Construction and Properties of Oil-Loaded Soybean Protein Isolate/Polysaccharide-Based Meat Analog Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPI/SA Oil-Loaded Composite Solution
2.3. Confocal Laser Scanning Microscopy (CLSM) of SPI/SA Oil-Loaded Composite Solutions
2.4. Droplet Size Distribution of SPI/SA Oil-Loaded Composite Solutions
2.5. Rheology Measurement of SPI/SA Oil-Loaded Composite Solutions
2.6. Physical Stability of SPI/SA Oil-Loaded Composite Solutions
2.7. Preparation of SPI/SA Oil-Loaded Composite Fibers
2.8. Microstructures of SPI/SA Oil-Loaded Composite Fibers
2.8.1. Confocal Laser Scanning Microscopy (CLSM)
2.8.2. Scanning Electron Microscope (SEM)
2.9. Color Evaluation of SPI/SA Oil-Loaded Composite Fibers
2.10. Water-Holding Capacity (WHC) of SPI/SA Oil-Loaded Composite Fibers
2.11. Low-Field Nuclear Magnetic Resonance (LF-NMR) of SPI/SA Oil-Loaded Composite Fibers
2.12. Mechanical Analysis of SPI/SA Oil-Loaded Composite Fibers
2.13. The Component Analysis of SPI/SA Oil-Loaded Composite Fibers
2.14. Statistical Analysis
3. Results and Discussion
3.1. Effect of Different Emulsifying Methods on Solution Behavior of SPI/SA Oil-Loaded Composite Solution
3.1.1. Microstructures and Droplet size Distribution of SPI/SA oil-Loaded Composite Solution
3.1.2. Rheological Properties of SPI/SA Oil-Loaded Composite Solution
3.2. Characterization of SPI/SA Oil-Loaded Composite Solution
3.2.1. CLSM Analysis
3.2.2. Droplet Size and Distribution
3.2.3. Stability Evaluation
3.2.4. Rheological Properties
3.3. Properties of SPI/SA Oil-Loaded Composite Fibers
3.3.1. Microstructures Analysis
3.3.2. Color Changes
3.3.3. Water Holding Capacity
3.3.4. Low-Field NMR Spin-Spin Relaxation (T2) Measurements
3.3.5. Mechanical Property
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tziva, M.; Negro, S.O.; Kalfagianni, A.; Hekkert, M.P. Understanding the Protein Transition: The Rise of Plant-Based Meat Substitutes. Environ. Innov. Soc. Transit. 2020, 35, 217–231. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Liu, H.; Li, T.; Wang, Q.; Kaplan, D.L. Microscopic Insight into the Interactions between Pea Protein and Fatty Acids during High-Moisture Extrusion Processing. Food Chem. 2023, 404, 134176. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant Protein-Based Alternatives of Reconstructed Meat: Science, Technology, and Challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat Analogues: Health Promising Sustainable Meat Substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef]
- Van Der Weele, C.; Feindt, P.; Jan Van Der Goot, A.; Van Mierlo, B.; Van Boekel, M. Meat Alternatives: An Integrative Comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Tuorila, H.; Hartmann, C. Consumer Responses to Novel and Unfamiliar Foods. Curr. Opin. Food Sci. 2020, 33, 1–8. [Google Scholar] [CrossRef]
- Manski, J.M.; Boom, R.M. Advances in Structure Formation of Anisotropic Protein-Rich Foods through Novel Processing Concepts. Trends Food Sci. Technol. 2007, 18, 546–557. [Google Scholar] [CrossRef]
- Cui, B.; Liang, H.; Li, J.; Zhou, B.; Chen, W.; Liu, J.; Li, B. Development and Characterization of Edible Plant-Based Fibers Using a Wet-Spinning Technique. Food Hydrocoll. 2022, 133, 107965. [Google Scholar] [CrossRef]
- Kendler, C.; Duchardt, A.; Karbstein, H.P.; Emin, M.A. Effect of Oil Content and Oil Addition Point on the Extrusion Processing of Wheat Gluten-Based Meat Analogues. Foods 2021, 10, 697. [Google Scholar] [CrossRef]
- Gandemer, G. Lipids in Muscles and Adipose Tissues, Changes during Processing and Sensory Properties of Meat Products. Meat Sci. 2002, 62, 309–321. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat Deposition, Fatty Acid Composition and Meat Quality: A Review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Trivedi, N.; Enamala, M.K.; Kuppam, C.; Parikh, P.; Nikolova, M.P.; Chavali, M. Plant-Based Meat Analogue (PBMA) as a Sustainable Food: A Concise Review. Eur. Food Res. Technol. 2021, 247, 2499–2526. [Google Scholar] [CrossRef]
- Manski, J.M.; Van Der Zalm, E.E.J.; Van Der Goot, A.J.; Boom, R.M. Influence of Process Parameters on Formation of Fibrous Materials from Dense Calcium Caseinate Dispersions and Fat. Food Hydrocoll. 2008, 22, 587–600. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Zhang, Y.; Wang, Q. Effect of Fatty Acid Saturation Degree on the Rheological Properties of Pea Protein and Its High-Moisture Extruded Product Quality. Food Chem. 2022, 390, 133139. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, Y.; Jia, F.; Chen, D.; Zhang, X.; Wang, Q.; Wang, J. Effect of Extrusion Temperature on the Protein Aggregation of Wheat Gluten with the Addition of Peanut Oil during Extrusion. Int. J. Biol. Macromol. 2021, 166, 1377–1386. [Google Scholar] [CrossRef]
- Ren, C. Effects of Thermal Sterilization on Soy Protein Isolate/Polyphenol Complexes: Aspects of Structure, In Vitro Digestibility and Antioxidant Activity. Food Res. Int. 2018, 112, 284–290. [Google Scholar] [CrossRef]
- Cui, B.; Mao, Y.; Liang, H.; Li, Y.; Li, J.; Ye, S.; Chen, W.; Li, B. Properties of Soybean Protein Isolate/Curdlan Based Emulsion Gel for Fat Analogue: Comparison with Pork Backfat. Int. J. Biol. Macromol. 2022, 206, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Li, Y.C.; Hu, G.S. Investigation on the Rheological Behavior of PA1010/POE/OMMT Nanocomposites. Adv. Mater. Res. 2012, 482–484, 2582–2586. [Google Scholar] [CrossRef]
- Wang, L.N.; Deng, Z.X.; Wang, Y.W. Rheological Behavior of Bamboo Charcoal PET. Adv. Mater. Res. 2011, 224, 240–244. [Google Scholar] [CrossRef]
- Qi, T.; Ren, J.; Li, X.; An, Q.; Zhang, N.; Jia, X.; Pan, S.; Fan, G.; Zhang, Z.; Wu, K. Structural Characteristics and Gel Properties of Pectin from Citrus Physiological Premature Fruit Drop. Carbohydr. Polym. 2023, 309, 120682. [Google Scholar] [CrossRef]
- Jiang, S.; Shang, L.; Liang, H.; Li, B.; Li, J. Preparation of Konjac Glucomannan/Xanthan Gum/Sodium Alginate Composite Gel by Freezing Combining Moisture Regulation. Food Hydrocoll. 2022, 127, 107499. [Google Scholar] [CrossRef]
- Liu, J.; Arner, A. Longitudinal Vibration Interferes with Cross-Bridge Attachment and Prevents Muscle Fibre Shrinkage under PSE-like Conditions. Meat Sci. 2021, 179, 108558. [Google Scholar] [CrossRef]
- Liu, J.; Puolanne, E.; Schwartzkopf, M.; Arner, A. Altered Sarcomeric Structure and Function in Woody Breast Myopathy of Avian Pectoralis Major Muscle. Front. Physiol. 2020, 11, 287. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis (Volume 1). Chem. Funct. Prop. Food Sacch. 1990, 1, 73–80. [Google Scholar]
- Zhang, M.; Fan, L.; Liu, Y.; Li, J. A Mechanistic Investigation of the Effect of Dispersion Phase Protein Type on the Physicochemical Stability of Water–in–Oil Emulsions. Food Res. Int. 2022, 157, 111293. [Google Scholar] [CrossRef]
- Yang, F.; Yang, J.; Qiu, S.; Xu, W.; Wang, Y. Tannic Acid Enhanced the Physical and Oxidative Stability of Chitin Particles Stabilized Oil in Water Emulsion. Food Chem. 2021, 346, 128762. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, B.; Guo, X.; Zhou, B.; Wang, S.; Pei, Y.; Li, B.; Liang, H. Fabrication and Characterization of Pickering Emulsions Stabilized by Desalted Duck Egg White Nanogels and Sodium Alginate. J. Sci. Food Agric. 2022, 102, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.H.V.; Snel, S.J.E.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G.M. Enhancing the Water Holding Capacity of Model Meat Analogues through Marinade Composition. J. Food Eng. 2021, 290, 110283. [Google Scholar] [CrossRef]
- Damez, J.-L.; Clerjon, S. Meat Quality Assessment Using Biophysical Methods Related to Meat Structure. Meat Sci. 2008, 80, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ma, C.; Yan, X.; Zeng, H.; McClements, D.J.; Liu, X.; Liu, F. Structure, Rheology and Functionality of Whey Protein Emulsion Gels: Effects of Double Cross-Linking with Transglutaminase and Calcium Ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Miklos, R.; Mora-Gallego, H.; Larsen, F.H.; Serra, X.; Cheong, L.-Z.; Xu, X.; Arnau, J.; Lametsch, R. Influence of Lipid Type on Water and Fat Mobility in Fermented Sausages Studied by Low-Field NMR. Meat Sci. 2014, 96, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tian, Y.; Sun, B.; Wang, J.; Tong, Q.; Jin, Z. Rapid, Accurate, and Simultaneous Measurement of Water and Oil Contents in the Fried Starchy System Using Low-Field NMR. Food Chem. 2017, 233, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Xu, D.; Li, D.; Guo, L.; Su, X.; Zhang, Y.; Wu, F.; Xu, X. Effect of Pigskin-Originated Gelatin on Properties of Wheat Flour Dough and Bread. Food Hydrocoll. 2019, 94, 183–190. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Li, T.; Zheng, H.; Khan, M.A.; Xu, X.; Sun, J.; Zhou, G. Effect of Protein Structure on Water and Fat Distribution during Meat Gelling. Food Chem. 2016, 204, 239–245. [Google Scholar] [CrossRef] [PubMed]



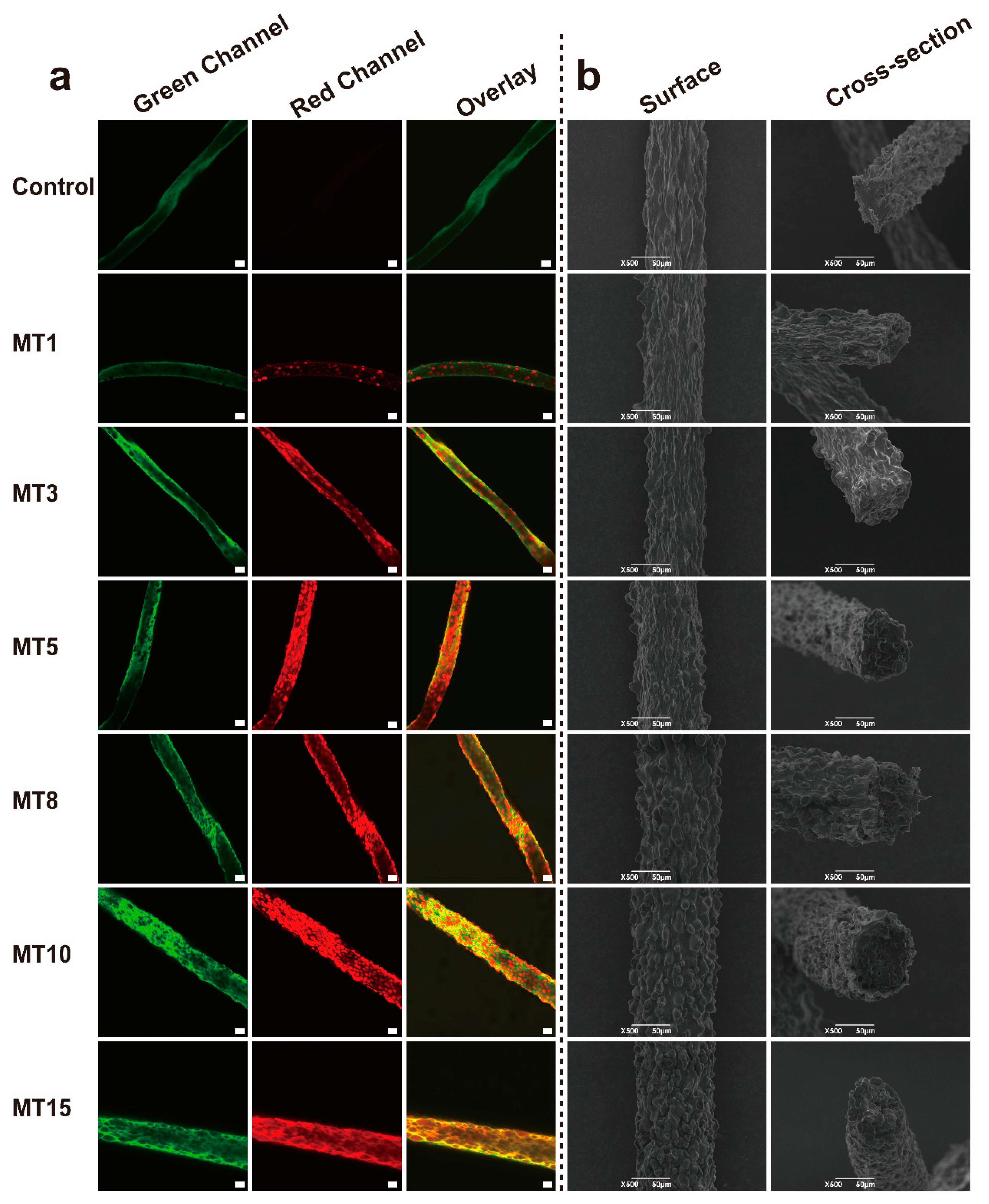



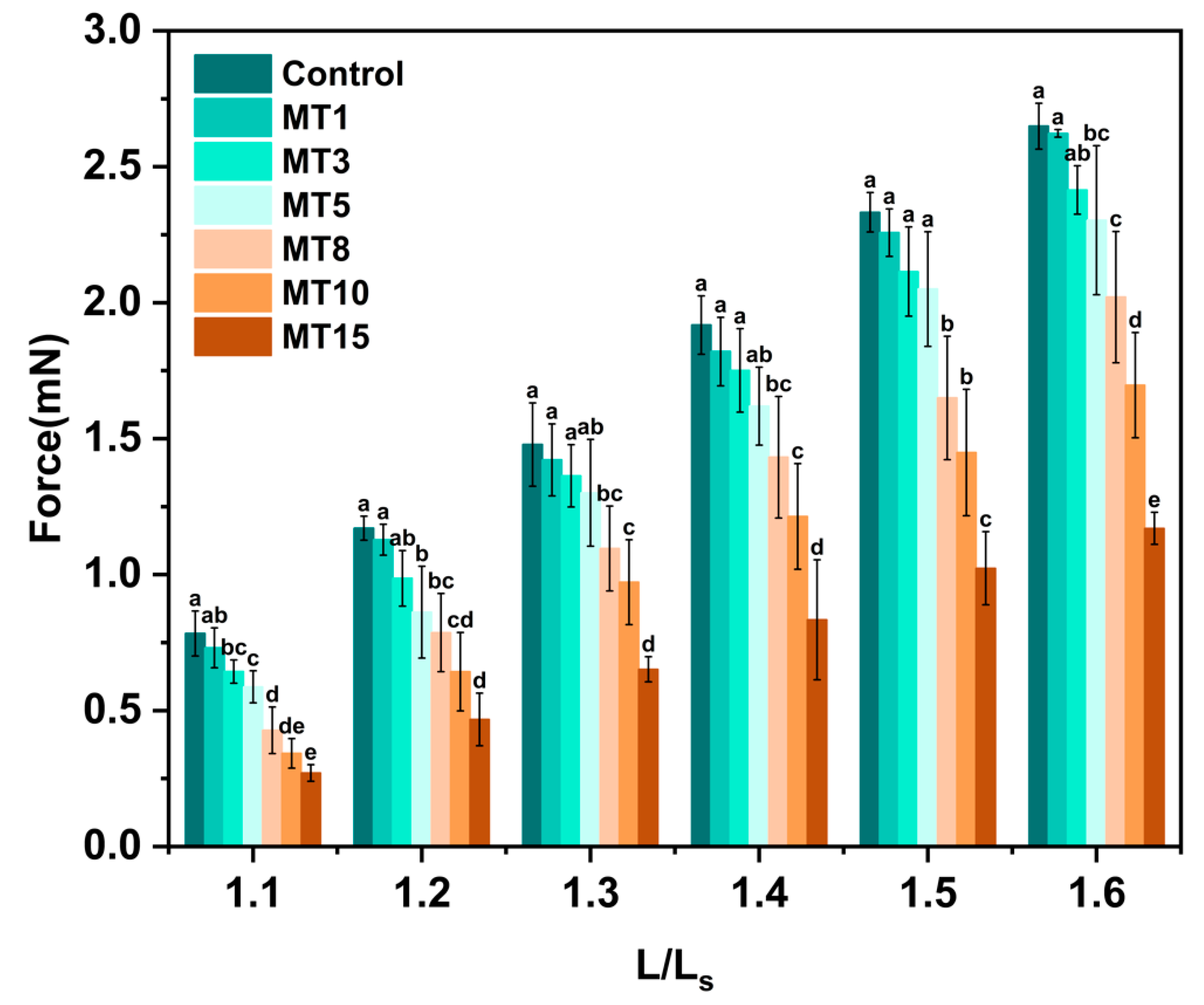
| Sample | n | R12 | Δη | R22 |
|---|---|---|---|---|
| Control | 0.7353 | 0.99 | 8.97 | 0.98 |
| MT1 | 0.7352 | 0.99 | 9.06 | 0.98 |
| HT1 | 0.6878 | 0.99 | 10.49 | 0.97 |
| Sample | n | R12 | Δη | R22 |
|---|---|---|---|---|
| MT3 | 0.7324 | 0.99 | 9.117 | 0.98 |
| MT5 | 0.7272 | 0.99 | 9.190 | 0.98 |
| MT8 | 0.7010 | 0.99 | 9.976 | 0.97 |
| MT10 | 0.6802 | 0.99 | 10.51 | 0.95 |
| MT15 | 0.6741 | 0.99 | 10.82 | 0.96 |
| Sample | Moisture (%) | Protein (%) | Oil (%) |
|---|---|---|---|
| Control | 74.30 ± 0.89 a | 15.50 ± 0.46 a | 0.11 ± 0.05 a |
| MT1 | 72.84 ± 0.54 a | 14.59 ± 0.32 a | 1.51 ± 0.08 a |
| MT3 | 70.67 ± 1.20 b | 13.29 ± 0.65 b | 5.06 ± 0.12 b |
| MT5 | 68.46 ± 0.68 c | 13.18 ± 0.48 b | 7.81 ± 0.35 c |
| MT8 | 63.31 ± 0.76 d | 13.03 ± 0.63 b | 13.72 ± 1.02 d |
| MT10 | 57.98 ± 0.57 e | 12.74 ± 0.53 b | 17.84 ± 0.89 e |
| MT15 | 55.20 ± 0.43 f | 10.87 ± 0.62 c | 24.71 ± 1.32 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Cui, B.; Wu, D.; Li, J.; Liang, H.; Zhou, B.; Li, B. Construction and Properties of Oil-Loaded Soybean Protein Isolate/Polysaccharide-Based Meat Analog Fibers. Foods 2024, 13, 1159. https://doi.org/10.3390/foods13081159
Zeng X, Cui B, Wu D, Li J, Liang H, Zhou B, Li B. Construction and Properties of Oil-Loaded Soybean Protein Isolate/Polysaccharide-Based Meat Analog Fibers. Foods. 2024; 13(8):1159. https://doi.org/10.3390/foods13081159
Chicago/Turabian StyleZeng, Xinyue, Bing Cui, Di Wu, Jing Li, Hongshan Liang, Bin Zhou, and Bin Li. 2024. "Construction and Properties of Oil-Loaded Soybean Protein Isolate/Polysaccharide-Based Meat Analog Fibers" Foods 13, no. 8: 1159. https://doi.org/10.3390/foods13081159





