Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Cultures
2.3. Chia Mucilage Extraction
2.4. Production of Chia Mucilage-Based Nanocapsules Loaded with Carvacrol and Carvacrol Emulsion
2.5. Film Preparation
2.6. Thickness and Mechanical Properties of the Films
2.7. Water Vapor Permeability (WVP)
2.8. Moisture and Solubility in Water
2.9. Optical Properties
2.10. Morphological Properties
2.11. Thermogravimetric Analysis
2.12. Antimicrobial Properties of the Films
2.13. Statistical Analyses
3. Results and Discussion
3.1. Thickness and Mechanical Properties
3.2. Water Vapor Permeability (WVP), Moisture Content (MC), and Water Solubility (WS)
3.3. Optical Properties of the Films
3.4. Morphological Properties
3.5. Thermogravimetric Analysis
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Saltmarsh, M. Essential Guide to Food Additives, 4th ed.; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Cansian, R.L.; Mossi, A.J.; Paroul, N.; Toniazzo, G.; Zboralski, F.; Prichoa, F.C.; Kubiak, G.B.; Lerin, L.A. Atividade antioxidante e antimicrobiana de extratos de canela-sassafras (Ocotea odorifera (vell.) Rowhwer). Perspectiva 2010, 34, 123–133. [Google Scholar]
- Julizan, N.; Ishmayana, S.; Zainuddin, A.; Hung, P.V.; Kurnia, D. Potential of Syzygnium polyanthum as Natural Food Preservative: A Review. Foods 2023, 12, 2275. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Keyata, E.O. Potentials of Natural Preservatives to Enhance Food Safety and Shelf Life: A Review. Sci. World J. 2022, 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef] [PubMed]
- Hodas, F.; Zorzenon, M.R.; Milani, P.G. Moringa oleifera potential as a functional food and a natural food additive: A biochemical approach. Acad Bras Cienc. 2021, 93, 18. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.Q.; Lopes, S.M.; Costa, T.M.H.; Flôres, S.H.; Rios, A.O. Active biodegradable cassava starch films incorporated lycopene nanocapsules. Ind. Crops Prod. 2017, 109, 818–827. [Google Scholar] [CrossRef]
- Pagno, C.H.; Farias, Y.B.; Costa, T.M.H.; Rios, A.O.; Flôres, S.H. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J. Food Sci. Technol. 2016, 53, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yoon, H.; Kang, D.H.; Chang, P.S.; Ryu, S. Endolysin LysSA97 is synergistic with carvacrol in controlling Staphylococcus aureus in foods. Int. J. Food Microbiol. 2017, 244, 19–26. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, L. Encapsulated natural antimicrobials: A promising way to reduce microbial growth in different food systems. Food Control 2021, 123, 107678. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Nunes, I.L. Oil nanoencapsulation: Development, application, and incorporation into the food market. Nanoscale Res. Lett. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Kurd, F.; Fathi, M.; Shekarchizadeh, H. Nanoencapsulation of hesperetin using basil seed mucilage nanofibers: Characterization and release modeling. Food Biosci. 2019, 32, 100475. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Goli, S.A.H.; Doost, A.S.; Roman, B.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Plant based Pickering stabilization of emulsions using soluble flaxseed protein and mucilage nano-assemblies. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 170–182. [Google Scholar] [CrossRef]
- Campo, C.; Santos, P.P.; Costa, T.M.H.; Paese, K.; Guterres, S.S.; Rios, A.O.; Flôres, S.H. Nanoencapsulation of chia seed oil with chia mucilage (Salvia hispanica L.) as wall material: Characterization and stability evaluation. Food Chem. 2017, 234, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.S.; Campo, C.; Paese, K.; Guterres, S.S.; Costa, T.M.H.; Flôres, S.H. Nanoencapsulation of linseed oil with chia mucilage as structuring material: Characterization, stability and enrichment of orange juice. Food Res. Int. 2019, 120, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Ixtaina, V.Y.; Nolasco, S.M.; Tomás, M.C. Physical properties of chia (Salvia hispanica L.) seeds. Ind. Crop. Prod. 2008, 28, 286–293. [Google Scholar] [CrossRef]
- Muñoz, L.A.; Aguilera, J.M.; Rodriguez-Turienzo, L.; Cobos, A.; Diaz, O. Characterization and microstructure of films made from mucilage of Salvia hispanica and whey protein concentrate. J. Food Eng. 2012, 111, 511–518. [Google Scholar] [CrossRef]
- Xing, X.; Hsieh, Y.S.Y.; Yap, K.; Ang, M.E.; Lahnstein, J.; Tucker, M.R.; Burton, R.A.; Bulone, V. Isolation and structural elucidation by 2D NMR of planteose, a major oligosaccharide in the mucilage of chia (Salvia hispanica L.) seeds. Carbohydr. Polym. 2017, 175, 231–240. [Google Scholar] [CrossRef]
- Froebel, L.K.; Froebel, L.E.; Duong, T. Refined functional carbohydrates reduce adhesion of Salmonella and Campylobacter to poultry epithelial cells in vitro. Poultry Sci. 2020, 99, 7027–7034. [Google Scholar] [CrossRef]
- Cruz, E.P.; Pires, J.B.; Santos, F.N.; Fonseca, L.M.; Radünz, M.; Dal Magro, J.; Gandra, E.A.; Zavareze, E.R.; Dias, A.R.G. Encapsulation of lemongrass essential oil into cassava starch fibers for application as antifungal agents in bread. Food Hydrocoll. 2023, 145, 109105. [Google Scholar] [CrossRef]
- Santacruz, S.; Rivandaneira, C.; Castro, M. Edible films based on starch and chitosan, Effect of starch source and concentration, plasticizer, surfactant’s hydrophobic tail and mechanical tratment. Food Hydrocoll. 2015, 49, 89–90. [Google Scholar] [CrossRef]
- Feng, M.; Yu, L.; Zhu, P.; Zhou, X.; Liu, H.; Yang, Y.; Zhou, J.; Gao, C.; Bao, X.; Chen, P. Development and preparation of active starch films carrying tea polyphenol. Carbohydr. Polym. 2018, 196, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, B.S.; Vasile, C. Encapsulation of natural bioactive compounds by electrospinning—Applications in food storage and safety. Polymers 2021, 13, 3771. [Google Scholar] [CrossRef]
- Caetano, K.S.; Lopes, N.A.; Costa, T.M.H.; Brandelli, A.; Rodrigues, E.; Flôres, S.H.; Cladera-Olivera, F. Characterization of active biodegradable films based on cassava starch and natural compounds. Food Packag. Shelf Life 2018, 16, 138–147. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Dobon, A.; Bermudez, J.M.; Lara-Lledo, M. The effect of active packaging on minimizing food losses: Life cycle assessment (LCA) of essential oil component-enabled packaging for fresh beef. Packag. Technol. Sci. 2015, 28, 761–774. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Interface Sci. 2020, 278, 102140. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, F.A.; Dalmása, M.; Maders, C.; Isaía, H.A.; Brandelli, A.; Malheiros, P.S. Carvacrol encapsulation into nanostructures: Characterization and antimicrobial activity against foodborne pathogens adhered to stainless steel. Food Res. Int. 2020, 133, 109143. [Google Scholar] [CrossRef] [PubMed]
- Dick, M.; Costa, T.M.H.; Gomaa, A.; Subirade, M.; Rios, A.O.; Flôres, S.H. Edible film production from chia seed mucilage: Effect of glycerol concentration on its physicochemical and mechanical properties. Carbohydr. Polym. 2015, 130, 198–205. [Google Scholar] [CrossRef]
- Cacciatore, F.A.; Maders, C.; Alexandre, B.; Pinilla, C.M.B.; Brandelli, A.; Malheiros, P.S. Carvacrol encapsulation into nanoparticles produced from chia and flaxseed mucilage: Characterization, stability and antimicrobial activity against Salmonella and Listeria monocytogenes. Food Microbiol. 2022, 108, 104116. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; Rios, A.O. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Assis, R.Q. Biodegradable Films with the Addition of Free and Nanoencapsulated Lycopene or β-Carotene. Master’s Thesis, Federal University of Rio Grande Do Sul (UFRGS), Porto Alegre, Brazil, 2017. [Google Scholar]
- ASTM. D882-12; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2012. [CrossRef]
- Pagno, C.H.; Costa, T.M.H.; Menezes, E.W.; Benvenutti, E.V.; Hertz, P.F.; Matte, C.R.; Tosati, J.V.; Monteiro, A.R.; Rios, A.O.; Flôres, S.H. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015, 173, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of microalgae addition on active biodegradable starch film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Pham, T.T.; Nguyen, T.N.H.; Le, N.T.; Dang, B.T.; Nguyen, B.Q.H. Post-harvest preservation of green grapes utilizing 405 nm light emitting diode. Case Stud. Chem. Environ. Eng. 2023, 8, 100463. [Google Scholar] [CrossRef]
- Tunç, S.; Duman, O. Preparation of active antimicrobial methyl cellulose/carvacrol/montmorillonite nanocomposite films and investigation of carvacrol release. LWT Food Sci. Technol. 2011, 44, 465–472. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.; Feng, X.; Wang, W.; Xu, F.; Zhang, L. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar] [CrossRef]
- Teles, V.; Roldi, M.; Luz, S.; Santos, W.; Andreani, L.; Valadares, L. Obtaining plasticized starch and microfibrillated cellulose from oil palm empty fruit bunches: Preparation and properties of the pure materials and their composites. BioResources 2021, 16, 3746–3759. [Google Scholar] [CrossRef]
- Martins, J.T.; Cerqueira, M.A.; Vicente, A.A. Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll. 2012, 27, 220–227. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- Jafari, S.M. An Overview of Nanoencapsulation Techniques and Their Classification; Elsevier Inc.: Gorgan, Iran, 2017. [Google Scholar]
- Bastos, M.D.S.R.; Laurentino, L.S.; Canuto, K.M.; Mendes, L.G.; Martins, C.M.; Silva, S.M.F.; Furtado, R.F.; Kim, S.; Biswas, A.; Cheng, H.N. Physical and mechanical testing of essential oil-embedded cellulose ester films. Polym. Test. 2016, 49, 156–161. [Google Scholar] [CrossRef]
- Monedero, F.M.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of oleic acid-beeswax mixtures on mechanical, optical and water barrier properties of soy protein isolate based films. J. Food Eng. 2009, 91, 509–515. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Arfa, A.B.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Grossmann, M.V.E.; Yamashita, F.; Pineda, E.A.G. Antimicrobial, mechanical, and barrier properties of cassava Starch–Chitosan films incorporated with oregano essential oil. J. Agric. Food Chem. 2009, 57, 7499–7504. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Rhim, J.W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Laroque, D.A.; Aragão, G.M.F.; Araújo, P.H.H.; Carciofi, B.A.M. Active cellulose acetate-carvacrol films: Antibacterial, physical and thermal properties. Packag. Technol. Sci. 2021, 34, 463–474. [Google Scholar] [CrossRef]
- Brasil. Surtos de Doenças de Transmissão Hídrica e Alimentar no Brasil. Brasilia: [s.n.], 2024. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/d/dtha/publicacoes/surtos-de-doencas-de-transmissao-hidrica-e-alimentar-no-brasil-informe-2024 (accessed on 8 March 2024).
- Center for Disease Control and Prevention (CDC). List of Selected Multistate Foodborne Outbreak Investigations. 2023. Available online: https://www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffoodsafety%2Foutbreaks%2Fmultistate-outbreaks%2Foutbreaks-list.html (accessed on 30 January 2024).
- European Food Safety Authority. The European Union One Health 2021. Zoonoses Report. EFSA J. 2022, 12, e07666. [Google Scholar]
- Lee, J.H.; Lee, J.; Song, K.B. Development of a chicken feet protein film containing essential oils. Food Hydrocoll. 2015, 46, 208–215. [Google Scholar] [CrossRef]
- Dannenberg, G.S.; Funck, G.D.; Cruxen, C.E.S.; Marques, J.L.; Silva, W.P.; Fiorentini, A.M. Essential oil from pink pepper as an antimicrobial component in cellulose acetate film: Potential for application as active packaging for sliced cheese. LWT Food Sci. Technol. 2017, 81, 314–318. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Marino, A.; Blanco, A.R.; Cellini, L.; Di Giulio, M.; Pizzimenti, F.; Roccaro, A.S.; Bisignano, G. In vitro activity of carvacrol against staphylococcal preformed biofilm by liquid and vapour contact. J. Med. Microbiol. 2009, 58, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Homayouni, H.; Kavoosi, G.; Nassiri, S.M. Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT Food Sci. Technol. 2017, 77, 503–509. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; Santana, L.C.A. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT Food Sci. Technol. 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and characterization of potato starch-thymol dispersion and film as potential antioxidant and antibacterial materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.E.B.; Santos, J.K.B.; Amorim, N.O.; Sousa, T.A.; Cavalcante, M.E.G.; Silva, J.C.S. Analysis of the development of the culture of the Panicum miliaceum L. (Panicum miliaceum L.) in soils where there was the cultivation of manioc (Manihot esculenta Crantz), with addition of the inoculation of the solution of Hoagland. Divers J. 2018, 3, 5–12. [Google Scholar] [CrossRef]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
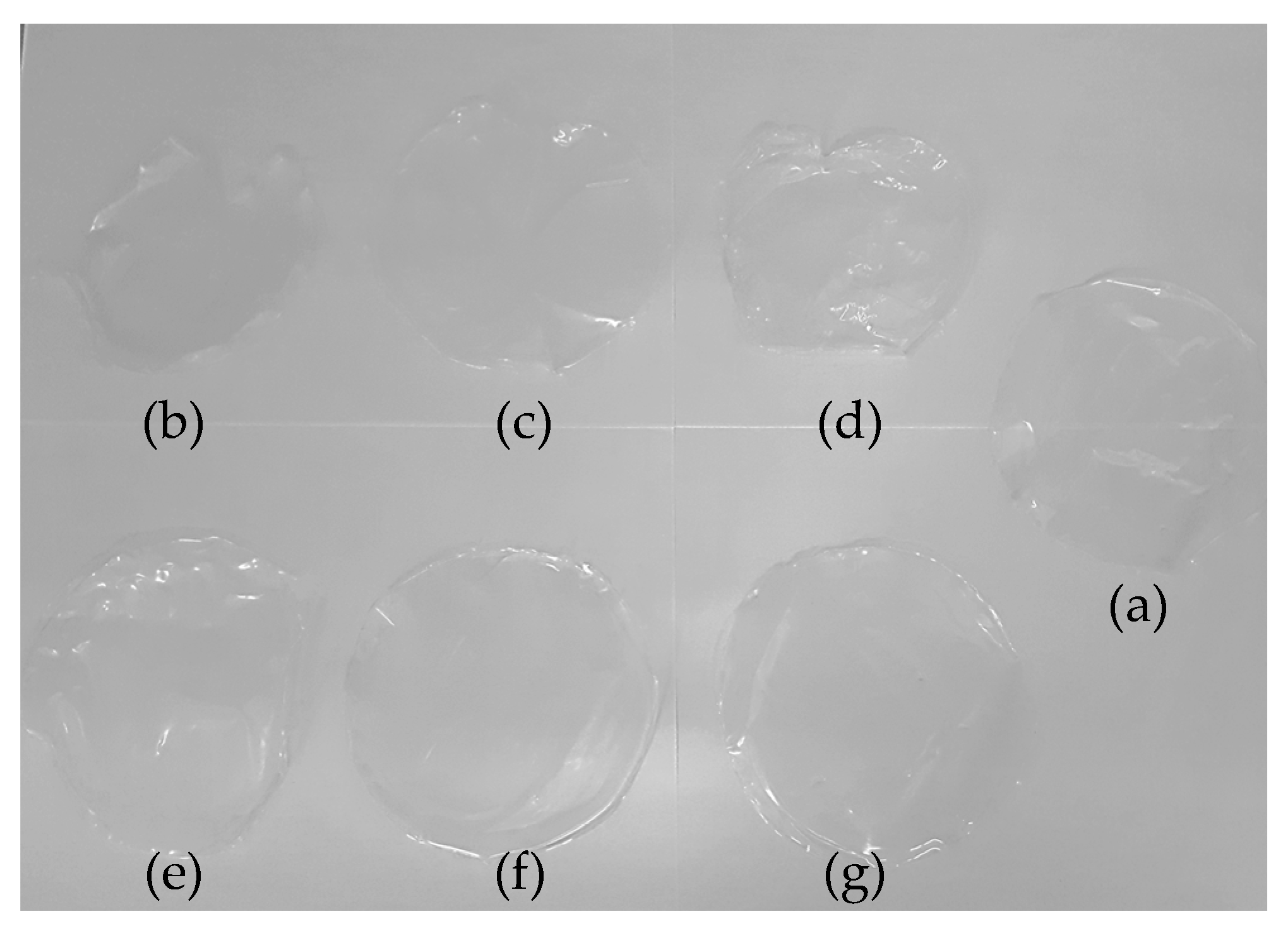
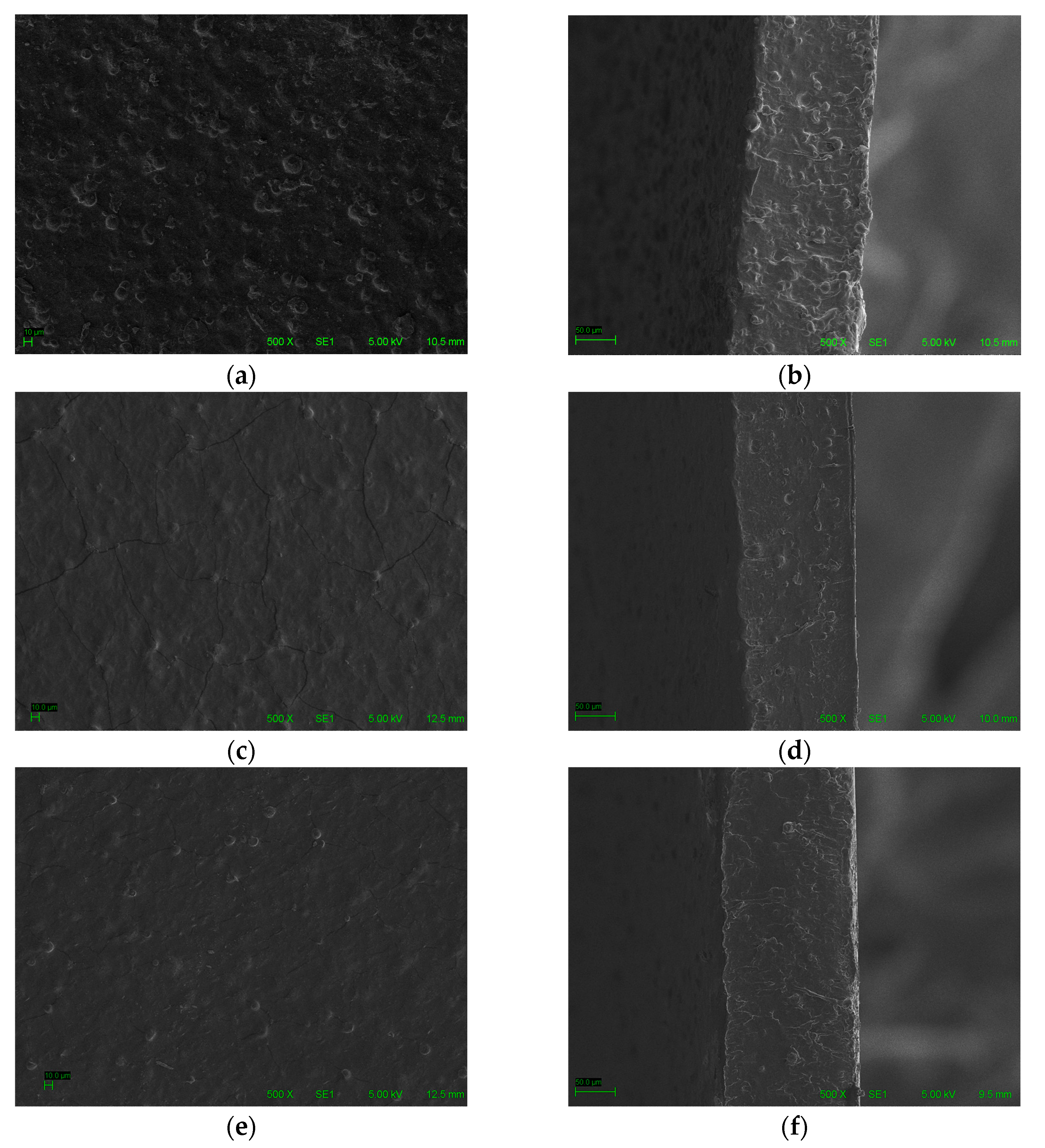
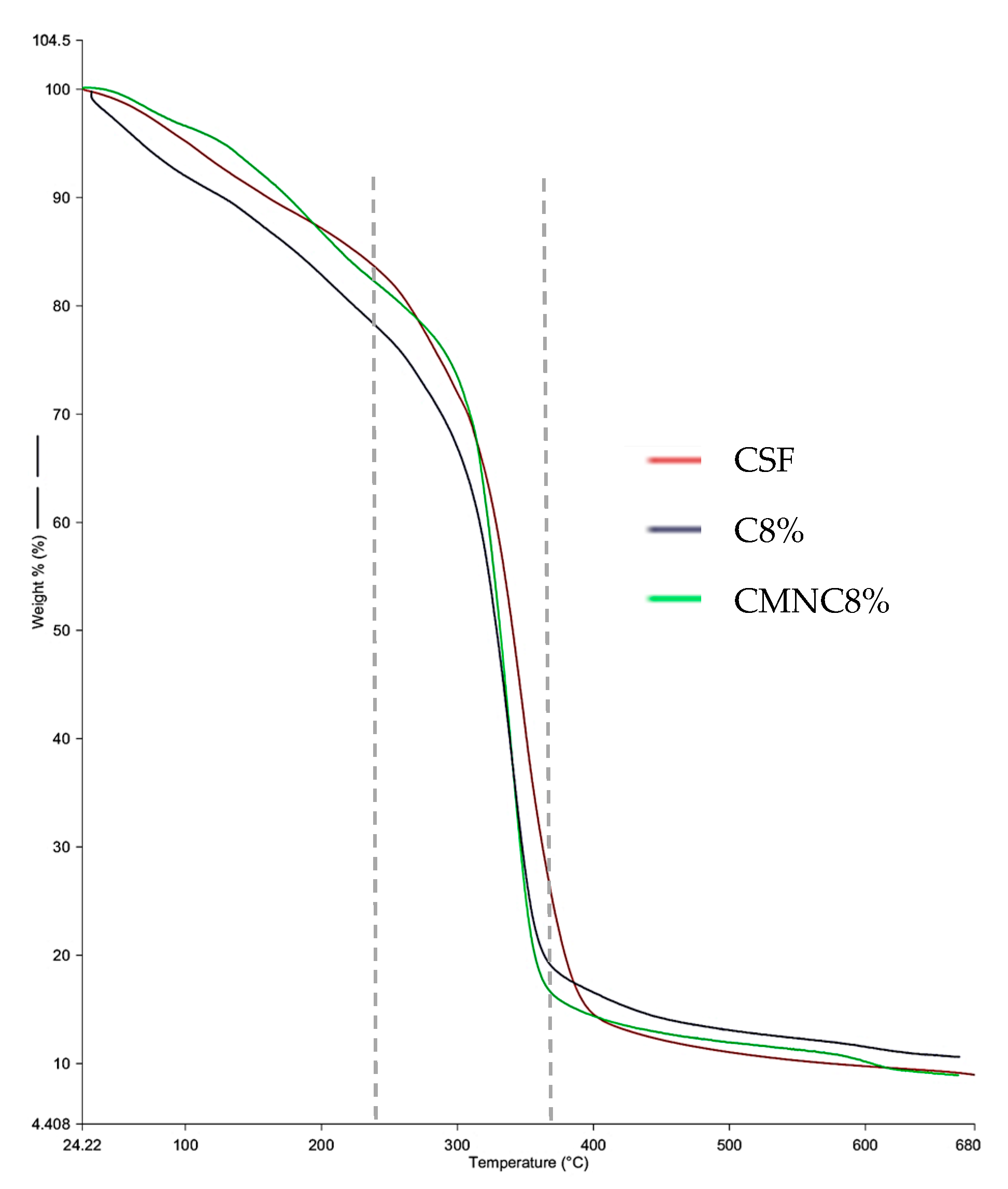

| Sample | Thickness (mm) | TS (MPa) | E (%) |
|---|---|---|---|
| CSF | 0.15 ± 0.02 ab | 0.73 ± 0.06 b | 191.14 ± 10.89 b |
| C2% | 0.12 ± 0.03 b | 1.32 ± 0.34 a | 157.82 ± 11.92 c |
| C5% | 0.14 ± 0.02 b | 1.33 ± 0.46 a | 156.51 ± 12.37 c |
| C8% | 0.13 ± 0.03 b | 1.34 ± 0.51 a | 157.98 ± 11.58 c |
| CMNC2% | 0.17 ± 0.01 a | 1.31 ± 0.11 a | 264.89 ± 13.01 a |
| CMNC5% | 0.17 ± 0.01 a | 1.30 ± 0.23 a | 265.54 ± 12.93 a |
| CMNC8% | 0.18 ± 0.02 a | 1.31 ± 0.17 a | 265.10 ± 13.56 a |
| Sample | WVP (g mm m−2 h−1 kPa−1) | MC (%) | WS (%) |
|---|---|---|---|
| CSF | 0.34 ± 0.03 b | 13.48 ± 0.22 b | 17.89 ± 0.42 a |
| C2% | 0.52 ± 0.05 a | 11.16 ± 0.34 c | 16.12 ± 0.29 b |
| C5% | 0.53 ± 0.05 a | 11.37 ± 0.25 c | 15.98 ± 0.33 b |
| C8% | 0.52 ± 0.06 a | 10.16 ± 0.14 d | 14.47 ± 0.76 c |
| CMCN2% | 0.43 ± 0.01 ab | 14.67 ± 0.86 a | 16.76 ± 0.55 b |
| CMCN5% | 0.43 ± 0.01 ab | 14.21 ± 0.33 a | 16.38 ± 0.81 b |
| CMCN8% | 0.44 ± 0.02 ab | 12.88 ± 0.81 b | 15.13 ± 0.64 c |
| Sample | Color Parameters | Opacity (A.mm−1) | |||
|---|---|---|---|---|---|
| L* | a* | b* | ∆E* | 600 nm | |
| CSF | 95.69 ± 0.79 a | 0.04 ± 0.01 b | 2.53 ± 0.17 c | 2.97 ± 0.67 a | 0.42 ± 0.07 c |
| C2% | 95.70 ± 0.39 a | −0.07 ± 0.01 d | 2.96 ± 0.14 a | 3.34 ± 0.36 a | 0.79 ± 0.24 a |
| C5% | 96.05 ± 0.12 a | −0.03 ± 0.02 c | 2.86 ± 0.04 ab | 2.92 ± 0.11 a | 0.86 ± 0.10 a |
| C8% | 95.28 ± 1.26 a | 0.14 ± 0.03 a | 2.65 ± 0.09 bc | 3.36 ± 1.06 a | 0.90± 0.13 a |
| CMNC2% | 96.79 ± 0.25 a | −0.03 ± 0.01 c | 2.65 ± 0.13 bc | 2.29 ± 0.24 a | 0.51± 0.18 b |
| CMNC5% | 96.21 ± 0.63 a | −0.04 ± 0.02 c | 2.63 ± 0.02 bc | 2.64 ± 0.58 a | 0.57± 0.02 b |
| CMNC8% | 95.86 ± 0.56 a | 0.02 ± 0.01 b | 2.77 ± 0.16 ab | 2.99 ± 0.50 a | 0.58± 0.03 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krümmel, A.; Pagno, C.H.; Malheiros, P.d.S. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods 2024, 13, 1141. https://doi.org/10.3390/foods13081141
Krümmel A, Pagno CH, Malheiros PdS. Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules. Foods. 2024; 13(8):1141. https://doi.org/10.3390/foods13081141
Chicago/Turabian StyleKrümmel, Aline, Carlos Henrique Pagno, and Patrícia da Silva Malheiros. 2024. "Active Films of Cassava Starch Incorporated with Carvacrol Nanocapsules" Foods 13, no. 8: 1141. https://doi.org/10.3390/foods13081141






