Abstract
The term Conjugated Linoleic Acid (CLA) refers generically to a class of positional and geometric conjugated dienoic isomers of linoleic acid. Among the isomers of linoleic acid cis9, trans11-CLA (c9, t11-CLA) and trans10, cis12-CLA (t10, c12-CLA) are found to be biologically active isomers, and they occur naturally in milk, dairy products and meat from ruminants. In addition, some vegetables and some seafoods have also been reported to contain CLA. Although the CLA levels in these natural sources are insufficient to confer the essential health benefits, anti-carcinogenic or anti-cancer effects are of current interest. In the rumen, CLA is an intermediate of isomerization and the biohydrogenation process of linoleic acid to stearic acid conducted by ruminal microorganisms. In addition to rumen bacteria, some other bacteria, such as Propionibacterium, Bifidobacterium and some lactic acid bacteria (LAB) are also capable of producing CLA. In this regard, Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) has demonstrated the ability to produce CLA isomers from linoleic acid by multiple enzymatic activities, including hydration, dehydration, and isomerization. L. plantarum is one of the most versatile species of LAB and the bacterium is widely used in the food industry as a microbial food culture. Thus, in this review we critically analyzed the literature produced in the last ten years with the aim to highlight the potentiality as well as the optimal conditions for CLA production by L. plantarum. Evidence was provided suggesting that the use of appropriate strains of L. plantarum, as a starter or additional culture in the production of some fermented foods, can be considered a critical factor in the design of new CLA-enriched functional foods.
1. Introduction
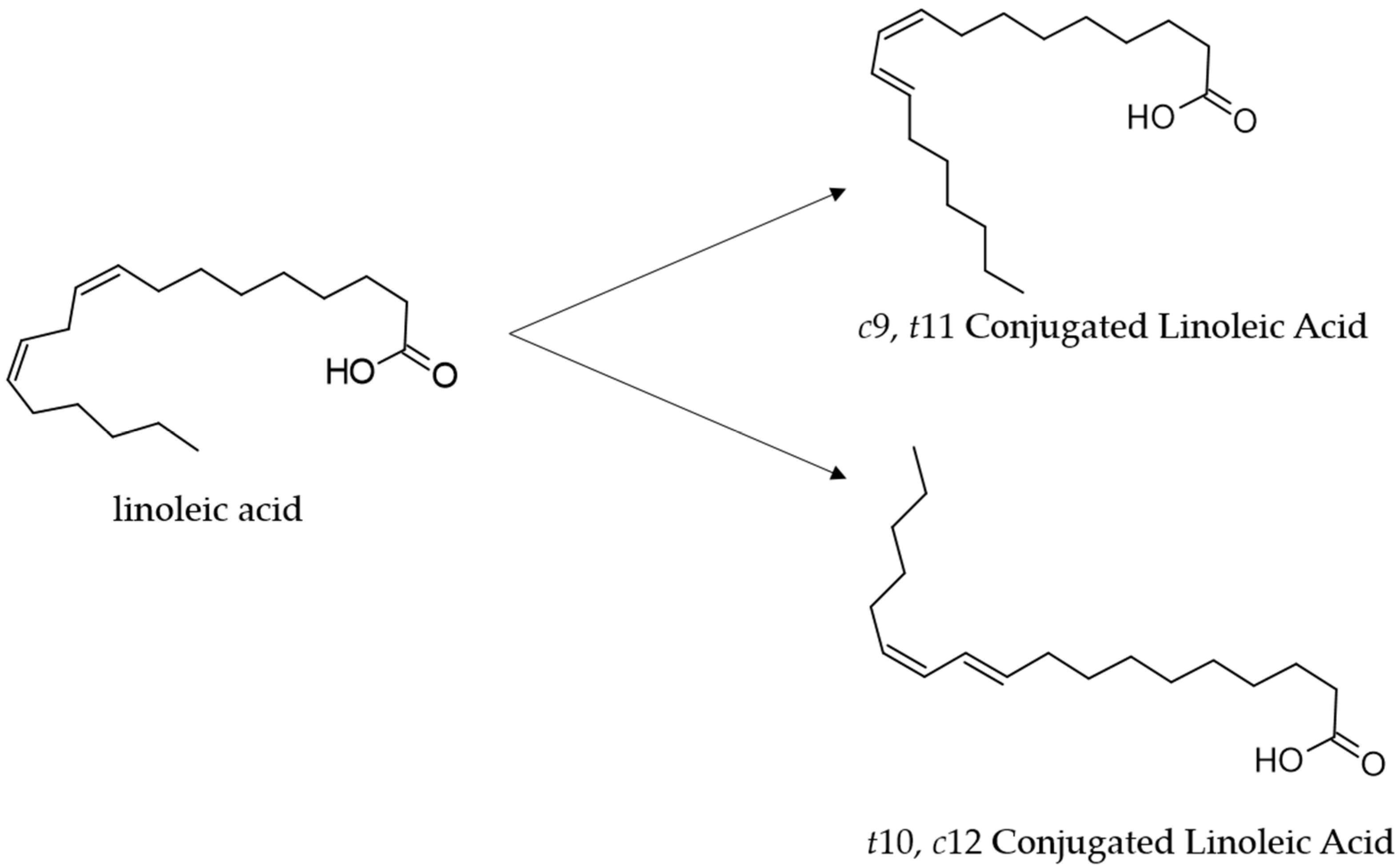
Conjugated linoleic acid (CLA) has received increasing attention in the last two decades for its potential health benefits [1]. CLAs, some of which are polyunsaturated fatty acids (PFA) of the ω-6 series, comprise a group of positional and geometric (cis or trans) isomers of linoleic acid (LA; cis-9,12-octadecadienoic acid 18:2) with a conjugated double bond [2]. The isomers cis-9, trans-11(c9t11, CLA1), commonly called rumenic acid, and trans-10, cis-12 (t10c12, CLA2) are the most abundant CLA isomers, naturally present in several foods [3] (Figure 1).
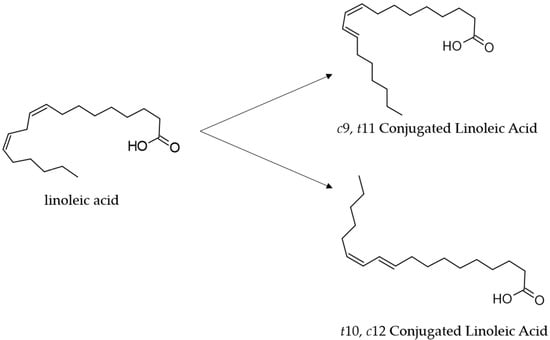
Figure 1.
Structures of the linoleic acid (18:2) and its major conjugated isomers cis 9, trans 11-CLA (rumenic acid, CLA1) and trans 10, cis 12-CLA (CLA2).
CLA is primarily a product of microbial metabolism in the digestive tract of ruminants, and it ultimately accumulates in milk, beef, and dairy products [4]. CLA is also present in vegetable oils (e.g., sunflower, soybean, castor, safflower, and sesame oils) and some fish oils (salmon and lake trout) [5,6].
Potential benefits to human health are the main reason for scientific interest in CLA. Recently, several properties have been attributed to CLA including anti-carcinogenic or anti-cancer effects [7], prevention and treatment of diabetes [8], anti-atherosclerosis [9] and anti-osteoporosis effects [10], prevention of increases in body fat [11], lowering of LDL-cholesterol [12], and anti-inflammatory and immunomodulatory properties [13].
As far as the voluminous literature on CLA is concerned, to date, a definitive cause-and-effect or biochemical relationship has not been established between the consumption of the CLA isomers and the aforementioned beneficial effects [14,15,16,17,18]. Currently, scientific interest in the consumption of CLA persists, and this interest is mainly aimed at verifying the safety and efficacy of CLA on human health [19].
A review by den Harting summarizes the pre-clinical and human studies conducted using CLA to date, which collectively suggest that CLA has efficacy against cancer, obesity, and atherosclerosis [20].
The main interest surrounding CLA is its anti-carcinogenic (preventing the onset of cancer) or anti-cancer (diminishing or eliminating cancerous growth) effects, and some recent studies have highlighted its effectiveness [7,21,22].
Recent insights support current recommendations of public health guidelines around the world that emphasize the consumption of ω-6 fatty acids (FA) [23,24,25] and advocate a reduction in dietary saturated fatty acids (SFAs) [26,27,28]. Therefore, CLA have become an object of study for applications in food production [1]. However, the beneficial effects of CLA at low concentrations may not be significant. The recommended dietary allowance of CLA for humans ranges from 1 to 3 g/d to accomplish desired health benefits [29,30].
Natural CLA is sourced mainly from meat and dairy products, which contain 0.6–10.0 mg CLA/g of fat and 3.4–9.4 mg CLA/g of fat, respectively [31,32]. Therefore, it is clear that the CLA content in these products is too low to be able to cause a beneficial effect in consumers. Thus, it may be of interest to look for strategies such as bacterial production [33,34,35] to increase CLA content in food [36,37].
The amount of CLA in food can be increased by using various enzymatic, chemical and biological methods [35]. The most environmentally friendly method for CLA synthesis is based on microbial biosynthesis [31,38], and many lactic acid bacteria (LAB) demonstrate the ability to produce CLA isomers from LA [39,40,41].
Among LAB, Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) have been identified as the most efficient CLA-producers among food-derived LAB [42,43].
L. plantarum is a LAB species with high ecological and metabolic adaptability [44,45,46] and is capable of inhabiting a variety of environments, including animal and human gastrointestinal tracts [47,48]. Some strains belonging to L. plantarum species are proposed as animal or human probiotics [49,50,51,52,53,54,55,56] and are extensively utilized as starter cultures in various fermented foods [57,58,59,60,61,62,63,64,65].
In this review, we highlight the role of L. plantarum in CLA production. To this aim, in the first section of the paper we described the molecular mechanisms of CLA biosynthesis in microorganisms, with a special focus on L. plantarum; subsequently, among the plethora of papers dealing with CLA production in LAB, we analyzed all the studies dealing with the synthesis of CLA isomers by L. plantarum reported in the literature (Scopus and PubMed as sources) of the last ten years. We trust that this review can provide support for the use of this bacterial species in the production of CLA-enriched foods.
2. Microbial Biosynthesis of CLA
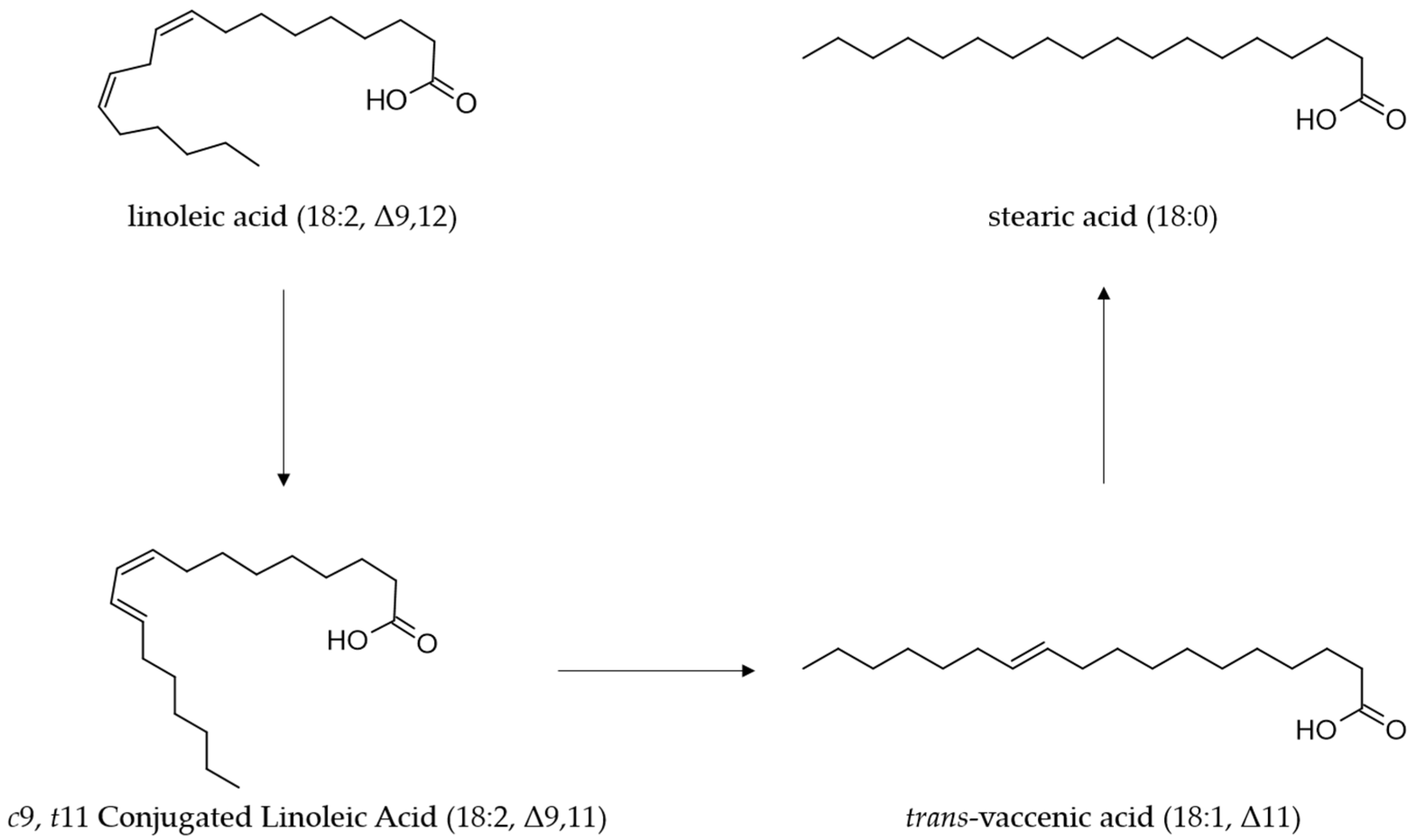
CLA, which is primarily a product of microbial metabolism in the digestive tract of ruminants, ultimately accumulates into ruminant-derived products such as meat, milk and other dairy products [66,67]. The PFA, present in the diet of ruminants are metabolized in the rumen by different species of microorganisms [68,69]. During this process, the PFA, including LA, are converted through isomerization and hydrogenation to stearic acid (C18:0) as the end-product [70,71]. LA is first isomerized to CLA, mainly rumenic acid (C18:2 c9t11, CLA1), and finally, through a biohydrogenation mechanism by reductase enzymes, the CLA isomers are converted to stearic acid in the rumen [72] (Figure 2).
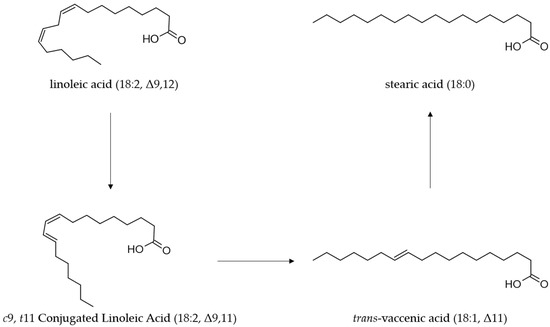
Figure 2.
Ruminal biohydrogenation process of linoleic by Butyrivibrio fibrisolvens.
All intermediates of this biohydrogenation process are absorbed in the gut and transported through the blood stream to different body tissues [73].
Rumen bacteria, especially Butyrivibrio fibrisolvens, are the initial microorganisms for CLA production, playing a pivotal role in the biotransformation and accumulation of CLA in ruminant-derived products [74]. Other bacteria such as LAB, Bifidobacterium, and Propionibacterium proved to efficiently synthesize CLA [33]. Among LAB, Lactobacillus strains and in particular L. plantarum have a high capacity to produce CLA [34,42,43,75,76,77].
It is unclear why bacteria convert LA into CLA-isomers. One of the best-supported hypotheses is that PFA such as LA are toxic to many bacteria by inhibiting cell growth, restructuring cell membranes, and interfering with biosynthesis of native fatty acids [78]. To survive such toxicities, CLA-producing bacteria may have evolved to carry out a biohydrogenation process which is the reduction of double bonds on the carbon chain, producing non-toxic SFAs as final products [33,34,79]. During biohydrogenation, various CLA isomers are formed [72,80].
The capability to produce CLA has been shown to vary among different LAB, and scientific data indicate strain-specificity in the ability to produce these isomers of LA [39,81,82].
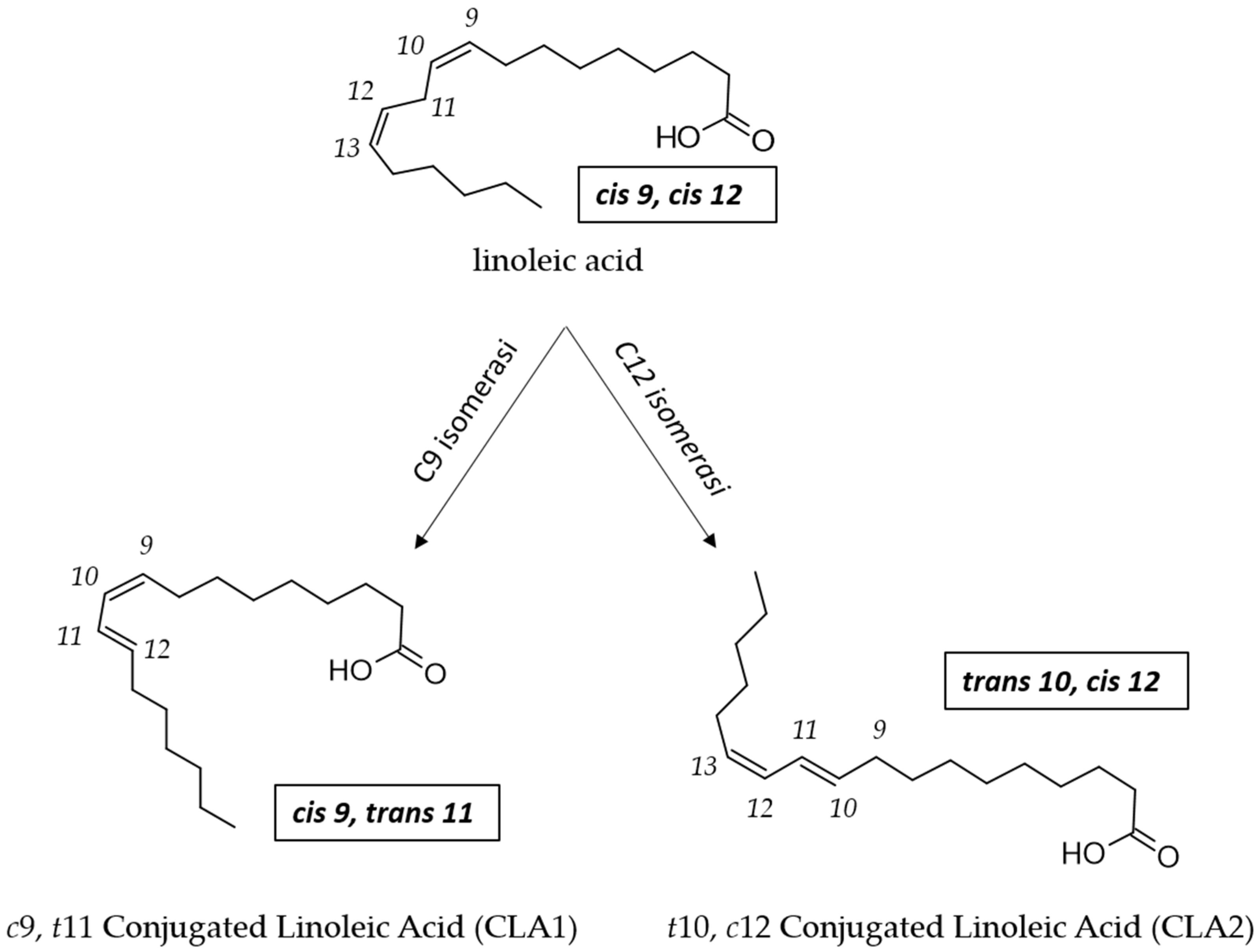
Rumen bacteria and Propionibacterium transform LA to CLA by a one-step reaction catalyzed by a LA isomerase (LAI) named PAI (Propionibacterium acnes isomerase; Figure 3) [83]. Also in LAB, LAI activity is considered to be the key factor involved in the bioconversion of LA to CLA [34], but there is a lack of information regarding the molecular mechanism behind the ability of LAB to convert LA to CLA [73,84].
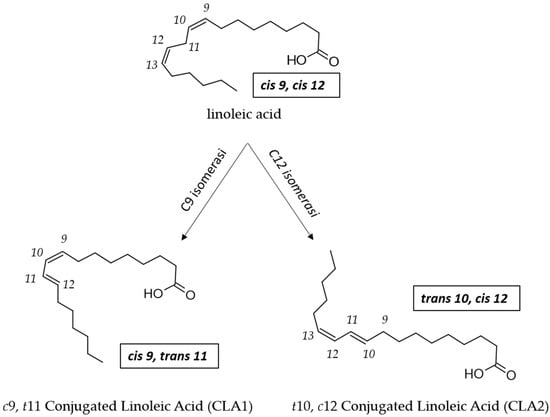
Figure 3.
Enzymatic conversion of LA to CLA1 and to CLA2 by LA isomerase (PAI) in Propionibacterium.
3. Molecular Mechanism of CLA Synthesis by L. plantarum
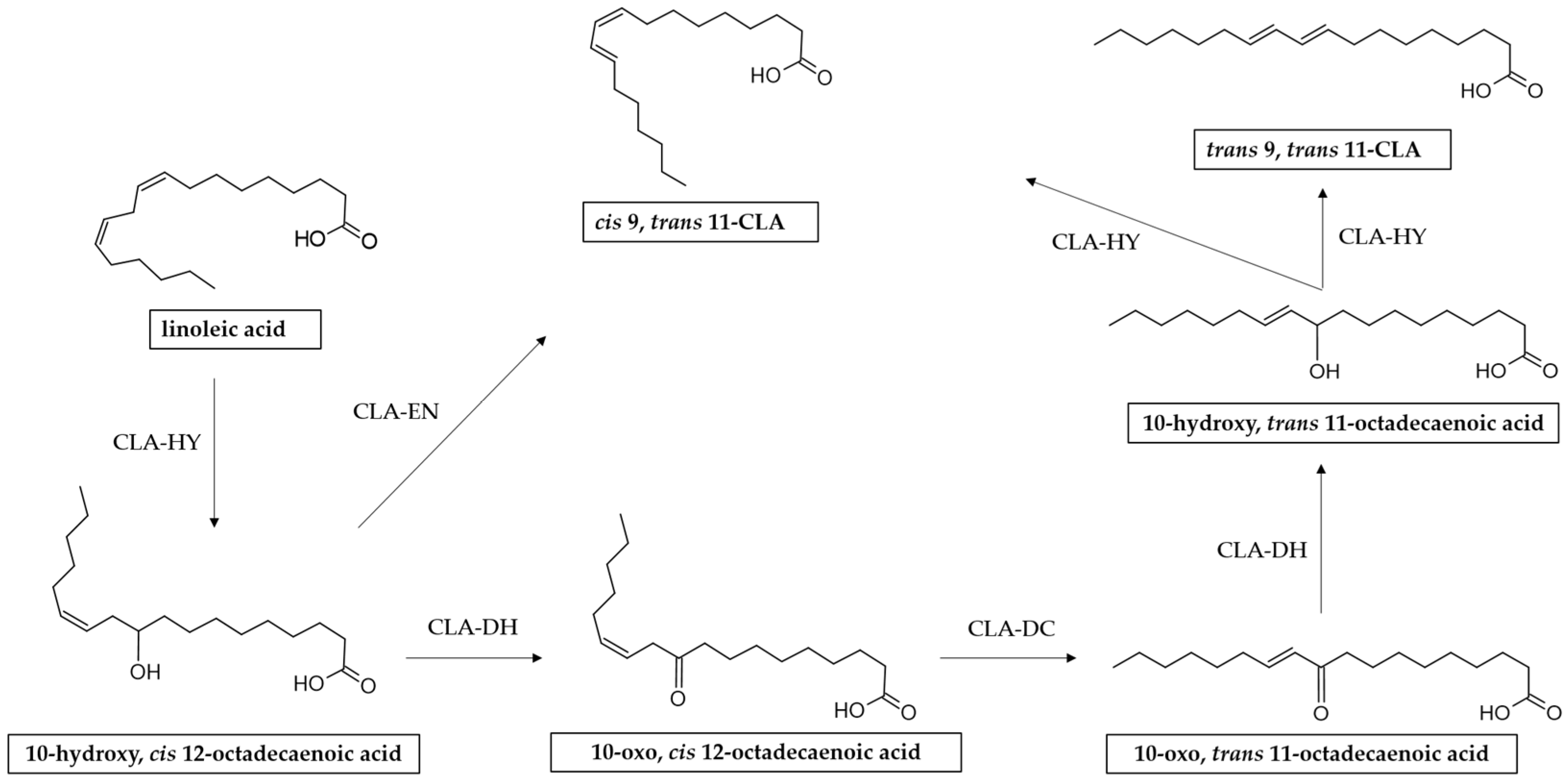
It has been reported that the process of CLA biosynthesis by L. plantarum is much more complicated [85]. Some studies have indicated that the mechanism for CLA production by L. plantarum involves multiple reactions including hydration, dehydration, and isomerization catalyzed by three enzymes, i.e., CLA hydratase (CLA-HY), CLA short-chain dehydrogenase (CLA-DH), and CLA acetoacetate decarboxylase (CLA-DC) [73,86,87,88,89] (Figure 4).
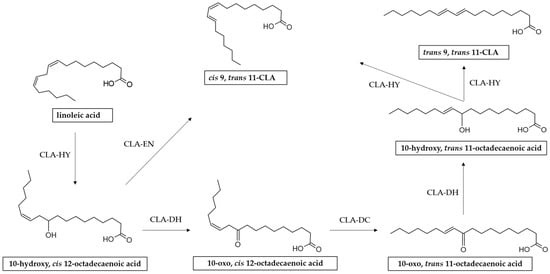
Figure 4.
Reaction scheme of LA isomerization to CLA by L. plantarum. CLA-HY: linoleate hydratase, a member of the Myosin Cross Reacting Antigen (MCRA) family; CLA-DH: short-chain hydrogenase/oxidoreductase; CLA-DC: acetoacetate decarboxylase.
These enzymes are separately encoded from three genes: CLA-HY is encoded by lp_0139 (cla-hy), while CLA-DH and CLA-DC are encoded by lp_0060 (cla-dh) and lp_0061 (cla-dc), respectively [90,91].
Liu and others showed that strains of L. plantarum with different CLA biosynthesis abilities possessed different transcriptional levels of cla-hy, cla-dh, and cla-dc, suggesting that the upregulation of the CLA yield may be achieved by regulating the transcription of these genes [91]. In another study, it was found that these three genes in L. plantarum WCFS1 are regulated by a LysR-type transcriptional regulator (LttR) [92]. LttR family proteins regulate a diverse set of genes, including those involved in stress responses, motility, virulence, amino acid metabolism, quorum sensing and motility [93]. In another recent study on L. plantarum AR195, it was shown that in addition to LttR, the arginine repressor ArgR2 positively regulated cla-dh and cla-dc transcription [92].
In addition to the complex enzyme system described above, in a study conducted by Ortega-Anaya and others it was demonstrated that L. plantarum CFQ-100 (subculture of L. plantarum ATCC 8014) possesses a multifunctional protein belonging to the enolase family (α-enolase). In addition to having a central role in glycolytic metabolism, α-enolase has a collateral role in the biohydrogenation metabolism of LA, being capable of catalyzing the formation of 9-cis, 11-trans-CLA through dehydration and isomerization 10-hydroxy-12-cis-octadecenoic acid [94].
4. CLA Production by L. plantarum in Different Culture Media
The ability to synthesize CLA not only differs among different bacterial species, but also between different strains belonging to the same species [39,81,82,84,95]. In addition, CLA production can be affected by several factors, such as added LA concentration, pH, temperature, and fermentation time [40,41,82,96]. In this context, for each individual bacterial strain, the optimization and standardization of fermentation conditions are necessary to maximize the production of CLA isomers.
The influence of the processing conditions to produce CLA in vitro using L. plantarum has been largely studied. The main results obtained in several studies on CLA production by several L. plantarum strains in different culture media with LA or ricinoleic acid (RA; 12-hydroxy-cis-9-octadecaenoic acid 18:1) as a CLA source are summarized in Table 1.

Table 1.
CLA production by Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) in different culture media.
Temperature is an important physical factor that can influence the growth of L. plantarum, a mesophilic bacterium that has an optimal growth temperature of approximately 37 °C [111]. In most of the studies cited below, the optimal conditions for maximum CLA production of L. plantarum were between 30 and 40 °C. It has been reported that regulating the temperature can induce variations in the lipid composition of microorganisms, resulting in the maintenance of the integrity of the cell membrane [112]. Therefore, temperature appears to be a critical factor in CLA production since LAI in L. plantarum was found to be a multi-component enzyme system widespread in both the soluble and the membrane fractions [86].
However, optimal temperatures for bacterial growth and CLA production do not always coincide. In fact, in a study conducted by Devi and Rashmi, the maximum growth of L. plantarum ATCC 8014 was observed at 37 °C, but the highest rate of CLA production was detected at 40 °C [98]. In other studies, maximum CLA production was achieved at 30 °C after 48 h [75,103], while two reports recorded the highest CLA production after 24 h at 37 °C and 40 °C, respectively [99,108]. As highlighted in these studies and discussed more later, in addition to temperature, fermentation time is another important factor that determines the growing phase of LAB and indirectly reflects the synthesis of LAI [113].
In general, all studies have shown that the production of CLA increases over time until it reaches a maximum, after which the amount of CLA tends to decrease progressively [98]. Therefore, the maximum amount of CLA can be obtained at a specific time for each individual strain, according to the different phases of bacterial growth (exponential and stationary phases), as well as LA concentration and LAI activity [2].
During fermentation, the pH of the medium significantly affects the shape and function of proteins, including enzymes responsible for the metabolic processes of fermentation. Each enzyme has an optimal range of pH, and the pH of fermentation outside of this range is associated with reduced enzyme activity. LAI is sensitive to pH and it has been shown that ruminal pH between 6.0 and 7.0 was associated with a high production of CLAs in rumen cultures [114].
All studies mentioned above indicate the significance of a range of pH around neutral for the synthesis of CLA from L. plantarum. This optimal pH range appears to result from a compromise between the optimal pH for the growth of L. plantarum [115] and the optimal pH for the activity of the key enzymes involved in the biosynthesis of CLA. The optimal initial culture pH for the production of CLA by different L. plantarum strains has been reported as 5.5 [104] and 6.0 [106,116,117], while Ando and others found that the maximum CLA synthesis from L. plantarum JCM 1551 occurred at pH 6.5 [100]. As stated above, CLA synthesis in L. plantarum includes the enzymes CLA-HY, CLA-DH and CLA-DC. In a study conducted by Takeuchi and others, it was found that the CLA-HY enzyme is optimally active at pH 5.5 [118].
In addition to pH and temperature, the substrate concentration (LA) is also crucial for the production of CLA. The LA probably inhibits growth by increasing the permeability of the bacterial membrane as a result of its surfactant action [119].
The amount of CLA in biomass depends on the initial LA concentration, cell growth state, and LAI activity for the bioconversion of LA to CLA [80]. As a general consideration, it can be said that CLA production increases with the increase in concentration of LA [78,97,104,106], provided that the concentration of LA does not exceed a tolerable limit. However, as we will see in some of the studies cited below, individual strains of L. plantarum differ in their tolerance to the initial concentration of LA.
Many LAB, including L. plantarum, demonstrate the ability to produce CLA isomers from the LA, and those isomers are mainly CLA1 and CLA2.
The washed cells of L. plantarum ZS2058, isolated from Chinese traditional fermented vegetables, in de Man–Rogosa–Sharpe (MRS) medium, containing 0.5 mg/mL of LA, produced a mixture of CLA1 and CLA2, 96.4% of which was CLA1. After 24 h at 37 °C under aerobic conditions, 312.4 μg/mL of CLA1 was produced [109].
In a subsequent work conducted by the same research group, it was found that the optimal pH and optimal temperature of bioconversion by L. plantarum ZS2058 yielding CLA were 6.5 and 40 °C, respectively [108]. Natural sauerkraut, a fermented food made primarily from fermentations of cabbage, contains a great number of LAB including L. plantarum, which is often predominant [120,121]. In a study reported in 2009 [105], fifteen CLA-producing LABs were isolated from natural fermentations of sauerkraut. In MRS 2.5 mL/L of LA was added and after 24 h at 30 °C L. plantarum NCUL005 showed the highest CLA-producing ability (0.623 mg/mL). The transformation efficiency of converting LA into CLA by NCUL005 was 26.67%, and the CLA produced by L. plantarum NCUL005 comprised a mixture of 32.2% CLA1 and 67.8% CLA2 isomers [105].
Lactobacillus plantarum WU-P19 isolated from a sample of a traditional fermented Indian trumpet (midnight horror, Oroxylum indicum) was investigated, with the aim of enhancing the LA conversion to CLA. Under static conditions at 37 °C and pH 6.0, after 36 h, MRS was supplemented with the cell permeabilizing agent chitosan, resulting in an increased cellular uptake of LA (37 mg/g) and production of 21 mg/g total CLA. Nearly 50% of total CLA was CLA1 and the remainder was CLA2 [106].
In a study conducted by Liu and others, forty-three LAB strains with a CLA-producing ability were isolated from three naturally fermented pickle brines [103]. At 48 h in MRS broth to which LA was added (100 µg/mL), L. plantarum lp15 exhibited the greatest capacity to produce CLA (26.1 μg/mL) and the highest tolerance to LA, up to 600 µg/mL. This strain converted about 25% of LA into CLA isomers, of which 75% was CLA1 [103].
Yang and others assessed the capability of some strains of food-derived lactobacilli to produce CLA from LA. They found that L. plantarum ZS2058 was the most efficient CLA producer in MRS broth with more than 50% LA conversion to CLA1 and t9, t11-CLA as dominant isomers [110].
The ability of different LAB species to produce CLA from LA has also been evaluated [99]. After 24 h at 37 °C in MRS broth, containing 1 mg/mL of LA and 1 mg/mL Tween 80, L. plantarum DSM 20179 showed the highest potential to produce CLA (95.25 μg/mL). An optimization analysis also showed that the maximum CLA production (240.69 μg/mL) by L. plantarum DSM 20179 can be obtained in skim milk (SM) supplemented with 1 mg/mL Tween 80, 7 g/L D-glucose, 3.0 mg/mL LA and 4.01 g/L yeast extract [99].
Sixty-four strains of food-grade lactobacilli and bifidobacteria were examined to verify their ability to produce CLA [41]. Lactobacilli were grown in MRS medium, and LA was added (500 μg/mL) at 37 °C for 48 h. In this case, more than 90% of the CLA was detected in the supernatant. L. plantarum CRL1920 isolated from chicha (fermented maize) and L. plantarum CRL1935 isolated from cheese, were able to conjugate LA with a conversion rate of 3.47% and 3.50%, respectively. In detail, L. plantarum CRL1920 and L. plantarum CRL1935 produced 6.95 and 7.26 μg/mL of CLA1; 5.11 and 5.22 μg/mL of CLA2; and 5.28 and 5.04 μg/mL of trans-9, trans-11 CLA, respectively [41].
Fifty-seven CLA producing LAB strains isolated from fermented dairy products were screened for their ability to produce CLA in MRS broth and SM, supplemented with 0.5 mg/mL of linoleic acid [95]. Positive strains were classified as L. plantarum (44%), L. gasseri (30%), L. fermentum (21%) and L. salivarius (5%) species. L. plantarum HIF15 was reported as the best producer of CLA (46.18 µg/mL in MRS and 52.61 µg/mL in SM) with a higher amount of CLA1 isomer (34.73 µg/mL in MRS; 38.31 in SM) [95].
Ribeiro and others conducted a study on 110 LAB isolated from a traditional Azorean cheese to test their ability to convert free LA to CLA. L. plantarum L2C21E8 and L. plantarum L3C1E8 were selected as CLA-producing strains. LABs were incubated in MRS broth containing free LA (0.5 mg/mL) and 2% (w/v) Tween 80, at 30 °C for 48 h. Preliminarily, CLA production was quantified by a spectrophotometric method [102]. L. plantarum L2C21E8 and L. plantarum L3C1E8 produced 17.94 and 15.36 μg/mL of CLA (expressed as CLA1 concentration) with a conversion of 3.59% and 3.07%, respectively. Afterwards, the CLA profiles were determined in cell supernatant and in cell pellet using gas chromatography–mass spectroscopy (GC–MS). CLA1 and CLA2 were the most abundant isomers generated, and they were mainly found in the cell supernatant.
These results are in agreement with previous studies that have shown that the production of CLA is located primarily in the extracellular phase [41], although it can also be found in smaller amounts in the cell membrane as a structural lipid [122].
In a study reported in 2009, six different strains of L. plantarum were examined for their ability to synthesize different metabolites including CLA [123]. L. plantarum strains were grown in MRS medium containing LA from 1% to 10% (w/v), and the LA metabolites formed in the medium were identified and quantitated by GC–MS. L. plantarum 2–3 showed maximum growth and conversion of LA to different metabolites from 1% to 10% (w/v) of LA supplied. The production of total LA metabolites gradually increased with the increase of LA concentration from 1% to 10% (w/v) [123]. This indicates that the L. plantarum showed high tolerance to LA by converting it into less toxic compounds. As previously reported, in fact, other studies have also suggested that the conversion of free LA metabolites to CLA could be a detoxification mechanism adopted by bacterial cells [33].
The aim of a subsequent study conducted by Aziz and others was to investigate the ability of L. plantarum YW1 to produce CLA. The results showed that L. plantarum YW11 is able to convert LA into a CLA isomer (rumenic acid) and stearic acid, at different concentrations of LA (also in this case1–10% w/v) [107]. L. plantarum YW11, isolated from kefir from Tibet, has been found to possess antimicrobial, anticancer, antioxidant, and immuno-regulatory activities [124,125].
In another study, some probiotic properties were evaluated using 10 strains of high CLA-producing LAB isolated from Jeotgal (a high-salt fermented seafood) [75]. The LAB were cultured in MRS broth containing LA (5 mg/mL) and 1% (v/v) Tween 80 at 30 °C for 48 h. The CLA isomers were quantified using GC–MS. L. plantarum JBCC105683 produced the highest concentration of CLA (748.8 μg/mL) and the ratio of CLA1 to CLA2 was approximately 80:20, whereas L. plantarum JBCC105645 produced an approximately equal proportion of CLA1 and CLA2 (~50:50 ratio). L. plantarum JBCC105683 strongly stimulated the immunological regulatory gene PMK-1 and a host defense antimicrobial peptide gene, clec-60, in Caenorhabditis elegans and produced significant induction of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, IL-12, and IL-10 in RAW 264.7 macrophages, indicating that they are good candidates for probiotics with a high CLA-converting activity [75].
Based on the results reported in this section, it is possible to highlight the following summary considerations:
- -
- the optimal temperature for CLA production is between 30 and 37 °C, a temperature range that also represents an optimal condition for the development of a mesophilic bacterium as L. plantarum;
- -
- conditions of the culture medium close to neutrality (pH 6–6.5) allows for the maximum amounts of produced CLA, with this optimal pH range resulting from a compromise between the optimal pH for the growth of L. plantarum and the optimal pH for the activity of the key enzymes involved in CLA biosynthesis;
- -
- the produced amount of the various CLA isomers showed a high variability among the different L. plantarum strains;
- -
- CLA production increases with the increase of concentration of LA provided up to a value of LA concentration not exceeding the tolerable limit for the bacterium, with this limit proved to depend on the strain.
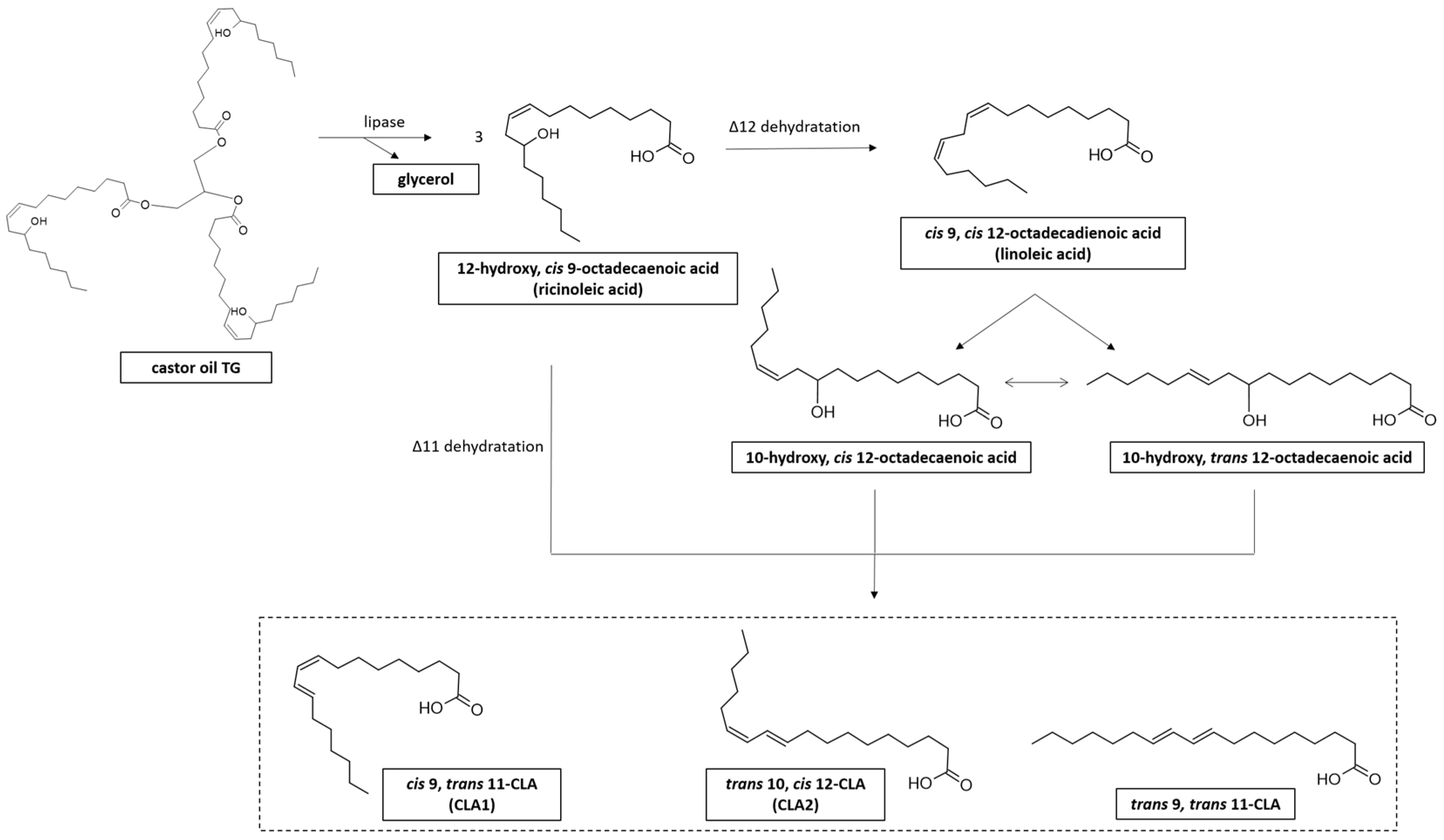
5. CLA Production from Vegetable Oils by L. plantarum
Castor, sunflower, safflower, and sesame oils are the most common vegetable oils used as a microbial substrate for CLA production [40] (Table 2). LAB can produce CLA from RA via its direct conversion into CLA through dehydration or through transformation of RA to LA followed by isomerization of LA to CLA (Figure 5) [126].
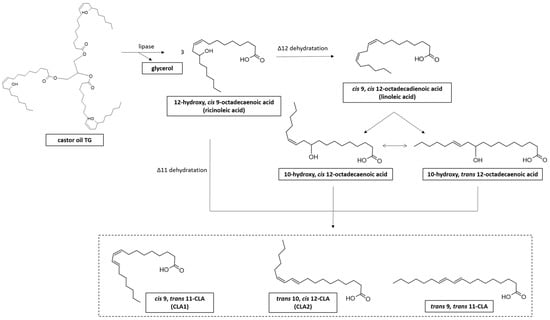
Figure 5.
Synthesis of CLA from castor oil and ricinoleic acid (RA) derivative. RA is the most abundant (around 90%) fatty acid (FA) present in castor oil triglyceride (TG), contained in castor bean (Ricinus communis L.) [127].
Based on these considerations, some authors have used castor oil as an alternative substrate for the production of CLA by L. plantarum. The lipase enzyme for castor oil hydrolysis has been used to release RA as a substrate for CLA production [42,100,116,128].

Table 2.
CLA production from vegetable oils by Lactiplantibacillus plantarum (formerly Lactobacillus plantarum).
Table 2.
CLA production from vegetable oils by Lactiplantibacillus plantarum (formerly Lactobacillus plantarum).
| L. plantarum Strain | Culture Medium, Environmental Conditions | CLA Source | Total CLA | CLA Isomers (%) | Ref. |
|---|---|---|---|---|---|
| AB20-961 (DSM2601) | sausage fermentation 79 h (73 h) | 5% SAO | 4.1 mg/g fat (7.5 mg/g fat) in sausage | n.d. | [129] |
| AB20-961 | subculture in MRS, 37 °C, 24 h sucuk fermentation 12 h | 2% HSO | 6.1 mg/g fat in sucuk | 69% CLA1 31% CLA2 in sucuk | [117] |
| AKU1009a | KPB, pH 6.5, 37 °C, washed cells, 24 h | 4.0 mg/mL CO (88% RA, 5% LA, 7% other) | 1.14 mg/mL | 17% CLA1 83% t9t11-CLA | [130] |
| ATCC8014 | MRS, 37 °C, pH of 6.5, 72 h, 2% washed cells | 8 mg/mL SO | 0.8 mg/mL | 48% CLA1 52% CLA2 | [78] |
| IMAU60042 | MRS, 37 °C, 20 h | 1.2 μg/mL SO (LA 67.3% of total FA) | 48.7 μg/g | n.d. | [131] |
| JCM 1551 | 1.0 M citrate buffer 37 °C, pH of 6.0, 99 h | 5.0 mg/mL CO | 2.7 mg/mL (up to 7.5 mg/mL with 30 mg/mL CO, 171 h) | 26% CLA1 74% t9t11-CLA | [116] |
| Lp in co-culture with L. acidophilus | SM, pH 6.4, 36 °C, 48 h 5% inoculum | 5% SAO | 316.5 μg/mL | n.d. | [132] |
| L. plantarum from buffalo milk | KPB, pH 6.5, 37 °C, 20 h 12% (w/v) washed cells, lipase | 8 mg/mL CO | 406 μg/mL | 56% CLA1 44% CLA2 | [128] |
| P1 | MRS, 37 °C, 24 h | 10 mg/mL SO | 400 μg/mL | n.d. | [133] |
| P1201 | soy-powder hydrolyzed milk 35 °C, 48 h | 1% SAO | 1.3 mg/g | 92% CLA1 8% CLA2 | [134] [135] |
| PTCC1058 | KPB, pH 6.5, 37 °C, 121 h 15% (w/v) washed cells, lipase | 4.6 mg/mL CO | 1.7 mg/mL | 44% CLA1 46% CLA2 | [136] |
| PTCC1745 | KPB, pH 6.5, 37 °C, 121 h 15% (w/v) washed cells, lipase | 9.6 mg/mL CO | 1.6 mg/mL | 41% CLA1 55% CLA2 | [136] |
| UALp-05 in co-culture with of L. lactis ssp. lactis and L. lactis ssp. cremoris | 91% milk fat to produce cheddar cheese | 9% SAO | 75 μg/g in 90 days ripened cheese | 25% CLA1 20% CLA2 20% c9c11-CLA 18% t9c11-CLA 16% c10t12-CLA | [43] |
Abbreviations: CLA1, cis-9, trans-11 CLA; CLA2, trans-10, cis-12 CLA; CO, castor oil; FA, fatty acids; HSO, hydrolyzed sunflower oil; KPB, potassium phosphate buffer; LA, linoleic acid; MRS, de Man, Rogosa and Sharp medium; RA, ricinoleic acid; SAO, safflower oil; SM, skim milk; SO, sunflower oil; n.d., not determined.
More than twenty years ago, Kishino and others conducted the first study on the biosynthesis of CLA from castor oil and RA by L. plantarum [130]. Using washed cells of L. plantarum AKU 1009a, 1.14 mg/mL of CLA were produced from 4.0 mg/mL castor oil in the presence of lipase. The CLA produced was a mixture of CLA1 (0.19 mg/mL) and trans-9, trans-11 octadecadienoic acids (0.95 mg/mL) [130].
In a separate study, L. plantarum JCM 1551 produced 2.4 mg/mL of CLA consisting of a mixture of two isomers, CLA1 (21% of total CLA) and trans-9, trans-11-octadecadienoic acid (79% of total CLA) [100].
In a subsequent study, the same research group found that a mixture of two isomers, CLA1 and trans-9, trans-11-octadecadienoic acid, was obtained by using as catalyst washed cells of L. plantarum JCM 1551 in the presence of lipase and 5.0 mg/mL of castor oil, 2.7 mg/mL of CLA was produced in 99 h, and from 30 mg/mL of castor oil, 7.5 mg/mL of CLA was produced in 171 h [116].
In another study, castor oil was used as substrate for the production of CLA using washed cells of L. plantarum strains and lipases as catalysts [128]. Mass spectral analysis showed that CLA1 (56.55%) and CLA2 (43.45%) isomers were produced [128].
In other research, CLA was produced from castor oil using washed cells of L. plantarum PTCC 1058 and L. plantarum subsp. plantarum PTCC1745 [136]. L. plantarum PTCC1058 has been distinguished by its ability to produce extracellular 1.7 g/L CLA from 4.6 mg/mL of castor oil. The resulting CLA was a mixture of CLA1 (44% of total CLA) and CLA2 (46% of total CLA) [136].
In the above studies, in which castor oil was used, the ability of L. plantarum to produce CLA isomers (mainly CLA1, CLA2, and trans-9, trans-11-octadecadienoic acid) from the RA was highlighted. This capacity has been optimized by adopting a temperature of 37 °C and a pH of the growing medium ranging between 6 and 6.5 (see Table 2 for refs).
It is important to point out that the castor oil seed is one of the well-known oil seeds in some areas of Africa where it forms an important part of the diet. Among traditional condiments used in the eastern part of Nigeria are Ogiri-Igbo and other Ogiri foods that are fermented products of Ricinus communis [137]. Ogiri foods have played major roles in the diets of communities in rural regions, serving not only as a nutritious non-meat protein substitute, but also as condiments and flavoring agents in soups and sauces [138].
Castor oil derived from castor beans contains toxic compounds such as ricin, a Type II ribosome-activating protein, and other related compounds as ricinine and ricinoleic acid. For this reason, it cannot be consumed directly but must be processed by fermentation to remove these toxic constituents [139]. Various studies of physical, chemical, and biological treatments have been conducted to establish efficient methods for castor meal detoxification [140]. LAB fermentation has been shown to be a technique that leads to a complete detoxification of castor oil and improves its nutritional value [141]. Additional research has shown that some LAB species, including L. plantarum, are part of the microbial community present during spontaneous fermentation of castor oil bean in Ogiri foods [142,143,144].
In developing products containing castor oil with improved quality and safety, the inclusion of pro-technological and probiotic microorganisms (such as L. plantarum) is crucial and microorganisms will serve as sustainable interventions for the development of African-specific starter cultures [145]. Therefore, it is desirable that in the future scientific investigations be conducted to select CLA-producing strains of L. plantarum able to perform a detoxification of fermented castor oil.
Sunflower (Helianthus annuus L.) is considered to be one of the most important oil plants having 22–55% oil content. Sunflower oil contains approximately 15% SFA and 85% unsaturated fatty acids (UFAs), and sunflower oil UFAs have been shown to consist of 14–43% oleic acid and 44–75% LA [146].
The following studies include investigations of sunflower oil, soy milk, castor oil, cod liver oil, flax oil, and linseed oil as sources of LA for the production of CLA by LAB [78,117,131,133].
In a study conducted by Li and others, six strains of L. plantarum (IMAU60042, IMAU60171, IMAU10156, IMAU30126, IMAU70089, and P8), after being isolated from traditional naturally fermented dairy products, were able to convert LA to CLA using sunflower oil as a substrate or during soy milk fermentation [131]. Sunflower oil used in this experiment contained LA that was 67.3% of total FA. Soymilk was added with 6.5% sucrose and after inoculation of LABs was incubated at 42 °C. The results showed that the six L. plantarum strains had different abilities to produce CLA. After 20 h at 37 °C, L. plantarum IMAU60042 produced the highest concentration of CLA (48.7 μg/g) in MRS supplemented with 1.2 μg/mL of sunflower oil. The same strain also produced the highest concentration of CLA in soy milk (122.4 μg/g) for 12 h. The CLA was composed of CLA1 and CLA2 isomers [131].
Hosseini’s research verified the production of CLA by L. plantarum ATCC 8014 from sunflower oil and castor oil as cost-effective substrates, compared to linoleic acid. The reaction mixture contained 1 mL of 100 mM potassium phosphate buffer (pH 6.5), 2% washed cells and different concentrations (1, 4, 8, 12 mg/mL) of LA, sunflower oil, and castor oil. The tests were carried out micro-aerobically at 37 °C for 72 h. The washed cells of L. plantarum ATCC 8014 produced the highest concentration of CLA isomers, compared to other LAB species examined. Analysis of the results revealed that the CLA produced was a mixture of two bioactive isomers including CLA1 (0.38 mg/mL) and CLA2 (0.42 mg/mL) from 8 mg/mL sunflower oil [78].
Al-Saman and others investigated the impact of oil type on the production capacity of CLA through eight LAB strains belonging to different species, including L. plantarum P1. Two vegetable oils (sunflower oil and linseed oil) and cod liver oil were used as substrates in MRS medium containing 1% of Tween 80. The oils were added in a concentration of 10 mg/mL to the medium and incubated for three days at 37 °C. CLA produced by L. plantarum P1 was 400.32 μg/mL from sunflower oil, 432.55 μg/mL from cod liver oil, and 488.12 μg/mL from flax oil. Based upon the results obtained, it can be deduced that the differences in CLA production may be due to the different fatty acid composition of the oils used [133]. The oil of Acer truncatum Bunge (ATB) seed is a novel, edible oil with a richer content of oleic and linoleic acids than other edible oils including rapeseed, peanut, grape and sunflower oils [147]. ATB is a tree species native to China [148].
In 2017, Chen and others published a study in which they developed a new method to produce two isomers of CLA from ATB-seed oil by fermentation using L. plantarum CGMCC8198. A novel probiotics strain L. plantarum CGMCC 8198 was inoculated (1%) in MRS broth with or without 0.5 mg/mL ATB-seed oil and then incubated anaerobically at 30 °C with a gaseous mixture of 80% nitrogen, 10% carbon dioxide, and 10% hydrogen. Analyses by GC–MS showed that the concentration of CLA1 and CLA2 in ATB-seed oil could be increased by about 9- and 2.25-fold, respectively, after being fermented by L. plantarum CGMCC 8198 [149].
6. CLA-Producing L. plantarum Strains in Fermented Food
The management of the CLA content in foods provides an important way to increase their nutritional and functional value and may significantly improve marketing, and possibly sales, by adding value to traditional products. In the absence of added CLA-producing bacteria, CLA is mostly found in the fatty meat and dairy products of ruminant animals and is derived from the metabolism of ruminal microorganisms [150].
The estimated daily human CLA intake ranges from 200 to 1000 mg per day [151,152,153]. A dietary increase in LA in the feed of dairy cows is one of the feeding strategies to increase the concentration of CLA in milk. The main sources of LA for animal feed are mainly cereals, oilseeds, and oils [74,154]. Because the natural concentrations of CLA in milk products are relatively low (normally ranges between 2 and 37 mg/g fat) to exert their health benefits [155], production of CLA by LAB can be achieved by microbial cultures to produce functional and fermented food products containing a higher amount of CLA [31].
Below and in Table 3, we report some results obtained using different CLA-producing strains of L. plantarum as added cultures in different fermented dairy and meat products.

Table 3.
CLA production by Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) in different fermented foods.
Ye and others tested the ability of L. plantarum, Lactobacillus acidophilus and Streptococcus thermophilus strains to produce conjugated linoleic acid (CLA) [132]. LAB were co-cultured in a medium containing skim milk supplemented with hydrolyzed safflower oil. More CLA was produced by co-culture than by using a single strain. Maximal CLA production (316.52 μg/mL) was obtained with an L. acidophilus–L. plantarum co-culture after 48 h at pH 6.4 and 36 °C [132].
The objective of another study was to examine the ability of different LAB strains isolated from artisanal cheese for their ability to produce CLA in skim milk and in simulated gastrointestinal conditions [156]. L. plantarum J25 bacteria were able to survive in simulated gastrointestinal conditions and to adhere to the intestinal mucosa. In skim milk from 2% LA, L. plantarum J25 produced 71.5% (13.72 μg/mL) CLA1 and 28.5% (9.81 μg/mL) CLA2 isomers. The tests were conducted at 37 °C for 48 h under aerobic conditions. In simulated intestinal juice solution containing 0.2% LA, L. plantarum J25 produced 15.05 μg/mL of CLA [156].
In our opinion, this result is of relevant interest, since it strongly suggests that potential probiotic L. plantarum strains preserve their ability to produce CLA even after having passed the gastro-intestinal tract; in this way, they could be able to express in situ its “postbiotic” capacities using CLA precursors contained in food.
In a study on 129 LAB strains [101], previously isolated from raw-milk, artisanal cheeses [158], L. plantarum L188 and L. plantarum L200 were recognized as producers of CLA. Miniature cheeses made with the addition of the L. plantarum L200 showed a higher content of CLA1 compared to the CLA1 content of the control cheeses, at 1.09% and 0.69% of total FAs, respectively [101]. Therefore, the authors of the present study suggest that the L. plantarum L200 strain could be used as an additional culture to increase the CLA content in cow’s milk cheeses.
A recent study investigated the characteristics of CLA-enriched cheddar cheese obtained using L. plantarum UALp-05 as a starter and safflower oil as a substrate for CLA synthesis [43]. The results obtained showed that L. plantarum UALp-05 and safflower oil did not negatively affect the composition of the cheddar cheese, contributing to a cheese in which the concentration of CLA increased even during the entire ripening phase.
Meat from ruminants generally has higher levels of CLA than does meat from non-ruminants. The highest CLA concentrations were found in lamb (4.3–19.0 mg/g lipid) and with slightly lower concentrations in beef (1.2–10.0 mg/g lipid) [152]. There is an increasing demand for meat and meat products with higher levels of polyunsaturated fatty acid (PUFA).
This also applies to CLA; in fact, even though animal source foods such as beef and dairy products naturally contain CLA, the concentration of CLA is generally considered low, especially in beef [159]. Therefore, studies investigating the enhancement of the concentration of CLA in meat and meat products by dietary manipulation and direct addition have increased in recent years [160]. LAB are a microbial group that contributes to the definition of the qualitative and sensory characteristics of fermented sausages [161,162]. L. plantarum has been shown as to be a dominant bacterium in many traditionally fermented sausages worldwide and is often proposed as a starter in the production of these products [57,163]. However, there are few studies related to enhancing microbial production of CLA by L. plantarum in the meat system [96,117,164].
Sucuk is a fermented dry and spicy sausage which is consumed in several Balkan, Middle Eastern and Central Asian cuisines [117]. Özer and others have used two CLA-producing strains of L. plantarum as starters in the fermentation of Sucuk. Preliminarily, twenty-three L. plantarum strains were screened in vitro for their ability to convert the LA of hydrolyzed sunflower oil (HSO) to CLA. The highest CLA production was obtained, after incubation at 37 °C for 24 h in MRS broth at pH 6.0 with 2% HSO, using L. plantarum AA1-2 and L. plantarum AB20-961 isolated from human sources. L. plantarum AB20-961 produced greater quantities of CLA isomers (CLA1 and CLA2) in sucuk during the first 24 h of fermentation, after beginning the fermentation initially at pH 5.8 or pH 6.0. In sucuk obtained using L. plantarum AB20-961 as starter, total CLA content increased from 4.9 to 5.4 mg/g of fat at initial pH of 5.8, and increased from 4.8 to 6.1 mg/g of fat at initial pH of 6.0. L. plantarum AA1-2 was not able to produce CLA during sucuk fermentation [117].
At the 63rd International Congress of Meat Science and Technology, results were presented regarding the use of L. plantarum AB20-961 and L. plantarum DSM2601 as starter cultures in sausage fermentation to enhance CLA contents of the final product [165]. These results showed that the CLA content of the sausage increased significantly during fermentation by both L. plantarum strains. While the CLA content of the sausage dough was 3.41 mg CLA/g fat, after the fermentation process, CLA contents of the sausages produced with L. plantarum AB20-961 and L. plantarum DSM2601 were 4.15 mg CLA/g fat and 7.54 mg CLA/g fat, respectively [165].
The aim of a subsequent study conducted by the same group [164] was to use optimized processing conditions for L. plantarum AB20-961 and L. plantarum DSM 2601 to obtain the highest CLA contents of semi-dry, fermented sausages. Results indicated that the CLA concentrations of the sausages were increased 21% by L. plantarum AB20–961 and 121% by L. plantarum DSM2601 after fermentation, compared to the initial concentration of CLA [164].
A follow-up study conducted by the same investigators determined the optimal pH, time, temperature, variety and concentration of the added dietary acid, and the initial bacterial cell density (L. plantarum AB20-961 and L. plantarum DSM2601) to maximize CLA production in fermented ground beef [96]. Using safflower oil, the greatest concentrations of CLA produced by L. plantarum AB20-961 and L. plantarum DSM2601 were 7.91 and 38.31 mg CLA/g fat, respectively [96].
As evidenced by these studies, different factors can affect the ability of L. plantarum to produce CLA in meat and meat products. Some of these factors include the pH of the meat, the fermentation time and temperature, the amount and variety of the FA added, and the initial starter cell density. The results obtained in the above-mentioned studies conducted by Özer and others have shown that it is possible to have microbial production of CLA during the fermentation of meat products using L. plantarum as a starter culture.
Soybean, Glycine max (L.), is an important crop that serves as a significant source of lipids and proteins, and soybean is the most commonly produced oil crop in the world. Soybean oil is primarily used in the production of shortening, margarines, cooking or frying oils, salad dressings, and mayonnaise. Soybeans, in addition to their nutritional value, contain specific bioactive phytochemicals [166,167] and approximately 18–24% of total lipids. The FA in soybean seed oil include palmitic acid (11%), stearic acid (4%), oleic acid (23%), LA (54%), and α-LA (8%) [168]. Due to their high LA content, soybeans have the potential to produce CLA-rich foods through LAB fermentation [2]. In a study reported in 2015 [157], L. plantarum S48 and L. plantarum P1201 produced CLA1 and CLA2 isomers from 8% skim milk medium supplemented with three different free LA concentrations (0.25, 0.5 and 1 mg/mL) at 35 °C for 48 h. Subsequently, the authors conducted comparative tests on the production of CLA in 10% fresh, steamed, and roasted soy-powder milk. After 48 h of fermentation at 37 °C, L. plantarum S48 and L. plantarum P1201 produced the greatest amounts of total CLA in steamed soy milk powder, i.e., 183.57 μg/mL and 198.72 μg/mL, respectively, of which 165 μg/mL and 180 μg/mL were CLA1. In other subsequent works, it has been shown that soy milk fermented by CLA-producing L. plantarum P1201 is enriched with both CLA and flavonoids and possesses some functional properties such as antioxidant activity and positive modulation of lipid metabolism [134,169].
Hwang and others conducted a study investigating the production of fermented soy milk using L. plantarum P1201. Soymilk with 2% sucrose was hydrolyzed with 10 U of cellulase, protease, and esterase at 37 °C for 24 h, and finally 1.0% safflower oil and the starter (2.0 × 107 CFU/mL) were added. Fermentation was carried out at 35 °C for 48 h. L. plantarum P1201 increased the content of isoflavones in the aglycone form (daidzein, glycitein and genistein) and produced CLA in fermented soymilk by improving some functional properties that positively influenced adipogenesis and lipid metabolism [135].
7. Conclusions
Thanks to the growth of scientific evidence, interest in the biological significance of CLA to human nutrition continues to increase. At present, different functional foods such as yogurt, cheese, and fermented soya milk, are manufactured with CLA-producing bacteria in order to obtain a final product with high CLA content. Although in vitro production of CLA has been intensively studied, few studies have verified the production of CLA in vivo by CLA-producing bacteria. The variation in CLA production among strains of LAB depends on many factors, such as the intrinsic characteristics of each particular strain and the environmental conditions in which the strain grows.
In this context, the optimization and standardization of fermentation conditions are necessary to optimize CLA synthesis in functional foods. Many strains of L. plantarum have been proposed as human probiotics and are widely used as starter cultures to produce various fermented foods. Among the LAB, specific strains belonging to this species have been identified as the most efficient producers of CLA. Therefore, when fermenting foods, the use of appropriate strains of L. plantarum as a starter or additional culture should be considered as a critical step. Progress in research on the use of various strains of L. plantarum and the conditions for their maximum efficiency can serve as a key factor in the design of new CLA-enriched functional foods.
Author Contributions
Conceptualization, M.I., C.D.M. and G.P.; writing—original draft preparation, M.I., F.L., C.D.M. and G.P.; writing—review and editing, M.I., G.P., C.D.M. and T.W.C.J.; visualization, C.D.M.; supervision, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
Affiliation Global Agronomy LLC for author Thomas W. Crawford Jr. refers to only the scientific entity: in this study, he acts, pro bono, as independent researcher. Neither, Global Agronomy, LLC has a role in financing, supporting, and/or influencing the current investigation. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kim, J.H.; Kim, Y.; Kim, Y.J.; Park, Y. Conjugated linoleic acid: Potential health benefits as a functional food ingredient. Annu. Rev. Food Sci. Technol. 2016, 7, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Meng, X.; Tong, P.; Liu, X. Biosynthesis of c9,t11-conjugated linoleic acid and the effect on characteristics in fermented soy milk. Food Chem. 2022, 368, 130866. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, E.B.T.; de Melo, I.L.P.; Mancini-Filho, J. Chemical and physiological aspects of isomers of conjugated fatty acids. Cienc. Tecnol. Aliment. 2010, 30, 295–307. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Machado, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Bioactive lipids: Chemistry, biochemistry, and biological properties. In Bioactive Lipids; Academic Press: Cambridge, MA, USA, 2022; pp. 1–35. ISBN 9780128240434. [Google Scholar]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Wawrzeńczyk, C. Production of concentrates of CLA obtained from sunflower and safflower and their application to the lipase-catalyzed acidolysis of egg yolk phosphatidylcholine. Eur. J. Lipid Sci. Technol. 2016, 118, 1566–1578. [Google Scholar] [CrossRef]
- Choi, B.-D.; Kang, S.-J.; Ha, Y.-L.; Ackman, R.G. Accumulation of conjugated linoleic acid (CLA) in tissues of fish fed diets containing various levels of CLA. In Quality Attributes of Muscle Foods; Springer: Boston, MA, USA, 1999; pp. 61–71. [Google Scholar]
- Dachev, M.; Bryndová, J.; Jakubek, M.; Moučka, Z.; Urban, M. The Effects of Conjugated Linoleic Acids on cancer. Processes 2021, 9, 454. [Google Scholar] [CrossRef]
- Malinska, H.; Hüttl, M.; Oliyarnyk, O.; Bratova, M.; Kazdova, L. Conjugated linoleic acid reduces visceral and ectopic lipid accumulation and insulin resistance in chronic severe hypertriacylglycerolemia. Nutrition 2015, 31, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Siennicka, A.; Baśkiewcz-Hałasa, M.; Bober, J.; Machalinski, B.; Chlubek, D. Conjugated linoleic acid isomers may diminish human macrophages adhesion to endothelial surface. Int. J. Food Sci. Nutr. 2012, 63, 30–35. [Google Scholar] [CrossRef]
- DeGuire, J.R.; Makarem, N.; Vanstone, C.A.; Morin, S.; Duque, G.; Weiler, H.A. Conjugated linoleic acid is related to bone mineral density but does not affect parathyroid hormone in men. Nutr. Res. 2012, 32, 911–920. [Google Scholar] [CrossRef]
- Lehnen, T.E.; da Silva, M.R.; Camacho, A.; Marcadenti, A.; Lehnen, A.M. A review on effects of conjugated linoleic fatty acid (CLA) upon body composition and energetic metabolism. J. Int. Soc. Sports Nutr. 2015, 12, 36. [Google Scholar] [CrossRef]
- Derakhshande-Rishehri, S.-M.; Mansourian, M.; Kelishadi, R.; Heidari-Beni, M. Association of foods enriched in conjugated linoleic acid (CLA) and CLA supplements with lipid profile in human studies: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 2041–2054. [Google Scholar] [CrossRef]
- Viladomiu, M.; Hontecillas, R.; Bassaganya-Riera, J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016, 785, 87–95. [Google Scholar] [CrossRef]
- Benjamin, S.; Prakasan, P.; Sreedharan, S.; Wright, A.-D.G.; Spener, F. Pros and cons of CLA consumption: An insight from clinical evidences. Nutr. Metab. 2015, 12, 4. [Google Scholar] [CrossRef]
- Rastgoo, S.; Shimi, G.; Shiraseb, F.; Karbasi, A.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Asbaghi, O.; Zamani, M. The effects of conjugated linoleic acid supplementation on inflammatory cytokines and adipokines in adults: A GRADE-assessed systematic review and dose–response meta-analysis. Front. Immunol. 2023, 14, 1092077. [Google Scholar] [CrossRef]
- Asbaghi, O.; Shimi, G.; Hosseini Oskouie, F.; Naseri, K.; Bagheri, R.; Ashtary-Larky, D.; Nordvall, M.; Rastgoo, S.; Zamani, M.; Wong, A. The effects of conjugated linoleic acid supplementation on anthropometrics and body composition indices in adults: A systematic review and dose-response meta-analysis. Br. J. Nutr. 2023, 131, 406–428. [Google Scholar] [CrossRef]
- Basak, S.; Duttaroy, A.K. Conjugated linoleic acid and its beneficial effects in obesity, cardiovascular disease, and cancer. Nutrients 2020, 12, 1913. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Feizi, A.; Heidari, Z.; Haghighatdoost, F. Association of omega-6 polyunsaturated fatty acids with blood pressure: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 2247–2259. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E.; et al. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: A review of pre-clinical and human trials with current perspectives. Nutrients 2018, 11, 370. [Google Scholar] [CrossRef]
- Yuce, M.; Gumuskaptan, C.; Con, A.H.; Yazici, F. Conjugated linoleic acid strengthens the apoptotic effect of cisplatin in A549 cells. Prostaglandins Other Lipid Mediat. 2023, 166, 106731. [Google Scholar] [CrossRef]
- Słowikowski, B.K.; Drzewiecka, H.; Malesza, M.; Mądry, I.; Sterzyńska, K.; Jagodziński, P.P. The influence of conjugated linoleic acid on the expression of peroxisome proliferator-activated receptor-γ and selected apoptotic genes in non-small cell lung cancer. Mol. Cell. Biochem. 2020, 466, 65–82. [Google Scholar] [CrossRef]
- Maki, K.C.; Eren, F.; Cassens, M.E.; Dicklin, M.R.; Davidson, M.H. ω-6 Polyunsaturated fatty acids and cardiometabolic health: Current evidence, controversies, and research gaps. Adv. Nutr. 2018, 9, 688–700. [Google Scholar] [CrossRef]
- Sellem, L.; Flourakis, M.; Jackson, K.G.; Joris, P.J.; Lumley, J.; Lohner, S.; Mensink, R.P.; Soedamah-Muthu, S.S.; Lovegrove, J.A. Impact of replacement of individual dietary SFAs on circulating lipids and other biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials in humans. Adv. Nutr. 2022, 13, 1200–1225. [Google Scholar] [CrossRef]
- Kim, H.K.; Kang, E.Y.; Go, G. Recent insights into dietary ω-6 fatty acid health implications using a systematic review. Food Sci. Biotechnol. 2022, 31, 1365–1376. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition (SACN). Satured Fats and Health. 2019; p. 443. Available online: https://www.gov.uk/government/publications/saturated-fats-and-health-sacn-report (accessed on 17 March 2024).
- MacDonald, H.B. Conjugated linoleic acid and disease prevention: A review of current knowledge. J. Am. Coll. Nutr. 2000, 19, 111S–118S. [Google Scholar] [CrossRef]
- Ritzenthaler, K.L.; McGuire, M.K.; Falen, R.; Shultz, T.D.; Dasgupta, N.; McGuire, M.A. Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology. J. Nutr. 2001, 131, 1548–1554. [Google Scholar] [CrossRef]
- Gorissen, L.; Leroy, F.; De Vuyst, L.; De Smet, S.; Raes, K. Bacterial production of conjugated linoleic and linolenic acid in foods: A technological challenge. Crit. Rev. Food Sci. Nutr. 2015, 55, 1561–1574. [Google Scholar] [CrossRef]
- Nunes, J.C.; Torres, A.G. Fatty acid and CLA composition of Brazilian dairy products, and contribution to daily intake of CLA. J. Food Compos. Anal. 2010, 23, 782–789. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Mei, Y.; Yang, B.; Zhao, J.; Stanton, C.; Chen, W. Advances in research on microbial conjugated linoleic acid bioconversion. Prog. Lipid Res. 2024, 93, 101257. [Google Scholar] [CrossRef]
- Yang, B.; Gao, H.H.; Stanton, C.; Ross, R.P.P.; Zhang, H.; Chen, Y.Q.; Chen, H.; Chen, W. Bacterial conjugated linoleic acid production and their applications. Prog. Lipid Res. 2017, 68, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Hu, Y.; Wei, W.; Jin, Q.; Wang, X. Production of conjugated fatty acids: A review of recent advances. Biotechnol. Adv. 2019, 37, 107454. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, T.R.; Nam, S.-H.; Ure, A.L. Factors affecting conjugated linoleic acid content in milk and meat. Crit. Rev. Food Sci. Nutr. 2005, 45, 463–482. [Google Scholar] [CrossRef]
- Siurana, A.; Calsamiglia, S. A metaanalysis of feeding strategies to increase the content of conjugated linoleic acid (CLA) in dairy cattle milk and the impact on daily human consumption. Anim. Feed Sci. Technol. 2016, 217, 13–26. [Google Scholar] [CrossRef]
- Salamon, R.; Vargáné-Visi, É.; András, C.D.; Csapóné Kiss, Z.; Csapó, J. Synthetic methods to obtain conjugated linoleic acids (CLAs) by catalysis—A review. Acta Aliment. 2015, 44, 229–234. [Google Scholar] [CrossRef]
- Kuhl, G.; De Dea Lindner, J. Biohydrogenation of linoleic acid by lactic acid bacteria for the production of functional cultured dairy products: A review. Foods 2016, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, A.; Mollaei Tavani, S.; Arjeh, E.; Jafari, S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X 2023, 20, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Terán, V.; Pizarro, P.L.; Zacarías, M.F.; Vinderola, G.; Medina, R.; Van Nieuwenhove, C. Production of conjugated dienoic and trienoic fatty acids by lactic acid bacteria and bifidobacteria. J. Funct. Foods 2015, 19, 417–425. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Omura, Y.; Matsumura, K.; Shimizu, S. Conjugated linoleic acid production from linoleic acid by lactic acid bacteria. J. Am. Oil Chem. Soc. 2002, 79, 159–163. [Google Scholar] [CrossRef]
- Khan, A.; Nadeem, M.; Al-Asmari, F.; Imran, M.; Ambreen, S.; Rahim, M.A.; Oranab, S.; Esatbeyoglu, T.; Bartkiene, E.; Rocha, J.M. Effect of Lactiplantibacillus plantarum on the conversion of linoleic acid of vegetable oil to conjugated linoleic acid, lipolysis, and sensory properties of cheddar cheese. Microorganisms 2023, 11, 2613. [Google Scholar] [CrossRef]
- Filannino, P.; De Angelis, M.; Di Cagno, R.; Gozzi, G.; Riciputi, Y.; Gobbetti, M. How Lactobacillus plantarum shapes its transcriptome in response to contrasting habitats. Environ. Microbiol. 2018, 20, 3700–3716. [Google Scholar] [CrossRef] [PubMed]
- Siezen, R.J.; van Hylckama Vlieg, J.E.T. Genomic diversity and versatility of Lactobacillus plantarum, a natural metabolic engineer. Microb. Cell Fact. 2011, 10, S3. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Lombardi, S.J.; Tremonte, P.; Succi, M.; Tipaldi, L.; Pannella, G.; Sorrentino, E.; Iorizzo, M.; Coppola, R. Biodiversity of Lactobacillus plantarum from traditional Italian wines. World J. Microbiol. Biotechnol. 2014, 30, 2299–2305. [Google Scholar] [CrossRef]
- Iorizzo, M.; Albanese, G.; Testa, B.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; D’andrea, M.; Iaffaldano, N.; Coppola, R. Presence of lactic acid bacteria in the intestinal tract of the mediterranean trout (Salmo macrostigma) in its natural environment. Life 2021, 11, 667. [Google Scholar] [CrossRef]
- Iorizzo, M.; Pannella, G.; Lombardi, S.J.; Ganassi, S.; Testa, B.; Succi, M.; Sorrentino, E.; Petrarca, S.; De Cristofaro, A.; Coppola, R.; et al. Inter-and intra-species diversity of lactic acid bacteria in apis mellifera ligustica colonies. Microorganisms 2020, 8, 1578. [Google Scholar] [CrossRef]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef]
- Letizia, F.; Albanese, G.; Testa, B.; Vergalito, F.; Bagnoli, D.; Di Martino, C.; Carillo, P.; Verrillo, L.; Succi, M.; Sorrentino, E.; et al. In vitro assessment of bio-functional properties from Lactiplantibacillus plantarum strains. Curr. Issues Mol. Biol. 2022, 44, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Ganassi, S.; Lombardi, S.J.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Probiotic properties and potentiality of Lactiplantibacillus plantarum strains for the biological control of chalkbrood disease. J. Fungi 2021, 7, 379. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Albanese, G.; Letizia, F.; Testa, B.; Tremonte, P.; Vergalito, F.; Lombardi, S.J.; Succi, M.; Coppola, R.; Sorrentino, E. Probiotic potentiality from versatile Lactiplantibacillus plantarum strains as resource to enhance freshwater fish health. Microorganisms 2022, 10, 463. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Battista, N.; Prete, R.; Corsetti, A. Health-promoting role of Lactiplantibacillus plantarum isolated from fermented foods. Microorganisms 2021, 9, 349. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, N.; Comas, J.C.; Harris, H.M.B.; Strain, C.; Stanton, C.; Hill, C.; Corsetti, A.; Gahan, C.G.M. Impact of food origin Lactiplantibacillus plantarum Strains on the human intestinal microbiota in an in vitro system. Front. Microbiol. 2022, 13, 832513. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Ganassi, S.; Albanese, G.; Letizia, F.; Testa, B.; Tedino, C.; Petrarca, S.; Mutinelli, F.; Mazzeo, A.; De Cristofaro, A. Antimicrobial activity from putative probiotic lactic acid bacteria for the biological control of american and european foulbrood diseases. Vet. Sci. 2022, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; Ganassi, S.; Ianiro, M.; Letizia, F.; Succi, M.; Tremonte, P.; Vergalito, F.; Cozzolino, A.; et al. Antimicrobial activity against Paenibacillus larvae and functional properties of Lactiplantibacillus plantarum strains: Potential benefits for honeybee health. Antibiotics 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C.; Zdolec, N. Lactobacillus plantarum with functional properties: An approach to increase safety and shelf-life of fermented foods. Biomed Res. Int. 2018, 2018, 9361614. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Bangar, S.P.; Echegaray, N.; Suri, S.; Tomasevic, I.; Lorenzo, J.M.; Melekoglu, E.; Rocha, J.M.; Ozogul, F.; Manuel Lorenzo, J.; et al. The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: A review of current knowledge. Microorganisms 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.; Testa, B.; Macciola, V.; Lombardi, S.J.; Iorizzo, M. Exploring enzyme and microbial technology for the preparation of green table olives. Eur. Food Res. Technol. 2016, 242, 363–370. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Iorizzo, M.; Testa, B.; Succi, M.; Tremonte, P.; Sorrentino, E.; Di Renzo, M.; Strollo, D.; Coppola, R. Inoculum strategies and performances of malolactic starter Lactobacillus plantarum M10: Impact on chemical and sensorial characteristics of fiano wine. Microorganisms 2020, 8, 516. [Google Scholar] [CrossRef]
- Arena, M.; Caggianiello, G.; Russo, P.; Albenzio, M.; Massa, S.; Fiocco, D.; Capozzi, V.; Spano, G. Functional starters for functional yogurt. Foods 2015, 4, 15–33. [Google Scholar] [CrossRef]
- Seddik, H.A.; Bendali, F.; Gancel, F.; Fliss, I.; Spano, G.; Drider, D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 2017, 9, 111–122. [Google Scholar] [CrossRef]
- Russo, P.; de Chiara, M.L.V.; Vernile, A.; Amodio, M.L.; Arena, M.P.; Capozzi, V.; Massa, S.; Spano, G. Fresh-cut pineapple as a new carrier of probiotic lactic acid bacteria. Biomed Res. Int. 2014, 2014, 309183. [Google Scholar] [CrossRef]
- Iorizzo, M.; Paventi, G.; Di Martino, C. Biosynthesis of Gamma-Aminobutyric Acid (GABA) by Lactiplantibacillus plantarum in fermented food production. Curr. Issues Mol. Biol. 2023, 46, 200–220. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT 2016, 73, 557–566. [Google Scholar] [CrossRef]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, H.; Zhang, J.; Mao, S. Variety of rumen microbial populations involved in biohydrogenation related to individual milk fat percentage of dairy cows. Front. Vet. Sci. 2023, 10, 1106834. [Google Scholar] [CrossRef] [PubMed]
- Klieve, A.V.; Hennessy, D.; Ouwerkerk, D.; Forster, R.J.; Mackie, R.I.; Attwood, G.T. Establishing populations of Megasphaera elsdenii YE 34 and Butyrivibrio fibrisolvens YE 44 in the rumen of cattle fed high grain diets. J. Appl. Microbiol. 2003, 95, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Liavonchanka, A.; Feussner, I. Biochemistry of PUFA double bond isomerases producing conjugated linoleic acid. ChemBioChem 2008, 9, 1867–1872. [Google Scholar] [CrossRef]
- Or-Rashid, M.M.; Wright, T.C.; McBride, B.W. Microbial fatty acid conversion within the rumen and the subsequent utilization of these fatty acids to improve the healthfulness of ruminant food products. Appl. Microbiol. Biotechnol. 2009, 84, 1033–1043. [Google Scholar] [CrossRef]
- Lashkari, S.; Bonefeld Petersen, M.; Krogh Jensen, S. Rumen biohydrogenation of linoleic and linolenic acids is reduced when esterified to phospholipids or steroids. Food Sci. Nutr. 2020, 8, 79–87. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of polyunsaturated fatty acids and their toxicity to the microflora of the rumen. Antonie Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Pimentel, L.L.; Fontes, A.L.; Gomes, A.M.; Rodríguez-Alcalá, L.M. Microbial production of conjugated linoleic acid and conjugated linolenic acid relies on a multienzymatic system. Microbiol. Mol. Biol. Rev. 2018, 82, e00019-18. [Google Scholar] [CrossRef]
- Wang, K.; Xin, Z.; Chen, Z.; Li, H.; Wang, D.; Yuan, Y. Progress of conjugated linoleic acid on milk fat metabolism in ruminants and humans. Animals 2023, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-E.; Kim, N.-J.; Kim, Y.-H.; Baik, S.-H. Probiotic properties of lactic acid bacteria with high conjugated linoleic acid converting activity isolated from jeot-gal, high-salt fermented seafood. Microorganisms 2021, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Kumar, S.; Choudhury, P.K.; Tyagi, B.; Tyagi, N. Conjugated linoleic acid producing potential of lactobacilli isolated from goat (AXB) rumen fluid samples. Asian-Australasian J. Anim. Sci. 2020, 33, 1233–1241. [Google Scholar] [CrossRef]
- Tapia, A.M.; Bautista, J.A.N.; Mendoza, B.C.; Pham, L.J.; Sarmago, I.G.; Oliveros, M.C.R. Production of conjugated linoleic acid by lactic acid bacteria: Screening and optimization. Philipp. J. Sci. 2019, 148, 457–464. [Google Scholar]
- Hosseini, E.S.; Kermanshahi, R.K.; Hosseinkhani, S.; Shojaosadati, S.A.; Nazari, M. Conjugated linoleic acid production from various substrates by probiotic Lactobacillus plantarum. Ann. Microbiol. 2015, 65, 27–32. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Callegari, M.L.; Lucini, L.; Morelli, L. Linoleic acid induces metabolic stress in the intestinal microorganism Bifidobacterium breve DSM 20213. Sci. Rep. 2020, 10, 5997. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Kim, Y.J.; Liu, R.H. Increase of conjugated linoleic acid content in milk by fermentation with lactic acid bacteria. J. Food Sci. 2002, 67, 1731–1737. [Google Scholar] [CrossRef]
- Andrade, J.C.; Ascencao, K.; Gullòn, P.; Henriques, S.M.S.; Pinto, J.M.S.; Rocha-Santos, T.A.P.; Freitas, A.C.; Gomes, A.M. Production of conjugated linoleic acid by food-grade bacteria: A review. Int. J. Dairy Technol. 2012, 65, 467–481. [Google Scholar] [CrossRef]
- Liavonchanka, A.; Hornung, E.; Feussner, I.; Rudolph, M.G. Structure and mechanism of the Propionibacterium acnes polyunsaturated fatty acid isomerase. Proc. Natl. Acad. Sci. USA 2006, 103, 2576–2581. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.L.; Soares, A.M.S.; Domingues, M.D.R.; Rodríguez-Alcalá, L.M.; Gomes, A.M. Study of the viability of using lipase-hydrolyzed commercial vegetable oils to produce microbially conjugated linolenic acid-enriched milk. Food Chem. 2023, 413, 135665. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Sarwar, A.; Naveed, M.; Shahzad, M.; Aqib Shabbir, M.; Dablool, A.S.; ud Din, J.; Ali Khan, A.; Naz, S.; Cui, H.; et al. Bio-Molecular analysis of selected food derived Lactiplantibacillus strains for CLA production reveals possibly a complex mechanism. Food Res. Int. 2022, 154, 111031. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Yokozeki, K.; Shimizu, S. Linoleic acid isomerase in Lactobacillus plantarum AKU1009a proved to be a multi-component enzyme system requiring oxidoreduction cofactors. Biosci. Biotechnol. Biochem. 2011, 75, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Qi, H.; Gu, Z.; Zhang, H.; Chen, W.; Chen, H.; Chen, Y.Q. Characterization of the triple-component linoleic acid isomerase in Lactobacillus plantarum ZS2058 by genetic manipulation. J. Appl. Microbiol. 2017, 123, 1263–1273. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kishino, S.; Park, S.-B.; Hirata, A.; Kitamura, N.; Saika, A.; Ogawa, J. Efficient enzymatic production of hydroxy fatty acids by linoleic acid Δ9 hydratase from Lactobacillus plantarum AKU 1009a. J. Appl. Microbiol. 2016, 120, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, H.; Gao, H.; Ren, Q.; Zhang, H.; Chen, W. Genetic determinates for conjugated linolenic acid production in Lactobacillus plantarum ZS2058. J. Appl. Microbiol. 2020, 128, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, H.; Tian, F.; Zhao, J.; Gu, Z.; Zhang, H.; Chen, Y.Q.; Chen, W. Complete genome sequence of Lactobacillus plantarum ZS2058, a probiotic strain with high conjugated linoleic acid production ability. J. Biotechnol. 2015, 214, 212–213. [Google Scholar] [CrossRef]
- Liu, X.-X.; Zhang, H.-Y.; Song, X.; Yang, Y.; Xiong, Z.-Q.; Xia, Y.-J.; Ai, L.-Z. Reasons for the differences in biotransformation of conjugated linoleic acid by Lactobacillus plantarum. J. Dairy Sci. 2021, 104, 11466–11473. [Google Scholar] [CrossRef]
- Liu, X.-X.; Liu, L.; Song, X.; Wang, G.-Q.; Xiong, Z.-Q.; Xia, Y.-J.; Ai, L.-Z. The arginine repressor ArgR 2 controls conjugated linoleic acid biosynthesis by activating the cla operon in Lactiplantibacillus plantarum. Microbiol. Spectr. 2022, 10, e0261921. [Google Scholar] [CrossRef]
- Maddocks, S.E.; Oyston, P.C.F. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef]
- Ortega-Anaya, J.; Hernández-Santoyo, A. Production of bioactive conjugated linoleic acid by the multifunctional enolase from Lactobacillus plantarum. Int. J. Biol. Macromol. 2016, 91, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J. Food Sci. Technol. 2017, 54, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Özer, C.O.; Kılıç, B. Optimization of pH, time, temperature, variety and concentration of the added fatty acid and the initial count of added lactic acid Bacteria strains to improve microbial conjugated linoleic acid production in fermented ground beef. Meat Sci. 2021, 171, 108303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Lv, J.P.; Li, S.R. Production of conjugated linoleic acid by whole-cell of Lactobacillus plantarum A6-1F. Biotechnol. Biotechnol. Equip. 2011, 25, 2266–2272. [Google Scholar] [CrossRef]
- Devi, P.U.M.; Rashmi, H.K. Effect of physico-chemical factors on the production of conjugated linoleic acid by Lactobacillus plantarum. Asian J. Microbiol. Biotechnol. Environ. Sci. 2017, 19, 412–418. [Google Scholar]
- Khosravi, A.; Safari, M.; Khodaiyan, F.; Gharibzahedi, S.M.T. Bioconversion enhancement of conjugated linoleic acid by Lactobacillus plantarum using the culture media manipulation and numerical optimization. J. Food Sci. Technol. 2015, 52, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Ando, A.; Ogawa, J.; Kishino, S.; Shimizu, S. CLA Production from ricinoleic acid by lactic acid bacteria. J. Am. Oil Chem. Soc. 2003, 80, 889–894. [Google Scholar] [CrossRef]
- Ares-Yebra, A.; Garabal, J.I.; Carballo, J.; Centeno, J.A. Formation of conjugated linoleic acid by a Lactobacillus plantarum strain isolated from an artisanal cheese: Evaluation in miniature cheeses. Int. Dairy J. 2019, 90, 98–103. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Stanton, C.; Yang, B.; Ross, R.P.; Silva, C.C.G. Conjugated linoleic acid production and probiotic assessment of Lactobacillus plantarum isolated from Pico cheese. LWT 2018, 90, 403–411. [Google Scholar] [CrossRef]
- Liu, P.; Shen, S.; Ruan, H.; Zhou, Q.; Ma, L.; He, G. Production of conjugated linoleic acids by Lactobacillus plantarum strains isolated from naturally fermented Chinese pickles. J. Zhejiang Univ. Sci. B 2011, 12, 923–930. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Demir, A.S.; Cebeci, A.; Jawaid, S. A highly selective whole cell biocatalysis method for the production of two major bioactive conjugated linoleic acid isomers. Biocatal. Agric. Biotechnol. 2013, 2, 328–332. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, J.; Gong, D. Identification of lactic acid bacterial strains with high conjugated linoleic acid-producing ability from natural sauerkraut fermentations. J. Food Sci. 2009, 74, M154–M158. [Google Scholar] [CrossRef] [PubMed]
- Palachum, W.; Choorit, W.; Chisti, Y. Accumulation of conjugated linoleic acid in Lactobacillus plantarum WU-P19 is enhanced by induction with linoleic acid and chitosan treatment. Ann. Microbiol. 2018, 68, 611–624. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Fahim, M.; Al Dalali, S.; Ud Din, Z.; Ud Din, J.; Xin, Z.; Jian, Z.; Pacheco Fill, T.; Zhennai, Y. In silico characterization of linoleic acid biotransformation to rumenic acid in food derived Lactobacillus plantarum YW11. Acta Biochim. Pol. 2020, 67, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xu, Q.; Ye, Q.; Chen, W.; Zhang, H. Bioconversion kinetics of conjugated linoleic acid by Lactobacillus plantarum ZS2058. Wei Sheng Wu Xue Bao 2009, 49, 174–179. [Google Scholar] [PubMed]
- Niu, X.Y.; Chen, W.; Tian, F.W.; Zhao, J.X.; Zhang, H. Bioconversion of conjugated linoleic acid by resting cells of Lactobacillus plantarum ZS2058 in potassium phosphate buffer system. Wei Sheng Wu Xue Bao 2007, 47, 244–248. [Google Scholar]
- Yang, B.; Chen, H.; Gu, Z.; Tian, F.; Ross, R.P.; Stanton, C.; Chen, Y.Q.; Chen, W.; Zhang, H. Synthesis of conjugated linoleic acid by the linoleate isomerase complex in food-derived lactobacilli. J. Appl. Microbiol. 2014, 117, 430–439. [Google Scholar] [CrossRef]
- Vera Pingitore, E.; Pessione, A.; Fontana, C.; Mazzoli, R.; Pessione, E. Comparative proteomic analyses for elucidating metabolic changes during EPS production under different fermentation temperatures by Lactobacillus plantarum Q823. Int. J. Food Microbiol. 2016, 238, 96–102. [Google Scholar] [CrossRef]
- Guerzoni, M.E.; Lanciotti, R.; Cocconcelli, P.S. Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiology 2001, 147, 2255–2264. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Puniya, A.K. Optimisation of fermentation variables for conjugated linoleic acid bioconversion by Lactobacillus fermentum DDHI 27 in modified skim milk. Int. J. Dairy Technol. 2018, 71, 46–55. [Google Scholar] [CrossRef]
- Troegeler-Meynadier, A.; Nicot, M.C.; Bayourthe, C.; Moncoulon, R.; Enjalbert, F. Effects of pH and Concentrations of linoleic and linolenic acids on extent and intermediates of ruminal biohydrogenation in vitro. J. Dairy Sci. 2003, 86, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Matejčeková, Z.; Spodniaková, S.; Dujmić, E.; Liptáková, D.; Valík, Ľ. Modelling growth of Lactobacillus plantarum as a function of temperature: Effects of media. J. Food Nutr. Res. 2019, 58, 125–134. [Google Scholar]
- Ando, A.; Ogawa, J.; Kishino, S.; Shimizu, S. Conjugated linoleic acid production from castor oil by Lactobacillus plantarum JCM 1551. Enzyme Microb. Technol. 2004, 35, 40–45. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B.; Kılıç, G.B. In-vitro microbial production of conjugated linoleic acid by probiotic L. plantarum strains: Utilization as a functional starter culture in sucuk fermentation. Meat Sci. 2016, 114, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Kishino, S.; Hirata, A.; Park, S.-B.; Kitamura, N.; Ogawa, J. Characterization of the linoleic acid Δ9 hydratase catalyzing the first step of polyunsaturated fatty acid saturation metabolism in Lactobacillus plantarum AKU 1009a. J. Biosci. Bioeng. 2015, 119, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Ren, D.; Yan, W.; Wang, Y.; Liu, H.; Shen, M. Linoleic acid inhibits Lactobacillus activity by destroying cell membrane and affecting normal metabolism. J. Sci. Food Agric. 2020, 100, 2057–2064. [Google Scholar] [CrossRef]
- Plengvidhya, V.; Breidt, F.; Lu, Z.; Fleming, H.P. DNA Fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl. Environ. Microbiol. 2007, 73, 7697–7702. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Guan, Q.; Song, S.; Hao, M.; Xie, M. Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control 2012, 26, 178–181. [Google Scholar] [CrossRef]
- Oh, D.-K.; Hong, G.-H.; Lee, Y.; Min, S.; Sin, H.-S.; Cho, S.K. Production of conjugated linoleic acid by isolated Bifidobacterium strains. World J. Microbiol. Biotechnol. 2003, 19, 907–912. [Google Scholar] [CrossRef]
- Aziz, T.; Sarwar, A.; Al-Dalali, S.; Din, Z.U.; Megrous, S.; ud Din, J.; Zou, X.; Zhennai, Y. Production of linoleic acid metabolites by different probiotic strains of Lactobacillus plantarum. Prog. Nutr. 2019, 21, 693–701. [Google Scholar]
- Min, Z.; Xiaona, H.; Aziz, T.; Jian, Z.; Zhennai, Y. Exopolysaccharides from Lactobacillus plantarum YW11 improve immune response and ameliorate inflammatory bowel disease symptoms. Acta Biochim. Pol. 2020, 67, 487–493. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Yang, Z. Immunomodulatory and antitumor activities of the exopolysaccharide produced by potential probiotic Lactobacillus plantarum YW11 in a HT-29 tumor-burdened nude mouse model. Food Sci. Technol. 2022, 42, e57822. [Google Scholar] [CrossRef]
- Ogawa, J.; Kishino, S.; Ando, A.; Sugimoto, S.; Mihara, K.; Shimizu, S. Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 2005, 100, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Nitbani, F.O.; Tjitda, P.J.P.; Wogo, H.E.; Detha, A.I.R. Preparation of ricinoleic acid from castor oil: A review. J. Oleo Sci. 2022, 71, ess21226. [Google Scholar] [CrossRef] [PubMed]
- Khaskheli, A.A.; Talpur, F.N.; Cebeci Aydin, A.; Jawaid, S.; Surhio, M.A.; Afridi, H.I. One-pot conjugated linoleic acid production from castor oil by Rhizopus oryzae lipase and resting cells of Lactobacillus plantarum. Biosci. Biotechnol. Biochem. 2017, 81, 2002–2008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Özer, C.O.; Kılıç, B. Conjugated linoleic acid production by L. plantarum AB20-961 and L. plantarum DSM2601 in fermented sausage. In 63rd International Congress of Meat Science and Technology; Troy, D., McDonnell, C., Hinds, L., Kerry, J., Eds.; Wageningen Academic: Leiden, The Netherlands, 2017; pp. 566–567. [Google Scholar]
- Kishino, S.; Ogawa, J.; Ando, A.; Omura, Y.; Shimizu, S. Ricinoleic acid and castor oil as substrates for conjugated linoleic acid production by washed cells of Lactobacillus plantarum. Biosci. Biotechnol. Biochem. 2002, 66, 2283–2286. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Bao, Y.; Liu, X.; Zhang, H. Conjugated linoleic acid conversion by six Lactobacillus plantarum strains cultured in MRS broth supplemented with sunflower oil and soymilk. J. Food Sci. 2012, 77, M330–M336. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Yu, T.; Yang, H.; Li, L.; Wang, H.; Xiao, S.; Wang, J. Optimal culture conditions for producing conjugated linoleic acid in skim-milk by co-culture of different Lactobacillus strains. Ann. Microbiol. 2013, 63, 707–717. [Google Scholar] [CrossRef]
- Al-Saman, M.A.E.-H.; Elsanhoty, R.M.; Elhadary, A.E. The impact of oil type and lactic acid bacteria on conjugated linoleic acid production. J. Biochem. Microbiol. Biotechnol. 2016, 4, 25–29. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, B.; Hwang, C.E.; Haque, M.A.; Kim, S.C.; Lee, C.S.; Kang, S.S.; Cho, K.M.; Lee, D.H. Changes in conjugated linoleic acid and isoflavone contents from fermented soymilks using Lactobacillus plantarum P1201 and screening for their digestive enzyme inhibition and antioxidant properties. J. Funct. Foods 2018, 43, 17–28. [Google Scholar] [CrossRef]
- Hwang, C.E.; Lee, D.H.; Kim, B.; Joo, O.S.; Kim, S.C.; Lee, J.H.; Hong, S.Y.; Choi, A.R.; Cho, K.M. Enhanced digestive enzyme activity and anti-adipogenic of fermented soy-powder milk with probiotic Lactobacillus plantarum P1201 through an increase in conjugated linoleic acid and isoflavone aglycone content. Korean J. Food Preserv. 2018, 25, 461–470. [Google Scholar] [CrossRef]
- Kouchak Yazdi, Z.; Alemzadeh, I.; Vossoughi, M. Comparison and optimization of conjugated linoleic acid production by Lactobacillus plantarum and Lactobacillus plantarum subsp. plantarum. Sci. Iran. 2017, 24, 1272–1280. [Google Scholar] [CrossRef]
- Okwunodulu, I.N.; Agha, E.F. Nutritional properties of indigenous fermented condiment (ogiri) produced from partial substitution of castor oil bean (Ricinus communis) with soybean (Glycine max) seeds. Niger. J. Biotechnol. 2021, 37, 32–46. [Google Scholar] [CrossRef]
- Achi, O.K. The potential for upgrading traditional fermented foods through biotechnology. African J. Biotechnol. 2005, 4, 375–380. [Google Scholar]
- Worbs, S.; Köhler, K.; Pauly, D.; Avondet, M.-A.; Schaer, M.; Dorner, M.B.; Dorner, B.G. Ricinus communis intoxications in human and veterinary medicine—A summary of real cases. Toxins 2011, 3, 1332–1372. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, M.; Guo, B.; Kan, Q.; Zhou, S.; Feng, K.; Lin, W.; Huang, Y.; Miao, J.; Cao, Y. Detoxification of three toxins in castor meal by a novel continuous phase-transition extraction in a pilot-scale. Ind. Crop. Prod. 2021, 172, 114076. [Google Scholar] [CrossRef]
- Ulanova, R.; Kravchenko, I. Lactic acid bacteria fermentation for detoxification of castor bean meal and processing of novel protein feeds supplement. Int. J. Eng. Sci. Innov. Technol. 2013, 2, 618–624. [Google Scholar]
- Ngene, A.C.; Onwuakor, C.E.; Aguiyi, J.C.; Ifeanyi, V.O.; Ohaegbu, C.G.; Okwuchukwu, C.P.; Kim, E.G.; Egbere, J.O. Screening of some lactic acid bacteria isolated from selected nigerian fermented foods for vitamin production. Adv. Microbiol. 2019, 9, 943–955. [Google Scholar] [CrossRef]
- Uzoh, C.V.; Orji, J.O.; Okeh, C.O.; Nworie, C.O.; Igwe, P.C.; Uwanta, L.I. Assessment of probiotic potentials of lactic acid bacteria isolated from some locally fermented foods. Asian J. Food Res. Nutr. 2022, 1, 11–16. [Google Scholar]
- Ojinnaka, M.C.; Ojimelukwe, P.C.; Ezeama, C.F. An assessment of the microbial and amino acid contents of ogiri produced by fermenting oil bean seeds of Ricinus communis. Sky J. Food Sci. 2013, 2, 10–18. [Google Scholar]
- Adesulu-Dahunsi, A.T.; Dahunsi, S.O.; Ajayeoba, T.A. Co-occurrence of Lactobacillus species during fermentation of african indigenous foods: Impact on food safety and shelf-life extension. Front. Microbiol. 2022, 13, 684730. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.R. Prediction of fatty acid composition of sunflower seeds by near-infrared reflectance spectroscopy. J. Food Sci. Technol. 2018, 55, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-H.; Zhang, J.-F.; Wang, Z.; Zhang, Y.-X.; Tian, W.-X. The extract of leaves of Acer truncatum Bunge: A natural inhibitor of fatty acid synthase with antitumor activity. J. Enzyme Inhib. Med. Chem. 2006, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lin, F.; Zhang, R.; Wang, M.; Gu, R.; Long, C. Acer truncatum Bunge: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 282, 114572. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-J.; Yan, L.-H.; Li, Q.; Zhang, C.; Si, C.-L.; Li, Z.-Y.; Song, Y.-J.; Zhou, H.; Zhang, T.-C.; Luo, X.-G. Bioconversion of conjugated linoleic acid by Lactobacillus plantarum CGMCC8198 supplemented with Acer truncatum bunge seeds oil. Food Sci. Biotechnol. 2017, 26, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Bach, A.; Calsamiglia, S.; Stern, M.D. Nitrogen metabolism in the rumen. J. Dairy Sci. 2005, 88, E9–E21. [Google Scholar] [CrossRef] [PubMed]
- Jutzeler van Wijlen, R.P.; Colombani, P.C. Grass-based ruminant production methods and human bioconversion of vaccenic acid with estimations of maximal dietary intake of conjugated linoleic acids. Int. Dairy J. 2010, 20, 433–448. [Google Scholar] [CrossRef]
- Schmid, A.; Collomb, M.; Sieber, R.; Bee, G. Conjugated linoleic acid in meat and meat products: A review. Meat Sci. 2006, 73, 29–41. [Google Scholar] [CrossRef]
- Bauman, D.E.; Lock, A.L. Conjugated linoleic acid: Biosynthesis and nutritional significance. In Advanced Dairy Chemistry Volume 2 Lipids; Springer: Boston, MA, USA; pp. 93–136.
- Gutiérrez, L.F. Conjugated linoleic acid in milk and fermented milks: Variation and effects of the technological processes. Rev. Vitae 2016, 23, 134–145. [Google Scholar] [CrossRef]
- Collomb, M.; Schmid, A.; Sieber, R.; Wechsler, D.; Ryhänen, E.-L. Conjugated linoleic acids in milk fat: Variation and physiological effects. Int. Dairy J. 2006, 16, 1347–1361. [Google Scholar] [CrossRef]
- Sosa-Castañeda, J.; Hernández-Mendoza, A.; Astiazarán-García, H.; Garcia, H.S.; Estrada-Montoya, M.C.; González-Córdova, A.F.; Vallejo-Cordoba, B. Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J. Dairy Sci. 2015, 98, 6651–6659. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, B.W.; Hwang, C.E.; Lee, Y.Y.; Lee, C.; Kim, B.J.; Park, J.Y.; Sim, E.Y.; Haque, M.A.; Lee, D.H.; et al. Screening of conjugated linoleic acid (CLA) producing Lactobacillus plantarum and production of CLA on soy-powder milk by these stains. Korean J. Microbiol. 2015, 51, 231–240. [Google Scholar] [CrossRef]
- Garabal, J.I.; Rodríguez-Alonso, P.; Centeno, J.A. Characterization of lactic acid bacteria isolated from raw cows’ milk cheeses currently produced in Galicia (NW Spain). LWT Food Sci. Technol. 2008, 41, 1452–1458. [Google Scholar] [CrossRef]
- Rule, D.C.; Broughton, K.S.; Shellito, S.M.; Maiorano, G. Comparison of muscle fatty acid profiles and cholesterol concentrations of bison, beef cattle, elk, and chicken1. J. Anim. Sci. 2002, 80, 1202–1211. [Google Scholar] [CrossRef]
- Marco, A.; Juarez, M.M.; Brunton, N.; Wasilewski, P.D.; Lynch, B.; Moon, S.-S.; Troy, D.J.; Mullen, A.M. Enriching breakfast sausages by feeding pigs with CLA supplemented diets. Food Chem. 2009, 114, 984–988. [Google Scholar] [CrossRef]
- Zdolec, N.; Mikuš, T.; Kiš, M. Lactic acid bacteria in meat fermentation: Dry sausage safety and quality. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–159. [Google Scholar]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research update on the impact of lactic acid bacteria on the substance metabolism, flavor, and quality characteristics of fermented meat products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; da Silva Barretto, A.C.; Santos, E.M.; Lorenzo, J.M. Functional fermented meat products with probiotics—A review. J. Appl. Microbiol. 2022, 133, 91–103. [Google Scholar] [CrossRef]
- Özer, C.O.; Kılıç, B. Utilization of optimized processing conditions for high yield synthesis of conjugated linoleic acid by L. plantarum AB20–961 and L. plantarum DSM2601 in semi-dry fermented sausage. Meat Sci. 2020, 169, 108218. [Google Scholar] [CrossRef] [PubMed]
- Ozer, C.O.; Kilic, B. Optimization of enhanced conjugated Linoleic Acid production by L. plantarum AB20-961 and L. plantarum DSM2601 in meat model system using response surface methodology. In 63rd International Congress of Meat Science and Technology; Troy, D., McDonnell, C., Hinds, L., Kerry, J., Eds.; Wageningen Academic: Leiden, The Netherlands, 2017; pp. 88–89. ISBN 9789086868605. [Google Scholar]
- Kim, I.-S.; Kim, C.-H.; Yang, W.-S. Physiologically Active Molecules and Functional Properties of Soybeans in Human Health—A Current Perspective. Int. J. Mol. Sci. 2021, 22, 4054. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Diao, J.; Wang, C. Recent advances in exploring and exploiting soybean functional peptides—A review. Front. Nutr. 2023, 10, 1185047. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Castilho, M.C.; Silveira, M.I.N.; Matallana, M.C.; Torija, M.E. Fatty acid profile of traditional soymilk. Eur. Food Res. Technol. 2004, 219, 251–253. [Google Scholar] [CrossRef]
- Xie, C.L.; Hwang, C.E.; Oh, C.K.; Yoon, N.A.; Ryu, J.H.; Jeong, J.Y.; Roh, G.S.; Kim, H.J.; Cho, G.J.; Choi, W.S.; et al. Fermented soy-powder milk with Lactobacillus plantarum P1201 protects against high-fat diet-induced obesity. Int. J. Food Sci. Technol. 2017, 52, 1614–1622. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).