Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review
Abstract
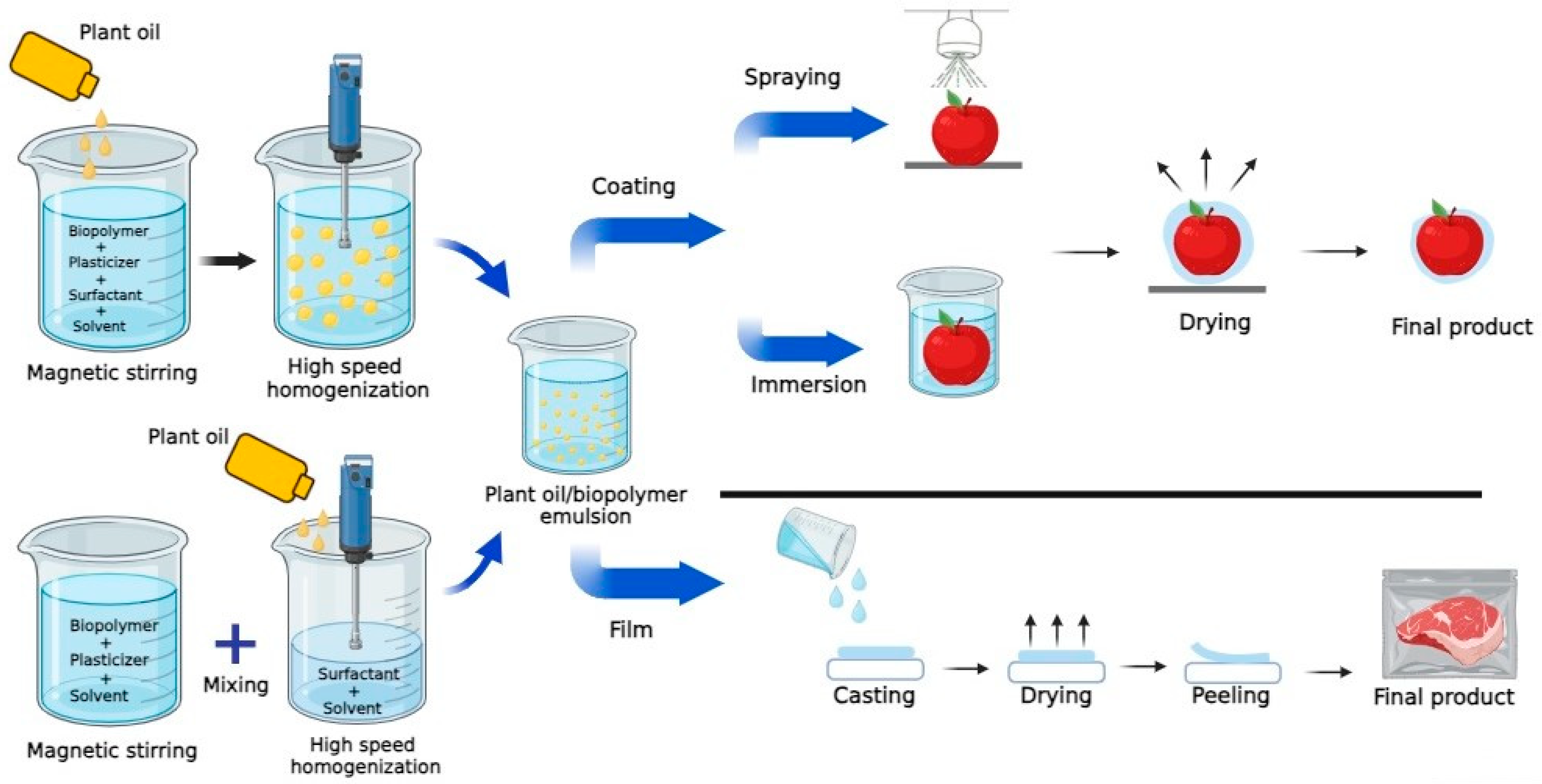
:1. Introduction
2. Biopolymer-Based Films and Coatings Incorporated with Plant Oils
3. Plant Oils and Biopolymers Interactions
4. Effect of Plant Oil Incorporation on Various Properties of Edible Packaging
4.1. Thickness
4.2. Mechanical Properties
4.3. Solubility in Water
| Matrix | Plant Oils | Matrix: Oil Proportion | Findings | Refs |
|---|---|---|---|---|
| Alginate | Linseed oil | Film coated with oil (thickness of the oil layer: 0.5 µm) | Decreased WVP; increased TS, UV barrier ability, thermal stability, and thickness | [3] |
| Sunflower seed oil | Film coated with oil (thickness of the oil layer: 0.5 µm) | Decreased WVP; increased UV barrier ability, total color value, thermal stability, and thickness | ||
| Alginate | Soybean oil | 1% (w/v): 0.5–1.5% (v/v) | Decreased WVP (over the range of 1% to 1.5% of soybean oil), TS, and EB; increased opacity | [35] |
| Carboxymethyl cellulose | Black cumin seed oil | 2% (w/v): 0–1% (w/v) | Decreased water solubility and EB; increased TS, antioxidant activity, thermal stability, and total color difference | [36] |
| Chitosan (medium MW) | Berberis crataegina seed oil | 1% (w/v): 0–1.6% (v/v) | Decreased water solubility, TS, and thermal stability; increased EB, opacity, antioxidant activity, UV barrier ability, and thickness | [37] |
| Chitosan (MW: 161 kDa) | Olive oil | 2% (w/v): 0–15% (w/w, based on the mass of chitosan) | Decreased WVP; increased TS, EB, thickness, and opacity | [28] |
| Chitosan (medium MW) | False flax seed oil | 2% (w/v): 0–2% (v/v) | Decreased water solubility; increased EB, opacity, thermal stability, and antioxidant activity | [33] |
| Corn starch | Sunflower oil | 3% (w/v): 0–10% (w/w, based on the mass of starch) | Decreased water solubility, WVP, and TS; increased opacity | [38] |
| Corn starch | Coffee oil | 4% (w/v): 0–1% (v/v) | Decreased WVP and opacity; increased TS, EB, UV barrier ability, and thickness | [14] |
| Corn starch/sodium alginate/gum Arabic | Coconut oil | 2% (w/w): 0–70% (w/w, based on the mass of gum Arabic) | Decreased OP, WVP, and TS; increased EB and thickness | [39] |
| Fenugreek galactomannan/xanthan gum | Grape seed oil | 1.5% (w/v):0–0.5% (v/v) | Decreased OP, WVP, TS, opacity, and thermal stability; increased EB and thickness | [21] |
| Konjak glucomanan/agar/gum Arabic | Coconut oil | 2.4% (w/w): 0–0.6% (w/w) | Decreased water solubility, WVP, and TS; increased EB and thickness | [16] |
| Mung bean starch/guar gum | Sunflower seed oil | 2.75% (w/w): 0–2% (w/w) | Decreased water solubility, WVP, TS, and EB; increased opacity, OP, and total color difference | [34] |
| Plantago major seed gum | Canola oil | 1% (w/v): 0–0.5% (v/v) | Decreased WVP, TS, thermal stability; increased EB and thickness | [23] |
| Maize oil | 1% (w/v): 0–0.5% (v/v) | Decreased WVP, TS, and thermal stability; increased EB and thickness | ||
| Olive oil | 1% (w/v): 0–0.5% (v/v) | Decreased WVP, TS, and thermal stability; increased EB and thickness | ||
| Potato starch | Coconut oil | 2.5% (w/w): 0–112% (w/w, based on the mass of potato starch) | Decreased WVP and thermal stability; increased TS (up to 14% of coconut oil) and transparency | [25] |
| Potato starch | Olive oil | 5% (w/v): 0–10% (w/w, based on the mass of potato starch) | Decreased WVP, TS, EB, and thermal stability; increased total color difference and UV barrier ability | [30] |
| Gelatin | Corn oil | 4% (w/v): 0–1.2% (w/v) | Decreased water solubility, WVP, TS, and transparency; increased EB, thermal stability, and UV barrier ability | [40] |
| Albumin | Olive oil | 9% (w/v): 0–1.5% (v/v) | Decreased water solubility, WVP, and OP; increased TS, EB, opacity, total color difference, and thickness | [32] |
| Gelatin | Camellia oil | 3% (w/v): 0–100% (w/w, based on the mass of gelatin) | Decreased WVP, TS, and thermal stability; increased EB, UV barrier ability, antioxidant activity, total color difference, opacity, and thickness | [15] |
| Gelatin | Olive oil | 5% (w/w):0–20% (w/w, based on the mass of gelatin) | Decreased WVP; increased TS, EB, thermal stability, opacity, and UV barrier ability | [31] |
| Gelatin | Sunflower oil | 4% (w/v):0–1% (w/v) | Decreased water solubility, WVP, and transparency | [41] |
| Soy protein isolate | Rapeseed oil | 10% (w/w): 0–3% (w/w) | Decreased WVP and TS; increased EB, total color difference, and opacity | [29] |
| Soy protein isolate | Flaxseed oil | 5% (w/w):1–10% (w/w) | Decreased WVP; increased TS (up to 5% of flaxseed oil), EB, and total color difference | [42] |
| Whey protein isolate | Sunflower oil | 8% (w/w): 0–0.15% (w/w) | Decreased water solubility, WVP, OP (up to 0.05% of sunflower oil), and TS; increased EB, thermal stability, and opacity | [43] |
| Whey protein isolate | Almond oil | 8% (w/w): 0–1% (w/w) | Decreased WVP; increased solubility in water, TS (up to 0.5% of almond oil), EB (at 1% of almond oil), total color difference, opacity, and OP | [44] |
| Walnut oil | 8% (w/w): 0–1% (w/w) | Decreased water solubility, WVP, and EB; increased TS (up to 0.5% of walnut oil), total color difference, OP, and opacity | ||
| Whey protein isolate | Rapeseed oil | 8% (w/w): 0–3% (v/v) | Decreased water solubility; increased TS, EB, total color difference, and opacity | [27] |
4.4. Water Vapor Permeability (WVP)
4.5. Oxygen Permeability (OP)
4.6. Ultraviolet Light Transmittance
4.7. Optical Properties
4.8. Thermal Properties
4.9. Antioxidant Properties
4.10. Antimicrobial Properties
5. Food Applications
5.1. Fruits and Vegetables
5.2. Meat Products
6. Challenges and Future Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Nehchiri, N.; Amiri, S.; Radi, M. Improving the water barrier properties of alginate packaging films by submicron coating with drying linseed oil. Packag. Technol. Sci. 2021, 34, 283–295. [Google Scholar] [CrossRef]
- Gupta, V.; Biswas, D.; Roy, S. A comprehensive review of biodegradable polymer-based films and coatings and their food packaging applications. Materials 2022, 15, 5899. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 66–105. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and incorporation of functional ingredients in edible films and coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- Sharma, P.; Shehin, V.P.; Kaur, N.; Vyas, P. Application of edible coating on fresh and minimally processed vegetables: A review. Int. J. Veg. Sci. 2019, 25, 295–314. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.U.; Majeed, Y.; Rebezov, M.; Khayrullin, M.; Bobkova, E.; Shariati, M.A.; Chung, I.M.; Thiruvengadam, M. Potentials of polysaccharides, lipids and proteins in biodegradable food packaging applications. Int. J. Biol. Macromol. 2021, 183, 2184–2198. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Yousuf, B.; Pandith, J.A.; Ahmad, S. Plant-derived active substances incorporated as antioxidant, antibacterial or antifungal components in coatings/films for food packaging applications. Food Biosci. 2023, 53, 102717. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Kaseke, T.; Opara, U.L.; Fawole, O.A. Effects of enzymatic pretreatment of seeds on the physicochemical properties, bioactive compounds, and antioxidant activity of pomegranate seed oil. Molecules 2021, 26, 4575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Yang, R.; Mao, J.; Jiang, J.; Wang, X.; Zhang, W.; Zhang, Q.; Li, P. Simultaneous determination of tocopherols, carotenoids and phytosterols in edible vegetable oil by ultrasound-assisted saponification, LLE and LC-MS/MS. Food Chem. 2019, 289, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Hu, G.; Al-Romaima, A.; Liu, X.; Bai, X.; Li, J.; Li, Z.; Qiu, M. Effect of green coffee oil as a natural active emulsifying agent on the properties of corn starch-based films. LWT 2022, 170, 114087. [Google Scholar] [CrossRef]
- Zong, X.; Zhang, X.; Bi, K.; Zhou, Y.; Zhang, M.; Qi, J.; Fu, M. Novel emulsion film based on gelatin/polydextrose/camellia oil incorporated with Lactobacillus pentosus: Physical, structural, and antibacterial properties. Food Hydrocoll. 2021, 121, 107063. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, L.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Physical, structural, and water barrier properties of emulsified blend film based on konjac glucomannan/agar/gum Arabic incorporating virgin coconut oil. LWT 2021, 154, 112683. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef]
- Medina, E.; De Castro, A.; Romero, C.; Brenes, M. Comparison of the concentrations of phenolic compounds in olive oils and other plant oils: Correlation with antimicrobial activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidants in food and food antioxidants. Food/Nahrung 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Khah, M.D.; Ghanbarzadeh, B.; Roufegarinejad Nezhad, L.; Ostadrahimi, A. Effects of virgin olive oil and grape seed oil on physicochemical and antimicrobial properties of pectin-gelatin blend emulsified films. Int. J. Biol. Macromol. 2021, 171, 262–274. [Google Scholar] [CrossRef]
- Niknam, R.; Soudi, M.R.; Mousavi, M. Biodegradable composite films based on Trigonella foenum-graceum galactomannan-xanthan gum: Effect of grape seed oil on various aspects of emulsified films. J. Am. Oil Chem. Soc. 2023, 100, 163–174. [Google Scholar] [CrossRef]
- Hanani, Z.A.N. Physicochemical characterization of kappa-carrageenan (Euchema cottoni) based films incorporated with various plant oils. Carbohydr. Polym. 2016, 157, 1479–1487. [Google Scholar]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Hamishehkar, H. Plantago major seed gum based biodegradable films: Effects of various plant oils on microstructure and physicochemical properties of emulsified films. Polym. Test. 2019, 77, 105868. [Google Scholar] [CrossRef]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Fangfang, Z.; Xinpeng, B.; Wei, G.; Wang, G.D.; Shi, Z.Z.; Jun, G. Effects of virgin coconut oil on the physicochemical, morphological and antibacterial properties of potato starch-based biodegradable films. Int. J. Food Sci. Technol. 2020, 55, 192–200. [Google Scholar] [CrossRef]
- Zhu, F. Polysaccharide based films and coatings for food packaging: Effect of added polyphenols. Food Chem. 2021, 359, 129871. [Google Scholar] [CrossRef]
- Galus, S.; Kadzinska, J. Moisture sensitivity, optical, mechanical and structural properties of whey protein-based edible films incorporated with rapeseed oil. Food Technol. Biotechnol. 2016, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocol. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- Farajpour, R.; Djomeh, Z.E.; Moeini, S.; Tavahkolipour, H.; Safayan, S. Structural and physico-mechanical properties of potato starch-olive oil edible films reinforced with zein nanoparticles. Int. J. Biol. Macromol. 2020, 149, 941–950. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Wang, Q.; Liu, F.; Wei, Z.-H. Characterization of gelatin-based edible films incorporated with olive oil. Food Res. Int. 2012, 49, 572–579. [Google Scholar] [CrossRef]
- Taqi, A.; Askar, K.A.; Nagy, K.; Mutihac, L.; Stamatin, I. Effect of different concentrations of olive oil and oleic acid on the mechanical properties of albumen (egg white) edible films. Afr. J. Biotechnol. 2011, 10, 12963–12972. [Google Scholar]
- Gursoy, M.; Sargin, I.; Mujtaba, M.; Akyuz, B.; Ilk, S.; Akyuz, L.; Kaya, M.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J. False flax (Camelina sativa) seed oil as suitable ingredient for the enhancement of physicochemical and biological properties of chitosan films. Int. J. Biol. Macromol. 2018, 114, 1224–1232. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, E.S.; Han, J. Enhancement of the water-resistance properties of an edible film prepared from mung bean starch via the incorporation of sunflower seed oil. Sci. Rep. 2020, 10, 13622. [Google Scholar] [CrossRef]
- Gutiérrez-Jara, C.; Bilbao-Sainz, C.; McHugh, T.; Chiou, B.-S.; Williams, T.; Villalobos-Carvajal, R. Physical, mechanical and transport properties of emulsified films based on alginate with soybean oil: Effects of soybean oil concentration, number of passes and degree of surface crosslinking. Food Hydrocoll. 2020, 109, 106133. [Google Scholar] [CrossRef]
- Biswas, A.; Ahmed, T.; Rana, M.R.; Hoque, M.M.; Ahmed, M.F.; Sharma, M.; Sridhar, K.; Ara, R.; Stephen Inbaraj, B. Fabrication and characterization of ZnO nanoparticles-based biocomposite films prepared using carboxymethyl cellulose, taro mucilage, and black cumin seed oil for evaluation of antioxidant and antimicrobial activities. Agronomy 2023, 13, 147. [Google Scholar] [CrossRef]
- Kaya, M.; Ravikumar, P.; Ilk, S.; Mujtaba, M.; Akyuz, L.; Labidi, J.; Salaberria, A.M.; Cakmak, Y.S.; Erkul, S.K. Production and characterization of chitosan based edible films from Berberis crataegina’s fruit extract and seed oil. Innov. Food Sci. Emerg. Technol. 2018, 45, 287–297. [Google Scholar] [CrossRef]
- Wardak, M.H.; Kingwascharapong, P.; Aryan, S.; Tanaka, F.; Tanaka, F. Preparation and characterization of corn starch-based film: Effect of citric acid or sunflower oil and its combination. J. Food. Meas. Charact. 2021, 15, 1907–1915. [Google Scholar] [CrossRef]
- Xiao, M.; Tang, B.; Qin, J.; Wu, K.; Jiang, F. Properties of film-forming emulsions and films based on corn starch/sodium alginate/gum Arabic as affected by virgin coconut oil content. Food Packag. Shelf Life 2022, 32, 100819. [Google Scholar] [CrossRef]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Effect of corn oil on physical, thermal, and antifungal properties of gelatin-based nanocomposite films containing nano chitin. LWT 2017, 76, 33–39. [Google Scholar] [CrossRef]
- Pérez-Mateos, M.; Montero, P.; Gómez-Guillén, M.C. Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends. Food Hydrocoll. 2009, 23, 53–61. [Google Scholar] [CrossRef]
- Hopkins, E.J.; Chang, C.; Lam, R.S.; Nickerson, M.T. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res. Int. 2015, 67, 418–425. [Google Scholar] [CrossRef]
- Erdem, B.G.; Dıblan, S.; Kaya, S. Development and structural assessment of whey protein isolate/sunflower seed oil biocomposite film. Food Bioproduct. Process. 2019, 118, 270–280. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Bahrami, A.; Mokkarram, R.R.; Khiabani, S.M.; Ghanbarzadeh, B.; Salehi, R. Physico-mechanical and antimicrobial properties of tragacanth/hydroxypropyl methylcellulose/beeswax edible films reinforced with silver nanoparticles. Int. J. Biol. Macromol. 2019, 129, 1103–1112. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Gas barrier and wetting properties of whey protein isolate-based emulsion films. Polym. Eng. Sci. 2019, 59, E376–E383. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.W. Biopolymer-based UV protection functional films for food packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Jusoh, N.; Isa, M.; Sarbon, N. Physical, mechanical and antioxidant properties of chicken skin gelatin films incorporated with virgin coconut oil. Biocatal. Agric. Biotechnol. 2022, 45, 102525. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Farshi, P.; Alizadeh, A.M.; Azadmard-Damirchi, S.; Torbati, M. Nutritional aspects of vegetable oils: Refined or unrefined? Eur. J. Lipid Sci. Technol. 2022, 124, 2100149. [Google Scholar] [CrossRef]
- Singh, G.P.; Bangar, S.P.; Yang, T.; Trif, M.; Kumar, V.; Kumar, D. Effect on the properties of edible starch-based films by the incorporation of additives: A Review. Polymers 2022, 14, 1987. [Google Scholar] [CrossRef] [PubMed]
- Vianna, T.C.; Marinho, C.O.; Júnior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef] [PubMed]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Liu, R.; Lu, M.; Zhang, T.; Zhang, Z.; Jin, Q.; Chang, M.; Wang, X. Evaluation of the antioxidant properties of micronutrients in different vegetable oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900079. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yilmaz, B.A.; Sargin, I. Effect of different animal fat and plant oil additives on physicochemical, mechanical, antimicrobial and antioxidant properties of chitosan films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Jafari, T.; Khaneghah, A.M. Vegetable oil-based nanoemulsions for the preservation of muscle foods: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 8554–8567. [Google Scholar] [CrossRef]
- Xuan, T.D.; Gu, G.Q.; Minh, T.N.; Quy, T.N.; Khanh, T.D. An overview of chemical profiles, antioxidant and antimicrobial activities of aommercial vegetable edible oils marketed in Japan. Foods 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Fernandes, A.; Calhelha, R.C.; Rouphael, Y.; Petrovic, J.; Sokovic, M.; Ferreira, I.C.F.R.; Barros, L. Antimicrobial properties, cytotoxic effects, and fatty acids composition of vegetable oils from purslane, linseed, luffa, and pumpkin Seeds. Appl. Sci. 2021, 11, 5738. [Google Scholar] [CrossRef]
- Yoon, B.; Jackman, J.; Valle-González, E.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Kingwascharapong, T.; Tanaka, F.; Tanaka, F. Effect of edible coatings developed from chitosan incorporated with tea seed oil on Japanese pear. Sci. Hortic. 2021, 288, 110314. [Google Scholar] [CrossRef]
- Cozmuta, A.M.; Turila, A.; Apjok, R.; Ciocian, A.; Cozmuta, L.M.; Peter, A.; Nicula, C.; Galić, N.; Benković, T. Preparation and characterization of improved gelatin films incorporating hemp and sage oils. Food Hydrocoll. 2015, 49, 144–155. [Google Scholar] [CrossRef]
- Hassani, F.; Garousi, F.; Javanmard, M. Edible coating based on whey protein concentrate-rice bran oil to maintain the physical and chemical properties of the kiwifruit Actinidia deliciosa. Trakia J. Sci. 2012, 10, 26–34. [Google Scholar]
- Phuong, N.T.H.; Koga, A.; Nkede, F.N.; Tanaka, F.; Tanaka, F. Application of edible coatings composed of chitosan and tea seed oil for quality improvement of strawberries and visualization of internal structure changes using X-ray computed tomography. Prog. Org. Coat. 2023, 183, 107730. [Google Scholar] [CrossRef]
- Mohammadi, M.; Rastegar, S.; Rohani, A. Enhancing shelf-life and quality of Mexican lime (Citrus aurantifolia cv.) fruit: Utilizing edible coating from wild sage seeds enriched with pomegranate seed oils. J. Food. Meas. Charact. 2023, 18, 331–344. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Tian, R.; Kuang, Y.; Wu, K.; Xiao, M.; Liu, Y.; Qian, H.; Jiang, F. Properties of konjac glucomannan/curdlan-based emulsion films incorporating camellia oil and the preservation effect as coatings on ‘Kyohógrapes. Int. J. Biol. Macromol. 2024, 258, 128836. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Hayatioğlu, N.; Hendekçi, M.C.; Tekin, İ.; Ersus, S. Development and characterization of edible films based on carboxymethyl cellulose enriched with pomegranate seed oil and the coating of strawberries. J. Food Process. Preserv. 2022, 46, 16607. [Google Scholar] [CrossRef]
- Nandane, A.S.; Dave, R.K.; Rao, T.V.R. Optimization of edible coating formulations for improving postharvest quality and shelf life of pear fruit using response surface methodology. J. Food Sci. Technol. 2017, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Uthairatanakij, A.; Wote, Y.H.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Whey protein incorporated with olive oil as novel edible coating for fresh cut pineapples. Acta Hortic. 2021, 1336, 351–356. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Dong, S.; Zhang, D.; Ma, C.; Chen, S.; Li, H. Biopolymer films based on chitosan/potato protein/linseed Oil/ZnO NPs to maintain the storage quality of raw meat. Food Chem. 2020, 332, 127375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zong, X.; Zhang, M.; Ge, Q.; Qi, J.; Liang, J.; Xu, X.; Xiong, G. Effect of konjac glucomannan/carrageenan-based edible emulsion coatings with camellia oil on quality and shelf-life of chicken meat. Int. J. Biol. Macromol. 2021, 183, 331–339. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Onopiuk, A.; Wojtasik-Kalinowska, I.; Zalewska, M.; Półtorak, A.; Wierzbicka, A. The influence of sage and hemp oils addition to gelatin-based edible coating on the quality features of pork. CyTA J. Food 2020, 18, 719–727. [Google Scholar] [CrossRef]
- Vargas, M.; Albors, A.; Chiralt, A. Application of chitosan-sunflower oil edible films to pork meat hamburgers. Procedia Food Sci. 2011, 1, 39–43. [Google Scholar] [CrossRef]
- Jeong, J.; Hur, S.; Yang, H.; Moon, S.; Hwang, Y.; Park, G.; Joo, S. Discoloration characteristics of 3 major muscles from cattle during cold storage. J. Food Sci. 2009, 74, C1–C5. [Google Scholar] [CrossRef] [PubMed]

| Antimicrobial Activity | |||||
|---|---|---|---|---|---|
| Matrix | Plant Oils | Matrix: Oil Proportion | Tested Microorganisms | Results | Refs |
| Carboxymethyl cellulose | Black cumin seed oil | 2% (w/v): 0–1% (w/v) | Staphylococcus aureus, Escherichia coli | The film containing black cumin seed oil showed higher antimicrobial activity against the tested microorganisms than the film without oil | [36] |
| Chitosan | Tea seed oil | 1% (w/v): 0–0.5% (w/v) | Botrytis cinera | Oil addition significantly reduced the growth of Botrytis cinerea | [59] |
| Chitosan (medium MW) | Berberis crataegina seed oil | 1% (w/v): 0–1.6% (w/v) | Escherichia coli, Salmonella typhmurium, Proteus microbilis, Proteus vulgaris, Pseudomonas aeruginosa, Enterobacter aerogenes, Staphylococcus aureus, Streptococcus mutans, and Bacillus thuringiensis | Antimicrobial activity of the film was improved against all tested microorganisms except Proteus vulgaris, Enterobacter aerogenes, and Streptococcus mutans | [37] |
| Chitosan (medium MW) | False flax seed oil | 2% (w/v): 0–2% (v/v) | Escherichia coli, Salmonella typhimurium, Proteus microbilis, Proteus vulgaris, Pseudomonas aeruginosa, Enterobacter aerogenes, Staphylococcus aureus, Streptococcus mutans, and Bacillus thuringiensis | Oil incorporation improved the antimicrobial activity of the film against all the tested microorganisms | [33] |
| Chitosan (medium MW) | Olive, corn, or sunflower oil | 1% (w/v): 0–0.25% (v/v) | Escherichia coli, Staphylococcus aureus, Proteus microbilis, Proteus vulgaris, Pseudomonas aeruginosa, Enterobacter aerogenes, Bacillus thuringiensis, Salmonella typhimurium, and Streptococcus mutans | Film with oil had higher antimicrobial activity than the film without oil The film incorporated with olive or sunflower oil showed higher antimicrobial activity than the film with corn oil | [54] |
| Pectin/gelatin | Grape seed or olive oil | 5% (w/v): 0.75% (w/w) | Salmonella typhimurium, Escherichia coli, Staphylococcus aureus, and Pseudomonas fluorescens | The films containing grape seed or olive oil showed antimicrobial activity against all tested microorganisms | [20] |
| Gelatin | Camellia oil | 3% (w/v): 0–100% (w/w, based on the mass of gelatin) | Staphylococcus aureus and Escherichia coli | The film with oil had higher antimicrobial activity against the tested microorganisms than the film without oil | [15] |
| Corn starch | Coffee oil | 4% (w/v): 0–1% (v/v) | Staphylococcus aureus, Escherichia coli, and Salmonella enterica | The addition of coffee oil gave corn starch films antibacterial activity against the tested microorganisms | [14] |
| Potato starch | Coconut oil | 2.5% (w/w): 0–112% (w/w, based on the mass of potato starch) | Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus | The addition of coconut oil gave potato starch films antibacterial activity against the tested microorganisms | [25] |
| Gelatin | Corn oil | 4% (w/v): 0–1.2% (w/v) | Aspergillus niger | Corn oil inhibited the antifungal activity of the film | [40] |
| Gelatin | Hemp seed oil | 4% (w/v): 0–2% (v/v) | Escherichia coli, Staphylococcus aureus, Listeria innocua, Saccharomyces cerevisiae, and Penicillium expansum | The addition of hemp seed oil gave gelatin films antimicrobial activity against Staphylococcus aureus and Listeria innocua. Oil addition had no effect on the growth of Escherichia coli, Saccharomyces cerevisiae, and Penicillium expansum | [60] |
| Matrix | Plant Oils | Matrix: Oil Proportion | Food Product and Packaging Conditions | Effect on Food Product | Refs |
|---|---|---|---|---|---|
| Fruits and vegetables | |||||
| Konjak glucomanan/agar/gum Arabic | Coconut oil | 2.4% (w/w): 0–0.6% (w/w) | Cucumber, stored for 12 days at 7 °C; the biodegradable container containing the sample was covered with the film | Decreased weight loss and firmness reductions | [16] |
| Whey protein isolate | Rice bran oil | 10% (w/v): 0–0.6% (w/v) | Kiwifruit, stored for 28 days at 4 °C, dipped in coating solution | Decreased weight loss; preserved firmness and taste; increased overall acceptability | [61] |
| Chitosan (MW: 96 kDa) | Tea seed oil | 1% (w/v): 0–0.1% (w/w) | Strawberry, stored for 24 days at 2 °C, dipped in coating solution | Reduced weight loss; retained firmness, color, moisture content and total soluble solids; delayed pH changes | [62] |
| Wild sage gum | Pomegranate seed oil | 0.1–0.2% (w/v): 0–0.05% (w/v) | Mexican lime fruit, stored for 24 days at 20 °C, dipped in coating solution | Decreased weight loss; preserved total phenols, flavonoids, color, antioxidant capacity, and sensory properties | [63] |
| konjac glucomannan/curdlan | Camellia oil | 1% (w/v): 0–0.15% (w/v) | ‘Kyoho’ grapes, stored for 10 days at room temperature, dipped in coating solution | Maintained the appearance, total soluble solids, and acid content; delayed weight loss and firmness decrease | [64] |
| Carboxymethyl cellulose | Pomegranate seed oil | 3% (w/v): 0–3% (v/v) | Strawberry, stored for 16 days at 5 °C, dipped in coating solution | Decreased weight loss; maintained highest level of total phenolic content | [65] |
| Chitosan | Tea seed oil | 1% (w/v): 0–0.5% (w/v) | Pear fruit, stored for 21 days at 25 °C, dipped in coating solution | Reduced respiration rate and fungal decay; maintained total soluble solids | [59] |
| Soy protein isolate | Olive oil | 2–6% (w/v): 0.7–1.1% (v/v) | Pear fruit, stored for 5 days at 28 °C, dipped in coating solution | The weight loss of the sample decreased as the olive oil concentration in the coating increased | [66] |
| Whey protein isolate | Olive oil | 10% (w/v): 0–1% (v/w) | Fresh cut pineapples, stored for 8 days at 4 °C, dipped in coating solution | Maintained ascorbic acid and total phenolic contents | [67] |
| Meat products | |||||
| Chitosan/potato protein | Linseed oil | 2.6%: 0–33% | Pork meat, stored for 7 days at 4 °C, wrapped with the film | Decreased change in pH; reduced microbial growth; preserved sensory properties | [68] |
| Konjak glucomannan/carrageenan | Camellia oil | 1% (w/v): 0–3.5% (w/v) | Chicken meat, stored for 10 days at 4 °C, dipped in coating solution | Decreased change in pH; reduced weight loss, total volatile nitrogen, thiobarbituric acid reactive substances, and microbial growth Extended shelf life of the chicken meat to 10 days by retarding the oxidation of lipids and proteins and microbial growth | [69] |
| Gelatin | Hemp seed oil | 4% (w/v): 0–2% (w/v) | Pork meat, stored for 12 days at 2 °C, dipped in coating solution | Improved oxidative stability; reduced microbial growth | [70] |
| Chitosan (high MW) | Sunflower oil | 1% (w/w): 0–1% (w/w) | Pork meat hamburger, stored for 8 days at 4 °C, coated with the film | Decreased formation of metmyoglobin | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghadas, H.C.; Chauhan, R.; Smith, J.S. Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review. Foods 2024, 13, 997. https://doi.org/10.3390/foods13070997
Moghadas HC, Chauhan R, Smith JS. Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review. Foods. 2024; 13(7):997. https://doi.org/10.3390/foods13070997
Chicago/Turabian StyleMoghadas, Hooman Chodar, Ruchi Chauhan, and J. Scott Smith. 2024. "Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review" Foods 13, no. 7: 997. https://doi.org/10.3390/foods13070997
APA StyleMoghadas, H. C., Chauhan, R., & Smith, J. S. (2024). Application of Plant Oils as Functional Additives in Edible Films and Coatings for Food Packaging: A Review. Foods, 13(7), 997. https://doi.org/10.3390/foods13070997







