Feruloyl Glyceride Mitigates Tomato Postharvest Rot by Inhibiting Penicillium expansum Spore Germination and Enhancing Suberin Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Materials and Treatments
2.2. Fruits and Treatments
2.3. Measurement of Relative Fungal Biomass
2.4. Analysis of Weight Loss and Fruit Firmness
2.5. Measurement of Soluble Solids Content (SSC) and Titratable Acid (TA)
2.6. Determination of Lycopene
2.7. Determination of Anthocyanin
2.8. Microscopy Observation of Suberin Polyphenols
2.9. Metabolites Profiling
2.10. Transcriptomic Profiling
2.11. Total RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.12. Statistical Analysis
3. Results
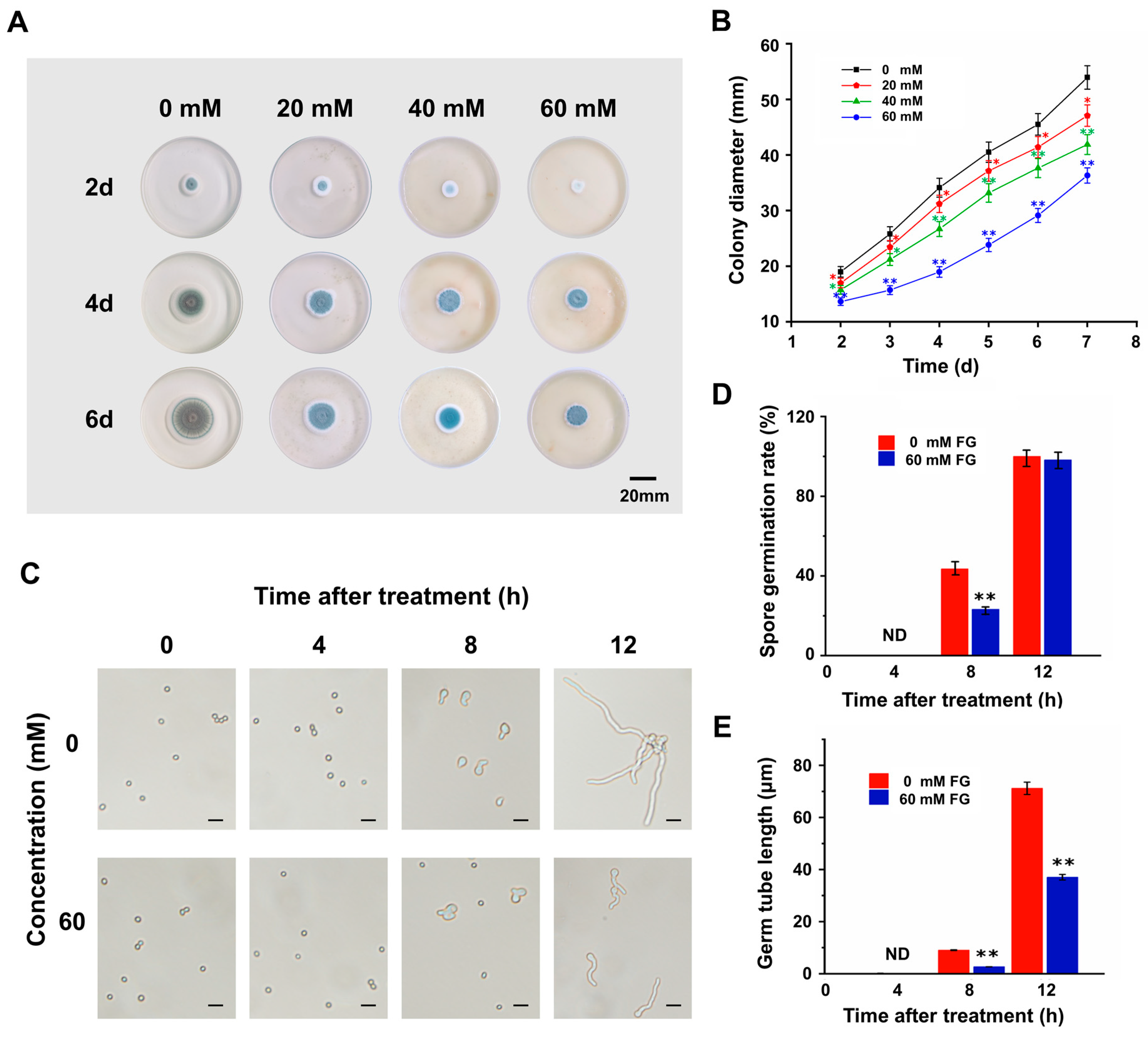
3.1. FG Inhibits the Growth of P. expansum In Vitro
3.2. FG Suppresses Penicillium Rot of Tomato Fruits without Affecting the Quality
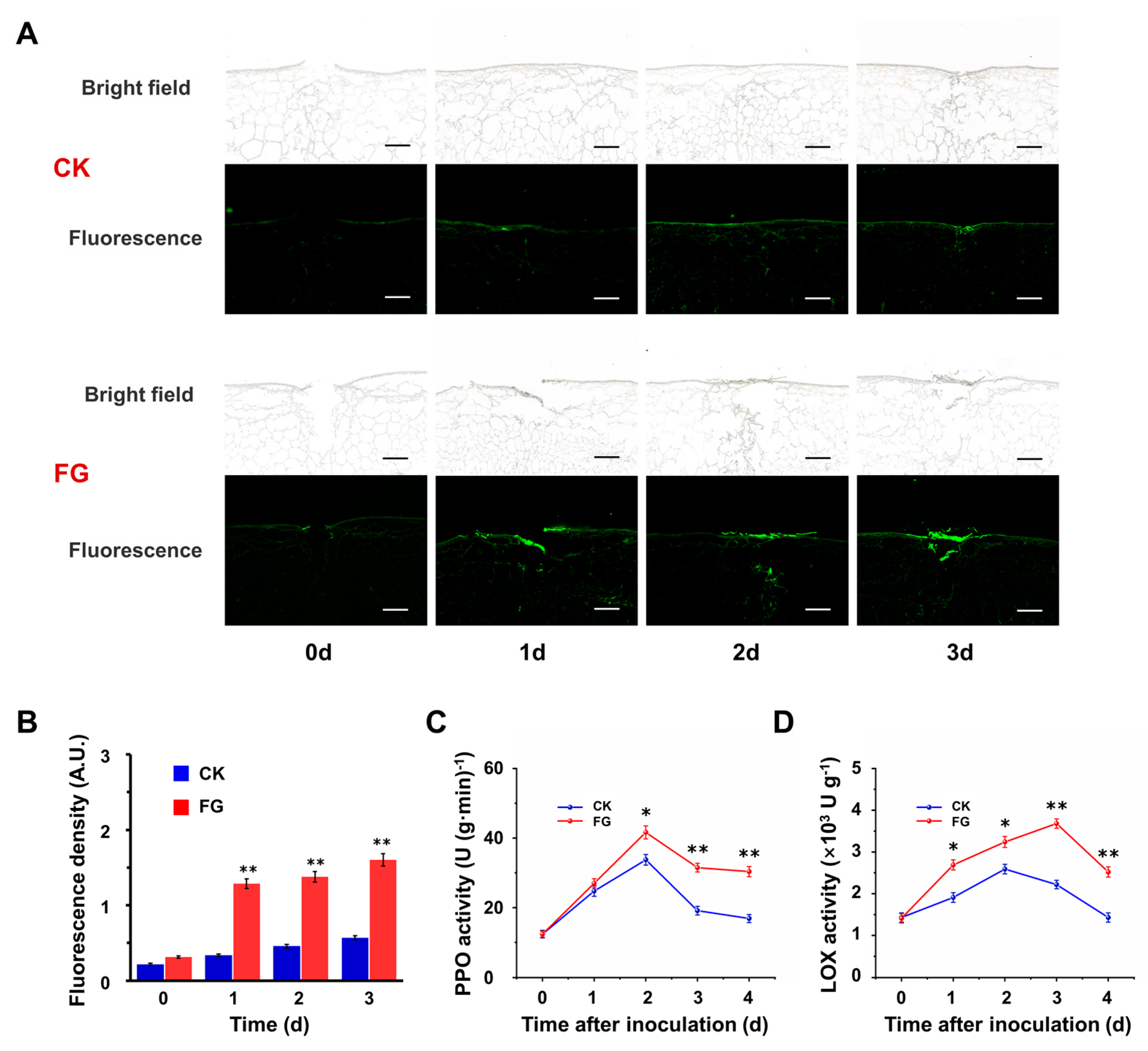
3.3. FG Enhances Suberin Accumulation on the Wound Surface
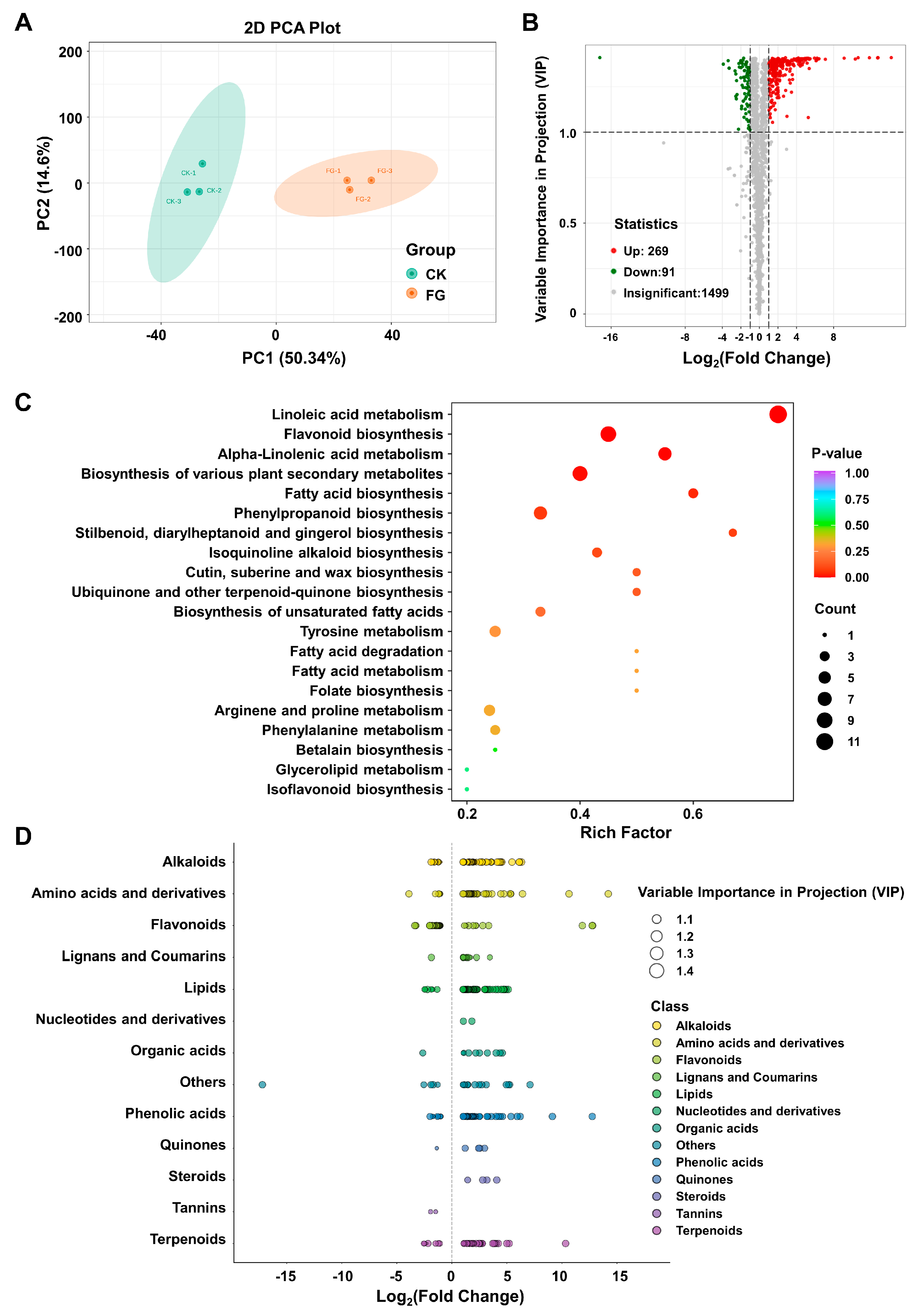
3.4. Impact of FG on Differential Modulation in Suberin Metabolites
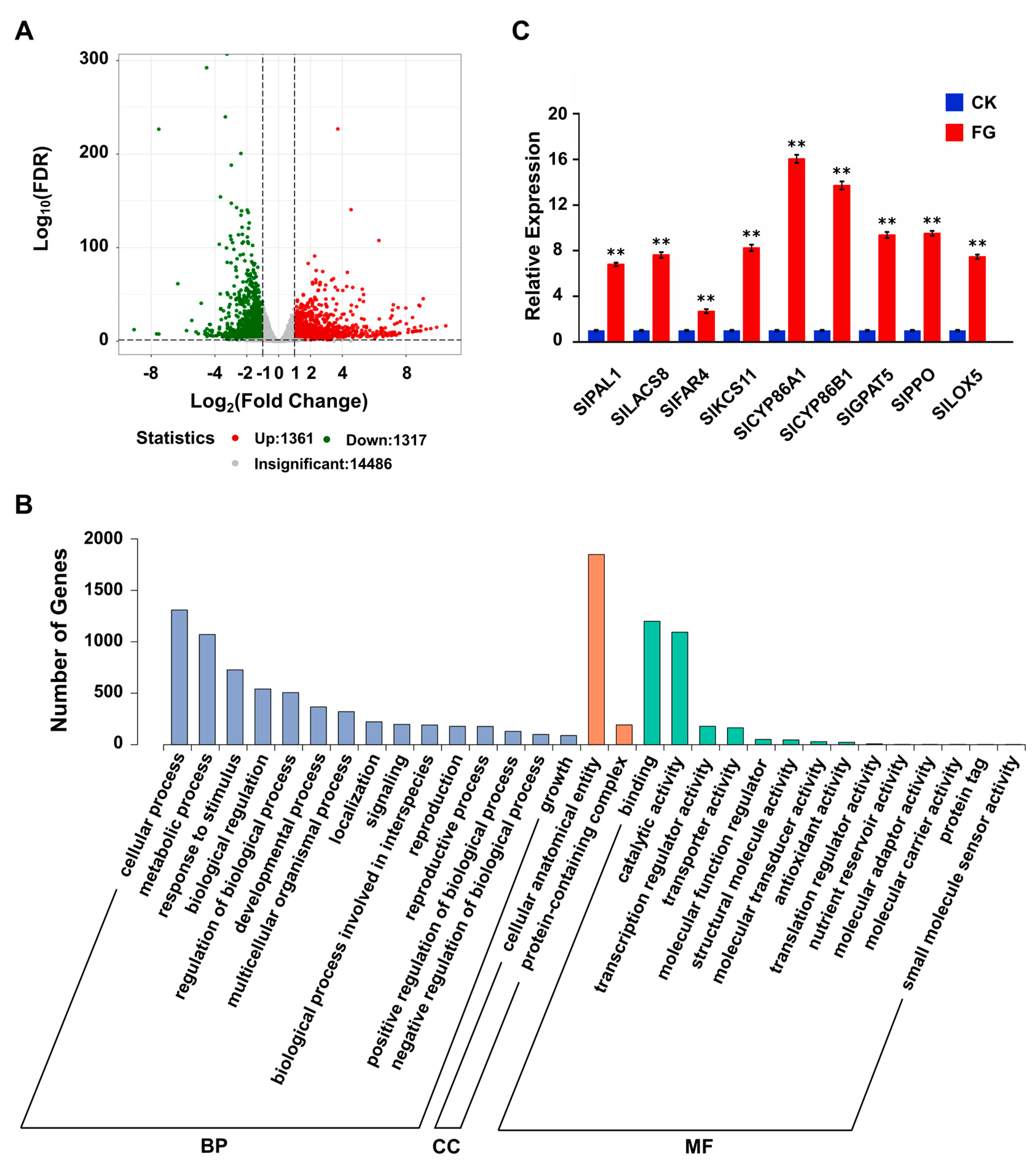
3.5. FG Stimulates the Transcription and Expression of Suberin-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nizamani, G.S.; Khaskheli, A.; Jiskani, A.M.; Khaskheli, S.; Khaskheli, A.; Poussio, G.; Jamro, H.-U.R.; Khaskheli, M. Isolation and Identification of the Fungi Causing Tomato Fruit Rot Disease in the Vicinity of Tandojam, Sindh. Agric. Sci. Dig. A Res. J. 2020, 41, 186–190. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Zhao, J.; Zhang, H.; Tian, F.; Zhai, Q.; Chen, W. Postharvest control of Penicillium expansum in fruits: A review. Food Biosci. 2020, 36, 100633. [Google Scholar] [CrossRef]
- Steinberg, G.; Gurr, S.J. Fungi, fungicide discovery and global food security. Fungal Genet. Biol. 2020, 144, 103476. [Google Scholar] [CrossRef]
- Garnault, M.; Duplaix, C.; Leroux, P.; Couleaud, G.; David, O.; Walker, A.-S.; Carpentier, F. Large-scale study validates that regional fungicide applications are major determinants of resistance evolution in the wheat pathogen Zymoseptoria tritici in France. New Phytol. 2021, 229, 3508–3521. [Google Scholar] [CrossRef]
- Souza, E.; Sales, C.; Oliveira, C.; Lopes, L.; Conceição, M.; Berger, L.; Stamford, T. Efficacy of a coating composed of chitosan from Mucor circinelloides and carvacrol to control Aspergillus flavus and the quality of cherry tomato fruits. Front. Microbiol. 2015, 6, 732. [Google Scholar] [CrossRef][Green Version]
- Nadia, N.; Fauconnier, M.-L.; Ennahli, N.; Abdessalem, T.; Mohammed, B.; Madani, I.; Ennahli, S.; Lahlali, R. Chemical Composition Profiling and Antifungal Activity of Saffron Petal Extract. Molecules 2022, 27, 8742. [Google Scholar] [CrossRef]
- Šentjurc, M.; Nemec, M.; Connor, H.D.; Abram, V. Antioxidant Activity of Sempervivum tectorum and Its Components. J. Agric. Food Chem. 2003, 51, 2766–2771. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saudagar, P. Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Thorston, UK, 2021; pp. 419–441. [Google Scholar]
- Li, Z.; Xue, S.; Xu, X.; Wang, B.; Zheng, X.; Li, B.; Xie, P.; Bi, Y.; Prusky, D. Preharvest multiple sprays with chitosan accelerate the deposition of suberin poly phenolic at wound sites of harvested muskmelons. Postharvest Biol. Technol. 2021, 179, 111565. [Google Scholar] [CrossRef]
- Machado, A.; Pereira, H.; Teixeira, R.T. Anatomy and development of the endodermis and phellem of Quercus suber L. roots. Microsc. Microanal. 2013, 19, 525–534. [Google Scholar] [CrossRef]
- Yang, R.; Han, Y.; Han, Z.; Ackah, S.; Li, Z.; Bi, Y.; Yang, Q.; Prusky, D. Hot water dipping stimulated wound healing of potato tubers. Postharvest Biol. Technol. 2020, 167, 111245. [Google Scholar] [CrossRef]
- Zhu, Y.; Zong, Y.; Liang, W.; Sabina, A.; Chai, X.; Li, Y.; Bi, Y.; Dov, P. β-Aminobutyric acid treatment accelerates the deposition of suberin polyphenolic and lignin at wound sites of potato tubers during healing. Postharvest Biol. Technol. 2021, 179, 111566. [Google Scholar] [CrossRef]
- Sanzani, S.M.; De Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Mechri, B.; Tekaya, M.; Hammami, M.; Chehab, H. Effects of drought stress on phenolic accumulation in greenhouse-grown olive trees (Olea europaea). Biochem. Syst. Ecol. 2020, 92, 104112. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.; Wang, X.; Yao, L.; Sheng, J.; Shen, L. Exogenous ferulic acid treatment increases resistance against Botrytis cinerea in tomato fruit by regulating nitric oxide signaling pathway. Postharvest Biol. Technol. 2021, 182, 111678. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Seo, Y.-H.; Oh, S.-W. Antibacterial activities of polyphenols against foodborne pathogens and their application as antibacterial agents. Food Sci. Biotechnol. 2022, 31, 985–997. [Google Scholar] [CrossRef]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef]
- Janus, E.; Pinheiro, L.R.; Nowak, A.; Kucharska, E.; Świątek, E.; Podolak, N.; Perużyńska, M.; Piotrowska, K.; Duchnik, W.; Kucharski, Ł.; et al. New Ferulic Acid and Amino Acid Derivatives with Increased Cosmeceutical and Pharmaceutical Potential. Pharmaceutics 2023, 15, 117. [Google Scholar] [CrossRef]
- Guo, M.; Li, C.; Huang, R.; Qu, L.; Liu, J.; Zhang, C.; Ge, Y. Ferulic acid enhanced resistance against blue mold of Malus domestica by regulating reactive oxygen species and phenylpropanoid metabolism. Postharvest Biol. Technol. 2023, 202, 112378. [Google Scholar] [CrossRef]
- Raj, N.D.; Singh, D. A critical appraisal on ferulic acid: Biological profile, biopharmaceutical challenges and nano formulations. Health Sci. Rev. 2022, 5, 100063. [Google Scholar] [CrossRef]
- Albuquerque, B.; Heleno, S.; Oliveira, M.; Barros, L.; Ferreira, I. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2020, 12, 14–29. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Atolani, O.; Banerjee, P.; Arolasafe, G.; Preissner, R.; Etukudoh, P.; Ibraheem, O. Computational and experimental validation of antioxidant properties of synthesized bioactive ferulic acid derivatives. Int. J. Food Prop. 2018, 21, 86–98. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-X.; Guo, S.-D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Rezaei, A.; Varshosaz, J.; Fesharaki, M.; Farhang, A.; Jafari, S. Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int. J. Nanomed. 2019, 14, 4589–4599. [Google Scholar] [CrossRef]
- Kashyap, A.; Jiménez-Jiménez, Á.; Figueras, M.; Serra, O.; Valls, M.; Sánchez Coll, N. The Tomato Feruloyl Transferase FHT Promoter Is an Accurate Identifier of Early Development and Stress-Induced Suberization. Plants 2023, 12, 1890. [Google Scholar] [CrossRef]
- Boher, P.; Serra, O.; Soler, M.; Molinas, M.; Figueras, M. The potato suberin feruloyl transferase FHT which accumulates in the phellogen is induced by wounding and regulated by abscisic and salicylic acids. J. Exp. Bot. 2013, 64, 3225–3236. [Google Scholar] [CrossRef]
- Jia, Y.; He, Y.; Lu, F. The structure-antioxidant activity relationship of dehydrodiferulates. Food Chem. 2018, 269, 480–485. [Google Scholar] [CrossRef]
- Nakayama, H.; Nakahara, M.; Matsugi, E.; Soda, M.; Hattori, T.; Hara, K.; Usami, A.; Kusumoto, C.; Higashiyama, S.; Kitaichi, K. Protective Effect of Ferulic Acid against Hydrogen Peroxide Induced Apoptosis in PC12 Cells. Molecules 2020, 26, 90. [Google Scholar] [CrossRef]
- Yao, N.; Sun, S. Hydrophilic Glyceryl Ferulates Preparation Catalyzed by Free Lipase B from Candida antartica. J. Oleo Sci. 2020, 69, 43–53. [Google Scholar] [CrossRef]
- Chen, D.; Li, G.; Liu, J.; Wisniewski, M.; Droby, S.; Levin, E.; Huang, S.; Liu, Y. Multiple transcriptomic analyses and characterization of pathogen-related core effectors and LysM family members reveal their differential roles in fungal growth and pathogenicity in Penicillium expansum. Mol. Genet. Genom. 2020, 295, 1415–1429. [Google Scholar] [CrossRef]
- Shi, X.; Long, Y.; He, F.; Zhang, C.; Wang, R.; Zhang, T.; Wu, W.; Hao, Z.; Wang, Y.; Wang, G.-L.; et al. The fungal pathogen Magnaporthe oryzae suppresses innate immunity by modulating a host potassium channel. PLoS Path. 2018, 14, e1006878. [Google Scholar] [CrossRef]
- Paul, V.; Singh, A.; Pandey, R. Determination of Titrable Acidity (TA). In Post-Harvest Physiology of Fruits and Flowers; Indian Agricultural Research Institute: New Delhi, India, 2010; pp. 44–45. [Google Scholar]
- Fish, W.; Perkins, P.; Collins, J. A Quantitative Assay for Lycopene That Utilizes Reduced Volumes of Organic Solvents. J. Food Compost. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing Lycopene Content in Fruits. Agric. Agric. Sci. Procedia 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Mustilli, A.C.; Fenzi, F.; Ciliento, R.; Alfano, F.; Bowler, C. Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 1999, 11, 145–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, C.; Zhang, Y.; Li, C.; Li, X.; Yu, Q.; Wang, S.; Wang, X.; Chen, X.; Feng, S. Transcriptomic and metabolomic analysis provides insights into anthocyanin and procyanidin accumulation in pear. BMC Plant Biol. 2020, 20, 129. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Liang, F.; Deng, B.-C.; Lai, G.-B.; Vicente Gonçalves, C.; Lu, H.-M.; Yan, J.; Huang, X.; Yi, L.-Z.; Liang, Y.-Z. Informative metabolites identification by variable importance analysis based on random variable combination. Metabolomics 2015, 11, 1539–1551. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCt Method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant Properties of Ferulic Acid and Its Related Compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Pinheiro, P.; Santiago, G.; Silva, F.; Araújo, A.; Oliveira, C.; Freitas, P.; Rocha, J.; Araújo Neto, J.; Costa, M.; Tintino, S.; et al. Ferulic acid derivatives inhibiting Staphylococcus aureus tetK and MsrA efflux pumps. Biotechnol. Rep. 2022, 34, e00717. [Google Scholar] [CrossRef]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef]
- Shalaby, S.; Larkov, O.; Lamdan, N.-L.; Goldshmidt-Tran, O.; Horwitz, B.A. Plant phenolic acids induce programmed cell death of a fungal pathogen: MAPK signaling and survival of Cochliobolus heterostrophus. Environ. Microbiol. 2016, 18, 4188–4199. [Google Scholar] [CrossRef]
- Song, W.; Xin, J.; Yu, C.; Xia, C.; Pan, Y. Alkyl ferulic acid esters: Evaluating their structure and antibacterial properties. Front. Microbiol. 2023, 14, 1135308. [Google Scholar] [CrossRef]
- Han, X.; Lu, W.; Wei, X.; Li, L.; Mao, L.; Zhao, Y. Proteomics analysis to understand the ABA stimulation of wound suberization in kiwifruit. J. Proteom. 2018, 173, 42–51. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Sengupta, S.; Burks, D.; Azad, R.K.; Mittler, R. Identification and characterization of a core set of ROS wave-associated transcripts involved in the systemic acquired acclimation response of Arabidopsis to excess light. Plant J. 2019, 98, 126–141. [Google Scholar] [CrossRef]
- Han, X.; Mao, L.; Wei, X.; Lu, W. Stimulatory involvement of abscisic acid in wound suberization of postharvest kiwifruit. Sci. Hortic. 2017, 224, 244–250. [Google Scholar] [CrossRef]
- Ren, H.; Bai, M.; Sun, J.; Liu, J.; Ren, M.; Dong, Y.; Wang, N.; Ning, G.; Changquan, W. RcMYB84 and RcMYB123 mediate jasmonate-induced defense responses against Botrytis cinerea in rose (Rosa chinensis). Plant J. 2020, 103, 1839–1849. [Google Scholar] [CrossRef]
- Zhu, Z.; Tian, S. Resistant responses of tomato fruit treated with exogenous methyl jasmonate to Botrytis cinerea infection. Sci. Hortic. 2012, 142, 38–43. [Google Scholar] [CrossRef]
- Pei, T.; Ma, P.; Ding, K.; Liu, S.; Jia, Y.; Ru, M.; Dong, J.; Liang, Z. SmJAZ8 acts as a core repressor regulating JA-induced biosynthesis of salvianolic acids and tanshinones in Salvia miltiorrhiza hairy roots. J. Exp. Bot. 2018, 69, 1663–1678. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Song, W.; Tang, X.; Liu, Y.; Miao, M. Feruloyl Glyceride Mitigates Tomato Postharvest Rot by Inhibiting Penicillium expansum Spore Germination and Enhancing Suberin Accumulation. Foods 2024, 13, 1147. https://doi.org/10.3390/foods13081147
Gao J, Song W, Tang X, Liu Y, Miao M. Feruloyl Glyceride Mitigates Tomato Postharvest Rot by Inhibiting Penicillium expansum Spore Germination and Enhancing Suberin Accumulation. Foods. 2024; 13(8):1147. https://doi.org/10.3390/foods13081147
Chicago/Turabian StyleGao, Jieyu, Wu Song, Xiaofeng Tang, Yongsheng Liu, and Min Miao. 2024. "Feruloyl Glyceride Mitigates Tomato Postharvest Rot by Inhibiting Penicillium expansum Spore Germination and Enhancing Suberin Accumulation" Foods 13, no. 8: 1147. https://doi.org/10.3390/foods13081147
APA StyleGao, J., Song, W., Tang, X., Liu, Y., & Miao, M. (2024). Feruloyl Glyceride Mitigates Tomato Postharvest Rot by Inhibiting Penicillium expansum Spore Germination and Enhancing Suberin Accumulation. Foods, 13(8), 1147. https://doi.org/10.3390/foods13081147





