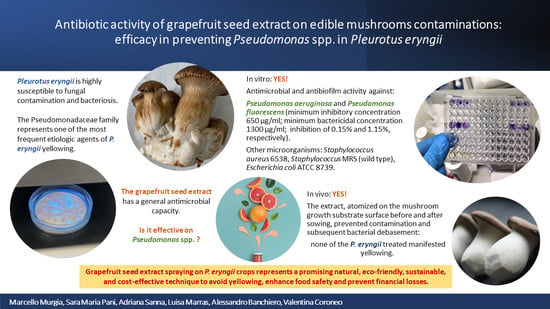
Antimicrobial Activity of Grapefruit Seed Extract on Edible Mushrooms Contaminations: Efficacy in Preventing Pseudomonas spp. in Pleurotus eryngii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapefruit Seed Extract
2.2. Culture Investigations
2.3. Antimicrobial Activity—Preliminary Assay
2.4. Inhibitory and Bactericidal Concentration–Broth Microdilution Susceptibility Test
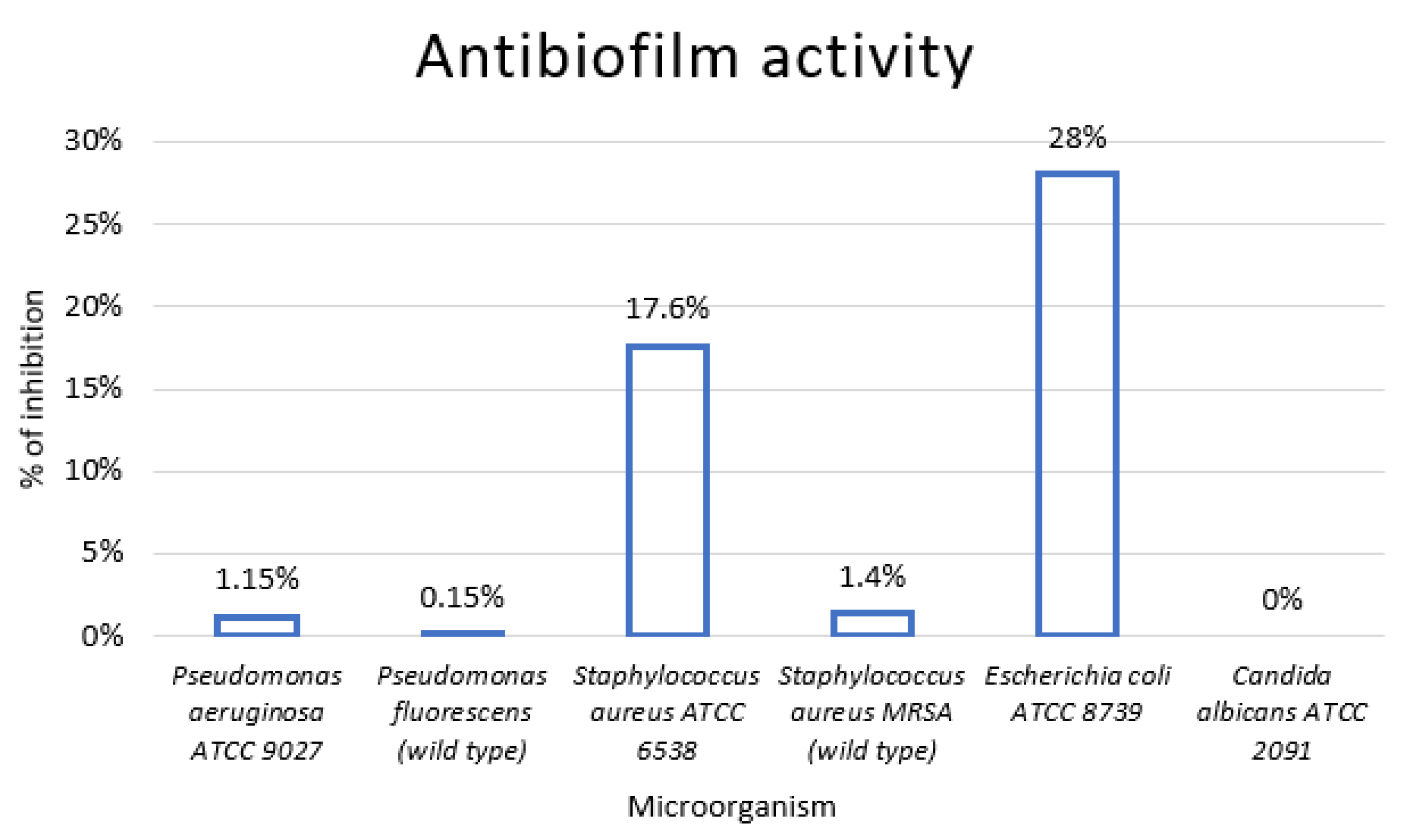
2.5. Antibiofilm Activity
2.6. Field Experiment
3. Results
3.1. Preliminary Inspections
3.2. GSE Analysis
3.3. In Vitro Analysis
3.4. In Vivo Analysis
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venturella, G.; Palazzolo, E.; Saiano, F.; Gargano, M.L. Notes on a New Productive Strain of King Oyster Mushroom, Pleurotus Eryngii (Higher Basidiomycetes), a Prized Italian Culinary-Medicinal Mushroom. Int. J. Med. Mushrooms 2015, 17, 199–206. [Google Scholar] [CrossRef]
- Carlavilla, J.R.; Manjón, J.L. The King Oyster Mushroom Pleurotus Eryngii Behaves as a Necrotrophic Pathogen of Eryngium Campestre. Ital. J. Mycol. 2023, 52, 22–31. [Google Scholar] [CrossRef]
- Lv, S.; Zhu, X.; Liu, Z.; Hu, L.; Xu, D.; Chitrakar, B.; Mo, H.; Li, H. Edible Pleurotus Eryngii Papery Food Prepared by Papermaking Process. Foods 2022, 11, 3514. [Google Scholar] [CrossRef] [PubMed]
- Teniou, S.; Bensegueni, A.; Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M.; Chovelon, B.; Bensouici, C.; Boumendjel, A.; Hininger-Favier, I. Biodriven Investigation of the Wild Edible Mushroom Pleurotus Eryngii Revealing Unique Properties as Functional Food. J. Funct. Foods 2022, 89, 104965. [Google Scholar] [CrossRef]
- Stajić, M.; Vukojević, J.; Duletić-Lauević, S. Biology of Pleurotus Eryngii and Role in Biotechnological Processes: A Review. Crit. Rev. Biotechnol. 2009, 29, 55–66. [Google Scholar] [CrossRef]
- Yu, A.; Ji, Y.; Ma, G.; Xu, J.; Hu, Q. Identification and Preparation of Selenium-Containing Peptides from Selenium-Enriched Pleurotus Eryngii and Their Protective Effect on Lead-Induced Oxidative Damage in NCTC1469 Hepatocytes. J. Sci. Food Agric. 2023, 103, 4522–4534. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Ma, N.; Zhao, L.; Zhao, E.; Gao, Z.; Wang, W.; Song, M.; Zhang, G.; Hu, Q.; Xiao, H. In Vitro and in Vivo Inhibitory Effects of a Pleurotus Eryngii Protein on Colon Cancer Cells. Food Funct. 2017, 8, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, Structure and Bioactivities of the Polysaccharides from Pleurotus Eryngii: A Review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yong, Y.; Xing, M.; Gu, Y.; Zhang, Z.; Zhang, S.; Lu, L. Characterization of Polysaccharides with Marked Inhibitory Effect on Lipid Accumulation in Pleurotus Eryngii. Carbohydr. Polym. 2013, 97, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Yoon, K.N.; Lee, J.S.; Cho, H.J.; Shim, M.J.; Lee, T.S. Dietary Effect of Pleurotus Eryngii on Biochemical Function and Histology in Hypercholesterolemic Rats. Saudi J. Biol. Sci. 2011, 18, 403–409. [Google Scholar] [CrossRef]
- Amerikanou, C.; Tagkouli, D.; Tsiaka, T.; Lantzouraki, D.Z.; Karavoltsos, S.; Sakellari, A.; Kleftaki, S.A.; Koutrotsios, G.; Giannou, V.; Zervakis, G.I.; et al. Pleurotus Eryngii Chips—Chemical Characterization and Nutritional Value of an Innovative Healthy Snack. Foods 2023, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Long, H.; Guo, Y.; Wang, S.; Chen, F.; Chen, X. Isolation, Structural Characterization, and Hypoglycemic Activities In Vitro of Polysaccharides from Pleurotus Eryngii. Molecules 2022, 27, 7140. [Google Scholar] [CrossRef] [PubMed]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial Mushrooms: Nutritional Quality and Effect of Cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Hess, J.M.; Jonnalagadda, S.S.; Slavin, J.L. What Is a Snack, Why Do We Snack, and How Can We Choose Better Snacks? A Review of the Definitions of Snacking, Motivations to Snack, Contributions to Dietary Intake, and Recommendations for Improvement. Adv. Nutr. 2016, 7, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.L.; Rana, G.L.; Sermani, S.; Scarola, L.; Cariddi, C. Control of Bacterial Yellowing of Cardoncello Mushroom Pleurotus Eryngii Using Acetic or Hydrochloric Acid Solutions. Crop Prot. 2013, 50, 24–29. [Google Scholar] [CrossRef]
- Gea, F.J.; Carrasco, J.; Suz, L.M.; Navarro, M.J. Characterization and Pathogenicity of Cladobotryum Mycophilum in Spanish Pleurotus Eryngii Mushroom Crops and Its Sensitivity to Fungicides. Eur. J. Plant Pathol. 2017, 147, 129–139. [Google Scholar] [CrossRef]
- Kim, M.K.; Seuk, S.W.; Lee, Y.H.; Kim, H.R.; Cho, K.M. Fungicide Sensitivity and Characterization of Cobweb Disease on a Pleurotus Eryngii Mushroom Crop Caused by Cladobotryum Mycophilum. Plant Pathol. J. 2014, 30, 82. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Diánez, F.; Gea, F.J. Incidence, Identification and Pathogenicity of Cladobotryum Mycophilum, Causal Agent of Cobweb Disease on Agaricus Bisporus Mushroom Crops in Spain. Ann. Appl. Biol. 2016, 168, 214–224. [Google Scholar] [CrossRef]
- Chen, J.T.; Huang, J.W. A Semiselective Medium for Detecting Gliocladium Roseum, the Causal Agent of King Oyster Mushroom Brown Spot. Plant Pathol. Bull. 2004, 13, 107–116. [Google Scholar]
- Kredics, L.; Kocsubé, S.; Nagy, L.; Komoń-Zelazowska, M.; Manczinger, L.; Sajben, E.; Nagy, A.; Vágvölgyi, C.; Kubicek, C.P.; Druzhinina, I.S.; et al. Molecular Identification of Trichoderma Species Associated with Pleurotus Ostreatus and Natural Substrates of the Oyster Mushroom. FEMS Microbiol. Lett. 2009, 300, 58–67. [Google Scholar] [CrossRef]
- González, A.J.; Gea, F.J.; Navarro, M.J.; Fernández, A.M. Identification and RAPD-Typing of Ewingella Americana on Cultivated Mushrooms in Castilla-La Mancha, Spain. Eur. J. Plant Pathol. 2012, 133, 517–522. [Google Scholar] [CrossRef]
- Reyes, J.E.; Venturini, M.E.; Oria, R.; Blanco, D. Prevalence of Ewingella Americana in Retail Fresh Cultivated Mushrooms (Agaricus Bisporus, Lentinula Edodes and Pleurotus Ostreatus) in Zaragoza (Spain). FEMS Microbiol. Ecol. 2004, 47, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.L.; De Corato, U.; Rana, G.L.; De Luca, P.; Pipoli, V.; Lops, R.; Scarola, L.; Mannerucci, F.; Piscitelli, L.; Cariddi, C. Suppressiveness of White Vinegar and Steam-Exploded Liquid Waste against the Causal Agents of Pleurotus Eryngii Yellowing. Crop Prot. 2015, 70, 61–69. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P. Pseudomonas “Reactans” a New Pathogen of Cultivated Mushrooms. In Pseudomonas Syringae and Related Pathogens; Springer: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Soler-Rivas, C.; Jolivet, S.; Arpin, N.; Olivier, J.M.; Wichers, H.J. Biochemical and Physiological Aspects of Brown Blotch Disease of Agaricus Bisporus. FEMS Microbiol. Rev. 1999, 23, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Bellettini, M.B.; Bellettini, S.; Fiorda, F.A.; Pedro, A.C.; Bach, F.; Fabela-Morón, M.F.; Hoffmann-Ribani, R. Diseases and Pests Noxious to Pleurotus Spp. Mushroom Crops. Rev. Argent. Microbiol. 2018, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Ryssel, H.; Kloeters, O.; Germann, G.; Schäfer, T.; Wiedemann, G.; Oehlbauer, M. The Antimicrobial Effect of Acetic Acid-An Alternative to Common Local Antiseptics? Burns 2009, 35, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Cvetnić, Z.; Vladimir-Knežević, S. Antimicrobial Activity of Grapefruit Seed and Pulp Ethanolic Extract. Acta Pharm. 2004, 54, 243–250. [Google Scholar]
- Kim, T.; Kim, J.H.; Oh, S.W. Grapefruit Seed Extract as a Natural Food Antimicrobial: A Review. Food Bioprocess Technol. 2021, 14, 626–633. [Google Scholar] [CrossRef]
- Yun, D.; Liu, J. Recent Advances on the Development of Food Packaging Films Based on Citrus Processing Wastes: A Review. J. Agric. Food Res. 2022, 9, 100316. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and Extraction Optimization of Citrus Seeds for Food and Functional Food Applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef]
- Reagor, L.; Gusman, J.; McCoy, L.; Carino, E.; Heggers, J.P. The Effectiveness of Processed Grapefruit-Seed Extract as an Antibacterial Agent: I. An in Vitro Agar Assay. J. Altern. Complement. Med. 2002, 8, 325–332. [Google Scholar] [CrossRef]
- Heggers, J.P.; Cottingham, J.; Gusman, J.; Reagor, L.; McCoy, L.; Carino, E.; Cox, R.; Zhao, J.G. The Effectiveness of Processed Grapefruit-Seed Extract as an Antibacterial Agent: II. Mechanism of Action and in Vitro Toxicity. J. Altern. Complement. Med. 2002, 8, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Barawi, S.; Hamzah, H.; Hamasalih, R.; Mohammed, A.; Abdalrahman, B.; Abdalaziz, S. Antibacterial Mode of Action of Grapefruit Seed Extract against Local Isolates of Beta-Lactamases-Resistant Klebsiella Pneumoniae and Its Potential Application. Int. J. Agric. Biol. 2021, 26, 499–508. [Google Scholar] [CrossRef]
- Çiçek Polat, D.; Eryilmaz, M.; Akalin, K.; Coşkun, M. Antimicrobial Activity of Grapefruit Seed. Hacettepe Univ. J. Fac. Pharm. 2018, 38, 1–3. [Google Scholar]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial Activity of Naringin Derivatives against Pathogenic Strains. J. Appl. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef]
- Zeng, X.; Zheng, Y.; He, Y.; Zhang, J.; Peng, W.; Su, W. Microbial Metabolism of Naringin and the Impact on Antioxidant Capacity. Nutrients 2022, 14, 3765. [Google Scholar] [CrossRef]
- Bugianesi, R.; Catasta, G.; Spigno, P.; D’Uva, A.; Maiani, G. Naringenin from Cooked Tomato Paste Is Bioavailable in Men. J. Nutr. 2002, 132, 3349–3352. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Jourdan, P.S.; McIntosh, C.A.; Mansell, R.L. Naringin Levels in Citrus Tissues: II. Quantitative Distribution of Naringin in Citrus Paradisi MacFad. Plant Physiol. 1985, 77, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic Potential of Naringin: An Overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Krzeminski, R.; Gralak, M.; Delgado-Licon, E.; Ayala, A.L.M.; Katrich, E.; Trakhtenberg, S. Changes in Plasma Lipid and Antioxidant Activity in Rats as a Result of Naringin and Red Grapefruit Supplementation. J. Agric. Food Chem. 2005, 53, 3223–3228. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef] [PubMed]
- Raja Kumar, S.; Mohd Ramli, E.S.; Abdul Nasir, N.A.; Ismail, N.H.M.; Mohd Fahami, N.A. Preventive Effect of Naringin on Metabolic Syndrome and Its Mechanism of Action: A Systematic Review. Evid. Based Complement. Altern. Med. 2019, 2019, 9752826. [Google Scholar] [CrossRef] [PubMed]
- Termkwancharoen, C.; Malakul, W.; Phetrungnapha, A.; Tunsophon, S. Naringin Ameliorates Skeletal Muscle Atrophy and Improves Insulin Resistance in High-Fat-Diet-Induced Insulin Resistance in Obese Rats. Nutrients 2022, 14, 4120. [Google Scholar] [CrossRef]
- Mir, I.A.; Tiku, A.B. Chemopreventive and Therapeutic Potential of “Naringenin,” a Flavanone Present in Citrus Fruits. Nutr. Cancer 2015, 67, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Massaro, L.; Raguzzini, A.; Aiello, P.; Valencia, D.V. The Potential Role of Naringin and Naringenin as Nutraceuticals Against Metabolic Syndrome. Endocr. Metab. Immune Disord. Drug Targets 2022, 23, 428–445. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, N.; Woodard, K.; Ramaraju, R.; Greenway, F.L.; Coulter, A.A.; Rebello, C.J. Naringenin Increases Insulin Sensitivity and Metabolic Rate: A Case Study. J. Med. Food 2020, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Staubach, P.; Tamai, I.; Langguth, P. High-Dose Short-Term Administration of Naringin Did Not Alter Talinolol Pharmacokinetics in Humans. Eur. J. Pharm. Sci. 2015, 68, 36–42. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, A.; Monga, V.; Bhatia, R. Compendium of Naringenin: Potential Sources, Analytical Aspects, Chemistry, Nutraceutical Potentials and Pharmacological Profile. Crit. Rev. Food Sci. Nutr. 2022, 63, 8868–8899. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Zhang, W.; Biswas, D.; Ramakrishnan, R.; Rhim, J.W. Grapefruit Seed Extract-Added Functional Films and Coating for Active Packaging Applications: A Review. Molecules 2023, 28, 730. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Standard Organization: Geneva, Switzerland, 2018.
- ISO 7218:2007/Amd 1:2013; Microbiology of Food and Animal Feeding Stuffs General Requirements and Guidance for Microbiological Examinations Amendment 1. International Standard Organization: Geneva, Switzerland, 2014.
- ISO 16266:2006; Water Quality Detection and Enumeration of Pseudomonas Aeruginosa Method by Membrane Filtration. International Standard Organization: Geneva, Switzerland, 2008.
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical Composition, Plant Genetic Differences, Antimicrobial and Antifungal Activity Investigation of the Essential Oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef] [PubMed]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of Biofilm in Microtiter Plates: Overview of Testing Conditions and Practical Recommendations for Assessment of Biofilm Production by Staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Osdaghi, E.; Martins, S.J.; Ramos-Sepulveda, L.; Vieira, F.R.; Pecchia, J.A.; Beyer, D.M.; Bell, T.H.; Yang, Y.; Hockett, K.L.; Bull, C.T. 100 Years since Tolaas: Bacterial Blotch of Mushrooms in the 21st Century. Plant Dis. 2019, 103, 2714–2732. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, Y.; Xie, J.; Zhao, S.; Qin, W.; Song, Q.; Wang, S.; Rong, C. Bacterial Infection Induces Ultrastructural and Transcriptional Changes in the King Oyster Mushroom (Pleurotus Eryngii). Microbiol. Spectr. 2022, 10, e01445-22. [Google Scholar] [CrossRef]
- Sajben, E.; Manczinger, L.; Nagy, A.; Kredics, L.; Vágvölgyi, C. Characterization of Pseudomonads Isolated from Decaying Sporocarps of Oyster Mushroom. Microbiol. Res. 2011, 166, 255–267. [Google Scholar] [CrossRef]
- Scales, B.S.; Dickson, R.P.; Lipuma, J.J.; Huffnagle, G.B. Microbiology, Genomics, and Clinical Significance of the Pseudomonas Fluorescens Species Complex, an Unappreciated Colonizer of Humans. Clin. Microbiol. Rev. 2014, 27, 927–948. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Jeong, K.S.; Cha, J.S. PCR Assays for Specific and Sensitive Detection of Pseudomonas Tolaasii, the Cause of Brown Blotch Disease of Mushrooms. Lett. Appl. Microbiol. 2002, 35, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.E.; Reyes, J.E.; Rivera, C.S.; Oria, R.; Blanco, D. Microbiological Quality and Safety of Fresh Cultivated and Wild Mushrooms Commercialized in Spain. Food Microbiol. 2011, 28, 1492–1498. [Google Scholar] [CrossRef]
- Takeoka, G.; Dao, L.; Wong, R.Y.; Lundin, R.; Mahoney, N. Identification of Benzethonium Chloride in Commercial Grapefruit Seed Extracts. J. Agric. Food Chem. 2001, 49, 3316–3320. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Dao, L.T.; Wong, R.Y.; Harden, L.A. Identification of Benzalkonium Chloride in Commercial Grapefruit Seed Extracts. J. Agric. Food Chem. 2005, 53, 7630–7636. [Google Scholar] [CrossRef]
- Han, H.W.; Kwak, J.H.; Jang, T.S.; Knowles, J.C.; Kim, H.W.; Lee, H.H.; Lee, J.H. Grapefruit Seed Extract as a Natural Derived Antibacterial Substance against Multidrug-Resistant Bacteria. Antibiotics 2021, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, Y.R.; Ha, Y.M.; Seo, H.J.; Kim, Y.H.; Park, S.M.; Sohn, J.H. Antibacterial Effect of Grapefruit Seed Extract (GSE) on Makgeolli-Brewing Microorganisms and Its Application in the Preservation of Fresh Makgeolli. J. Food Sci. 2014, 79, M1159–M1167. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Lim, P.N.; Tong, S.Y.; Thian, E.S. Development of Grapefruit Seed Extract-Loaded Poly(ε-Caprolactone)/Chitosan Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2019, 22, 100396. [Google Scholar] [CrossRef]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Anti-Biofilm Activity of Grapefruit Seed Extract against Staphylococcus Aureus and Escherichia Coli. J. Microbiol. Biotechnol. 2019, 29, 1177–1183. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Ioannou, I.; M’hiri, N.; Chaaban, H.; Boudhrioua, N.M.; Ghoul, M. Effect of the Process, Temperature, Light and Oxygen on Naringin Extraction and the Evolution of Its Antioxidant Activity. Int. J. Food Sci. Technol. 2018, 53, 2754–2760. [Google Scholar] [CrossRef]


| Flavonoids | RT (min) | Formula | m/z Experimental | m/z Theoretical | Δ (ppm) | Major Fragmentaion | mg/L |
|---|---|---|---|---|---|---|---|
| Rutin | 6.49 | C27H30O16 | 611.1606 | 611.1607 | −0.16 | 303.0496 | 99.03 |
| Naringin | 9.75 | C27H32O14 | 581.1864 | 581.1865 | −0.17 | 273.0752 | 46.57 |
| Hesperidin | 9.25 | C28H34O15 | 611.1967 | 611.1970 | −0.49 | 303.0857 | 45.76 |
| Neohesperidin | 16.11 | C28H34O15 | 611.1972 | 611.1970 | 0.33 | 303.0874 | 168.29 |
| Naringenin | 19.23 | C15H12O5 | 273.0758 | 273.0757 | 0.36 | 287.0904 | 3515.05 |
| RT | Compounds | % | Calculated Kováts Retention Indexes | Theoretical Kováts Retention Indexes |
|---|---|---|---|---|
| 18.944 | Lactic Acid, 2TMS derivative | 14.69208 | 1055 | 1057 |
| 21.066 | Diacetin, TMS | 3.489502 | 1105 | NA |
| 22.808 | Glycerol, 3TMS derivative | 56.27466 | 1279 | 1282 |
| 26.651 | Diacetin, TMS | 5.599487 | 1105 | NA |
| 26.952 | 1,2,3-Butanetriol-3TMS | 10.27547 | 1285 | 1286 |
| 27.853 | Butane, 1,2,3-tris(trimethylsiloxy)-TMS | 0.131453 | 1285 | 1285 |
| 28.013 | Monocaproin, 2TMS | 0.13052 | 1886 | 1886 |
| 28.613 | Diglycerol, 4TMS derivative | 0.3968 | 1902 | NA |
| 30.696 | Ascorbic acid, 4TMS derivative | 5.920048 | 1968 | 1971 |
| 31.777 | 9-Octadecenenitrile | 0.23135 | 2315 | NA |
| 33.338 | Citric acid, 4TMS derivative | 1.752973 | 2618 | 2622 |
| 34.64 | Oleamide, TMS derivative | 1.105656 | 2763 | 2765 |
| Target Microorganisms | MIC | MBC |
|---|---|---|
| Staphylococcus aureus ATCC 6538 | 162,5 µg/mL | 650 µg/mL |
| Staphylococcus aureus MRSA wild type | 325 µg/mL | 325 µg/mL |
| Pseudomonas aeruginosa ATCC 9027 Pseudomonas fluorescens wild type | 650 µg/mL | 1300 µg/mL |
| Escherichia coli 8739 | 650 µg/mL | 1300 µg/mL |
| Candida albicans ATCC 2091 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, M.; Pani, S.M.; Sanna, A.; Marras, L.; Manis, C.; Banchiero, A.; Coroneo, V. Antimicrobial Activity of Grapefruit Seed Extract on Edible Mushrooms Contaminations: Efficacy in Preventing Pseudomonas spp. in Pleurotus eryngii. Foods 2024, 13, 1161. https://doi.org/10.3390/foods13081161
Murgia M, Pani SM, Sanna A, Marras L, Manis C, Banchiero A, Coroneo V. Antimicrobial Activity of Grapefruit Seed Extract on Edible Mushrooms Contaminations: Efficacy in Preventing Pseudomonas spp. in Pleurotus eryngii. Foods. 2024; 13(8):1161. https://doi.org/10.3390/foods13081161
Chicago/Turabian StyleMurgia, Marcello, Sara Maria Pani, Adriana Sanna, Luisa Marras, Cristina Manis, Alessandro Banchiero, and Valentina Coroneo. 2024. "Antimicrobial Activity of Grapefruit Seed Extract on Edible Mushrooms Contaminations: Efficacy in Preventing Pseudomonas spp. in Pleurotus eryngii" Foods 13, no. 8: 1161. https://doi.org/10.3390/foods13081161