Hovenia dulcis Fruit Peduncle Polysaccharides Reduce Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders in Alcohol-Exposed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction and Characterization of HDPs
2.2. Animal Exposure
2.3. Serum Biochemical Analysis
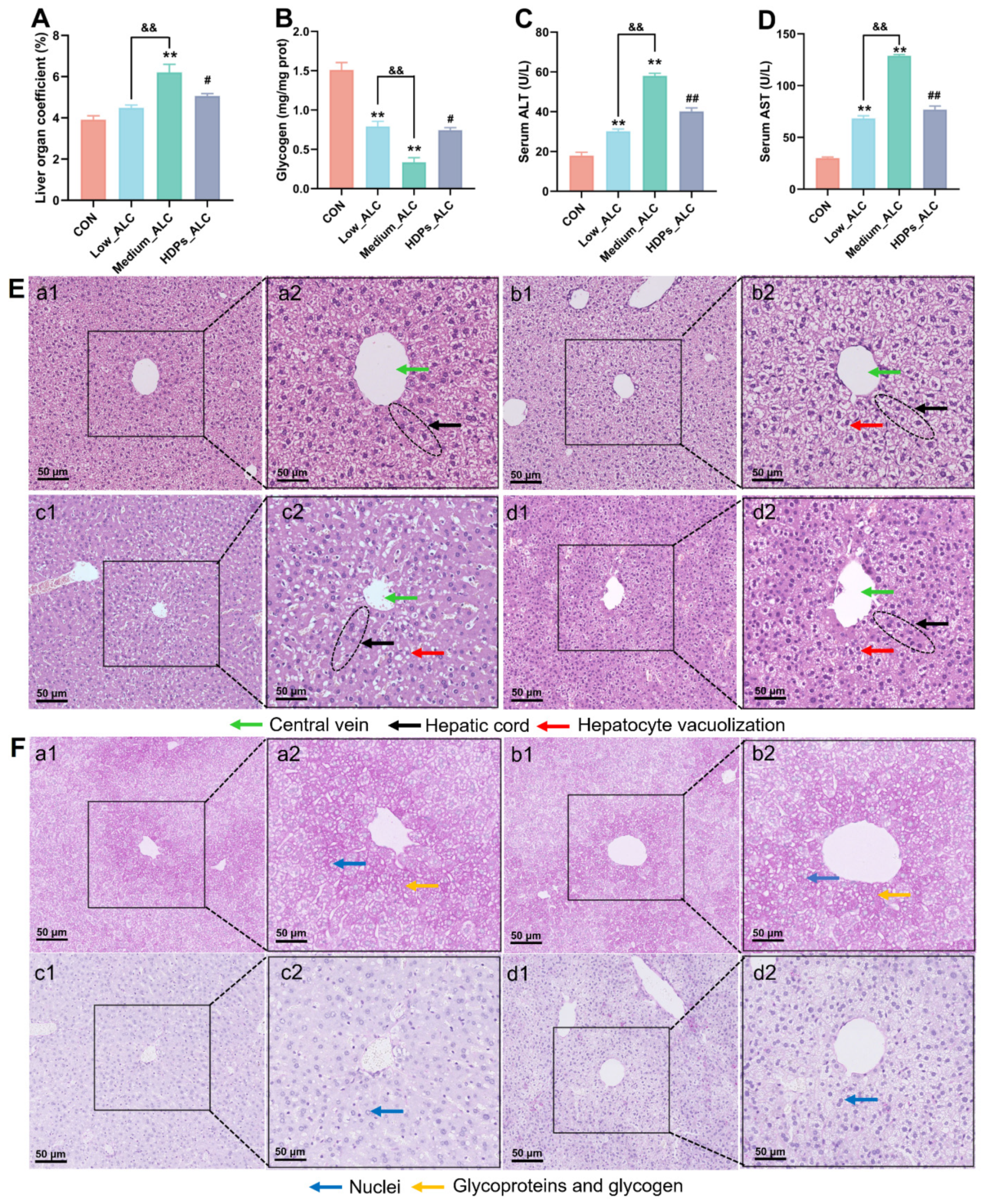
2.4. Histological Examination
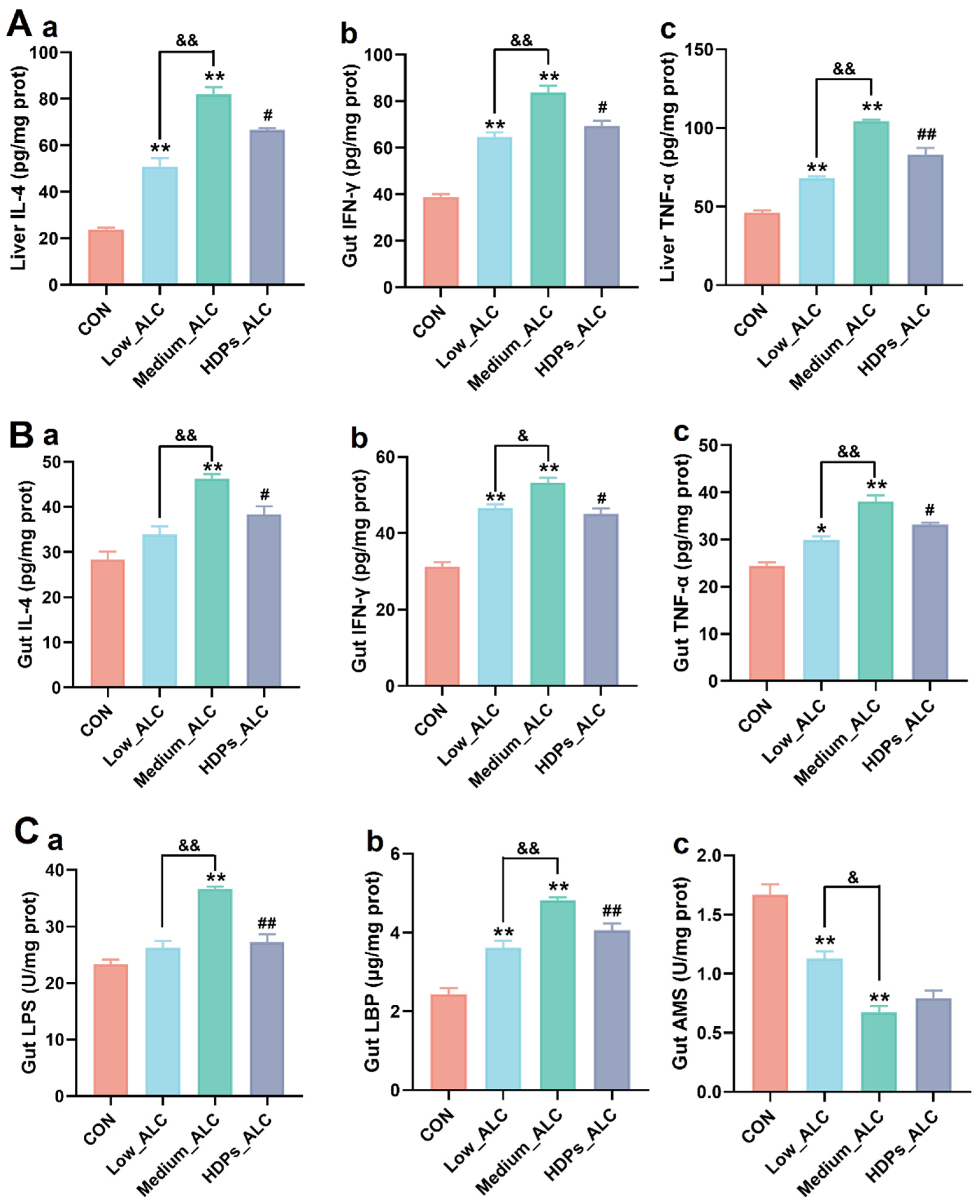
2.5. Biological Determination of Injury-Related Parameters in Liver and Intestine
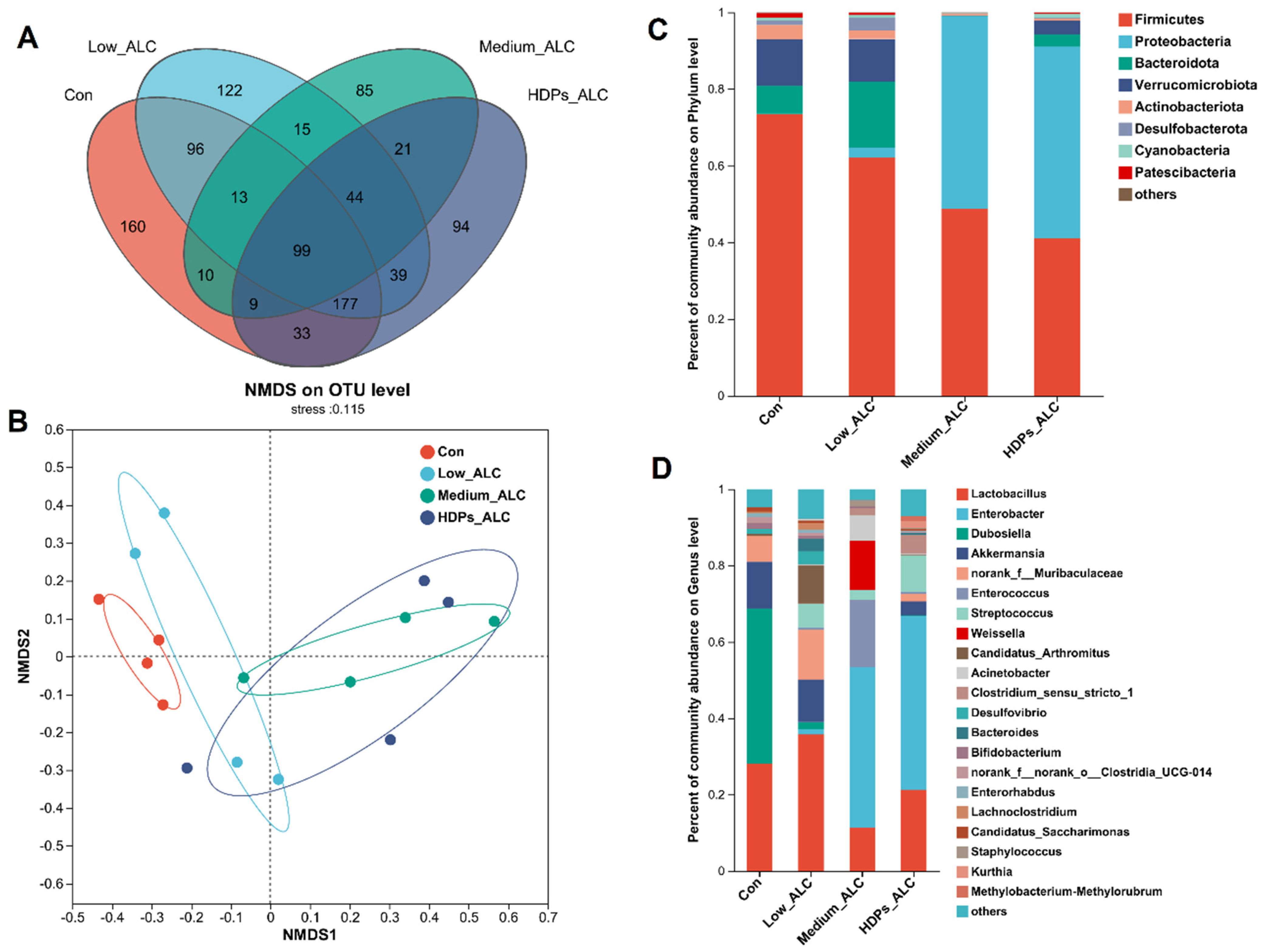
2.6. Gut Microbiota Analysis
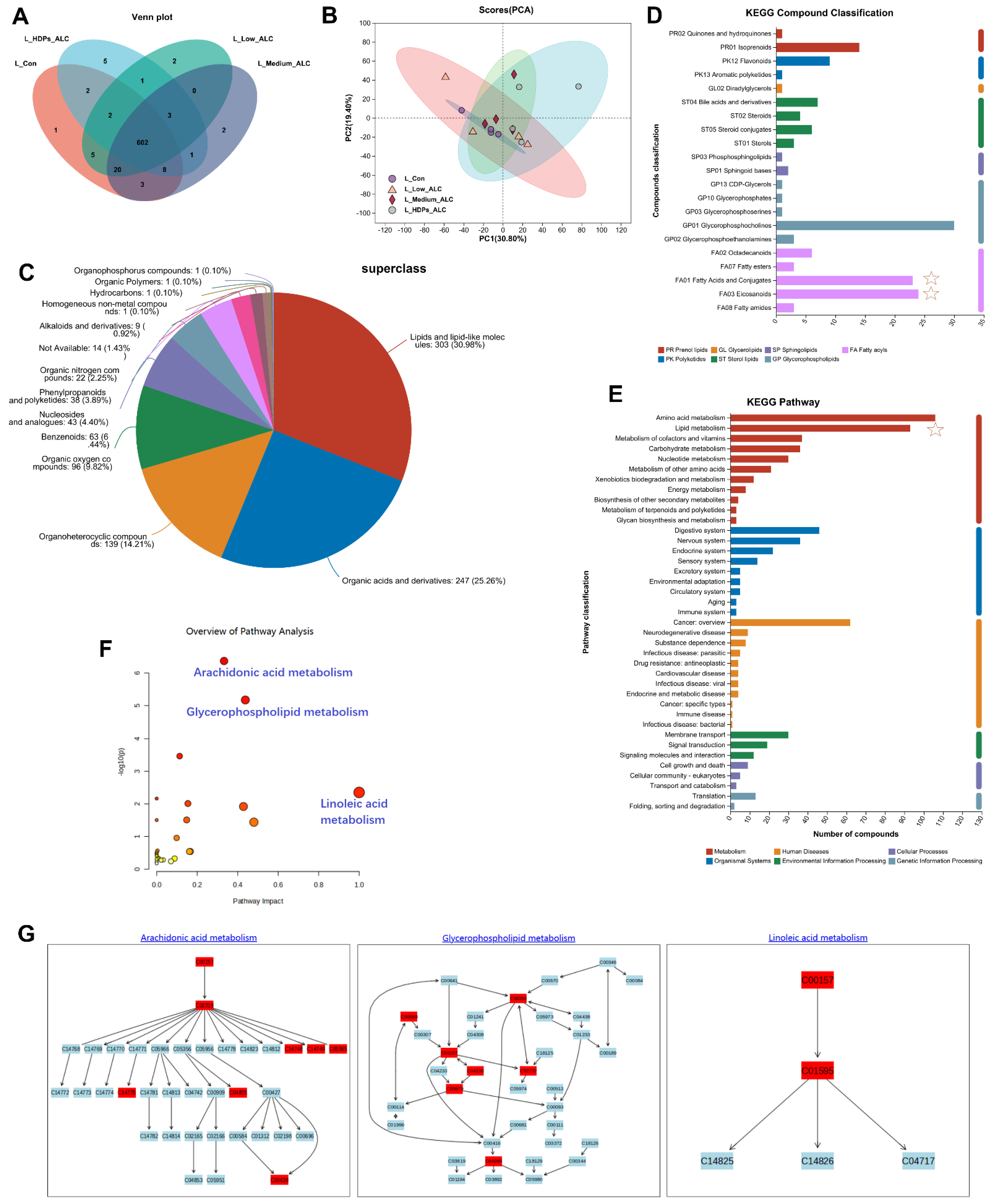
2.7. Hepatic Metabolomics Analysis
2.8. Statistical Analysis
3. Results
3.1. Structural Characterization of HDPs
3.2. Changes in Serum Lipid Levels and Liver Damage
3.3. Changes in Hepatic and Intestinal Inflammatory Cytokines and Intestinal Enzyme Activities
3.4. Dysbiosis of Gut Microbiota
3.5. Hepatic Metabolomics Changes
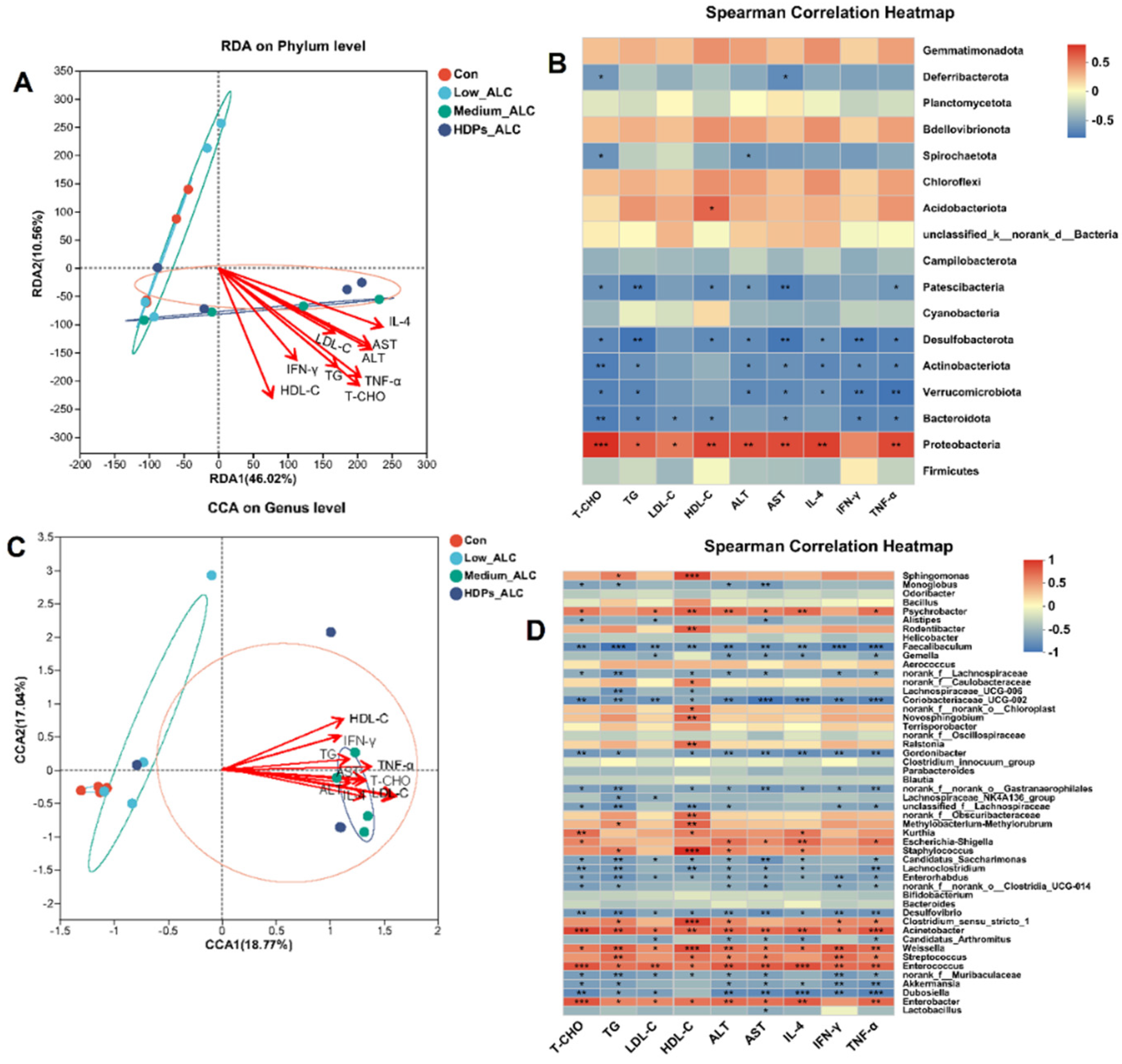
3.6. Correlations of Lipid Changes and Liver Metabolites with Gut Microbiota in Alcohol-Exposed Mice
3.6.1. Relationship between Lipid Changes and Gut Microbiota
3.6.2. Relationship between Hepatic Metabolites and Gut Microbiota
4. Discussion
4.1. HDPs Reduced Alcohol-Caused Lipid Abnormalities
4.2. HDPs Alleviated Alcohol-Exposed Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders
4.3. Comparison of HDPs Studies
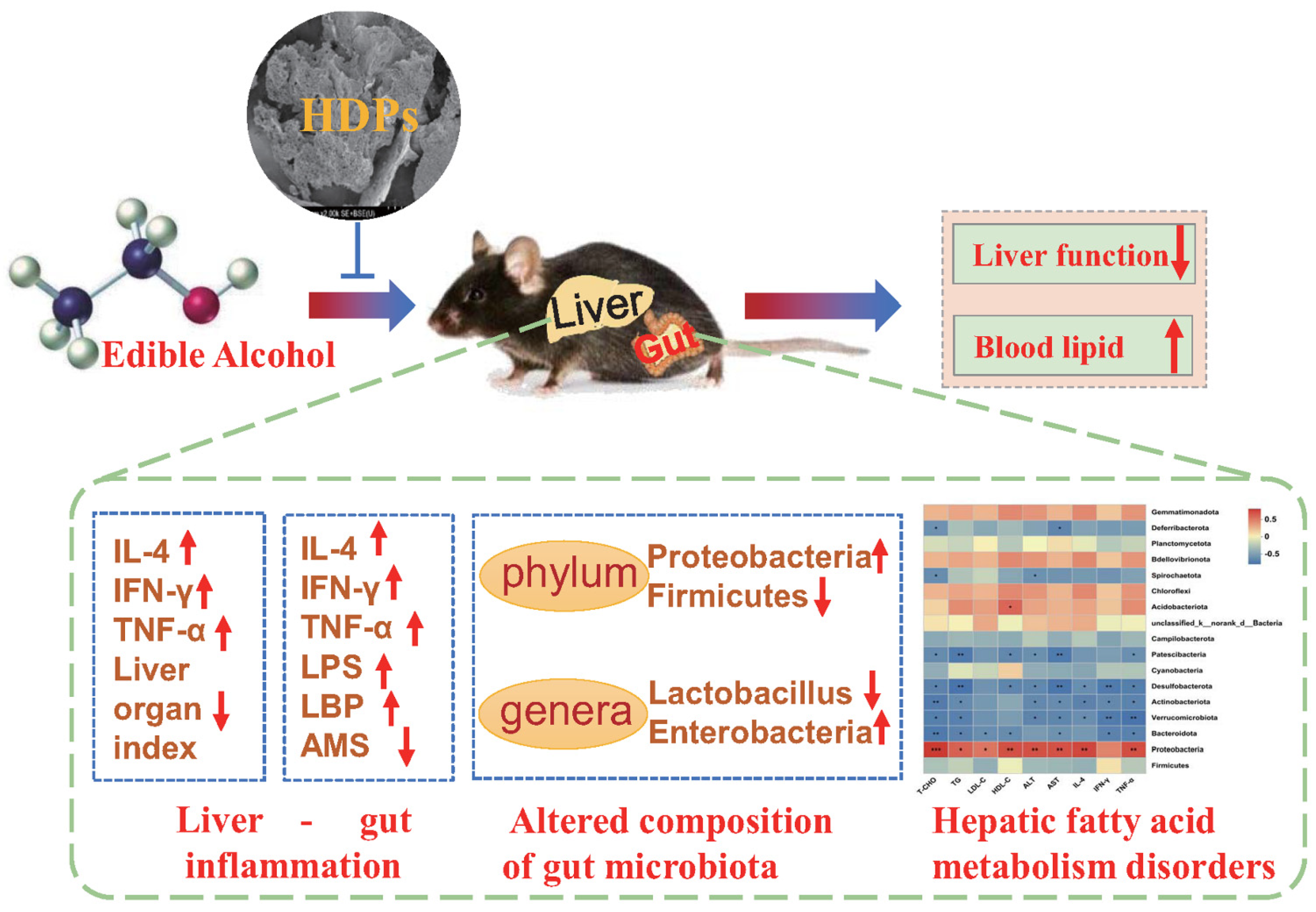
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Alanine aminotransferase | ALT |
| Aspartate aminotransferase | AST |
| Control | CON |
| Fourier transform infrared | FT-IR |
| High-density lipoprotein cholesterol | HDL-C |
| Hovenia dulcis fruit peduncle polysaccharides | HDPs |
| HDPs + Medium dose of alcohol | HDPs_ALC |
| Interferon-Gamma | IFN-γ |
| Low-density lipoprotein cholesterol | LDL-C |
| Low dose of alcohol | Low_ALC |
| Medium dose of alcohol | Medium_ALC |
| National center for biotechnology information | NCBI |
| Orthogonal partial least squares discriminant analysis | OPLS-DA |
| Principal component analysis | PCA |
| Triglycerides | TG |
| Total cholesterol | T-CHO |
| Tumor Necrosis Factor-Alpha | TNF-α |
| Ultra-High Performance Liquid Chromatography | UHPLC |
| World Health Organization | WHO |
References
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization Publications: Geneva, Switzerland, 2018; pp. 38–60.
- Zhao, C.L.; Wu, X.L.; Chen, J.; Qian, G.Q. The therapeutic effect of IL-21 combined with IFN-γ inducing CD4+CXCR5+CD57+T cells differentiation on hepatocellular carcinoma. J. Adv. Res. 2021, 36, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.G.; Wang, J.; Li, L.Z.; Hu, W.J.; Qu, Y.D.; Ding, Y.P.; Meng, L.N.; Teng, L.R.; Wang, D. Hepatoprotective Effects of Antrodia cinnamomea: The Modulation of oxidative stress signaling in a mouse model of alcohol-induced acute liver injury. Oxid. Med. Cell. Longev. 2017, 2017, 7841823. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.V.; Barker, D.F.; Zhang, J.; McClain, C.J.; Barve, S.; Gobejishvili, L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J. Pathol. 2016, 240, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.; Hochrath, K.; Horvath, A.; Chen, P.; Seebauer, C.T.; Llorente, C.; Wang, L.R.; Alnouti, Y.; Fouts, D.E.; Peter Stärkel, P.; et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology 2018, 67, 2150–2166. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Stärkel, P.; Turner, J.R.; Ho, S.B.; Schnabl, B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015, 61, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Duan, Y.; Liu, J.; Torralba, M.G.; Kuelbs, C.; Ventura-Cots, M.; Abraldes, J.G.; Bosques-Padilla, F.; Verna, E.C.; Brown, R.S.J.; et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 2020, 71, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Zhong, Y.J.; Cheng, Q.; Wang, Y.Z.; Fan, Y.Y.; Yang, C.F.; Ma, Z.H.; Li, Z.H.; Li, L. miR-378b regulates insulin sensitivity by targeting insulin receptor and p110α in alcohol-induced hepatic steatosis. Front. Pharmacol. 2020, 11, 717. [Google Scholar] [CrossRef]
- Ambade, A.; Lowe, P.; Kodys, K.; Catalano, D.; Gyongyosi, B.; Cho, Y.; Vellve, A.I.; Adejumo, A.; Saha, B.; Calenda, C. Pharmacological inhibition of ccr2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis, and inflammation in mice. Hepatology 2019, 69, 1105–1121. [Google Scholar] [CrossRef]
- Pi, A.W.; Jiang, K.; Ding, Q.C.; Lai, S.L.; Yang, W.W.; Zhu, J.Y.; Guo, R.; Fan, Y.B.; Chi, L.F.; Li, S.T. Alcohol abstinence rescues hepatic steatosis and liver injury via improving metabolic reprogramming in chronic alcohol-fed mice. Front. Pharmacol. 2021, 12, 752148. [Google Scholar] [CrossRef]
- Caslin, B.; Mohler, K.; Thiagarajan, S.; Melamed, E. Alcohol as friend or foe in autoimmune diseases: A role for gut microbiome? Gut Microbes 2021, 13, 1916278. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, Q.J.; Luo, Y.X.; Yang, Q.; Wei, X.Y.; Kan, J.Q. High-pressure ultrasonic-assisted extraction of polysaccharides from Hovenia dulcis: Extraction, structure, antioxidant activity and hypoglycemic. Int. J. Biol. Macromol. 2019, 137, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhu, W.; Li, Z.; Ling, S. Effect of juice and fermented vinegar from Hovenia dulcis peduncles on chronically alcohol-induced liver damage in mice. Food Funct. 2012, 3, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, M.Y.; Lee, Y.J.; Kim, J.H.; Kim, J.Y.; Kwon, O. Effect of Hovenia dulcis fruit extract on alcohol-induced metabolism, inflammation and hangover following acute alcohol consumption: A randomized, double-blind, placebo-controlled study. FASEB J. 2016, 30, lb209. [Google Scholar] [CrossRef]
- Qiu, P.; Dong, Y.; Zhu, T.; Luo, Y.Y.; Kang, X.J.; Pang, M.X.; Li, H.Z.; Xu, H.; Gu, C.; Pan, S.H. Semen hoveniae extract ameliorates alcohol-induced chronic liver damage in rats via modulation of the abnormalities of gut-liver axis. Phytomedicine 2019, 52, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sferrazza, G.; Brusotti, G.; Zonfrillo, M.; Temporini, C.; Tengattini, S.; Bononi, M.; Tateo, F.; Calleri, E.; Pierimarchi, P. Hovenia dulcis Thumberg: Phytochemistry, pharmacology, toxicology and regulatory framework for its use in the European Union. Molecules 2021, 26, 903. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Tang, G.Y.; Zhao, C.N.; Liu, Q.; Xu, X.Y.; Cao, S.Y. Hepatoprotective effects of Hovenia dulcis seeds against alcoholic liver injury and related mechanisms investigated via network pharmacology. World J. Gastroenterol. 2020, 26, 3432–3446. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Eom, S.H.; Yu, C.Y.; Roitsch, T. Hovenia dulcis—An Asian traditional herb. Planta Med. 2010, 76, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Luo, Y.X.; Sang, Y.X.; Kan, J.Q. Isolation, purification, structural characterization, and hypoglycemic activity assessment of polysaccharides from Hovenia dulcis (Guai Zao). Int. J. Biol. Macromol. 2022, 208, 1106–1115. [Google Scholar] [CrossRef]
- Yang, B.; Luo, Y.X.; Wei, X.Y.; Kan, J.Q. Polysaccharide from Hovenia dulcis (Guaizao) improves pancreatic injury and regulates liver glycometabolism to alleviate STZ-induced type 1 diabetes mellitus in rats. Int. J. Biol. Macromol. 2022, 214, 655–663. [Google Scholar] [CrossRef]
- Tang, H.H.; Zhu, S.L. Research progress of Hovenia dulcis’ antialcoholism and liver protection effect. Food Nutr. China 2012, 18, 69–72, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Choi, R.Y.; Woo, M.J.; Ham, J.R.; Lee, M.K. Anti-steatotic and anti-inflammatory effects of Hovenia dulcis Thunb extracts in chronic alcohol-fed rats. Biomed. Pharmacother. 2017, 90, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiang, M.L.; Sun, Z.G.; Du, Y.Q. Optimization of ultrasonic extraction of polysaccharides from Hovenia dulcis peduncles and their antioxidant potential. Int. J. Biol. Macromol. 2015, 80, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Zhang, Y.C.; Zhu, S.J.; Song, Y.; Zhang, Z.H.; Chen, G.X. Optimization of extraction process of polysaccharides from Hovenia dulcis fruit pedicels and its antioxidant activity. Sci. Technol. Food Ind. 2023, 44, 230–237, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Wu, J.C.; Li, C.Y.; Bai, L.S.; Wu, J.; Rui, B.; Ye, M.Z.; Huang, L.; Chen, H.Y.; Rui, W. Structural differences of polysaccharides from Astragalus before and after honey processing and their effects on colitis mice. Int. J. Biol. Macromol. 2021, 182, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wu, Q.J.; Luo, Y.X.; Yang, Q.; Chen, G.J.; Wei, X.Y.; Kan, J.Q. Japanese grape (Hovenia dulcis) polysaccharides: New insight into extraction, characterization, rheological properties, and bioactivities. Int. J. Biol. Macromol. 2019, 134, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhao, Z.J.; Chen, H.J.; Pan, X.L.; Li, R.S.; Wu, D.W.; Hu, X.C.; Zhang, L.L.; Wu, H.W.; Li, X.H. Dynamic Analysis of Physicochemical Properties and Polysaccharide Composition during the Pile-Fermentation of Post-Fermented Tea. Foods 2022, 11, 3376. [Google Scholar] [CrossRef]
- Li, C. Replication of Animal Models for Human Diseases; People’s Medical Publishing House: Beijing, China, 2008; pp. 60–62. [Google Scholar]
- Wong, H.L.X.; Qin, H.Y.; Tsang, S.W.; Zuo, X.; Che, S.; Chow, C.F.W.; Li, X.; Xiao, H.T.; Zhao, L.; Huang, T. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.D.; Bu, F.T.; Li, X.F.; Chen, Y.; Zhu, S.; Wang, J.N.; Chen, S.Y.; Sun, Y.Y.; Pan, X.Y.; et al. Circular RNA circFBXW4 suppresses hepatic fibrosis via targeting the miR-18b-3p/FBXW7 axis. Theranostics 2020, 10, 4851–4870. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, X.B.; Liu, Y.C.; Yu, W.Q.; Si, Y.H.; Guo, S.D. Fenofibrate enhances lipid deposition via modulating PPARγ, SREBP-1c, and gut microbiota in ob/ob mice fed a high-fat diet. Front. Nutr. 2020, 9, 971581. [Google Scholar] [CrossRef]
- Han, H.; Wang, M.Y.; Zhong, R.Q.; Yi, B.; Schroyen, M.; Zhang, H.F. Depletion of gut microbiota inhibits hepatic lipid accumulation in high-fat diet-fed mice. Int. J. Mol. Sci. 2022, 23, 9350–9371. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.R.; Ma, N.; Liu, X.W.; Li, S.H.; Qin, Z.; Bai, L.X.; Yang, Y.J.; Li, J.Y. Untargeted and targeted metabolomics reveal the underlying mechanism of aspirin eugenol ester ameliorating rat hyperlipidemia via inhibiting fxr to induce cyp7a1. Front. Pharmacol. 2021, 12, 733789. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinform. 2016, 55, 11–14. [Google Scholar] [CrossRef]
- Arab, J.P.; Cabrera, D.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Verma, V.K.; Simonetto, D.; Cao, S.; Yaqoob, U.; Leon, J.; Freire, M. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J. Hepatol. 2020, 73, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Clugston, R.D.; Huang, L.S.; Blaner, W.S. Chronic alcohol consumption has a biphasic effect on hepatic retinoid loss. FASEB J. 2015, 29, 3654–3667. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liao, A.M.; Cui, Y.; Yu, G.; Hou, Y.; Pan, L.; Chen, W.J.; Zheng, S.N.; Li, X.X.; Ma, J.R.; et al. Wheat embryo globulin protects against acute alcohol-induced liver injury in mice. Food Chem. Toxicol. 2021, 153, 112240. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.L.; You, S.P.; Liu, T.; Xu, F.; Ji, T.F.; Gu, Z.Y. Hepatoprotective effects of nicotiflorin from nymphaea candida against concanavalin a-induced and d-galactosamine-induced liver injury in mice. Int. J. Mol. Sci. 2017, 18, 587. [Google Scholar] [CrossRef]
- Gu, C.W.; Zhou, Z.H.; Yu, Z.H.; He, M.L.; He, L.Q.; Luo, Z.A.; Xiao, W.D.; Yang, Q.; Zhao, F.F.; Li, L.H.; et al. The microbiota and it’s correlation with metabolites in the gut of mice with nonalcoholic fatty liver disease. Front. Cell. Infect. Microbiol. 2022, 12, 870785. [Google Scholar] [CrossRef]
- Frandsen, H.S.; Vej-Nielsen, J.M.; Smith, L.E.; Sun, L.; Mikkelsen, K.L.; Thulesen, A.P.; Hagensen, C.E.; Yang, F.Q.; Rogowska-Wrzesinska, A. Mapping proteome and lipidome changes in early-onset non-alcoholic fatty liver disease using hepatic 3d spheroids. Cells 2022, 11, 3216. [Google Scholar] [CrossRef]
- Samuelson, D.R.; Shellito, J.E.; Maffei, V.J.; Tague, E.D.; Campagna, S.R.; Blanchard, E.E.; Luo, M.; Taylor, C.M.; Ronis, M.J.J.; Molina, P.E.; et al. Alcohol-associated intestinal dysbiosis impairs pulmonary host defense against Klebsiella pneumoniae. PLoS Pathog 2017, 13, e1006426. [Google Scholar] [CrossRef]
- Duan, Y.; Chu, H.K.; Brandl, K.; Jiang, L.; Zeng, S.L.; Meshgin, N.; Papachristoforou, E.; Argemi, J.; Mendes, B.G.; Wang, Y.H.; et al. CRIg on liver macrophages clears pathobionts and protects against alcoholic liver disease. Nat. Commun. 2021, 12, 7172. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wojciech, L.; Png, C.W.; Koh, E.Y.; Aung, T.T.; Kioh, D.Y.Q.; Chan, E.C.Y.; Malleret, B.; Zhang, Y.L.; Peng, G.N.; et al. Experimental colonization with Blastocystis ST4 is associated with protective immune responses and modulation of gut microbiome in a DSS-induced colitis mouse model. Cell. Mol. Life Sci. 2022, 79, 245. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.W.; Liu, X.F.; Liang, L.H.; Wang, G.Q.; Xiong, Z.Q.; Zhang, H.; Song, X.; Ai, L.Z.; Xia, Y.J. Antrodin a from antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.L.; Wu, Y.Z.; Ye, X.L.; Ma, L.; Qi, J.Y.; Yu, D.; Wei, Y.Q.; Lin, G.G.; Ren, G.P.; Li, D.S. FGF21 ameliorates nonalcoholic fatty liver disease by inducing autophagy. Mol. Cell. Biochem. 2016, 420, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.L.; Chen, Q.Y.; Wei, T.F.; Dou, N.; Shang, D.; Yuan, D. Systematic characterization of Puerariae Flos metabolites in vivo and assessment of its protective mechanisms against alcoholic liver injury in a rat model. Front. Pharmacol. 2022, 13, 915535. [Google Scholar] [CrossRef] [PubMed]
- Bartel, J.; Krumsiek, J.; Schramm, K.; Adamski, J.; Gieger, C.; Herder, C.; Carstensen, M.; Peters, A.; Rathmann, W.; Roden, M.; et al. The human blood metabolome-transcriptome interface. PLoS Genet. 2015, 11, e1005274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wei, Y.L.; Karras, I.; Cai, P.J.; Xiao, Y.H.; Jia, C.L.; Qian, X.L.; Zhu, S.Y.; Zheng, L.J.; Hu, X.; et al. Modulation of the gut microbiota and lipidomic profiles by black chokeberry (Aronia melanocarpa L.) polyphenols via the glycerophospholipid metabolism signaling pathway. Front. Nutr. 2022, 9, 913729. [Google Scholar] [CrossRef] [PubMed]
- Du, L.J.; Wang, Q.; Ji, S.; Sun, Y.F.; Huang, W.J.; Zhang, Y.P.; Li, S.S.; Yan, S.K.; Jin, H.Z. Metabolomic and microbial remodeling by shanmei capsule improves hyperlipidemia in high fat food-induced mice. Front. Cell. Infect. Microbiol. 2022, 12, 729940. [Google Scholar] [CrossRef] [PubMed]
- Niiya, M.; Shimato, Y.; Ohno, T.; Makino, T. Effects of Hovenia dulcis fruit and peduncle extract on alcohol metabolism. J. Ethnopharmacol. 2023, 321, 117541. [Google Scholar] [CrossRef]
- Wang, M.C.; Zhu, P.L.; Jiang, C.X.; Ma, L.P.; Zhang, Z.J.; Zeng, X.X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhu, S.; Zhang, Y.; Zhu, Z.; Xue, Y.; Liu, X. Hovenia dulcis Fruit Peduncle Polysaccharides Reduce Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders in Alcohol-Exposed Mice. Foods 2024, 13, 1145. https://doi.org/10.3390/foods13081145
Liu L, Zhu S, Zhang Y, Zhu Z, Xue Y, Liu X. Hovenia dulcis Fruit Peduncle Polysaccharides Reduce Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders in Alcohol-Exposed Mice. Foods. 2024; 13(8):1145. https://doi.org/10.3390/foods13081145
Chicago/Turabian StyleLiu, Liangyu, Sijie Zhu, Yuchao Zhang, Zhenyuan Zhu, Yong Xue, and Xudong Liu. 2024. "Hovenia dulcis Fruit Peduncle Polysaccharides Reduce Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders in Alcohol-Exposed Mice" Foods 13, no. 8: 1145. https://doi.org/10.3390/foods13081145
APA StyleLiu, L., Zhu, S., Zhang, Y., Zhu, Z., Xue, Y., & Liu, X. (2024). Hovenia dulcis Fruit Peduncle Polysaccharides Reduce Intestinal Dysbiosis and Hepatic Fatty Acid Metabolism Disorders in Alcohol-Exposed Mice. Foods, 13(8), 1145. https://doi.org/10.3390/foods13081145






