Valorization of Agricultural By-Products (Fragaria vesca) through the Production of Value-Added Micro/Nanostructures Using Electrohydrodynamic Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Fragaria vesca Leaf Extract Production
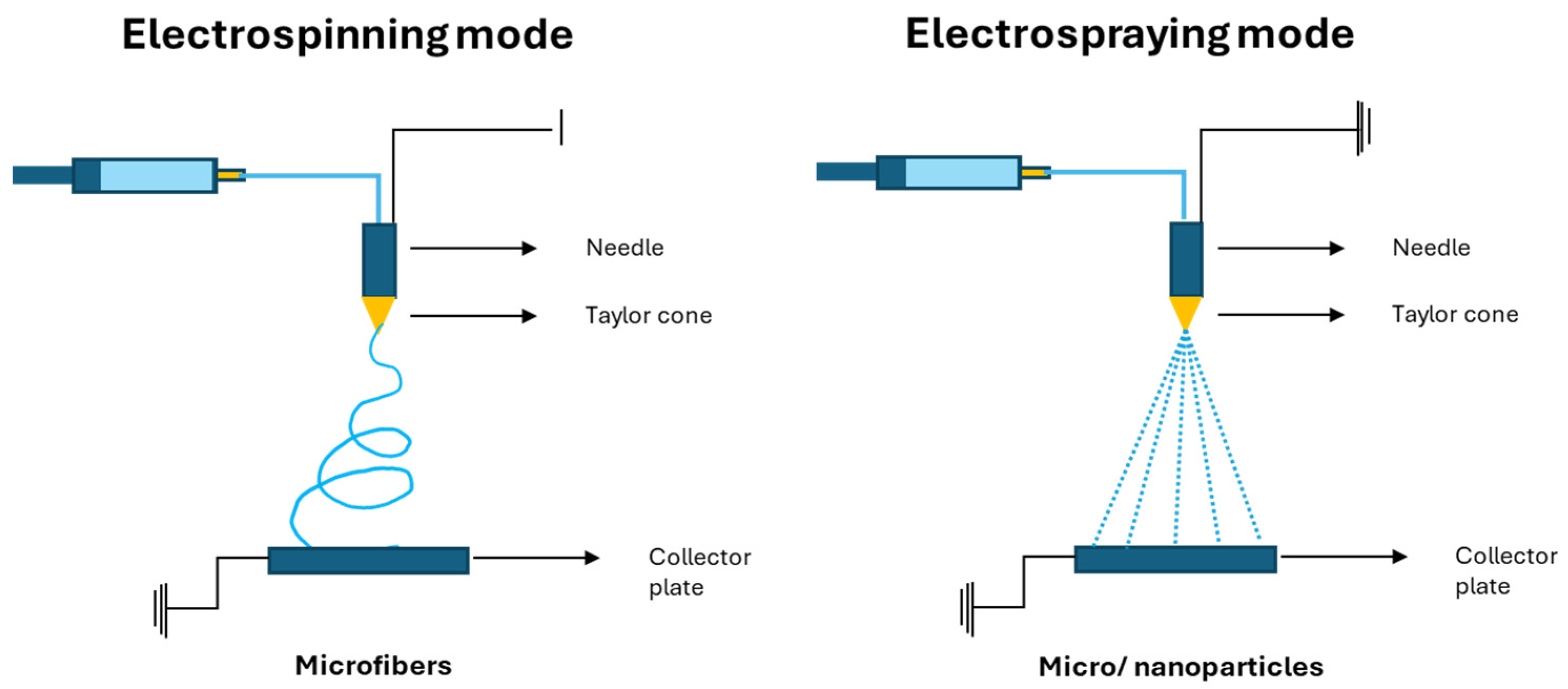
2.3. Micro/Nanostructure Production
2.4. Conductivity of the Zein Solutions
2.5. Microstructure Morphology
2.6. In Vitro Release Studies
2.7. In Vitro Antioxidant Activity Experiment
2.8. Statistical Analysis
3. Results and Discussion
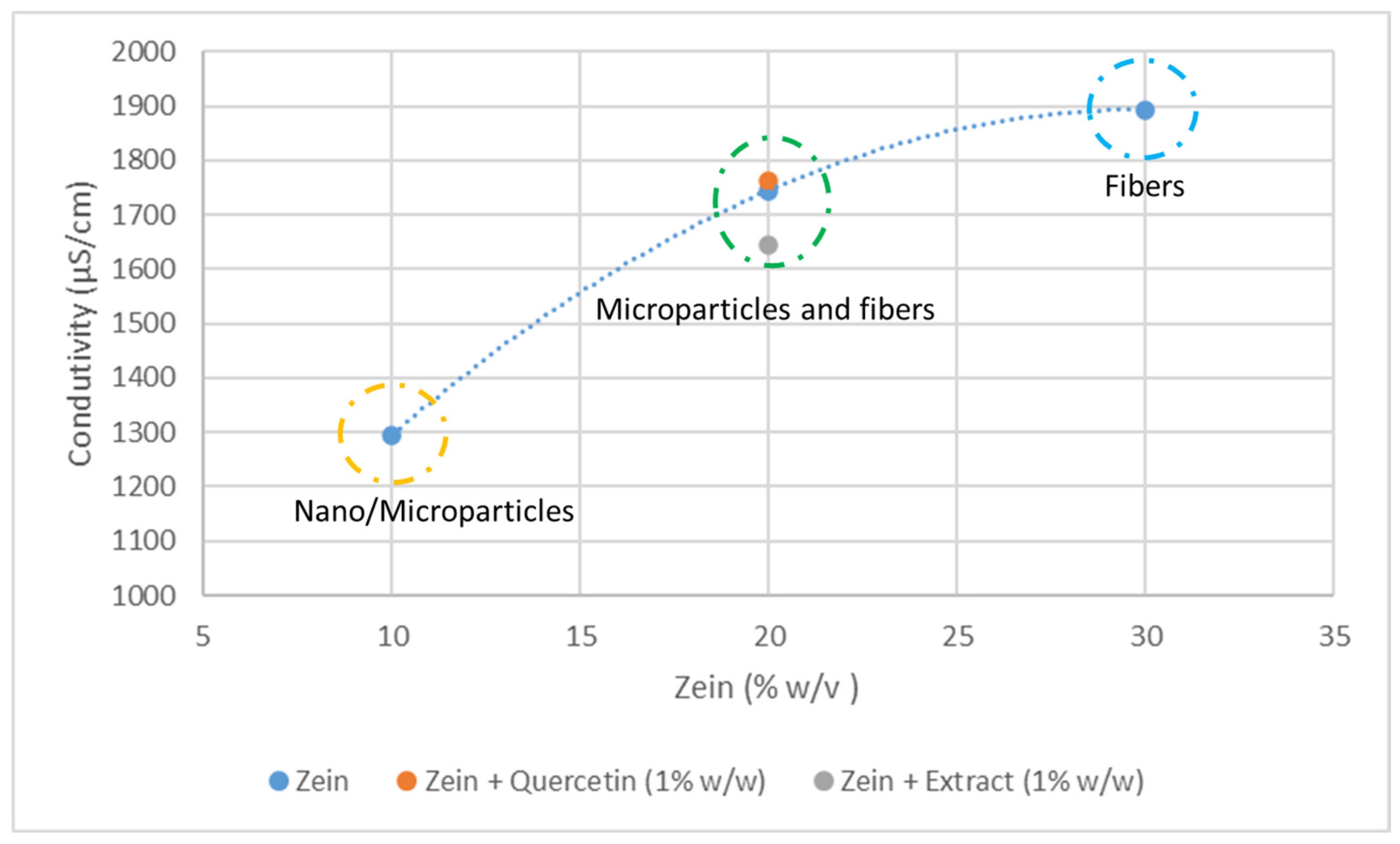
3.1. Characterization of the Microstructures
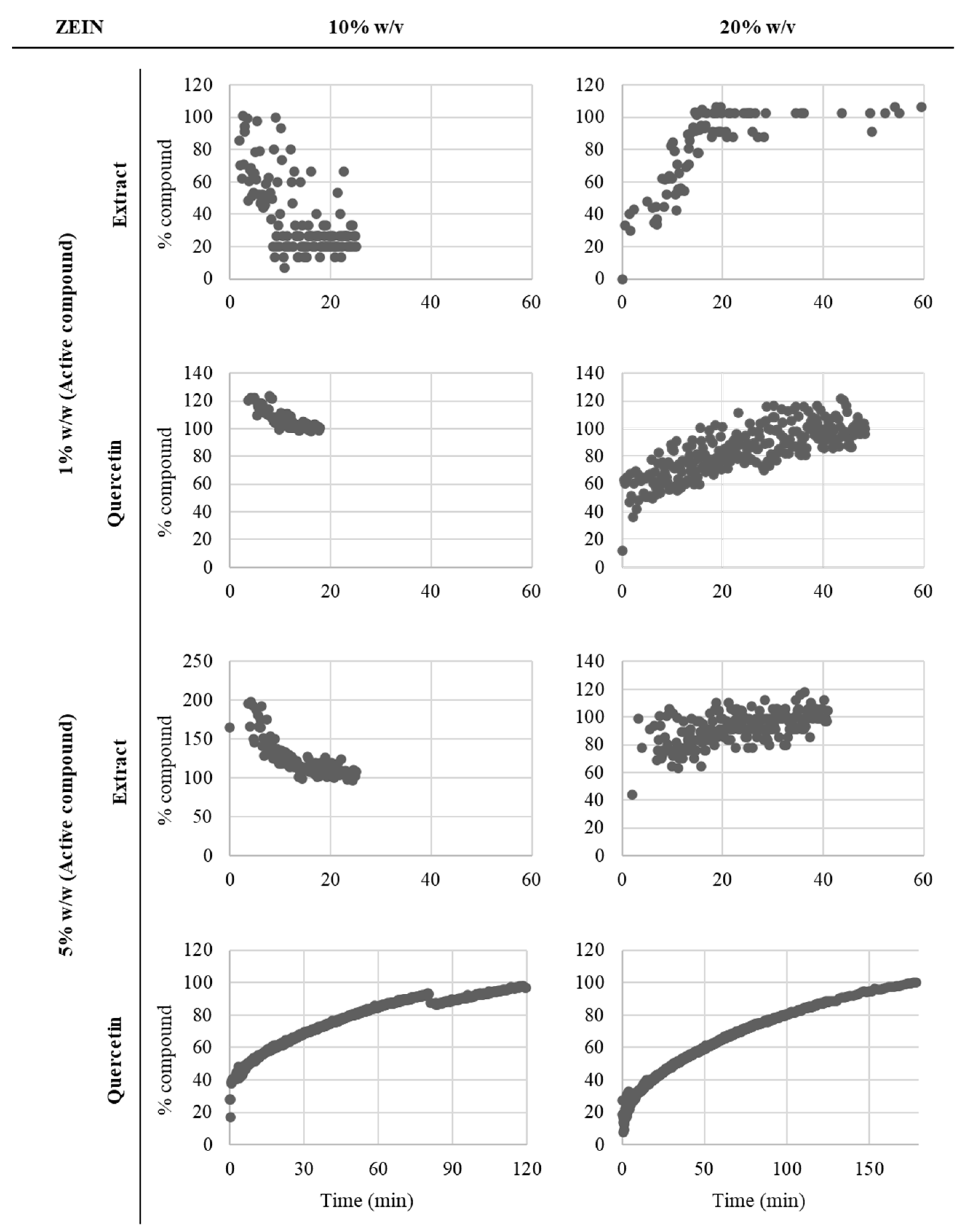
3.2. In Vitro Release Assays
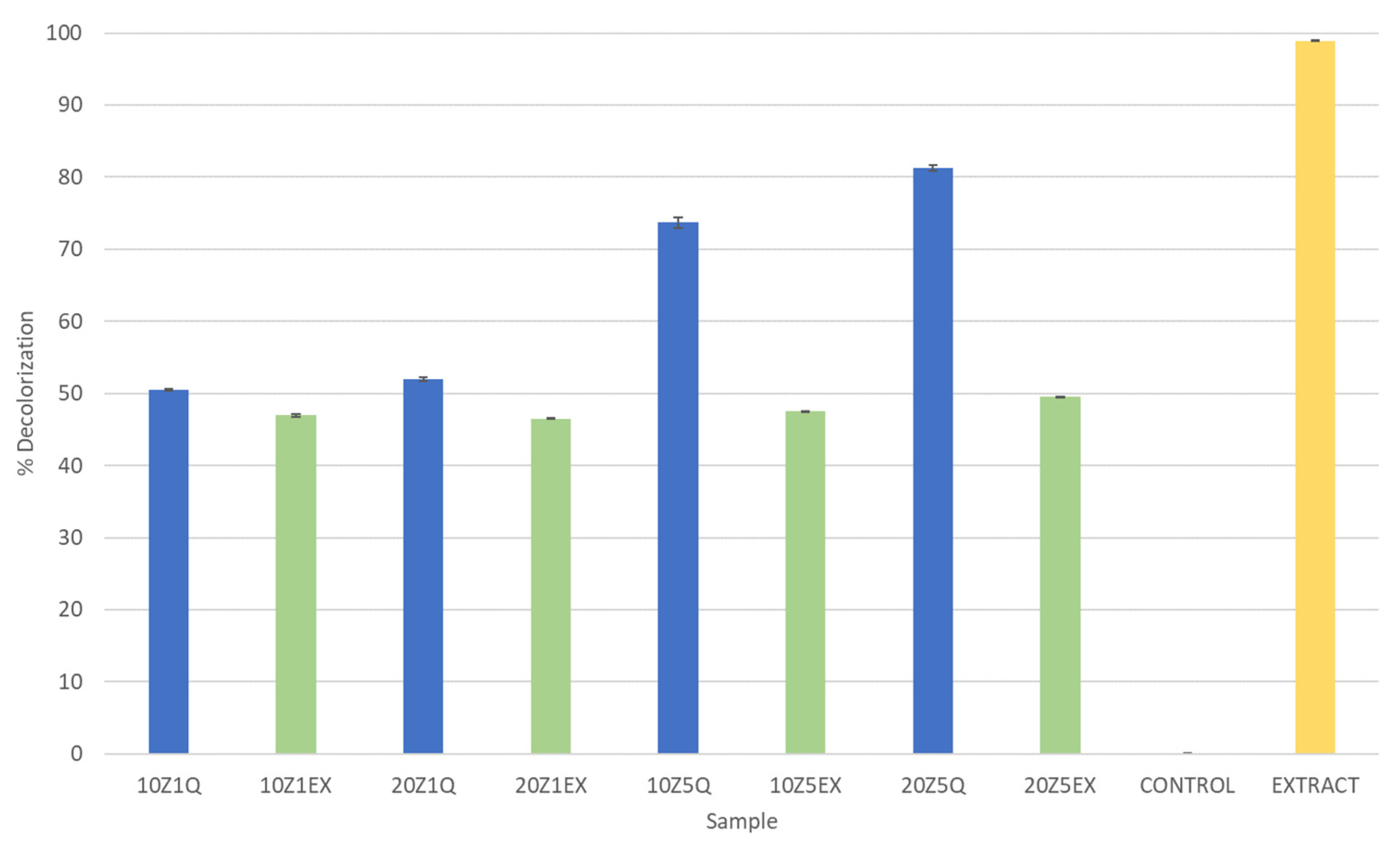
3.3. Antioxidant Capacity Assessment (ABTS Method)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant By-Product Antioxidants: Control of Protein-Lipid Oxidation in Meat and Meat Products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Graciano-Verdugo, A.Z.; Moreno-Vásquez, M.J.; Lagarda-Díaz, I.; Barreras-Urbina, C.G.; Armenta-Villegas, L.; Olguín-Moreno, A.; Tapia-Hernández, J.A. Trends in Sustainable Green Synthesis of Silver Nanoparticles Using Agri-Food Waste Extracts and Their Applications in Health. J. Nanomater. 2022, 2022, 8874003. [Google Scholar] [CrossRef]
- Castro, V.T.G.; Rubiano, E.F.L.; Carreño, D.K.L.; Marín, F.G.M.; Lozano, J.E.S.; Montenegro-Marin, C.E.; Garcia, P.A.G.; Victor, J.A.M. Strawberry Fragaria Cultivation in the Municipality of Sibaté, Cundinamarca. Characterization of the Production and Marketing System to Incorporate; Springer Nature: Singapore, 2024; Volume 344, ISBN 9789819903320. [Google Scholar]
- Dias, M.I.; Barros, L.; Fernandes, I.P.; Ruphuy, G.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C.F.R. A Bioactive Formulation Based on Fragaria vesca L. Vegetative Parts: Chemical Characterisation and Application in κ-Carrageenan Gelatin. J. Funct. Foods 2015, 16, 243–255. [Google Scholar] [CrossRef]
- Ni, N.; Dumont, M.J. Protein-Based Hydrogels Derived from Industrial Byproducts Containing Collagen, Keratin, Zein and Soy. Waste Biomass Valoriz. 2017, 8, 285–300. [Google Scholar] [CrossRef]
- Anwar, M.A.; Galal, D.; Khalifa, I.; Zahran, H.A.; Capanoglu, E.; Farag, M.A. Metabolomics: A Compilation of Applications for Enhancing Agricultural Traits, Disease Resistance, Biotic Interaction, Byproducts Valorization, and Quality Control Purposes of Olive. Trends Food Sci. Technol. 2024, 143, 104311. [Google Scholar] [CrossRef]
- Majeed, T.; Shabir, I.; Srivastava, S.; Maqbool, N.; Dar, A.H.; Jan, K.; Pandey, V.K.; Shams, R.; Bashir, I.; Dash, K.K.; et al. Valorization of Food Wastes by Implementation of Subcritical Water Extraction: A Comprehensive Review. Trends Food Sci. Technol. 2024, 143, 104316. [Google Scholar] [CrossRef]
- Tachie, C.; Nwachukwu, I.D.; Aryee, A.N.A. Trends and Innovations in the Formulation of Plant-Based Foods. Food Prod. Process. Nutr. 2023, 5, 16. [Google Scholar] [CrossRef]
- Poponi, S.; Ruggieri, A.; Pacchera, F.; Arcese, G. The Circular Potential of a Bio-District: Indicators for Waste Management. Br. Food J. 2024, 126, 290–308. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girão, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria Vesca Leaves through Inflammation, Proteasome and Autophagy Modulation. J. Ethnopharmacol. 2014, 158 Pt A, 113–122. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Akamova, A.V.; Nemyatykh, O.D.; Flisyuk, E.V.; Luzhanin, V.G.; Povydysh, M.N.; Mikhailova, I.V.; Pozharitskaya, O.N. Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharmacol. 2021, 12, 697411. [Google Scholar] [CrossRef]
- Vega, E.N.; García-Herrera, P.; Ciudad-Mulero, M.; Dias, M.I.; Matallana-González, M.C.; Cámara, M.; Tardío, J.; Molina, M.; Pinela, J.; Pires, T.C.S.P.; et al. Wild Sweet Cherry, Strawberry and Bilberry as Underestimated Sources of Natural Colorants and Bioactive Compounds with Functional Properties. Food Chem. 2023, 414, 135669. [Google Scholar] [CrossRef]
- Seke, F.; Adiamo, O.Q.; Sultanbawa, Y.; Sivakumar, D. In Vitro Antioxidant Activity, Bioaccessibility, and Thermal Stability of Encapsulated Strawberry Fruit (Fragaria × Ananassa) Polyphenols. Foods 2023, 12, 4045. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular Effects in Vitro of Aqueous Extract of Wild Strawberry (Fragaria vesca, L.) Leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Ziewiecki, R.; Capek, P.; Matulová, M.; Wilk, K.A. New Isolation Process for Bioactive Food Fiber from Wild Strawberry Leaf. Biochem. Eng. J. 2020, 161, 107639. [Google Scholar] [CrossRef]
- Pawlaczyk-Graja, I.; Balicki, S.; Wilk, K.A. Effect of Various Extraction Methods on the Structure of Polyphenolic-Polysaccharide Conjugates from Fragaria vesca L. Leaf. Int. J. Biol. Macromol. 2019, 130, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Gonçalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zofia, N.-Ł.; Martyna, Z.-D.; Bujak, T. Evaluation of cosmetic and dermatological properties of kombucha-fermented berry leaf extracts considered to be by-products. Molecules 2022, 27, 2345. [Google Scholar] [CrossRef]
- Coelho, S.C.; Laget, S.; Benaut, P.; Rocha, F.; Estevinho, B.N. A New Approach to the Production of Zein Microstructures with Vitamin B12, by Electrospinning and Spray Drying Techniques. Powder Technol. 2021, 392, 47–57. [Google Scholar] [CrossRef]
- Couto, A.F.; Favretto, M.; Paquis, R.; Estevinho, B.N. Co-Encapsulation of Epigallocatechin-3-Gallate and Vitamin B12 in Zein Microstructures by Electrospinning/ Electrospraying Technique. Molecules 2023, 28, 2544. [Google Scholar] [CrossRef]
- Castro Coelho, S.; Nogueiro Estevinho, B.; Rocha, F. Encapsulation in Food Industry with Emerging Electrohydrodynamic Techniques: Electrospinning and Electrospraying—A Review. Food Chem. 2021, 339, 127850. [Google Scholar] [CrossRef]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Electrohydrodynamic Processing for the Production of Zein-Based Microstructures and Nanostructures. Curr. Opin. Colloid Interface Sci. 2021, 56, 101504. [Google Scholar] [CrossRef]
- Mendes, A.C.; Chronakis, I.S. Electrohydrodynamic Encapsulation of Probiotics: A Review. Food Hydrocoll. 2021, 117, 106688. [Google Scholar] [CrossRef]
- Giteru, S.G.; Ali, M.A.; Oey, I. Recent Progress in Understanding Fundamental Interactions and Applications of Zein. Food Hydrocoll. 2021, 120, 106948. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, Proanthocyanidins, Flavones and Flavonols in Some Plant Materials and Their Antioxidant Activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Chaumun, M.; Goëlo, V.; Ribeiro, M.; Rocha, F.; Estevinho, B.N. In Vitro Evaluation of Microparticles with Laurus nobilis L. Extract Prepared by Spray-Drying for Application in Food and Pharmaceutical Products. Food Bioprod. Process. 2020, 122, 124–135. [Google Scholar] [CrossRef]
- Coelho, S.C.; Rocha, F.; Estevinho, B.N. Electrospinning of Microstructures Incorporated with Vitamin B9 for Food Application: Characteristics and Bioactivities. Polymers 2022, 14, 4337. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, B.N.; Rocha, F. Kinetic Models Applied to Soluble Vitamins Delivery Systems Prepared by Spray Drying. Dry. Technol. 2017, 35, 1249–1257. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the Conditions for Determination of Antioxidant Activity by Abts and Dpph Assays—A Practical Approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Benaut, P.; Laget, S.; Estevinho, B.N.; Rocha, F. Optimization of Electrospinning Parameters for the Production of Zein Microstructures for Food and Biomedical Applications. Micron 2022, 152, 103164. [Google Scholar] [CrossRef]
- Li, Y.; Lim, L.-T.; Kakuda, Y. Electrospun Zein Fibers as Carriers to Stabilize (−)-Epigallocatechin Gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Miri, M.A.; Najafi, M.; Soleimanifard, S.; Aran, M. Encapsulation of Rosemary Essential Oil in Zein by Electrospinning Technique. J. Food Sci. 2021, 86, 4070–4086. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N. Fundamentals of Electrospinning as a Novel Delivery Vehicle for Bioactive Compounds in Food Nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- do Evangelho, J.A.; Crizel, R.L.; Chaves, F.C.; Prietto, L.; Pinto, V.Z.; de Miranda, M.Z.; Dias, A.R.G.; da Rosa Zavareze, E. Thermal and Irradiation Resistance of Folic Acid Encapsulated in Zein Ultrafine Fibers or Nanocapsules Produced by Electrospinning and Electrospraying. Food Res. Int. 2019, 124, 137–146. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.P.; Dos, T.; Acunha, S.; De Oliveira, J.P.; Fonseca, L.M.; Tavares Da Silva, F.; Guimarães, V.M.; Da, E.; Zavareze, R. Electrospun Protein Fibers Loaded with Yerba Mate Extract for Bioactive Release in Food Packaging. J. Sci. Food Agric. 2020, 100, 3341–3350. [Google Scholar] [CrossRef]
- Padmaa Paarakh, M.; Ani Jose, P.; Setty, C.M.; Christoper, G.V.P. Release kinetics-concepts and applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Faki, R.; Gursoy, O.; Yilmaz, Y. Effect of Electrospinning Process on Total Antioxidant Activity of Electrospun Nanofibers Containing Grape Seed Extract. Open Chem. 2019, 17, 912–918. [Google Scholar] [CrossRef]
- Achilladelis, P.; Petsas, A.S.; Karantonis, H.C. Effect of Fortification of Tahini with Natural Plant Origin Raw Materials on Its Bioactivity. Appl. Sci. 2023, 13, 9626. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary Reference Intake (DRI) Value for Dietary Polyphenols: Are We Heading in the Right Direction? Br. J. Nutr. 2008, 99, 55–58. [Google Scholar] [CrossRef]
- García-Cortés, M.; Robles-Díaz, M.; Ortega-Alonso, A.; Medina-Caliz, I.; Andrade, R.J. Hepatotoxicity by Dietary Supplements: A Tabular Listing and Clinical Characteristics. Int. J. Mol. Sci. 2016, 17, 537. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]

| Biopolymer | Biopolymer Concentration (% w/v) | Core Material | Total Solid Content (% w/w) | Flow Rate (mL/h) | Sample Code |
|---|---|---|---|---|---|
| Zein | 10 | Quercetin | 1 | 1.0 | 10Z1Q |
| 5 | 1.0 | 10Z5Q | |||
| Extract | 1 | 1.0 | 10Z1E | ||
| 5 | 1.0 | 10Z5E | |||
| 20 | Quercetin | 1 | 2.0 | 20Z1Q | |
| 5 | 2.0 | 20Z5Q | |||
| Extract | 1 | 2.0 | 20Z1E | ||
| 5 | 2.0 | 20Z5E |
| Compound | Solvent | Equation | R2 | LOD (mg/mL) | LOQ (mg/mL) |
|---|---|---|---|---|---|
| Quercetin | Deionized Water | y = 59.67x + 0.005 | 0.998 | 0.000043 | 0.000144 |
| Active Compound | Zein (% w/v) | Particle Average Size (µm) | |
|---|---|---|---|
| 1% w/w | Extract | 10 | 0.22 ± 0.02 |
| 20 | 1.87 ± 0.13 | ||
| Quercetin | 10 | 0.11 ± 0.01 | |
| 20 | 1.00 ± 0.06 | ||
| 5% w/w | Extract | 10 | 0.16 ± 0.01 |
| 20 | 1.15 ± 0.07 | ||
| Quercetin | 10 | 0.19 ± 0.01 | |
| 20 | 1.37 ± 0.08 | ||
| Korsmeyer–Peppas | Weibull | |||||
|---|---|---|---|---|---|---|
| Sample | Kk (min−1) | n | R2 | Td (min) | β | R2 |
| 10Z1Q | * | |||||
| 10Z1EX | * | |||||
| 20Z1Q | 0.495 | 0.158 | 0.419 | 5.83 | 0.333 | 0.524 |
| 20Z1EX | 0.309 | 0.275 | 0.446 | 15.57 | 0.408 | 0.352 |
| 10Z5Q | 0.354 | 0.194 | 0.921 | 18.34 | 0.524 | 0.933 |
| 10Z5EX | * | |||||
| 20Z5Q | 0.151 | 0.354 | 0.985 | 63.32 | 0.486 | 0.963 |
| 20Z5EX | 0.346 | 0.372 | 0.283 | 0.82 | 0.219 | 0.557 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, A.F.; Estevinho, B.N. Valorization of Agricultural By-Products (Fragaria vesca) through the Production of Value-Added Micro/Nanostructures Using Electrohydrodynamic Techniques. Foods 2024, 13, 1162. https://doi.org/10.3390/foods13081162
Couto AF, Estevinho BN. Valorization of Agricultural By-Products (Fragaria vesca) through the Production of Value-Added Micro/Nanostructures Using Electrohydrodynamic Techniques. Foods. 2024; 13(8):1162. https://doi.org/10.3390/foods13081162
Chicago/Turabian StyleCouto, Ana Francisca, and Berta N. Estevinho. 2024. "Valorization of Agricultural By-Products (Fragaria vesca) through the Production of Value-Added Micro/Nanostructures Using Electrohydrodynamic Techniques" Foods 13, no. 8: 1162. https://doi.org/10.3390/foods13081162
APA StyleCouto, A. F., & Estevinho, B. N. (2024). Valorization of Agricultural By-Products (Fragaria vesca) through the Production of Value-Added Micro/Nanostructures Using Electrohydrodynamic Techniques. Foods, 13(8), 1162. https://doi.org/10.3390/foods13081162






