Growth Reduction of Vibrionaceae and Microflora Diversity in Ice-Stored Pacific White Shrimp (Penaeus vannamei) Treated with a Low-Frequency Electric Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
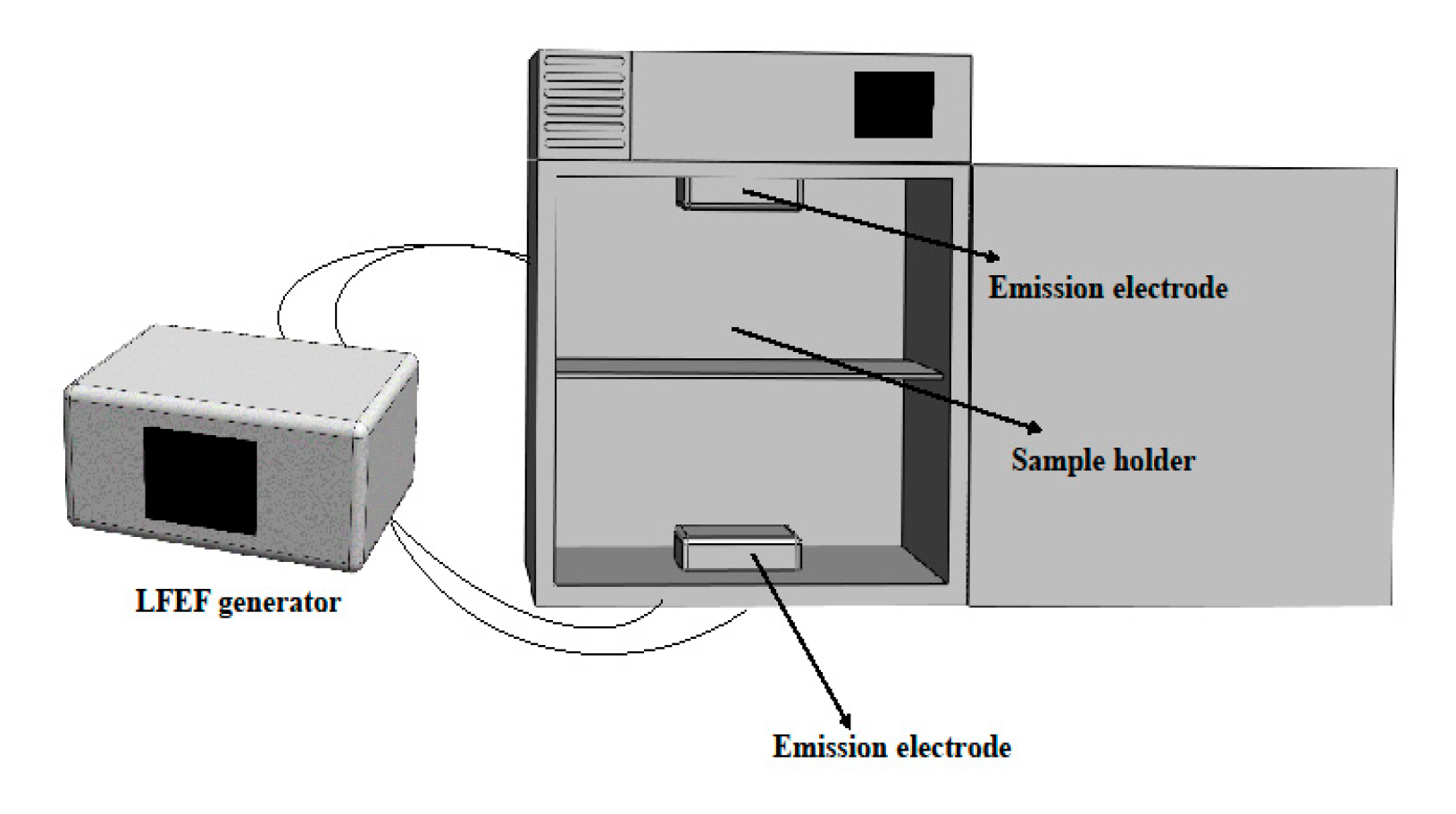
2.2. LFEF Experiment Apparatus
2.3. Determination of pH during Storage
2.4. Determination of TVC during Storage
2.5. Determination of TVB-N during Storage
2.6. DNA Extraction
2.7. PCR Amplification and Illumina Sequencing
2.8. Processing of the Sequencing Data
2.9. Statistical Analysis
3. Results and Discussion
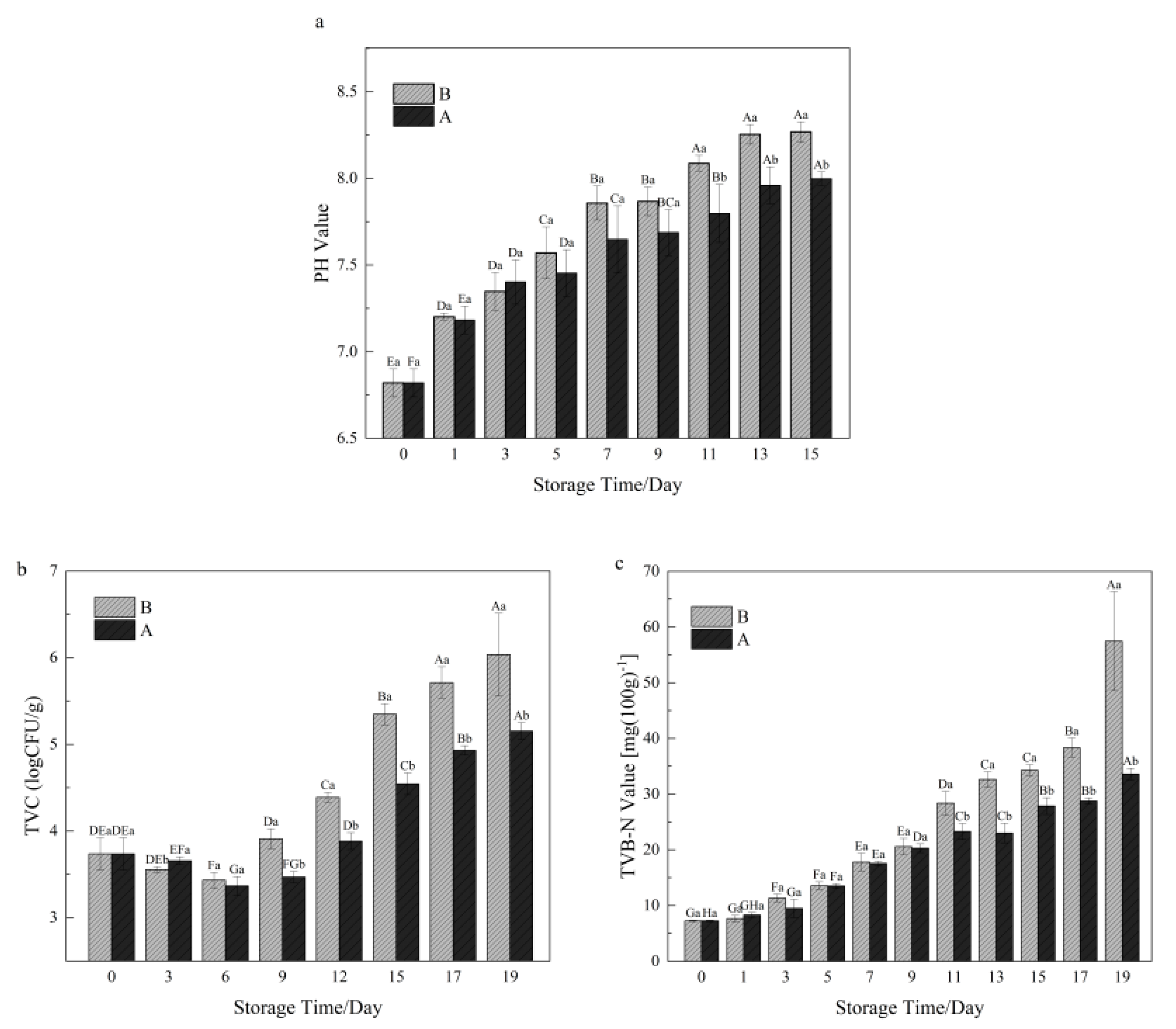
3.1. pH Value in Shrimp during Storage
3.2. TVC in Shrimp during Storage
3.3. TVB-N Value in Shrimp during Storage
3.4. Sequencing Data Analysis
3.5. Species Annotation and Composition Analysis
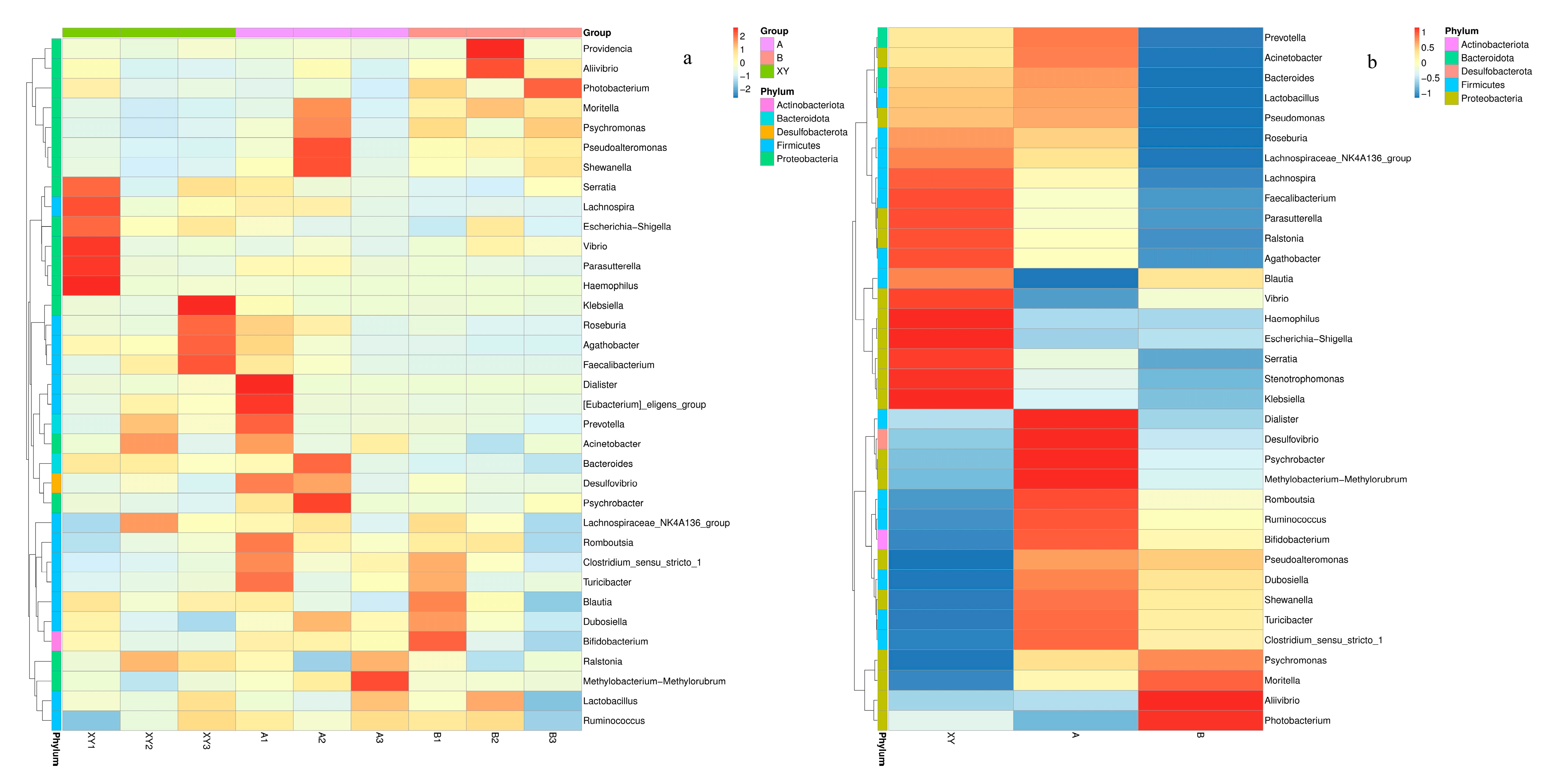
3.6. Heatmaps of Species Composition and Key Species Differences
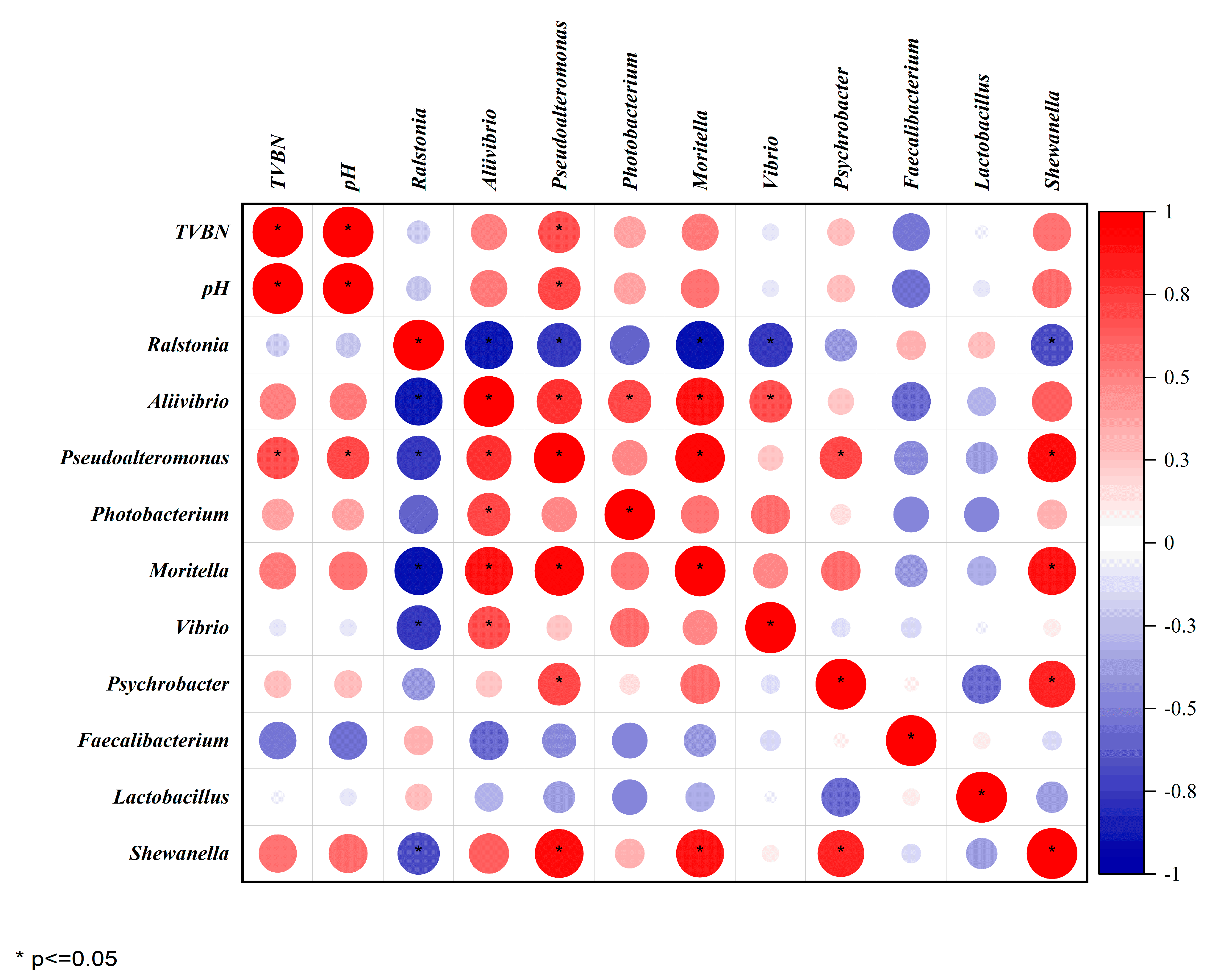
3.7. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fennema, O.R.; Powrie, W.D.; Marth, E.H. Low Temperature Preservation of Foods and Living Matter; Taylor & Francis: Abingdon, UK, 1973; Volume 76. [Google Scholar]
- Ando, M.; Nakamura, H.; Harada, R.; Yamane, A. Effect of super chilling storage on maintenance of freshness of kuruma prawn. Food Sci. Technol. Res. 2004, 10, 25–31. [Google Scholar] [CrossRef]
- Chong, C.Y.; Abu Bakar, F.; Rahman, R.A.; Bakar, J.; Zaman, M.Z. Biogenic amines, amino acids and microflora changes in Indian mackerel (Rastrellinger kanagurta) stored at ambient (25–29 °C) and ice temperature (0 °C). J. Food Sci. Technol. 2014, 51, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, G.G.; Evans, J.A. The use of supercooling for fresh foods: A review. J. Food Eng. 2015, 148, 74–79. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Zhang, X.-L.; Wang, G.-B.; Mo, F.-Y.; Zhang, X.-R. Application of super-cooled storage of aquatic products: A review. Int. J. Refrig. 2023, 154, 66–72. [Google Scholar] [CrossRef]
- Wu, C.-h.; Yuan, C.-h.; Ye, X.-q.; Hu, Y.-q.; Chen, S.-g.; Liu, D.-h. A critical review on superchilling preservation technology in aquatic product. J. Integr. Agric. 2014, 13, 2788–2806. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, A.; Bin, L. Application status and prospect of ice temperature storage technology in food storage. J. Refrig. Technol. 2021, 44, 52–55. [Google Scholar]
- Fallah-Joshaqani, S.; Hamdami, N.; Keshavarzi, E.; Keramat, J.; Dalvi-Isfahan, M. Evaluation of the static electric field effects on freezing parameters of some food systems. Int. J. Refrig. 2019, 99, 30–36. [Google Scholar] [CrossRef]
- James, C.; Purnell, G.; James, S.J. A review of novel and innovative food freezing technologies. Food Bioprocess Technol. 2015, 8, 1616–1634. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Lu, L.; Huang, F.; Chen, W.; Zhang, C.; Zhang, H.; Goto, K. Effects of the combination of moderate electric field and high-oxygen modified atmosphere packaging on pork meat quality during chill storage. J. Food Process. Preserv. 2020, 44, e14299. [Google Scholar] [CrossRef]
- Suwandy, V.; Carne, A.; van de Ven, R.; Bekhit, A.E.-D.A.; Hopkins, D.L. Effect of repeated pulsed electric field treatment on the quality of cold-boned beef loins and topsides. Food Bioprocess Technol. 2015, 8, 1218–1228. [Google Scholar] [CrossRef]
- Kang, T.; You, Y.; Jun, S. Supercooling preservation technology in food and biological samples: A review focused on electric and magnetic field applications. Food Sci. Biotechnol. 2023, 32, 1453. [Google Scholar] [CrossRef] [PubMed]
- Yun, O.; Zeng, X.-A.; Brennan, C.; Han, Z. Effect of pulsed electric field on membrane lipids and oxidative injury of Salmonella typhimurium. Int. J. Mol. Sci. 2016, 17, 1374. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, X.; Zhang, D.; Schroyen, M.; Wang, D.; Li, X.; Hou, C. Changes in the Freshness and bacterial community of fresh pork in controlled freezing point storage assisted by different electrostatic field usage frequencies. Food Bioprocess Technol. 2023, 17, 939–954. [Google Scholar] [CrossRef]
- Qian, C.; Pan, H.; Shao, H.; Yu, Q.; Lou, Y.; Li, Y. Effects of HVEF treatment on the physicochemical properties and bacterial communities of Larimichthys crocea fillets during refrigerated storage. Int. J. Food Sci. Technol. 2022, 57, 7691–7700. [Google Scholar] [CrossRef]
- Zhang, J.; Fei, L.; Cui, P.; Walayat, N.; Ji, S.; Chen, Y.; Lyu, F.; Ding, Y. Effect of low voltage electrostatic field combined with partial freezing on the quality and microbial community of large yellow croaker. Food Res. Int. 2023, 169, 112933. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, H.-M.; Chang, M.B. Inactivation of aquatic microorganisms by low-frequency AC discharges. IEEE Trans. Plasma Sci. 2008, 36, 215–219. [Google Scholar] [CrossRef]
- Wu, G.; Yang, C.; Bruce, H.L.; Roy, B.C.; Li, X.; Zhang, C. Effects of alternating electric field during freezing and thawing on beef quality. Food Chem. 2023, 419, 135987. [Google Scholar] [CrossRef] [PubMed]
- Kunmei, W.; Han, W.; Yue, W.; Chong, Y.; Yanxia, L.; Hongyu, L.; Tao, Y. The antibacterial mechanism of compound preservatives combined with low voltage electric fields on the preservation of steamed mussels (Mytilus edulis) stored at ice-temperature. Front. Nutr. 2023, 10, 1126456. [Google Scholar]
- He, Y.; Xie, Z.; Xu, Y.; Guo, C.; Zhao, X.; Yang, H. Effect of slightly acid electrolysed water ice on metabolite and volatilome profile of shrimp (Penaeus vannamei) during cold storage. Food Control 2023, 145, 109421. [Google Scholar] [CrossRef]
- Mousavi, M.; Hosseini, S.M.; Hosseini, H.; Abedi, A.-S.; Khani, M.; Heshmati, A.; Abhari, K.; Shahraz, F.; Taghizadeh, M.; Akhavan, A. Gliding arc plasma discharge conditions on microbial, physicochemical, and sensory properties of shrimp (LitoPenaeus vannamei): In Vivo and In Vitro Studies. Food Bioprocess Technol. 2022, 15, 2327–2343. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Hozzein, W.N.; Benjakul, S. Effect of pulsed electric field and modified atmospheric packaging on melanosis and quality of refrigerated Pacific white shrimp treated with leaf extract of Chamuang (Garcinia cowa Roxb.). Food Packag. Shelf Life 2020, 25, 100544. [Google Scholar] [CrossRef]
- Yang, C.; Wu, G.; Li, Y.; Zhang, C.; Liu, C.; Li, X. Effect of Low-voltage electrostatic field on oxidative denaturation of myofibrillar protein from lamb-subjected freeze-thaw cycles. Food Bioprocess Technol. 2023, 16, 2070–2081. [Google Scholar] [CrossRef]
- De Filippis, F.; Parente, E.; Ercolini, D. Recent Past, Present, and future of the food microbiome. Annu. Rev. Food Sci. Technol. 2018, 9, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Kaszab, E.; Farkas, M.; Rado, J.; Micsinai, A.; Nyiro-Fekete, B.; Szabo, I.; Kriszt, B.; Urbanyi, B.; Szoboszlay, S. Novel members of bacterial community during a short-term chilled storage of common carp (Cyprinus carpio). Folia Microbiol. 2022, 67, 299–310. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Martínez-Alvarez, O.; Gómez-Guillén, M.d.C.; Montero, P. Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis-inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 2007, 42, 1029–1038. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Wu, T.; Bai, Y.; Yuan, C.; Chen, S.; Gakushi, I.; Hu, Y. The preservation effect of CGA-Gel combined with partial freezing on sword prawn (Parapenaeopsis hardwickii). Food Chem. 2019, 313, 126078. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Duan, X.; Wu, M.; Li, T.; Lin, H.; Hu, J. Analysis of microbial community composition and changes in Lito Penaeus vannamei in low-Voltage electrostatic field. Food Ind. 2022, 43, 160–165. [Google Scholar]
- Brandfass, C.; Karlovsky, P. Upscaled CTAB-based DNA extraction and real-time PCR assays for Fusarium culmorum and F. graminearum DNA in plant material with reduced sampling error. Int. J. Mol. Sci. 2008, 9, 2306–2321. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Li, L.; Dong, Y.; Qian, G.; Hu, X.; Ye, L. Performance and microbial community analysis of bio-electrocoagulation on simultaneous nitrification and denitrification in submerged membrane bioreactor at limited dissolved oxygen. Bioresource Technol. 2018, 258, 168–176. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, A.; Basu, B. Vertical electric field induced bacterial growth inactivation on amorphous carbon electrodes. Carbon 2015, 81, 193–202. [Google Scholar] [CrossRef]
- Lan, W.; Sun, Y.; Liu, S.; Guan, Y.; Zhu, S.; Xie, J. Effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality and microbial composition of pompano during ice storage. Ultrason. Sonochem. 2022, 86, 106032. [Google Scholar] [CrossRef]
- Qiu, L.; Zhao, Y.; Ma, H.; Tian, X.; Bai, C.; Liao, T. The quality and bacterial community changes in freshwater crawfish stored at 4 °C in vacuum packaging. Molecules 2022, 27, 8618. [Google Scholar] [CrossRef] [PubMed]
- Chaillou, S.; Chaulot-Talmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Desmonts, M.H.; Dousset, X.; Feurer, C.; Hamon, E.; et al. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. ISME J. 2015, 9, 1105–1118. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, L.; Sun, Y.; Ren, F.; Chen, S.; Zhang, H.; Guo, H. Changes in the microbiota of lamb packaged in a vacuum and in modified atmospheres during chilled storage analysed by high-throughput sequencing. Meat Sci. 2016, 121, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sun, W.; Xiong, G.; Shi, L.; Jiao, C.; Wu, W.; Li, X.; Qiao, Y.; Liao, L.; Ding, A.; et al. Effects of HVEF treatment on microbial communities and physicochemical properties of catfish fillets during chilled storage. LWT-Food Sci. Technol. 2020, 131, 109667. [Google Scholar] [CrossRef]
- Shi, T.-T.; Xie, C.; Zhang, J.-W.; Li, Y.-H.; Zhu, Y.-M.; Wu, Y.-T.; Zhou, Z.-Y.; Yu, Z. Effect of low-voltage alteenating frequency electric field on microbial community of hairtail Trichiurus lepturus during preservation. Oceanol. Limnol. Sin. 2022, 53, 133–140. [Google Scholar]
- Fojt, L.; Strasák, L.; Vetterl, V.; Smarda, J. Comparison of the low-frequency magnetic field effects on bacteria Escherichia coli, Leclercia adecarboxylata and Staphylococcus aureus. Bioelectrochemistry 2004, 63, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xie, J.; Qian, Y. Comparative analysisi of microbial communities of fresh and spoiled pacific white shrimp stored at 4 °C by 16s rRNA-based pyrosequencing and DGGE. J. Chin. Inst. Food Sci. Technol. 2018, 18, 280–287. [Google Scholar]
- Jia, S.; Liu, Y.; Zhuang, S.; Sun, X.; Li, Y.; Hong, H.; Lv, Y.; Luo, Y. Effect of ε-polylysine and ice storage on microbiota composition and quality of Pacific white shrimp (LitoPenaeus vannamei) stored at 0 °C. Food Microbiol. 2019, 83, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kergourlay, G.; Taminiau, B.; Daube, G.; Champomier Vergès, M.-C. Metagenomic insights into the dynamics of microbial communities in food. Int. J. Food Microbiol. 2015, 213, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, X.; Chen, H.; He, B.; Mei, Y.; Wang, D.; Li, J. Effect of the combination of vanillin and chitosan coating on the microbial diversity and shelf-Life of refrigerated turbot (Scophthalmus maximus) filets. Front. Microbiol. 2020, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T. The Family Burkholderiaceae. Prokaryotes Alphaproteobacteria Betaproteobacteria 2014, 343, 759–776. [Google Scholar]
- Bekaert, K.; Devriese, L.; Maes, S.; Robbens, J. Characterization of the dominant bacterial communities during storage of Norway lobster and Norway lobster tails (Nephrops norvegicus) based on 16S rDNA analysis by PCR-DGGE. Food Microbiol. 2015, 46, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mittenzwey, R.; Süßmuth, R.; Mei, W. Effects of extremely low-frequency electromagnetic fields on bacteria—The question of a co-stressing factor. Bioelectrochem. Bioenerg. 1996, 40, 21–27. [Google Scholar] [CrossRef]
- Yimeng, C.; Chaorong, G.; Wei, L.; Huaiying, Y. The Intestinal Bacterial Community and Functional Potential of LitoPenaeus vannamei in the Coastal Areas of China. Microorganisms 2021, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T.; Adley, C.C. Ralstonia pickettii: A persistent gram-negative nosocomial infectious organism. J. Hosp. Infect. 2006, 62, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiol. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, K.; Heyndrickx, M.; Herman, L.; Devlieghere, F.; Vlaemynck, G. Seafood quality analysis: Molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol. 2011, 28, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Rohling, A.; Calero, S.; Halaihel, N.; Marquina, P.; Raso, J.; Calanche, J.; Antonio Beltran, J.; Alvarez, I.; Cebrian, G. Characterization of the spoilage microbiota of hake fillets packaged under a modified atmosphere (MAP) rich in CO2 (50% CO2/50% N2) and stored at different temperatures. Foods 2019, 8, 489. [Google Scholar] [CrossRef] [PubMed]
- Basak, P.; Biswas, A.; Bhattacharyya, M. Chapter 24—Exploration of extremophiles genomes through gene study for hidden biotechnological and future potential. In Physiological and Biotechnological Aspects of Extremophiles; Salwan, R., Sharma, V., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 315–325. [Google Scholar]
- Daisuke, K.; Yuri, F.; Waka, M.; Yui, N.; Tsutomu, Y.; Kazuki, S.; Shun, Y.; Marie, K.; Kai, S.; Taketo, K.; et al. Identification of changes in the microflora composition of Japanese horse mackerel (Trachurus japonicus) during storage to identify specific spoilageorganisms. Curr. Res. Food Sci. 2022, 5, 1216–1224. [Google Scholar]
- Rego, D.; Costa, L.; Pereira, M.T.; Redondo, L.M. Cell membrane permeabilization studies of Chlorella sp. by pulsed electric fields. IEEE Trans. Plasma Sci. 2015, 43, 3483–3488. [Google Scholar] [CrossRef]
- Wang, T.-Q.; Li, L.-R.; Tan, C.-X.; Yang, J.-W.; Shi, G.-X.; Wang, L.-Q.; Hu, H.; Liu, Z.-S.; Wang, J.; Wang, T.; et al. Effect of electroacupuncture on gut microbiota in participants with knee osteoarthritis. Front. Cell. Infect. Microbiol. 2021, 11, 597431. [Google Scholar] [CrossRef]
- Broekaert, K.; Heyndrickx, M.; Herman, L.; Devlieghere, F.; Vlaemynck, G. Molecular identification of the microbiota of peeled and unpeeled brown shrimp (Crangon crangon) during storage on ice and at 7.5 °C. Food Microbiol. 2013, 36, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Di, P.; Wang, H.; Li, B.; Pan, Y.; Yan, S.; Wang, Y. Bacterial community associated with the intestinal tract of chinese mitten crab (Eriocheir sinensis) farmed in lake tai, China. PLoS ONE 2015, 10, e0123990. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Washio, K.; Okahara, R.; Morikawa, M. Biofilm formation and proteolytic activities of Pseudoalteromonas bacteria that were isolated from fish farm sediments. Microb. Biotechnol. 2009, 2, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, K.; Noseda, B.; Heyndrickx, M.; Vlaemynck, G.; Devlieghere, F. Volatile compounds associated with Psychrobacter spp. and Pseudoalteromonas spp., the dominant microbiota of brown shrimp (Crangon crangon) during aerobic storage. Int. J. Food Microbiol. 2013, 166, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, R.-M.; Sun, J.; Bougouffa, S.; Ding, W.; Cai, L.; Lan, Y.; Tong, H.; Li, Y.; Jamieson, A.J.; et al. Genome reduction in Psychromonas species within the gut of an amphipod from the ocean’s deepest point. Msystems 2018, 3, e00009–18. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef] [PubMed]
- Mace, S.; Cardinal, M.; Jaffres, E.; Cornet, J.; Lalanne, V.; Chevalier, F.; Serot, T.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Evaluation of the spoilage potential of bacteria isolated from spoiled cooked whole tropical shrimp (Penaeus vannamei) stored under modified atmosphere packaging. Food Microbiol. 2014, 40, 9–17. [Google Scholar] [CrossRef]
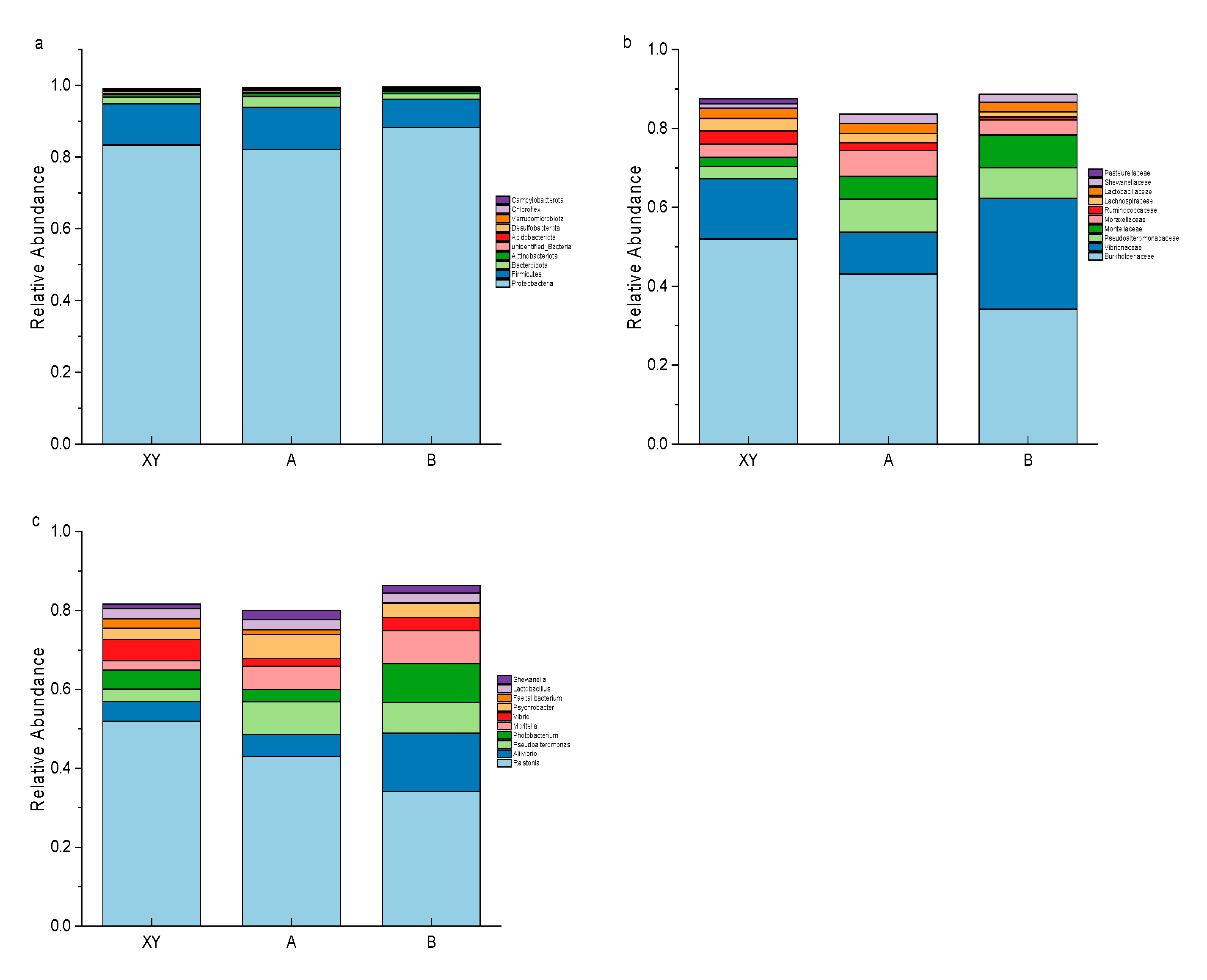

| Alpha Diversity | ACE | Chao1 | Shannon | Simpson | Observed Species | Goods Coverage | PD_Whole_Tree |
|---|---|---|---|---|---|---|---|
| XY | 1162.905 ± 48.073 | 1113.229 ± 41.160 | 4.003 ± 0.590 | 0.709 ± 0.1 | 984.000 ± 48.073 | 0.995 ± 0.001 | 125.648 ± 13.310 |
| B | 1034.974 ± 88.8 | 997.573 ± 84.427 | 4.243 ± 0.421 | 0.835 ± 0.0 | 838.333 ± 88.861 | 0.995 ± 0.001 | 83.566 ± 10.884 |
| A | 1207.065 ± 66.651 | 1162.186 ± 73.308 | 4.390 ± 0.999 | 0.765 ± 0.172 | 1005.667 ± 66.651 | 0.995 ± 0.000 | 96.578 ± 13.408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Chen, H.; Liang, Z.; Chen, S.; Xia, Y.; Zhu, S.; Yu, M. Growth Reduction of Vibrionaceae and Microflora Diversity in Ice-Stored Pacific White Shrimp (Penaeus vannamei) Treated with a Low-Frequency Electric Field. Foods 2024, 13, 1143. https://doi.org/10.3390/foods13081143
Xu L, Chen H, Liang Z, Chen S, Xia Y, Zhu S, Yu M. Growth Reduction of Vibrionaceae and Microflora Diversity in Ice-Stored Pacific White Shrimp (Penaeus vannamei) Treated with a Low-Frequency Electric Field. Foods. 2024; 13(8):1143. https://doi.org/10.3390/foods13081143
Chicago/Turabian StyleXu, Lijuan, Haiqiang Chen, Zuanhao Liang, Shanshan Chen, Yu Xia, Siming Zhu, and Ming Yu. 2024. "Growth Reduction of Vibrionaceae and Microflora Diversity in Ice-Stored Pacific White Shrimp (Penaeus vannamei) Treated with a Low-Frequency Electric Field" Foods 13, no. 8: 1143. https://doi.org/10.3390/foods13081143





