Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
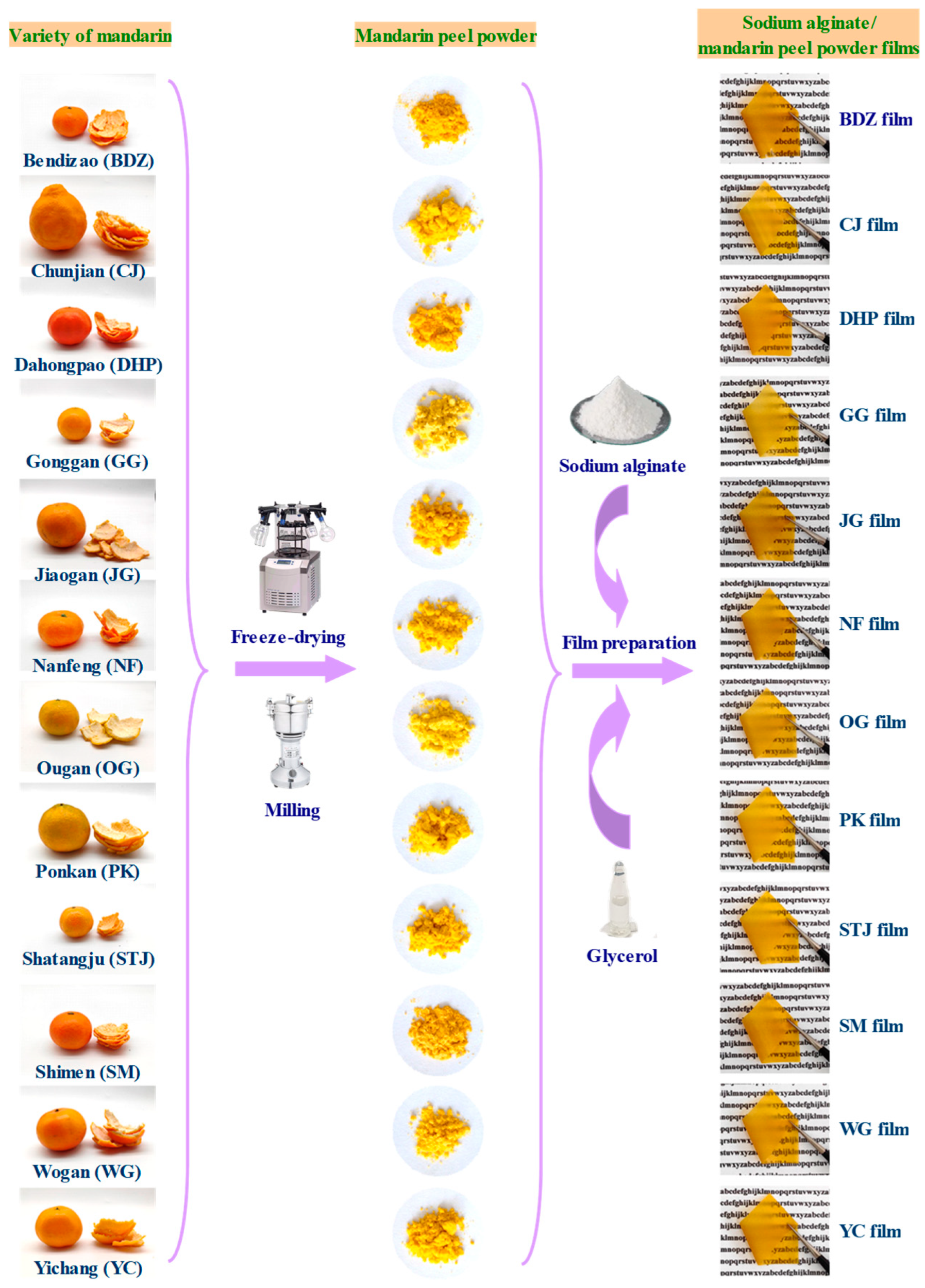
2.2. Preparation and Compositional Analysis of Mandarin Peel Powder
2.3. Preparation of Active Packaging Films
2.4. Structural Characterization of Films
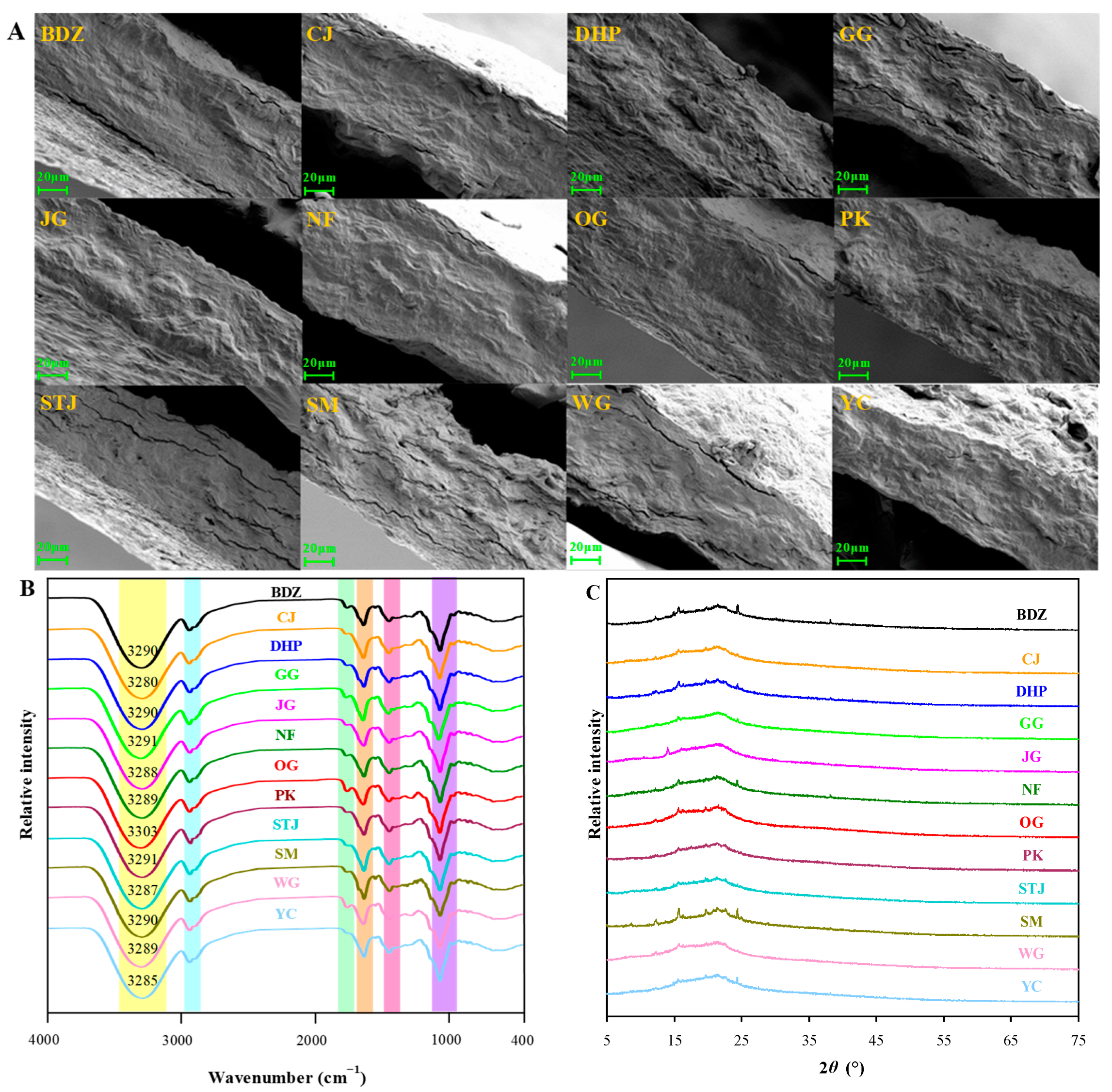
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. Infrared Spectroscopy
2.4.3. X-ray Diffraction (XRD)
2.5. Determination of the Physical and Functional Properties of Films
2.5.1. Optical Properties
2.5.2. Thickness
2.5.3. Water Blocking Ability
2.5.4. Oxygen Permeability (OP)
2.5.5. Mechanical Properties
2.5.6. Thermogravimetric Analysis (TGA)
2.5.7. Antioxidant-Releasing Ability
2.5.8. Antimicrobial-Releasing Ability
2.5.9. Biodegradability
2.6. Corn Oil Packaging Test of Films
2.7. Statistical Analysis
3. Results and Discussion
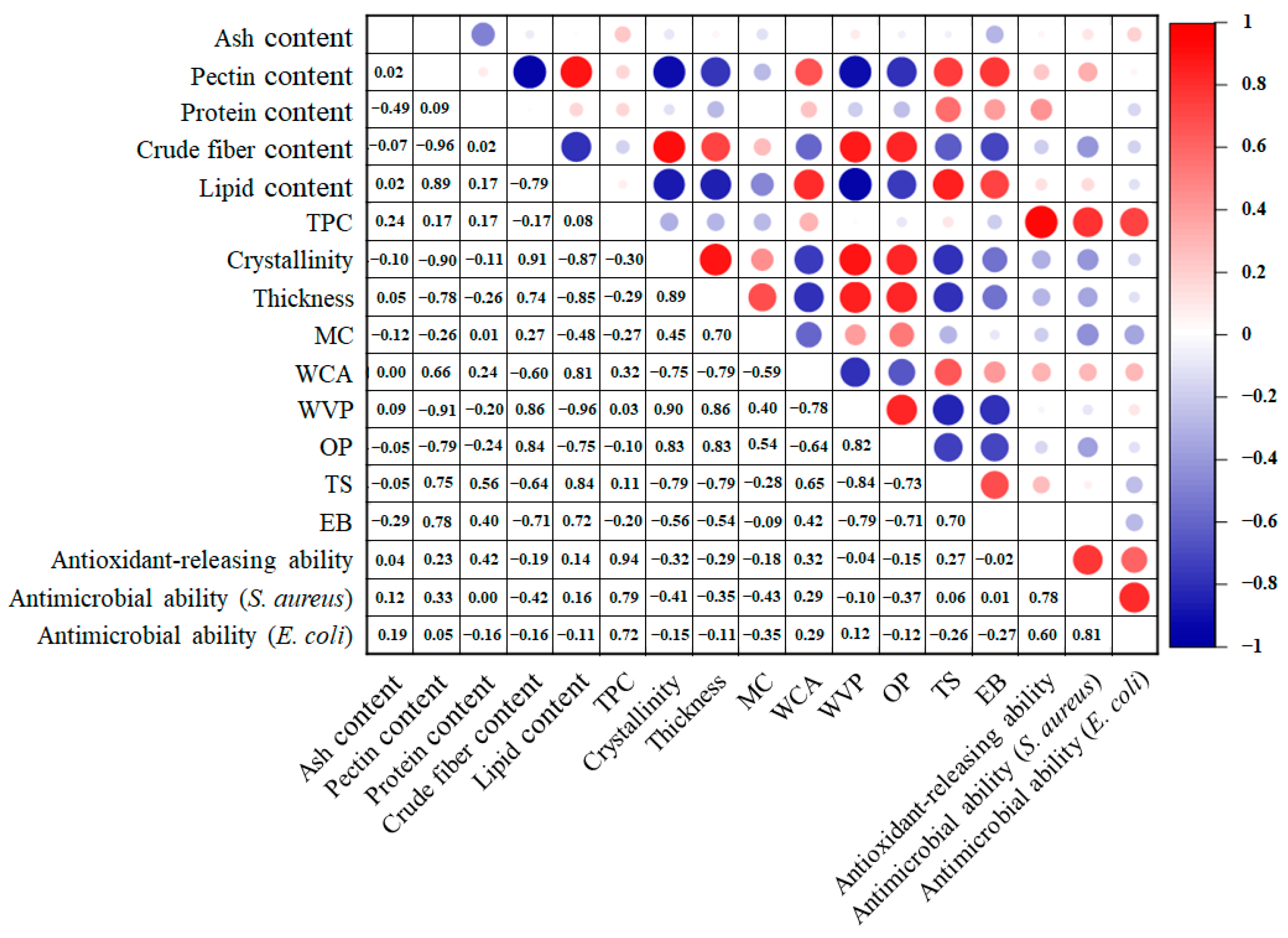
3.1. Proximate Composition of Mandarin Peel Powder
3.2. Structural Characteristics of Films
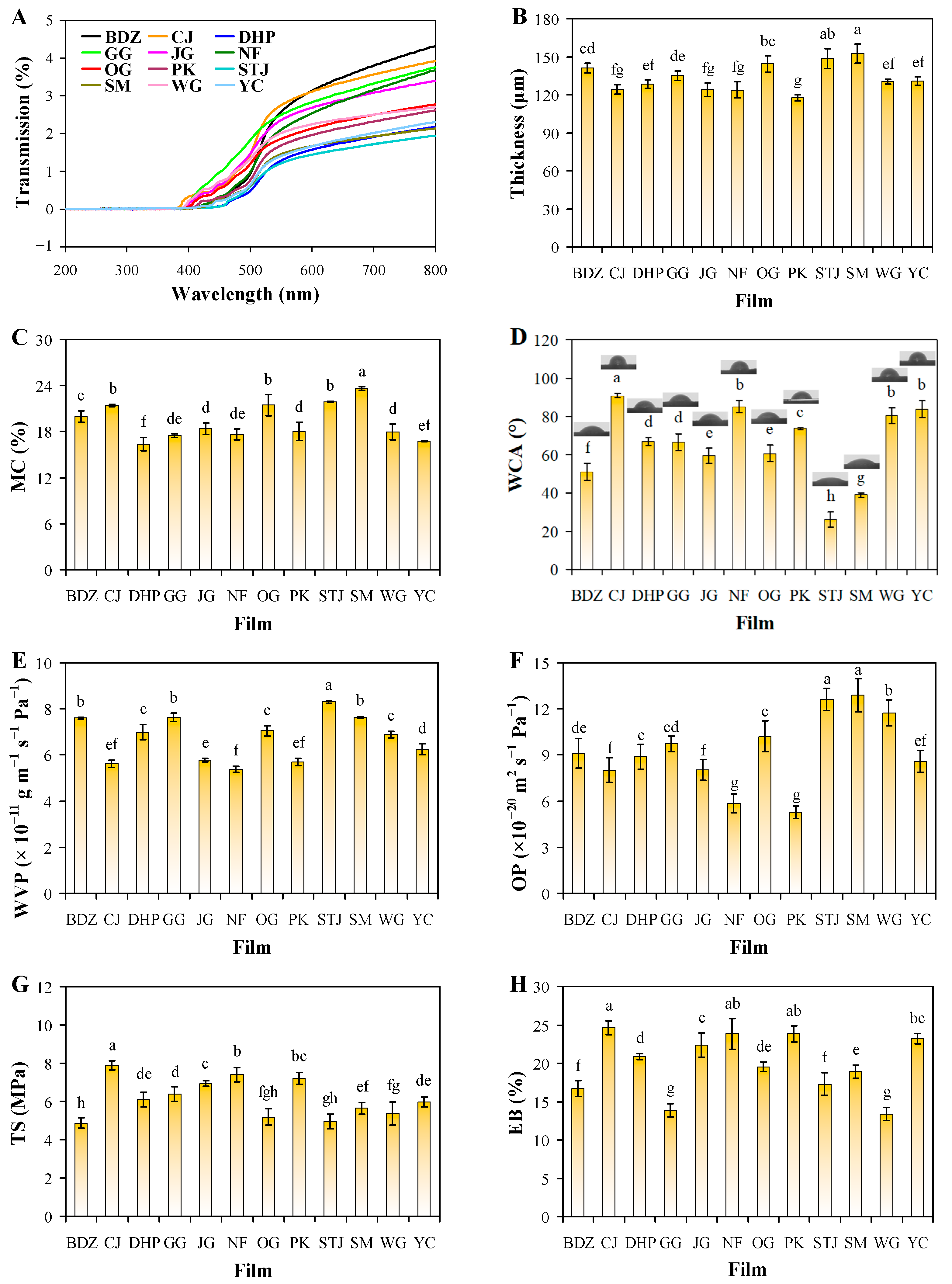
3.3. Optical Properties of Films
3.4. Thickness and Water Blocking Ability of Films
3.5. Oxygen Blocking Ability of Films
3.6. Mechanical Properties of Films
3.7. Thermal Properties of Films
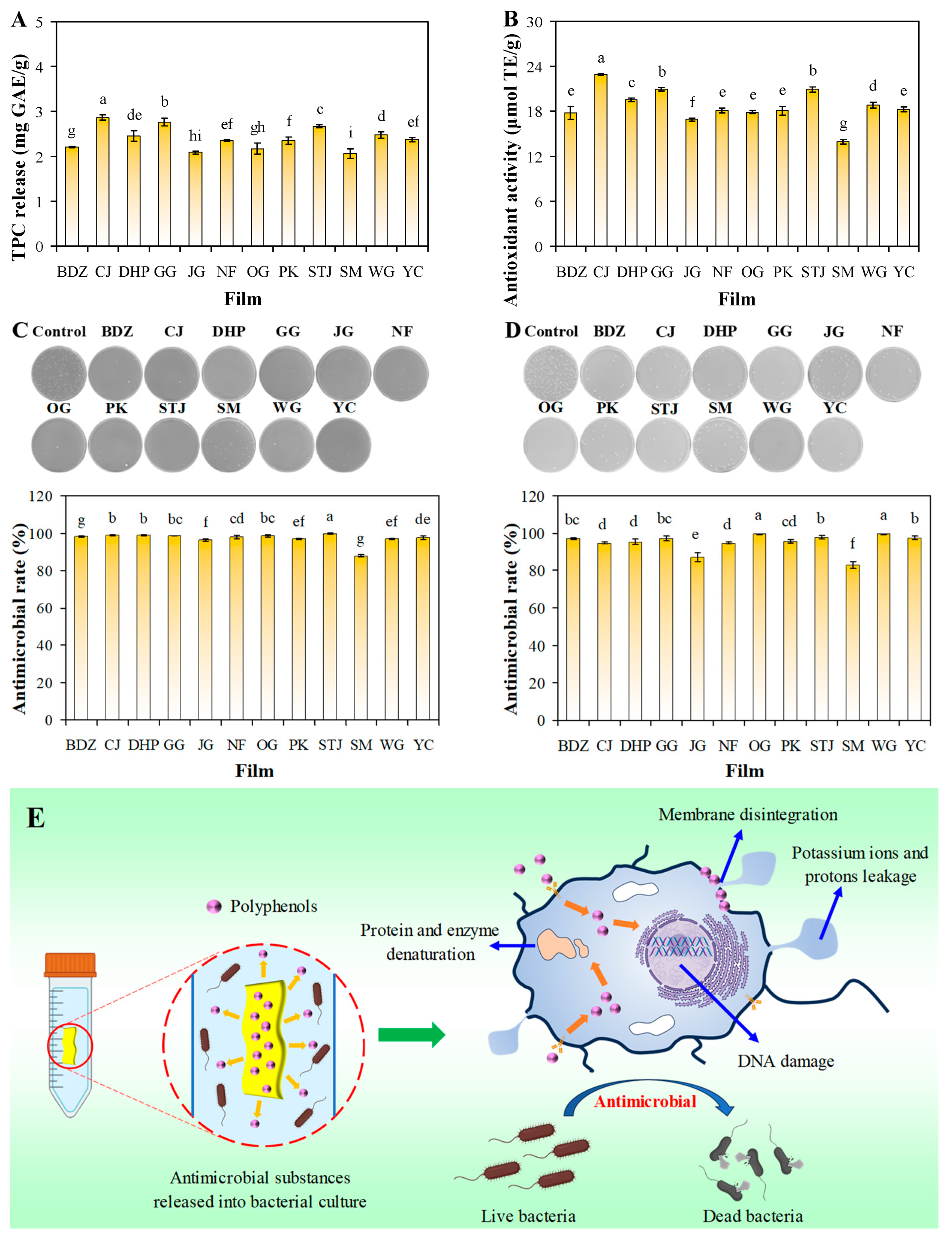
3.8. Antioxidant-Releasing Ability of Films
3.9. Antimicrobial-Releasing Ability of Films
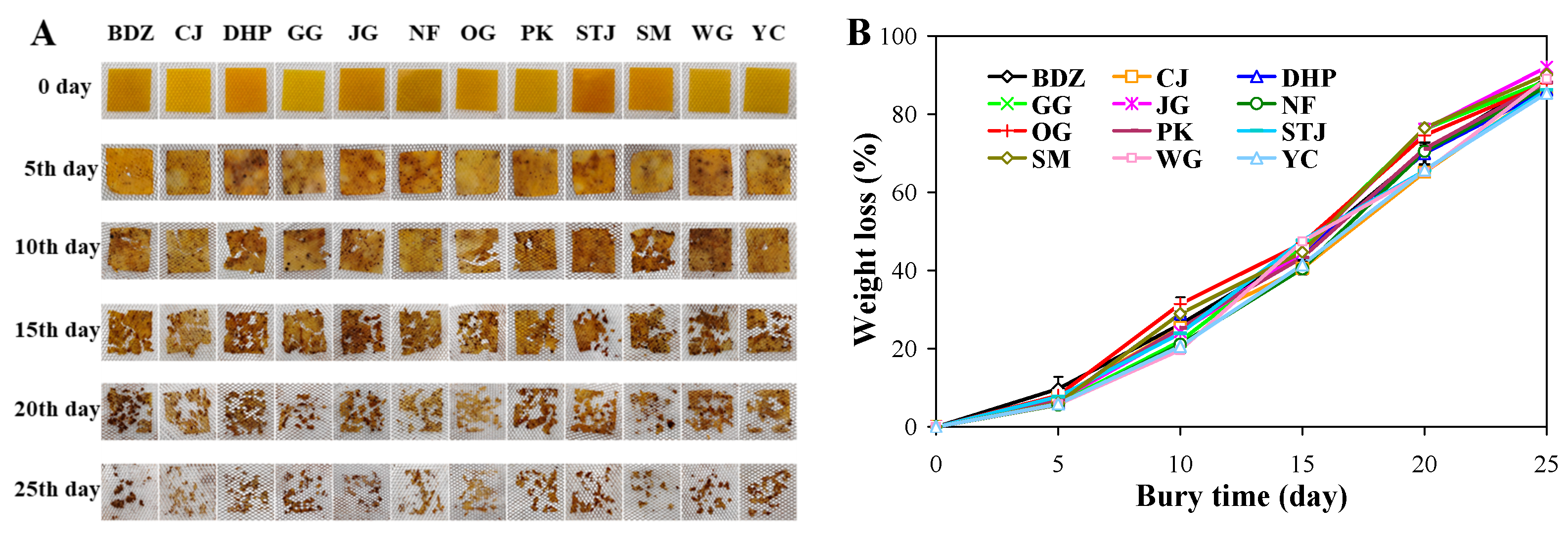
3.10. Biodegradability of Films
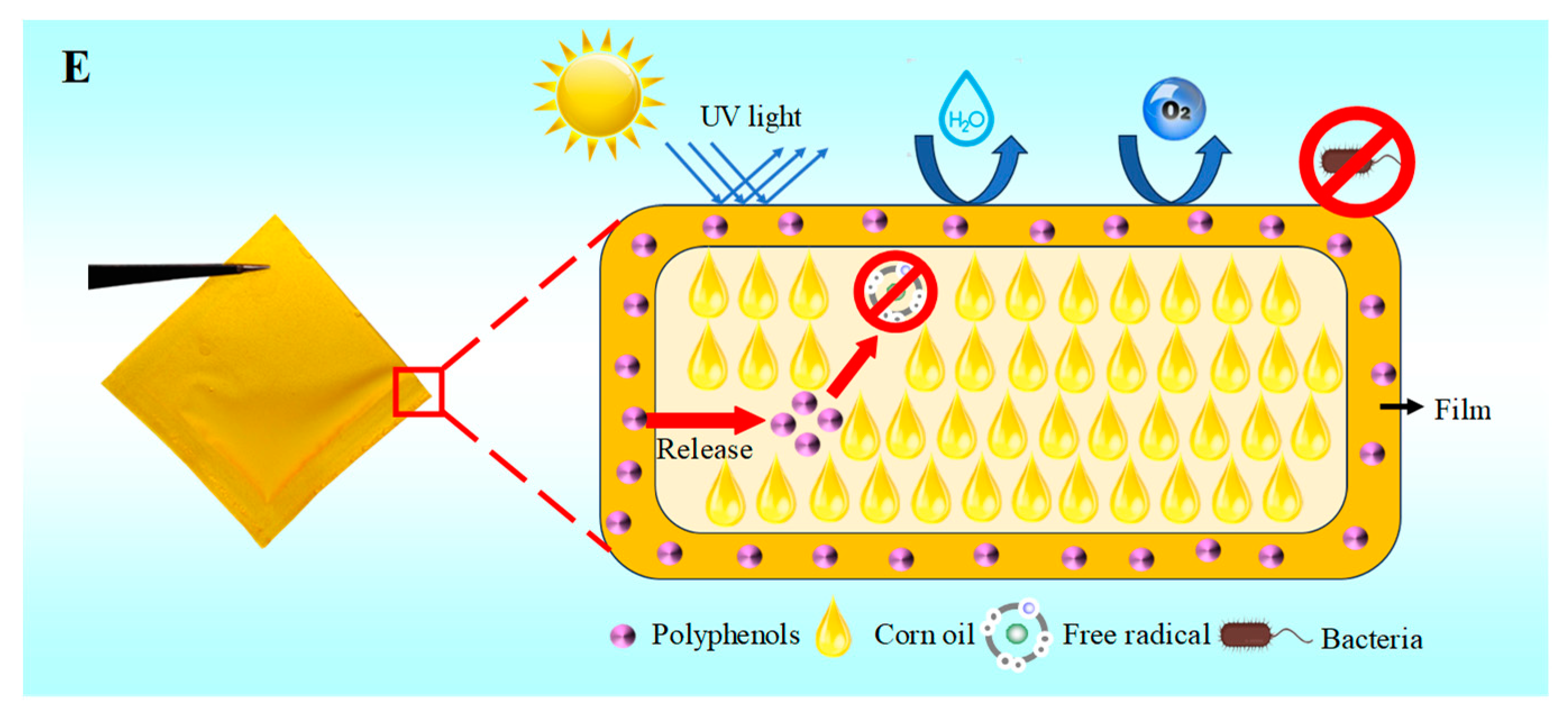
3.11. Application of Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, M.R.; Hsieh, S.; Ricacho, N. Innovative food packaging, food quality and safety, and consumer perspectives. Processes 2022, 10, 747. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Kishore, A.; Aravind, S.M.; Kumar, P.; Singh, A.; Kumari, K.; Kumar, N. Innovative Packaging Strategies for Freshness and Safety of Food Products: A Review. Packag. Technol. Sci. 2024; 1–29, Early View. [Google Scholar]
- Alves, J.; Gaspar, P.D.; Lima, T.M.; Silva, P.D. What is the role of active packaging in the future of food sustainability? A systematic review. J. Sci. Food Agric. 2023, 103, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Baican, M. Progresses in food packaging, food quality, and safety—Controlled-release antioxidant and/or antimicrobial packaging. Molecules 2021, 26, 1263. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; de Carvalho, A.P.A.D.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Liu, D.; Zhang, X.; Yin, Z.; Ismail, B.B.; Ye, X.; Guo, M. A review of active packaging in bakery products: Applications and future trends. Trends Food Sci. Technol. 2021, 114, 459–471. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Tasopoulos, N.; Chatzopoulou, P.; Mourtzinos, I. Current status, technology, regulation and future perspectives of essential oils’ usage in food and drink industry. J. Sci. Food Agric. 2023, 103, 6727–6751. [Google Scholar] [CrossRef] [PubMed]
- Singaram, A.J.V.; Guruchandran, S.; Ganesan, N.D. Review on functionalized pectin films for active food packaging. Packag. Technol. Sci. 2024, 37, 237–262. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Gaikwad, K.K. Natural antimicrobial and antioxidant compounds for active food packaging applications. Biomass Convers. Biorefin. 2022, 14, 4419–4440. [Google Scholar] [CrossRef]
- Phiri, R.; Rangappa, S.M.; Siengchin, S.; Oladijo, O.P.; Dhakal, H.N. Development of sustainable biopolymer-based composites for lightweight applications from agricultural waste biomass: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 436–450. [Google Scholar] [CrossRef]
- Birania, S.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Rohilla, P.; Kumar, R. Advances in development of biodegradable food packaging material from agricultural and agro-industry waste. J. Food Process Eng. 2022, 45, e13930. [Google Scholar] [CrossRef]
- Thakur, N.; Raposo, A. Development and application of fruit and vegetable based green films with natural bio-actives in meat and dairy products: A review. J. Sci. Food Agric. 2023, 103, 6167–6179. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent advances on the development of food packaging films based on citrus processing wastes: A review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Ahmad, A.; Dubey, P.; Younis, K.; Yousuf, O. Mosambi (Citrus limetta) peel and Sago based biodegradable film: Development and characterization of physical, water barrier and biodegradation properties. Bioresour. Technol. Rep. 2022, 18, 101016. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, D.; Lu, J.; Jiao, C.; Li, S.; Lei, Y.; Liu, D.; Wang, J.; Zhang, Z.; Liu, Y.; et al. Effect of high-pressure homogenization on microstructure and properties of pomelo peel flour film-forming dispersions and their resultant films. Food Hydrocoll. 2020, 102, 105628. [Google Scholar] [CrossRef]
- Wu, H.; Lei, Y.; Zhu, R.; Zhao, M.; Lu, J.; Xiao, D.; Jiao, C.; Zhang, Z.; Shen, G.; Li, S. Preparation and characterization of bioactive edible packaging films based on pomelo peel flours incorporating tea polyphenol. Food Hydrocoll. 2019, 90, 41–49. [Google Scholar] [CrossRef]
- Soofi, M.; Alizadeh, A.; Hamishehkar, H.; Almasi, H.; Roufegarinejad, L. Preparation of nanobiocomposite film based on lemon waste containing cellulose nanofiber and savory essential oil: A new biodegradable active packaging system. Int. J. Biol. Macromol. 2021, 169, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Terzioğlu, P.; Güney, F.; Parın, F.N.; Şen, İ.; Tuna, S. Biowaste orange peel incorporated chitosan/polyvinyl alcohol composite films for food packaging applications. Food Packag. Shelf Life 2021, 30, 100742. [Google Scholar] [CrossRef]
- Taghavi Kevij, H.; Salami, M.; Mohammadian, M.; Khodadadi, M.; Emam-Djomeh, Z. Mechanical, physical, and bio-functional properties of biopolymer films based on gelatin as affected by enriching with orange peel powder. Polym. Bull. 2021, 78, 4387–4402. [Google Scholar] [CrossRef]
- Sambudi, N.S.; Lin, W.Y.; Harun, N.Y.; Mutiari, D. Modification of poly (lactic acid) with orange peel powder as biodegradable composite. Polymers 2022, 14, 4126. [Google Scholar] [CrossRef]
- Yun, D.; Wang, Z.; Li, C.; Chen, D.; Liu, J. Antioxidant and antimicrobial packaging films developed based on the peel powder of different citrus fruits: A comparative study. Food Biosci. 2023, 51, 102319. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, W.; Li, Z.; Zhang, N.; Wang, S.; Shi, J.; Zhai, X.; Zhang, J.; Shen, T. Preparation of a Dual-Functional Active Film Based on Bilayer Hydrogel and Red Cabbage Anthocyanin for Maintaining and Monitoring Pork Freshness. Foods 2023, 12, 4520. [Google Scholar] [CrossRef]
- Bi, F.; Zhang, X.; Liu, J.; Yong, H.; Gao, L.; Liu, J. Development of antioxidant and antimicrobial packaging films based on chitosan, D-α-tocopheryl polyethylene glycol 1000 succinate and silicon dioxide nanoparticles. Food Packag. Shelf Life 2020, 24, 100503. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Karim, A.A.; Ariffin, F. Enhancing the functional properties of fish gelatin mats by dual encapsulation of essential oils in β-cyclodextrins/fish gelatin matrix via coaxial electrospinning. Food Hydrocoll. 2023, 137, 108324. [Google Scholar] [CrossRef]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohydr. Polym. 2021, 254, 117410. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, J.; Yun, D.; Yong, H.; Liu, J. Antioxidant packaging films developed based on chitosan grafted with different catechins: Characterization and application in retarding corn oil oxidation. Food Hydrocoll. 2022, 133, 107970. [Google Scholar] [CrossRef]
- Xu, Q.D.; Jing, Z.; He, Q.; Zeng, W.C. A novel film based on gluten, pectin and polyphenols and its potential application in high-fat food. J. Sci. Food Agric. 2023, 103, 6119–6127. [Google Scholar] [CrossRef] [PubMed]
- Marey, S.; Shoughy, M. Effect of temperature on the drying behavior and quality of citrus peels. Int. J. Food Eng. 2016, 12, 661–671. [Google Scholar] [CrossRef]
- Rafiq, S.; Singh, B.; Gat, Y. Effect of different drying techniques on chemical composition, color and antioxidant properties of kinnow (Citrus reticulata) peel. J. Food Sci. Technol. 2019, 56, 2458–2466. [Google Scholar] [CrossRef]
- Anticona, M.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J.; Blesa, J. Comprehensive analysis of polyphenols from hybrid Mandarin peels by SPE and HPLC-UV. LWT 2022, 165, 113770. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of phenolic composition and antioxidant properties of different varieties of Chinese citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of peel essential oils from four selected Tunisian Citrus species: Evidence for the genotypic influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Liu, N.; Yang, W.; Li, X.; Zhao, P.; Liu, Y.; Guo, L.; Huang, L.; Gao, W. Comparison of characterization and antioxidant activity of different citrus peel pectins. Food Chem. 2022, 386, 132683. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yuan, F.; Pan, Q.; Fan, R.; Gao, Y. Physicochemical characterization of five types of citrus dietary fibers. Biocatal. Agric. Biotechnol. 2015, 4, 250–258. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, J.; Tian, X.; Xu, Z.; Huang, L.; Xiao, H.; Ren, X.; Kong, Q. Alginate with citrus pectin and pterostilbene as healthy food packaging with antioxidant property. Int. J. Biol. Macromol. 2021, 193, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Meydanju, N.; Pirsa, S.; Farzi, J. Biodegradable film based on lemon peel powder containing xanthan gum and TiO2–Ag nanoparticles: Investigation of physicochemical and antibacterial properties. Polym. Test. 2022, 106, 107445. [Google Scholar] [CrossRef]
- Li, C.; Sun, J.; Yun, D.; Wang, Z.; Tang, C.; Liu, J. A new method to prepare color-changeable smart packaging films based on the cooked purple sweet potato. Food Hydrocoll. 2023, 137, 108397. [Google Scholar] [CrossRef]
- Bigi, F.; Maurizzi, E.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Waste orange peels as a source of cellulose nanocrystals and their use for the development of nanocomposite films. Foods 2023, 12, 960. [Google Scholar] [CrossRef]
- Ali, A.; Chen, Y.; Liu, H.; Yu, L.; Baloch, Z.; Khalid, S.; Zhu, J.; Chen, L. Starch-based antimicrobial films functionalized by pomegranate peel. Int. J. Biol. Macromol. 2019, 129, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Ren, X.; Zhu, S.; Gao, Y. Fabrication and characterization of an economical active packaging film based on chitosan incorporated with pomegranate peel. Int. J. Biol. Macromol. 2021, 192, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Younis, H.G.; Abdellatif, H.R.; Ye, F.; Zhao, G. Tuning the physicochemical properties of apple pectin films by incorporating chitosan/pectin fiber. Int. J. Biol. Macromol. 2020, 159, 213–221. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquézar, B.; Alós, E.; Lado, J.; Zacarías, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Anesi, A.; Martens, S. Flavedo and albedo of five citrus fruits from Southern Italy: Physicochemical characteristics and enzyme-assisted extraction of phenolic compounds. J. Food Meas. Charact. 2021, 15, 1754–1762. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and lipid-containing composite edible coatings and films. Food Rev. Int. 2022, 38, 574–597. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.W.; Lee, S.H.; Kim, S.S.; Park, S.H.; Jeon, J.K.; Kim, S.; Park, Y.K. Pyrolysis properties and kinetics of mandarin peel. Korean J. Chem. Eng. 2011, 28, 2012–2016. [Google Scholar] [CrossRef]
- Garde, J.A.; Catala, R.; Gavara, R.; Hernandez, R.J. Characterizing the migration of antioxidants from polypropylene into fatty food simulants. Food Addit. Contam. 2001, 18, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Chun, Y.; Lee, J.H.; Lee, J.U.; Lee, T.; Yoo, H.Y. Sustainable utilization strategy of organic waste via fabrication of bioelastomer with antibacterial and antioxidant activities using mandarin peel extracts. Agriculture 2023, 13, 161. [Google Scholar] [CrossRef]
- Aguilar-Veloz, L.M.; Calderón-Santoyo, M.; Vazquez Gonzalez, Y.; Ragazzo-Sánchez, J.A. Application of essential oils and polyphenols as natural antimicrobial agents in postharvest treatments: Advances and challenges. Food Sci. Nutr. 2020, 8, 2555–2568. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; De Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, antioxidant, antibacterial, and biofilm inhibitory activities of peel and pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; González-Martínez, C.; Chiralt, A. Biodegradable antimicrobial films for food packaging: Effect of antimicrobials on degradation. Foods 2021, 10, 1256. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of antioxidants for enhancing oxidative stability of bulk edible oils: A review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible oil parameters during deterioration processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef]

| Variety | Ash (%) | Pectin (%) | Protein (%) | Crude Fiber (%) | Lipid (%) | TPC (mg GAE/g) |
|---|---|---|---|---|---|---|
| BDZ | 4.00 ± 0.16 b | 14.24 ± 0.57 de | 4.44 ± 0.50 fg | 4.08 ± 0.79 cd | 8.72 ± 0.11 fg | 6.08 ± 0.16 bc |
| CJ | 3.05 ± 0.31 e | 18.13 ± 0.79 a | 7.90 ± 0.13 a | 3.52 ± 0.23 e | 12.82 ± 0.74 b | 6.70 ± 0.14 a |
| DHP | 2.82 ± 0.16 e | 14.75 ± 0.57 cd | 6.75 ± 0.50 b | 4.43 ± 0.28 bc | 10.43 ± 0.40 e | 6.10 ± 0.02 bc |
| GG | 3.87 ± 0.25 bc | 12.37 ± 0.64 f | 7.01 ± 0.63 b | 4.72 ± 0.22 ab | 9.26 ± 0.08 f | 6.44 ± 0.24 ab |
| JG | 2.98 ± 0.11 e | 16.77 ± 0.71 b | 5.33 ± 0.25 de | 3.64 ± 0.17 de | 12.51 ± 0.29 b | 5.58 ± 0.08 d |
| NF | 4.74 ± 1.48 a | 18.43 ± 0.50 a | 4.79 ± 0.63 ef | 3.32 ± 0.28 e | 13.58 ± 0.39 a | 5.95 ± 0.01 cd |
| OG | 2.81 ± 0.26 e | 14.85 ± 0.36 cd | 5.41 ± 0.37 d | 4.00 ± 0.34 cd | 9.16 ± 0.16 f | 5.70 ± 0.39 cd |
| PK | 3.22 ± 0.11 de | 16.81 ± 0.64 b | 6.84 ± 0.63 b | 3.52 ± 0.34 e | 11.17 ± 0.11 d | 5.96 ± 0.08 cd |
| STJ | 3.86 ± 0.08 bc | 13.64 ± 0.57 e | 5.33 ± 0.50 de | 4.44 ± 0.17 bc | 7.73 ± 0.87 h | 6.54 ± 0.13 a |
| SM | 3.31 ± 0.21 cde | 12.27 ± 0.79 f | 6.13 ± 0.38 c | 5.00 ± 0.40 a | 8.54 ± 0.15 g | 4.97 ± 0.28 e |
| WG | 3.78 ± 0.18 bcd | 14.65 ± 0.71 cd | 4.17 ± 0.38 g | 4.27 ± 0.17 c | 10.38 ± 0.30 e | 6.44 ± 0.24 ab |
| YC | 3.28 ± 0.25 cde | 15.30 ± 0.50 c | 6.03 ± 0.25 c | 4.00 ± 0.22 cd | 11.74 ± 0.39 c | 6.00 ± 0.84 cd |
| Films | L* | a* | b* | ΔE |
|---|---|---|---|---|
| BDZ | 70.11 ± 0.25 i | 12.51 ± 0.19 a | 75.53 ± 0.52 d | 83.48 ± 0.38 a |
| CJ | 78.28 ± 0.23 b | 3.62 ± 0.45 h | 78.35 ± 0.37 b | 83.26 ± 0.30 a |
| DHP | 74.06 ± 0.14 g | 12.74 ± 0.20 a | 77.50 ± 0.05 c | 84.27 ± 0.02 a |
| GG | 78.88 ± 0.22 a | 0.60 ± 0.42 i | 64.99 ± 0.23 h | 70.04 ± 0.17 b |
| JG | 74.09 ± 0.02 g | 7.51 ± 0.27 d | 72.81 ± 0.22 e | 79.15 ± 0.24 c |
| NF | 71.61 ± 0.13 h | 9.88 ± 0.10 c | 77.50 ± 0.08 c | 84.57 ± 0.05 c |
| OG | 74.22 ± 0.30 g | 4.92 ± 0.06 g | 66.05 ± 0.11 g | 68.47 ± 0.19 b |
| PK | 77.31 ± 0.31 c | 5.93 ± 0.21 f | 75.07 ± 0.57 d | 80.40 ± 0.64 c |
| STJ | 76.29 ± 0.25 e | 6.45 ± 0.17 e | 75.17 ± 0.11 d | 83.68 ± 0.17 c |
| SM | 74.33 ± 0.02 g | 10.70 ± 0.07 b | 79.92 ± 0.31 a | 86.26 ± 0.30 c |
| WG | 76.75 ± 0.03 d | 5.94 ± 0.18 f | 68.34 ± 0.71 f | 74.03 ± 0.66 b |
| YC | 74.72 ± 0.26 f | 6.58 ± 0.47 e | 72.70 ± 0.16 e | 78.80 ± 0.26 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, D.; Liu, J. Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder. Foods 2024, 13, 1174. https://doi.org/10.3390/foods13081174
Yun D, Liu J. Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder. Foods. 2024; 13(8):1174. https://doi.org/10.3390/foods13081174
Chicago/Turabian StyleYun, Dawei, and Jun Liu. 2024. "Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder" Foods 13, no. 8: 1174. https://doi.org/10.3390/foods13081174
APA StyleYun, D., & Liu, J. (2024). Preparation, Characterization and Application of Active Food Packaging Films Based on Sodium Alginate and Twelve Varieties of Mandarin Peel Powder. Foods, 13(8), 1174. https://doi.org/10.3390/foods13081174








