharoenaudomrung, N.; Posridee, K.; Oonsivilai, A.; Oonsivilai, R. Golden Barrel Cactus: Unveiling Its Potential as a Functional Food and Nutraceutical Source. Foods 2024, 13, 1137. https://doi.org/10.3390/foods13071137
Chaicharoenaudomrung N, Posridee K, Oonsivilai A, Oonsivilai R. Golden Barrel Cactus: Unveiling Its Potential as a Functional Food and Nutraceutical Source. Foods. 2024; 13(7):1137. https://doi.org/10.3390/foods13071137
Chicago/Turabian StyleChaicharoenaudomrung, Nipha, Kakanang Posridee, Anant Oonsivilai, and Ratchadaporn Oonsivilai. 2024. "Golden Barrel Cactus: Unveiling Its Potential as a Functional Food and Nutraceutical Source" Foods 13, no. 7: 1137. https://doi.org/10.3390/foods13071137
APA StyleChaicharoenaudomrung, N., Posridee, K., Oonsivilai, A., & Oonsivilai, R. (2024). Golden Barrel Cactus: Unveiling Its Potential as a Functional Food and Nutraceutical Source. Foods, 13(7), 1137. https://doi.org/10.3390/foods13071137










