Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Pecan Samples
2.3. Lipid Extraction Procedure
2.4. Instruments and Methods
2.5. Lipid Identification
2.6. Quality Assurance
2.7. Statistical Analysis
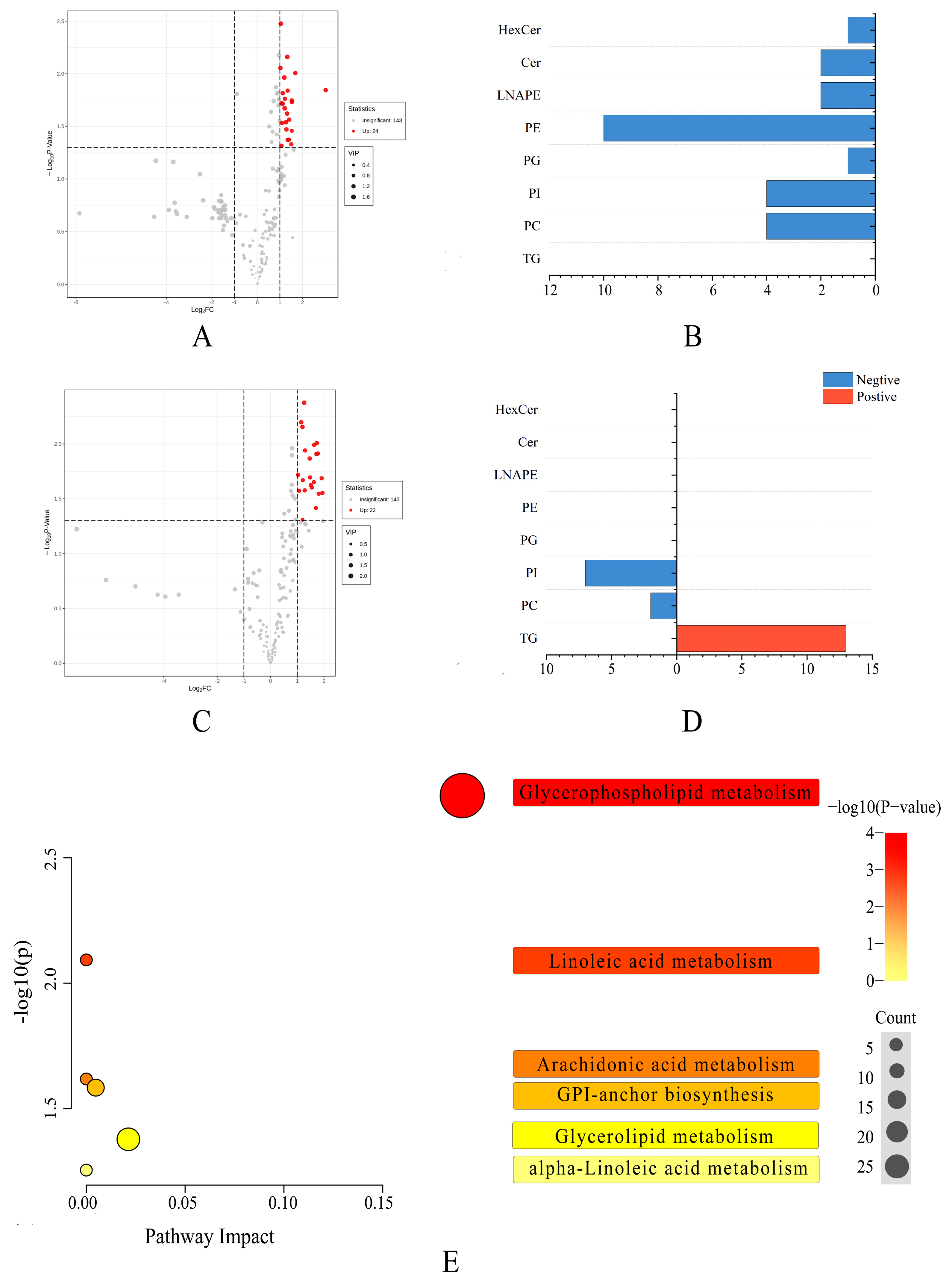
3. Results
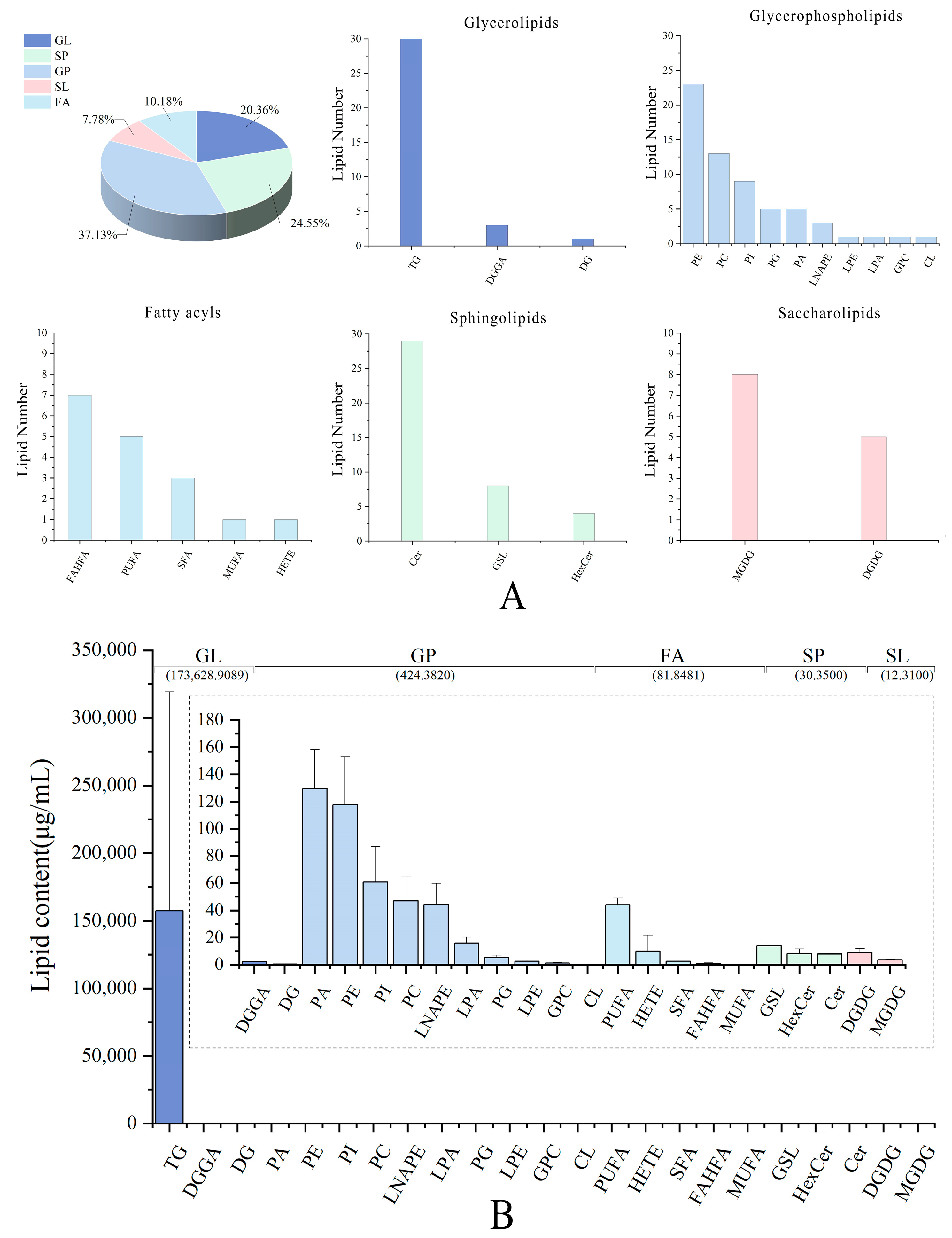
3.1. Lipid Profiles of Pecan
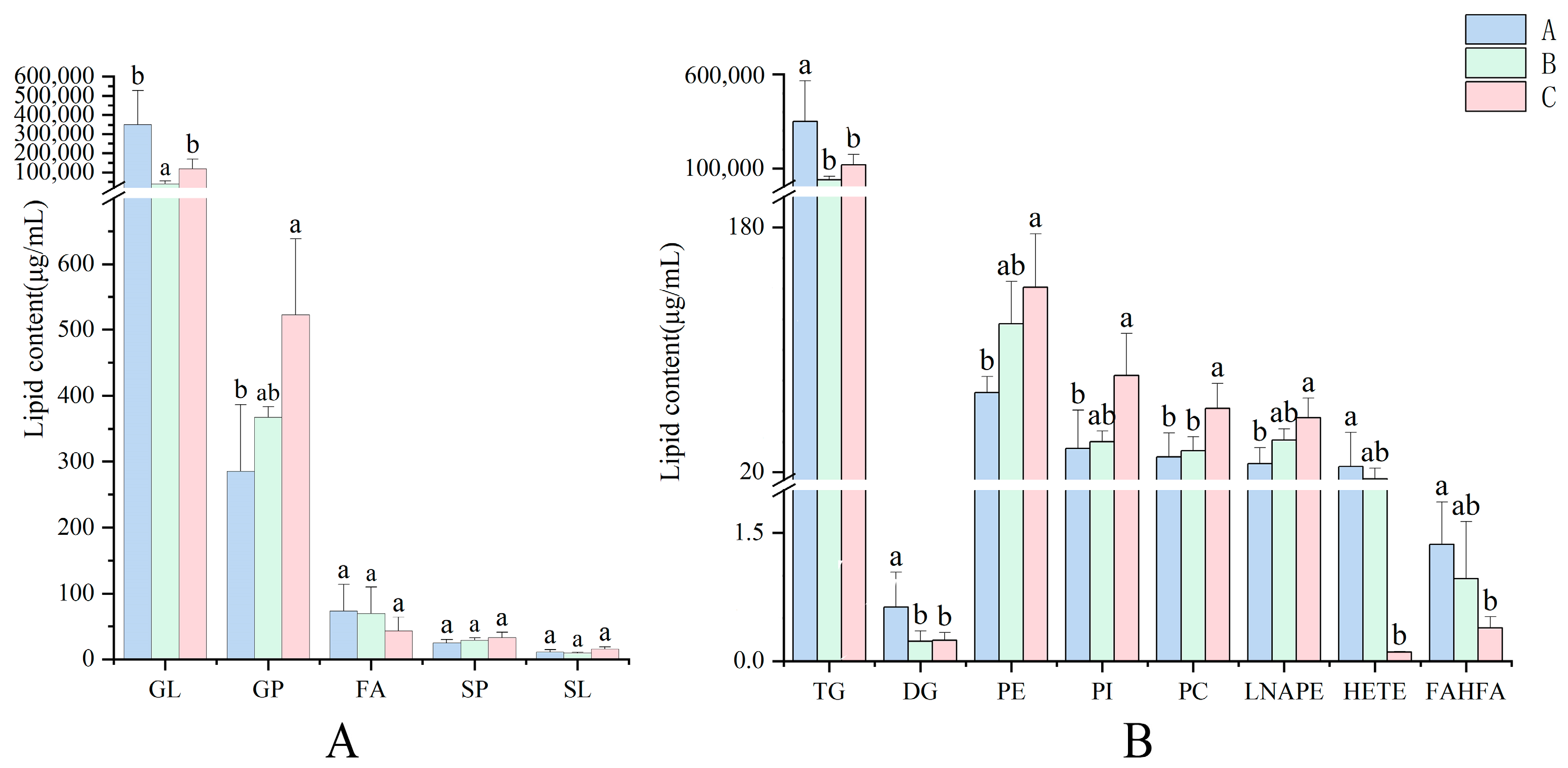
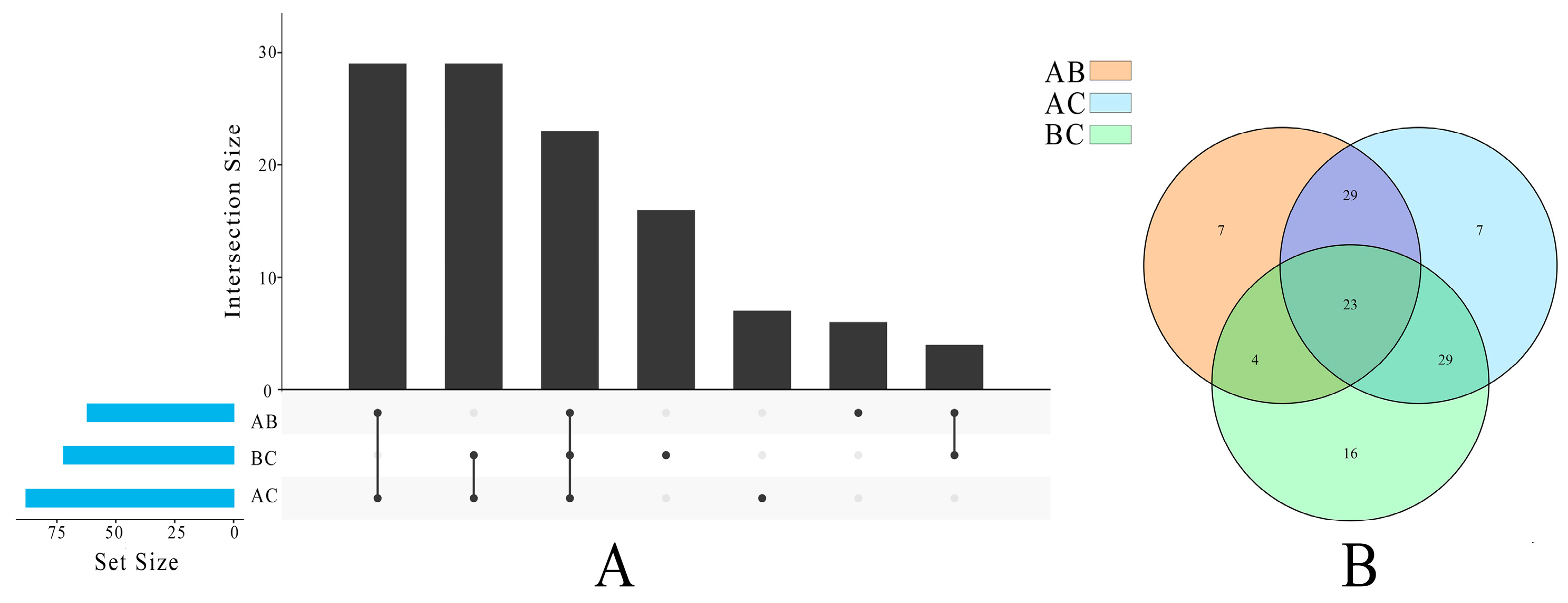
3.2. The Lipid Compositions of Pecan in Response to Biotic Stress
3.3. The Lipid Chain Length and Unsaturation of Pecan in Response to Biotic Stress
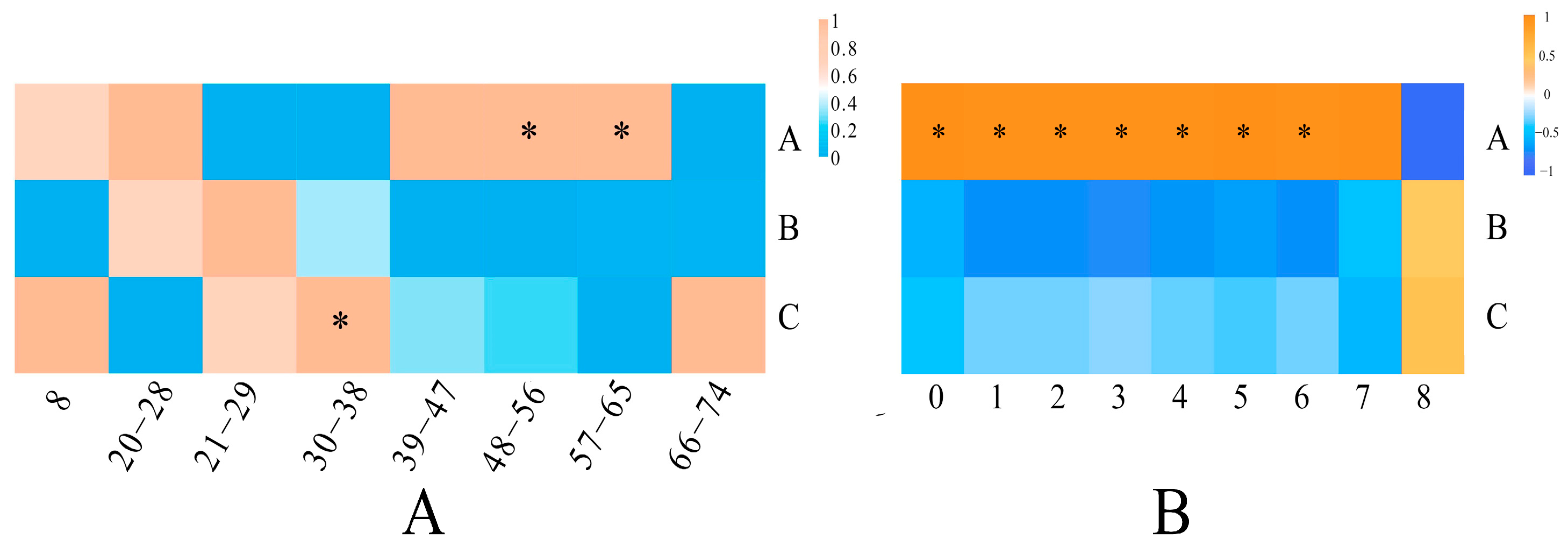
3.4. Multivariate Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, T.; Zaaboul, F.; Yin, S.; Ye, Z.; Sun, Y.; Zhao, J.; Xu, Y.J.; Liu, Y. Studies on the lipid oxidation and oleosomes behavior in raw pecan kernels during storage. Food Chem. 2023, 405, 134867. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.R.; Machadoribeiro, Q.; Klein, B.; Santos, I.D.D.; Wagner, R. Effect of low oxygen on quality attributes of ‘barton’ pecan nuts after long-term storage at different temperatures. Sci. Hortic.-Amst. 2020, 263, 1090. [Google Scholar] [CrossRef]
- Ribeiro, S.R.; Klein, B.; Ribeiro, Q.M.; Santos, I.D.D.; Wagner, R. Chemical composition and oxidative stability of eleven pecan cultivars produced in Southern Brazil. Food Res. Int. 2020, 136, 109596. [Google Scholar] [CrossRef]
- Siebeneichler, T.J.; Hoffmann, J.F.; Galli, V.; Zambiazi, R.C. Composition and impact of pre- and post-harvest treatments/factors in pecan nuts quality. Trends Food Sci. Tech. 2022, 131, 46–60. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Alvarez-Parrilla, E.; López-Díaz, J.A.; Maldonado-Mendoza, I.E.; Gómez-García, M.d.C.; de la Rosa, L.A. The pecan nut (Carya illinoinensis) and its oil and polyphenolic fractions differentially modulate lipid metabolism and the antioxidant enzyme activities in rats fed high-fat diets. Food Chem. 2015, 168, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Guarneiri, L.L.; Paton, C.M.; Cooper, J.A. Pecan-enriched diets decrease postprandial lipid peroxidation and increase total antioxidant capacity in adults atrisk for cardiovascular disease. Nutr. Res. 2021, 93, 69–78. [Google Scholar] [CrossRef]
- Tanwar, B.; Modgil, R.; Goyal, A. Nutritional and phytochemical composition of pecan nut [Carya illinoinensis (Wangenh.) K. Koch] and its hypocholesterolemic effect in an animal model. Br. Food J. 2021, 123, 1433–1448. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, F.; Hu, T.; Zhou, C. Lipidomic analyses of five Carya illinoinensis cultivars. Food Sci. Nutr. 2023, 11, 6336–6348. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.R.; Klein, B.; Santos, I.D.D.; Thewes, F.R.; Brackmann, A.; Both, V.; Wagner, R. Effects of controlled atmosphere and storage temperature on the quality of shelled ‘Barton’ pecan nuts during long-term storage. Food Res. Int. 2022, 158, 111498. [Google Scholar] [CrossRef]
- Jia, X.; Yang, X.; Xu, M.; Tan, W.; Yin, M.; Liu, P.; Tong, H. Lipidomics reveals temporal changes in pecan kernel lipids during storage with or without sodium erythorbate treatment. Postharvest Biol. Technol. 2023, 199, 112297. [Google Scholar] [CrossRef]
- Antonelli, M.; Benedetti, B.; Cavaliere, C.; Cerrato, A.; Montone, C.M.; Piovesana, S.; Lagana, A.; Capriotti, A.L. Phospholipidome of extra virgin olive oil: Development of a solid phase extraction protocol followed by liquid chromatography-high resolution mass spectrometry for its software-assisted identification. Food Chem. 2020, 310, 125860. [Google Scholar] [CrossRef]
- Song, S.; Cheong, L.Z.; Wang, H.; Man, Q.Q.; Pang, S.J.; Li, Y.Q.; Ren, B.; Wang, Z.; Zhang, J. Characterization of phospholipid profiles in six kinds of nut using HILIC-ESI-IT-TOFMS system. Food Chem. 2018, 240, 1171–1178. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, Z.; Zhang, L.; Wu, W.; Huang, Y.; Jia, Z.; Zhou, L.; Gao, L.; Long, H.; Lei, M.; et al. Spatial lipidomics of eight edible nuts by desorption electrospray ionization with ion mobility mass spectrometry imaging. Food Chem. 2022, 371, 130893. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, K.; Xi, J.; Xu, Y.; Si, X.; Pei, D.; Lyu, S.; Xia, G.; Wang, J.; et al. Analysis of lipidomics profile of Carya cathayensis nuts and lipid dynamic changes during embryonic development. Food Chem. 2022, 370, 130975. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H.; Chen, D.; Tan, H.; Huang, Y.; Cozzolino, D. Lipidomic changes in banana (Musa cavendish) during ripening and comparison of extraction by Folch and Bligh–Dyer methods. J. Agric. Food Chem. 2020, 68, 11309–11316. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, W.; Shen, D.; Li, Z.; Shu, J.; Liu, Y. Comprehensive lipidomics analysis reveals the changes in lipid profile of camellia oil affected by insect damage. Front. Nutr. 2022, 9, 993334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Peng, F.; Li, Y. Pecan production in china. Sci. Hortic-Amst. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Du, Y.L.; Li, J.; Wang, Z.Y. Research Progress of Conogethes punctiferalis (Lepidoptera: Crambidae) in China. In The Black spotted, Yellow Borer, Conogethes punctiferalis Guenée and Allied Species; Chakravarthy, A.K., Ed.; Springer: Singapore, 2018; pp. 45–66. [Google Scholar]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Minami, Y.; Gowda, D.; Chiba, H.; Hui, S.P. Detection and characterization of lipids in eleven species of fish by non-targeted liquid chromatography/mass spectrometry. Food Chem. 2022, 393, 133402. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Guo, L.; Zhang, Y.; Walzem, R.L.; Pendergast, J.S.; Printz, R.L.; Morris, L.C.; Matafonova, E.; Stien, X.; Kang, L.; et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J. Clin. Investig. 2014, 124, 3391–3406. [Google Scholar] [CrossRef]
- May-Zhang, L.S.; Chen, Z.; Dosoky, N.S.; Yancey, P.G.; Boyd, K.L.; Hasty, A.H.; Linton, M.F.; Davies, S.S. Administration of N-Acyl-Phosphatidylethanolamine Expressing Bacteria to Low Density Lipoprotein Receptor−/− Mice Improves Indices of Cardiometabolic Disease. Sci. Rep. 2019, 9, 420. [Google Scholar] [CrossRef]
- Jia, Y.; Gan, Y.; He, C.; Chen, Z.; Zhou, C. The mechanism of skin lipids influencing skin status. J. Dermatol. Sci. 2018, 89, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Fu, L.; Song, Y.; Li, J.; Xue, X.; Li, S.; Li, L. Wax composition and concentration in jujube (Ziziphus jujuba Mill.) cultivars with differential resistance to fruit cracking. J. Plant Physiol. 2020, 255, 153294. [Google Scholar] [CrossRef] [PubMed]
- Zoong Lwe, Z.S.; Welti, R.; Anco, D.; Naveed, S.; Rustgi, S.; Narayanan, S. Heat stress elicits remodeling in the anther lipidome of peanut. Sci. Rep. 2020, 10, 22163. [Google Scholar] [CrossRef]
- Li, Z.G.; Zeng, H.Z.; Ao, P.X.; Gong, M. Lipid response to short-term chilling shock and long-term chill hardening in Jatropha curcas L. seedlings. Acta Physiol. Plant. 2014, 36, 2803–2814. [Google Scholar] [CrossRef]
- Mueller, S.P.; Krause, D.M.; Mueller, M.J.; Fekete, A. Accumulation of extrachloroplastic triacylglycerols in Arabidopsis seedlings during heat acclimation. J. Exp. Bot. 2015, 66, 4517–4526. [Google Scholar] [CrossRef]
- Sun, J.; Feng, X.; Lyu, C.; Zhou, S.; Liu, Z. Effects of different processing methods on the lipid composition of hazelnut oil: A lipidomics analysis. Food Sci. Human. Well. 2022, 11, 427–435. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, L.; Yang, H.; Zhang, J.; Hou, X.; Wu, C.; Zhang, R.; Cheng, Y. Comprehensive comparative analysis of lipid profile in dried and fresh walnut kernels by UHPLC-Q-Exactive Orbitrap/MS. Food Chem. 2022, 386, 132706. [Google Scholar] [CrossRef]
- Lin, F.; Qu, Y.; Zhang, Q. Phospholipids. Plant Signal Behav. 2014, 9, 5. [Google Scholar] [CrossRef]
- Gu, Y.; He, L.; Zhao, C.; Wang, F.; Yan, B.; Gao, Y.; Li, Z.; Yang, K.; Xu, J. Biochemical and Transcriptional Regulation of Membrane Lipid Metabolism in Maize Leaves under Low Temperature. Front. Plant Sci. 2017, 8, 2053. [Google Scholar] [CrossRef]
- Mehta, S.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Fight Hard or Die Trying: Current Status of Lipid Signaling during Plant-Pathogen Interaction. Plants 2021, 10, 1098. [Google Scholar] [CrossRef]
- Reina-Pinto, J.J.; Yephremov, A. Surface lipids and plant defenses. Plant Physiol. Bioch. 2009, 47, 540–549. [Google Scholar] [CrossRef]
- Wang, R.; Li, B.; Lam, S.M.; Shui, G. Integration of lipidomics and metabolomics for in-depth understanding of cellular mechanism and disease progression. J. Genet. Genom. 2020, 47, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Simoes, A.; Domingues, M.R. Fruit seeds and their oils as promising sources of value-added lipids from agro-industrial byproducts: Oil content, lipid composition, lipid analysis, biological activity and potential biotechnological applications. Crit. Rev. Food Sci. 2021, 61, 1305–1339. [Google Scholar] [CrossRef]
- Tosin, M.O.; Cao, W. Exploring foods as natural sources of FAHFAs—A review of occurrence, extraction, analytical techniques and emerging bioactive potential. Trends Food Sci. Tech. 2022, 129, 591–607. [Google Scholar]
- Su, Y.; Zhang, J.; Xu, Z.; Li, J.; Wang, J. Integrative analysis of metabolome and transcriptome reveals the mechanism of color formation in white root (salvia miltiorrhiza). Ind. Crop Prod. 2021, 170, 113784. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Wang, L.; Liu, X.; Zhang, J.; He, Z. Stereoselective metabolomic and lipidomic responses of lettuce (Lactuca sativa L.) exposing to chiral triazole fungicide tebuconazole. Food Chem. 2022, 371, 131209. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, P.; Lyu, C.; Tian, J.; Meng, X.; Tan, H.; Dong, W. Comprehensive lipidomics analysis of the lipids in hazelnut oil during storage. Food Chem. 2022, 378, 132050. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V., Jr.; Walther, T.C. Glycerolipid Synthesis and Lipid Droplet Formation in the Endoplasmic Reticulum. Cold Spring Harb. Perspect. Biol. 2023, 15, a041246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhang, W.; Li, Q.; Cui, M.; Shen, D.; Shu, J.; Mo, R.; Liu, Y. Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis). Foods 2024, 13, 974. https://doi.org/10.3390/foods13070974
Zhou L, Zhang W, Li Q, Cui M, Shen D, Shu J, Mo R, Liu Y. Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis). Foods. 2024; 13(7):974. https://doi.org/10.3390/foods13070974
Chicago/Turabian StyleZhou, Lingyuan, Wei Zhang, Qingyang Li, Maokai Cui, Danyu Shen, Jinping Shu, Runhong Mo, and Yihua Liu. 2024. "Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis)" Foods 13, no. 7: 974. https://doi.org/10.3390/foods13070974
APA StyleZhou, L., Zhang, W., Li, Q., Cui, M., Shen, D., Shu, J., Mo, R., & Liu, Y. (2024). Evaluation of Lipid Quality in Fruit: Utilizing Lipidomic Approaches for Assessing the Impact of Biotic Stress on Pecans (Carya illinoinensis). Foods, 13(7), 974. https://doi.org/10.3390/foods13070974




