The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts
Abstract
:1. Introduction
2. Compositional Significance of Cruciferous Vegetable Waste and Byproducts
2.1. Varieties of Cruciferous Vegetable Waste and Byproducts
2.2. Nutritional and Phytochemical Composition
2.2.1. Nutritional Profile
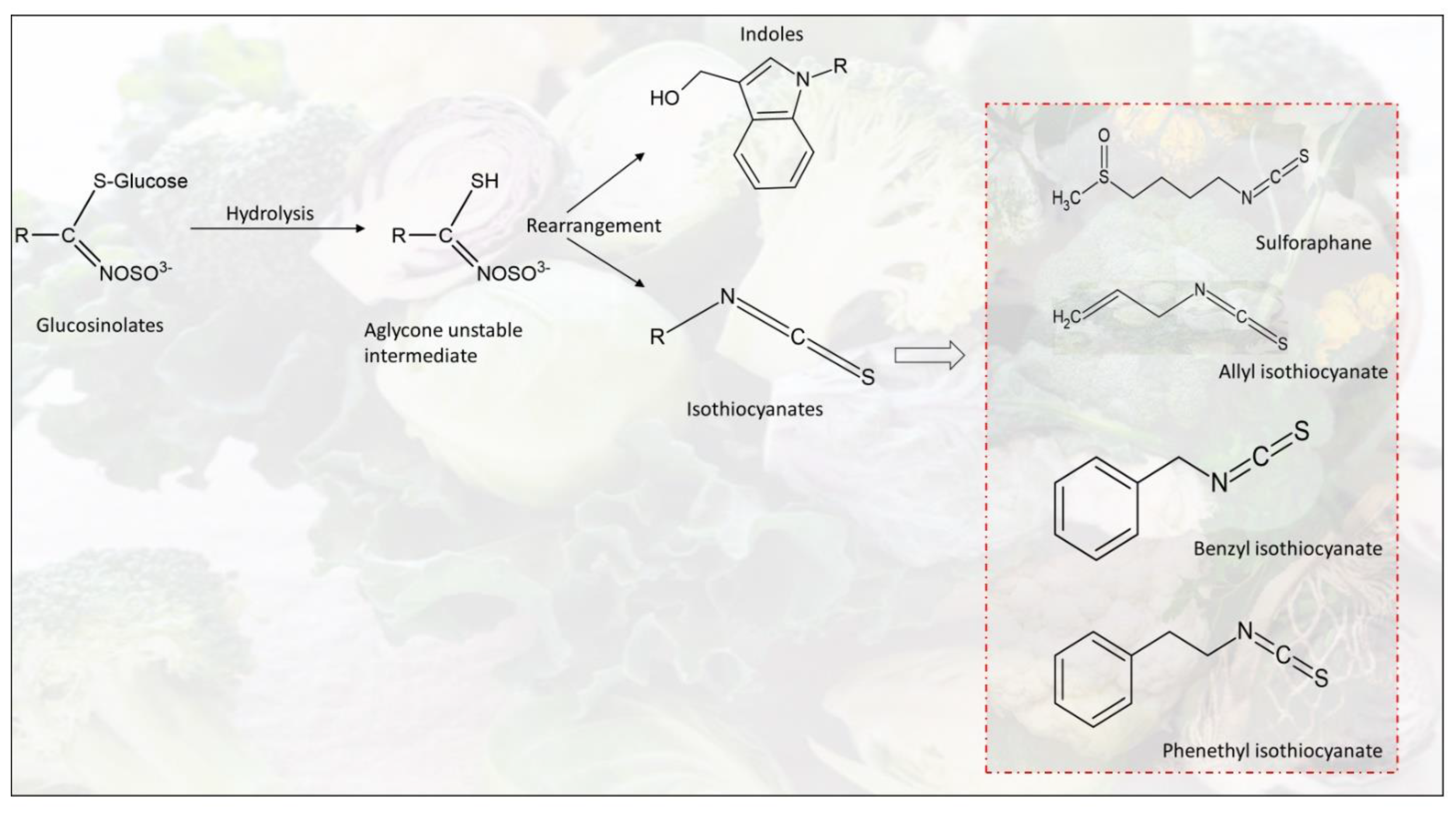
2.2.2. Phytochemical Composition
| Source | Characterization | Significant Compounds | Reference |
|---|---|---|---|
| Cauliflower stem and leaves | Ultrasound-assisted extraction (UAE) | Isothiocyanates | [28] |
| Cauliflower byproducts | High-resolution Q-TOF-LC–MS/MS | ACE inhibitory peptide | [29] |
| Green and red cabbage | High-performance liquid chromatography–mass spectrometry (HPLC-MS) and HPLC | 6 aliphatic glucosinolates (glucoiberin, glucoiberverin, sinigrin, gluconapin, glucoraphanin, and pro-goitrin), three indolyl glucosinolates (glucobrassicin, neoglucobrassicin, and 4-methoxy glucobrassicin), and one aromatic glucosinolates (gluconasturtiin) | [24] |
| Cauliflower byproducts (outer leaves) | Ultra(high)-pressure liquid chromatography–electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry | 19 flavonoid glycosides (8 non-acylated and 11 acylated), major aglycones: kaempferol and quercetin, major phenolic acids: sinapic acid and ferulic acid | [30] |
| Industrial broccoli discards | Ultra-high performance liquid chromatography–MS/MS (UPLC MS/MS) | Aliphatic and indolic glucosinolates, flavonoids (kaempherol-3-o-sophoroside, quercetin-3-diglucoside-7-glucoside), hydroxycinnamic acids | [13] |
| Fermented broccoli stalk byproduct | Liquid Chromatography–Electrospray Ionization-Triple Quadrupole Mass Spectrometry (LC-ESI-QqQ-MS/MS) | Glucoerucin, indolic glucosinolates (glucobrassicin, 4-methoxy-glucobrassicin, and 4-hidroxy-glucobrassicin), phenolic acids, and flavonoids (sinapic acid, 4-O-feruloyl quinic acid, and quercetin-3-O-diglucoside) | [31] |
2.3. Significant Characteristics of Cruciferous Byproducts
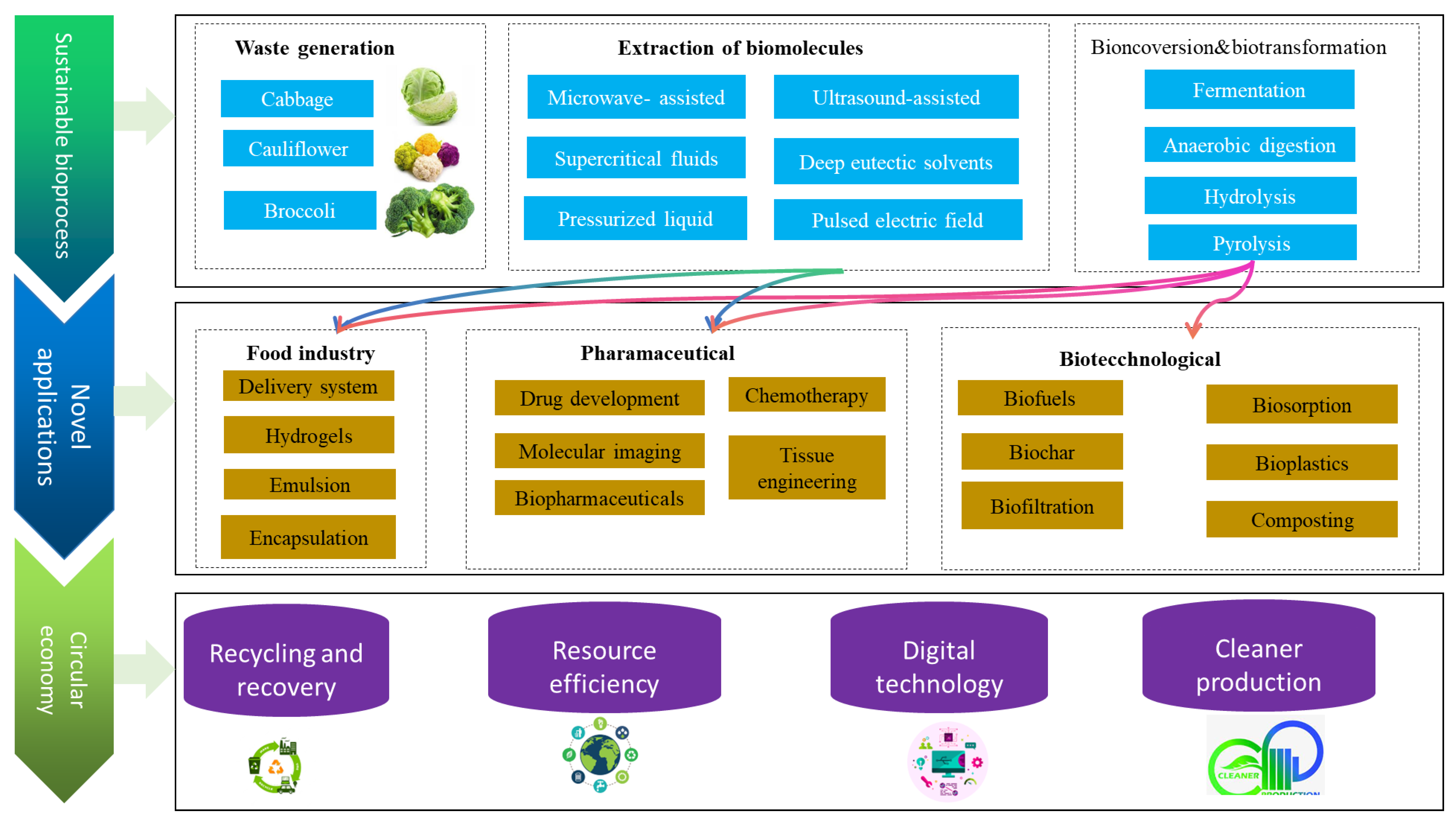
3. Important Technological Aspects in Byproduct Valorization
3.1. Influence of Processing Conditions to Be Used in Potential Applications
3.2. Technologies Involved in Waste Utilization
| Source | Technology Used | Treatment Conditions | Pre-Treatment | Important Facts | Research Output | Reference |
|---|---|---|---|---|---|---|
| Cabbage byproducts (leaves) | Biotransformation via lactic acid bacteria fermentation (Lactiplantibacillus, Lacticaseibacillus) | Mixture of 180 g cabbage leaf, 40 g rice straw, and 40 g corn flour; 5 mL of Lactiplantibacillus plantarum 3.3 × 108 CFU/mL vacuum-packed and stored at 30 °C for 3, 7, 15, and 30 days | NA | Preserved dry matter (DM) content, decreased pH, accumulation of acetic acid, butyric acid, and ammonia positively improved microflora in final silage | Cabbage byproducts as an added value silage DM 283.4 g/kg on day 30; lactic acid 52.1 g/kg DM on day 15 | [21] |
| Cabbage and cauliflower waste (CCW) (1:1 w/w) | Anaerobic co-digestion (mesophilic) | 3 main treatment steps: start-up, inoculum acclimatisation, and treatment of the waste mixture; 65 days | CCW mixture was subjected to mechanical treatment to reduce size and stored at 4 °C until experiment | Maximum 60% biodegradability, 37 g nitrogen/kg dry weight in the residual mixture | 250 mL/g VS of methane yield (VS: volatile solids) | [55] |
| Vegetable waste (80% broccoli, 5% cabbage, and other) | Fermentation (Fed-batch bioreactor) | Vegetable hydrolylate total sugar 300 g/L, 10 g/L yeast extract pH 6.3, dissolved oxygen 5%, 150–250 rpm | Macerated broccolisuspension in water (50 g/L) was treated with H2SO4 (0.5%, 1%, 3%, and 7% v/v) at 121 °C for 30 min | The most efficient strain was identified as Enterobacter ludwigii FMCC 204 | 17.6 g/L of 2,3-butanediol yield | [65] |
| Broccoli stalks (BS), cauliflower cores (CC) | Pyrolysis and carbonization | Heating rate of 10 °C/min; pyrolysis/carbonization temperatures of 500; 600, 700, and 800 °C, dwell time of 2 and 4 h; N2 and CO2 flows of 100 mL/min | Dried biomass particle size of 0.67 mm | Preparation of adsorbents from vegetable waste for heavy metal removal;adsorption capacity of adsorbent was effective for the removal of Cd2+, Zn2+, Ni2+, and Cu2+ from solutions; adsorption capacities were higher for adsorbents obtained from BS | Yields of obtained adsorbents from biomass pyrolysis and carbonization ranged from BS 25 to 29% and CC 26 to 30% | [66] |
| Vegetable waste | Thermostatic anaerobic digestion followed by thermostatic aerobic digestion | Anaerobic digestion: 37 °C, 30 days; aerobic digestion: 30 °C, 48 h | NA | Novel fast-treated recycling approach for vegetables, abundance of N, P, K, and no heavy metals in the treated waste | 96% COD removal efficiency; 81.75% of Germination Index in treated biogas slurry | [54] |
| Cauliflower outer leaves (CL) | Fungal fermentation(filamentous fungi: Aspergillus niger, A. oryzae, Aspergillus sojae, Rhizopus oryzae, Rhizopus azygosporus, and Phanerochaete chrysoporium) | Initial inoculum 106 spores/mL, mix 120 g of CL with 2 mL of inoculum in 20 mL sterilized water, fermentation at 30 °C, 7 days | NA | Fermentation facilitates releasing phenolic compounds, fermentation with Aspergillus sojae showed the highest level of total phenolic compounds (TPC), and kaempferol-3-O-diglucoside was dominant; fungal fermentation results in a shift in phenolic profile to a profile with less or no carbohydrate moieties at the 3-or 7-carbon position | TPC in Aspergillus sojae fermented samples after 1 day: 321 mg rutin equivalents (RE)/100 g fresh weight (FW)), kaempferol-3-O-diglucoside: 38–126 mg RE/100 g FW | [67] |
| Broccoli stems | Fermentation with Lactiplantibacillus plantarum | 200 g of blanched samples, 2 mL of 8.6 log CFU/mL inoculum, fermentation at 37 °C for 96 h | Disruption to a maximum particle size of 5 or 10 mm followed by hot water blanching; 72 °C for 1 min | Fermentation facilitates faster drying rates and enhances phenol and flavonoid retention. | Total Phenol Content: 3.7 mg GAE/gdm. Total Flavonoid Content: 1.2 mg QE/gdm | [68] |
| Broccoli stalks | Solid–liquid extraction | Total fiber (TF) extraction with 80% ethanol; insoluble fiber extraction (IF) with water at a ratio of 1/2.5 (m/v), 70 °C, 30 min | Freeze drying | IF possessed a good prebiotic effect | TF and IF yields: 67% and 70% | [69] |
| Waste Chinese cabbage (CW) | Two-phase anaerobic digestion | Phase 1: 1 month, 0.2–0.7 kg VS·d−1 of CW slurry, 37 °C, 40% fresh pig manure, 20% sludge water; Methanococcus and Methanosarcina were main methanogens in anaerobic digester Phase 2: >2 months; daily feed rate of 3–11 kg CW from reactor 1 | NA | In phase 2, every feed rate was used for a week, and the feed was increased steadily; higher biogas and methane productivity without nitrogen substrate and bicarbonate; fast gas production with stable operation | 0.62 m3·(kg VS)−1 of biogas; 65–68% methane | [70] |
| Cauliflower waste | Bacteria-assisted solid–liquid extraction | Rapid solid–liquid dynamic extraction: 60 extraction cycles, with each cycle having a 3 min static phase, followed by a 2 min dynamic phase; 5 h | Enzymatic pre-treatment with Bacillus subtilis culture | Bacillus subtilis exhibits enhanced production of the essential xylanases and cellulases enzymes for breaking down plant cell walls | 0.245 mg/g of polyphenol content, 0.006 g/g of isothiocyanates, 2.711 g/g of chlorogenic acid, 3.071 g/g of epigallocatechin gallate | [71] |
4. Potential Applications of Cabbage, Broccoli, and Cauliflower Byproducts
4.1. Food Industry Applications
4.1.1. Antimicrobial Properties
4.1.2. Cruciferous Component-Based Functional Delivery Systems
4.1.3. Other Functional Properties
4.2. Medicinal Applications
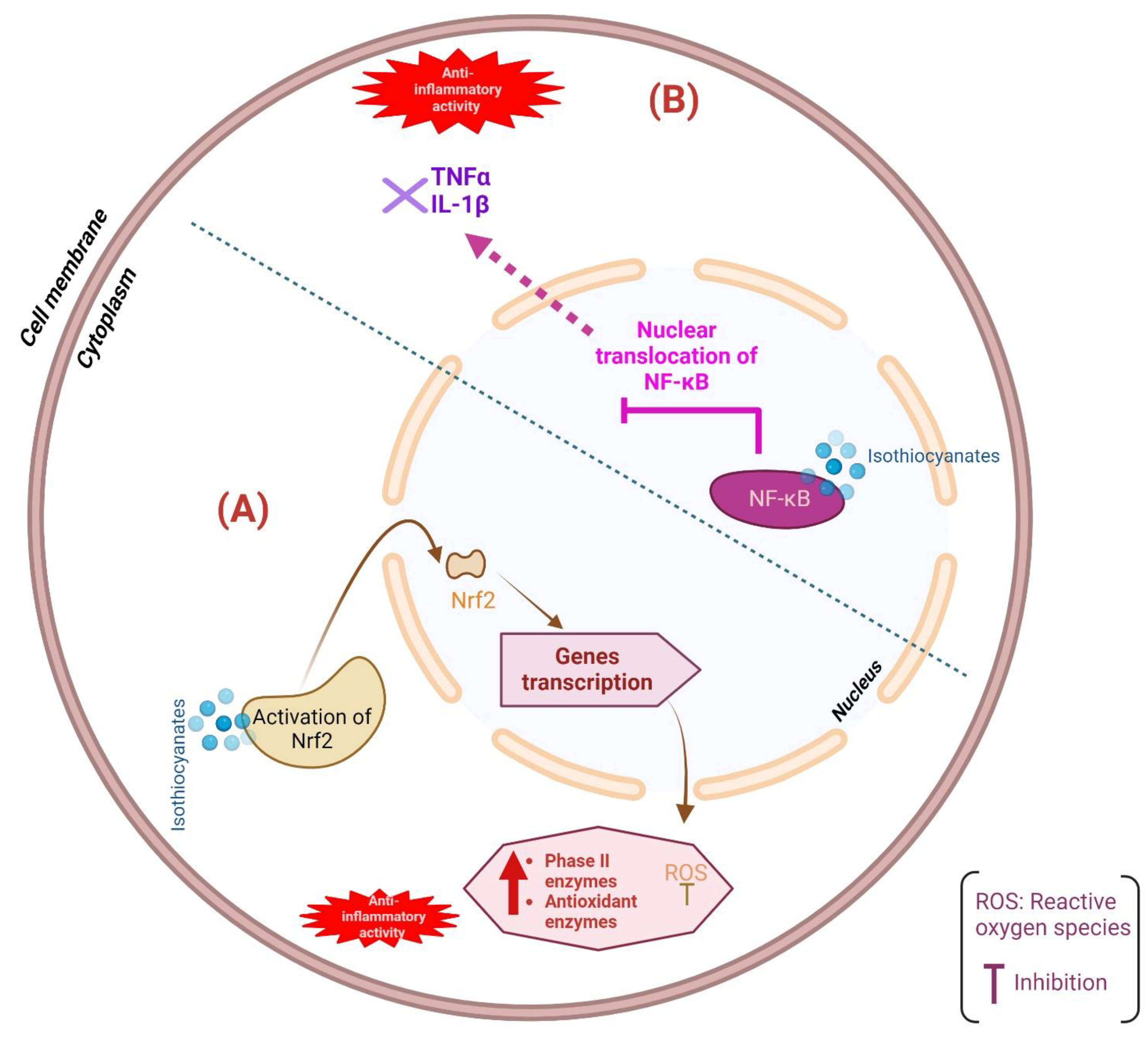
4.2.1. Anti-Inflammatory Properties
4.2.2. Anticancer Properties
4.2.3. Other Medicinally Beneficial Properties
4.3. Other Applications
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Read, Q.D.; Brown, S.; Cuéllar, A.D.; Finn, S.M.; Gephart, J.A.; Marston, L.T.; Meyer, E.; Weitz, K.A.; Muth, M.K. Assessing the environmental impacts of halving food loss and waste along the food supply chain. Sci. Total Environ. 2020, 712, 136255. [Google Scholar] [CrossRef] [PubMed]
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing agroindustry by-products for obtaining value-added products and reducing environmental impact. J. Chem. 2022, 2022, 3656932. [Google Scholar] [CrossRef]
- Belaud, J.-P.; Prioux, N.; Vialle, C.; Sablayrolles, C. Big data for agri-food 4.0: Application to sustainability management for by-products supply chain. Comput. Ind. 2019, 111, 41–50. [Google Scholar] [CrossRef]
- Komesu, A.; da Silva Martins, L.H.; Pandey, P.; Kuila, A.; Penteado, C.F.A.; Penteado, E.D.; de Oliveira, J.A.R. Fruit and Vegetable Waste an Economic Alternate to Costlier Raw Materials for Value Added Products. In Waste Management; CRC Press: Boca Raton, FL, USA, 2022; pp. 60–82. [Google Scholar]
- Manchali, S.; Chidambara Murthy, K.N.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Murillo, G.; Mehta, R.G. Cruciferous Vegetables and Cancer Prevention. Nutr. Cancer 2001, 41, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, S.; Singh, B. Antioxidant enzymes in cabbage: Variability and inheritance of superoxide dismutase, peroxidase and catalase. Sci. Hortic. 2010, 124, 9–13. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem. 2004, 88, 503–509. [Google Scholar] [CrossRef]
- Mago, M.; Gupta, R.; Yadav, A.; Garg, V.K. Sustainable treatment and nutrient recovery from leafy waste through vermicomposting. Bioresour. Technol. 2022, 347, 126390. [Google Scholar] [CrossRef] [PubMed]
- Son, A.-R.; Kim, S.-H.; Valencia, R.A.; Jeong, C.-D.; Islam, M.; Yang, C.-J.; Lee, S.-S. Kimchi cabbage (Brassica rapa L.) by-products treated with calcium oxide and alkaline hydrogen peroxide as feed ingredient for Holstein steers. J. Anim. Sci. Technol. 2021, 63, 841. [Google Scholar] [CrossRef]
- Petkowicz, C.L.; Williams, P.-A. Pectins from food waste: Characterization and functional properties of a pectin extracted from broccoli stalk. Food Hydrocoll. 2020, 107, 105930. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Wang, L.; Xu, L. Extraction optimization, antioxidant, and hypoglycemic activities in vitro of polysaccharides from broccoli byproducts. J. Food Biochem. 2017, 41, e12387. [Google Scholar] [CrossRef]
- Thomas, M.; Badr, A.; Desjardins, Y.; Gosselin, A.; Angers, P. Characterization of industrial broccoli discards (Brassica oleracea var. italica) for their glucosinolate, polyphenol and flavonoid contents using UPLC MS/MS and spectrophotometric methods. Food Chem. 2018, 245, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Bao, T.; Zheng, X.; Chen, W.; Wang, J. A recyclable protein resource derived from cauliflower by-products: Potential biological activities of protein hydrolysates. Food Chem. 2017, 221, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Chaisamlitpol, S.; Hiranvarachat, B.; Srichumpoung, J.; Devahastin, S.; Chiewchan, N. Bioactive compositions of extracts from cabbage outer leaves as affected by drying pretreatment prior to microwave-assisted extraction. Sep. Purif. Technol. 2014, 136, 177–183. [Google Scholar] [CrossRef]
- Moreb, N.; Murphy, A.; Jaiswal, S.; Jaiswal, A.K. Cabbage. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 33–54. [Google Scholar]
- Li, H.; Xia, Y.; Liu, H.-Y.; Guo, H.; He, X.-Q.; Liu, Y.; Wu, D.-T.; Mai, Y.-H.; Li, H.-B.; Zou, L. Nutritional values, beneficial effects, and food applications of broccoli (Brassica oleracea var. italica Plenck). Trends Food Sci. Technol. 2022, 119, 288–308. [Google Scholar] [CrossRef]
- Schäfer, J.; Stanojlovic, L.; Trierweiler, B.; Bunzel, M. Storage related changes of cell wall based dietary fiber components of broccoli (Brassica oleracea var. italica) stems. Food Res. Int. 2017, 93, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Ekenci, D.; Yılmaz, B.; Capasso, R.; Ozer, D. Cruciferous Vegetables and Their Bioactive Metabolites: From Prevention to Novel Therapies of Colorectal Cancer. Evid.-Based Complement. Altern. Med. 2022, 2022, 1534083. [Google Scholar]
- Du, G.; Zhang, G.; Shi, J.; Zhang, J.; Ma, Z.; Liu, X.; Yuan, C.; Li, X.; Zhang, B. Keystone taxa Lactiplantibacillus and Lacticaseibacillus directly improve the ensiling performance and microflora profile in co-ensiling cabbage byproduct and rice straw. Microorganisms 2021, 9, 1099. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pest infestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef]
- Favela-González, K.M.; Hernández-Almanza, A.Y.; De la Fuente-Salcido, N.M. The value of bioactive compounds of cruciferous vegetables (Brassica) as antimicrobials and antioxidants: A review. J. Food Biochem. 2020, 44, e13414. [Google Scholar] [CrossRef]
- Choi, S.-H.; Park, S.; Lim, Y.P.; Kim, S.-J.; Park, J.-T.; An, G. Metabolite Profiles of Glucosinolates in Cabbage Varieties (Brassica oleracea var. capitata) by Season, Color, and Tissue Position Introduction. Environ. Biotechnol. 2014, 55, 237–247. [Google Scholar] [CrossRef]
- Llorach, R.; Gil-Izquierdo, A.; Ferreres, F.; Tomás-Barberán, F.A. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. var. botrytis) agroindustrial byproducts. J. Agric. Food Chem. 2003, 51, 3895–3899. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Rößle, C.; Brunton, N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009, 116, 202–207. [Google Scholar] [CrossRef]
- Zanoni, F.; Primiterra, M.; Angeli, N.; Zoccatelli, G. Microencapsulation by spray-drying of polyphenols extracted from red chicory and red cabbage: Effects on stability and color properties. Food Chem. 2020, 307, 125535. [Google Scholar] [CrossRef] [PubMed]
- Amofa-Diatuo, T.; Anang, D.M.; Barba, F.J.; Tiwari, B.K. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compos. Anal. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, T.; Han, W.; Chen, W.; Zheng, X.; Wang, J. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from cauliflower by-products protein hydrolysate. Process Biochem. 2016, 51, 1299–1305. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Raes, K.; Coelus, S.; Struijs, K.; Smagghe, G.; Van Camp, J. Ultra(high)-pressure liquid chromatography-electrospray ionization-time-of-flight-ion mobility-high definition mass spectrometry for the rapid identification and structural characterization of flavonoid glycosides from cauliflower waste. J. Chromatogr. A 2014, 1323, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Salas-Millán, J.-Á.; Aznar, A.; Conesa, E.; Conesa-Bueno, A.; Aguayo, E. Functional food obtained from fermentation of broccoli by-products (stalk): Metagenomics profile and glucosinolate and phenolic compounds characterization by LC-ESI-QqQ-MS/MS. LWT 2022, 169, 113915. [Google Scholar] [CrossRef]
- Shi, M.; Ying, D.Y.; Ye, J.H.; Sanguansri, L.; Augustin, M.A. Broccoli byproducts for protection and co-delivery of EGCG and tuna oil. Food Chem. 2020, 326, 126963. [Google Scholar] [CrossRef]
- Cruz, A.B.; Pitz, H.d.S.; Veber, B.; Bini, L.A.; Maraschin, M.; Zeni, A.L.B. Assessment of bioactive metabolites and hypolipidemic effect of polyphenolic-rich red cabbage extract. Pharm. Biol. 2016, 54, 3033–3039. [Google Scholar] [CrossRef]
- Gudiño, I.; Martín, A.; Casquete, R.; Prieto, M.H.; Ayuso, M.C.; Córdoba, M.d.G. Evaluation of Broccoli (Brassica oleracea VAR. Italica) Crop By-Products as Sources of Bioactive Compounds. SSRN Electron. J. 2022, 304, 111284. [Google Scholar] [CrossRef]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Use of postharvest UV-B and UV-C radiation treatments to revalorize broccoli byproducts and edible florets. Innov. Food Sci. Emerg. Technol. 2017, 43, 77–83. [Google Scholar] [CrossRef]
- Kowalski, A.; Agati, G.; Grzegorzewska, M.; Kosson, R.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Tuccio, L.; Grifoni, D.; Vågen, I.M.; et al. Valorization of waste cabbage leaves by postharvest photochemical treatments monitored with a non-destructive fluorescence-based sensor. J. Photochem. Photobiol. B Biol. 2021, 222, 112263. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. UV-B radiation as abiotic elicitor to enhance phytochemicals and development of red cabbage sprouts. Horticulturae 2021, 7, 567. [Google Scholar] [CrossRef]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Effect of processing on antioxidants and their activity in dietary fiber powder from cabbage outer leaves. Dry. Technol. 2010, 28, 1063–1071. [Google Scholar] [CrossRef]
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009, 113, 964–969. [Google Scholar] [CrossRef]
- Mrkìc, V.; Cocci, E.; Rosa, M.D.; Sacchetti, G. Effect of drying conditions on bioactive compounds and antioxidant activity of broccoli (Brassica oleracea L.). J. Sci. Food Agric. 2006, 86, 1559–1566. [Google Scholar] [CrossRef]
- Abdul, P.M.; Jahim, J.M.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd Nor, M.T. Effects of changes in chemical and structural characteristic of ammonia fibre expansion (AFEX) pretreated oil palm empty fruit bunch fibre on enzymatic saccharification and fermentability for biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Kim, S.-H.; Pandey, A. Microbial strategies for bio-transforming food waste into resources. Bioresour. Technol. 2020, 299, 122580. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Martínez-Zamora, L.; Cano-Lamadrid, M.; Hashemi, S.; Castillejo, N. Genus Brassica By-Products Revalorization with Green Technologies to Fortify Innovative Foods: A Scoping Review. Foods 2023, 12, 561. [Google Scholar] [CrossRef]
- González, F.; Quintero, J.; Del Río, R.; Mahn, A. Optimization of an extraction process to obtain a food-grade sulforaphane-rich extract from broccoli (Brassica oleracea var. italica). Molecules 2021, 26, 4042. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez García, S.L.; Raghavan, V. Microwave-assisted extraction of phenolic compounds from broccoli (Brassica oleracea) stems, leaves, and florets: Optimization, characterization, and comparison with maceration extraction. Recent Prog. Nutr. 2022, 2, 11. [Google Scholar]
- Maity, M.; Bhattacharyya, D.K.; Bhowal, J. Improvement of β-galactosidase production by solid-state fermentation using cauliflower (Brassica oleraceae var. botrytis) waste by Enterobacter aerogenes KCTC2190. Res. J. Biotechnol. 2023, 18, 18–23. [Google Scholar] [CrossRef]
- Das, A.; Ghosh, U. Solid-state fermentation of waste cabbage by Penicillium notatum NCIM NO-923 for production and characterization of cellulases. J. Sci. Ind. Res. 2009, 68, 714–718. [Google Scholar]
- Zdziobek, P.; Jodłowski, G.S.; Strzelec, E.A. Biopreservation and Bioactivation Juice from Waste Broccoli with Lactiplantibacillus plantarum. Molecules 2023, 28, 4594. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Naha, A.; Bhattacharyya, D.; Bhowal, J. Effective delignification and decrystallization of cauliflower wastes by using dilute phosphoric acid for efficient enzymatic digestibility to produce fermentable sugars. Biomass Bioenergy 2019, 125, 169–179. [Google Scholar] [CrossRef]
- Beniche, I.; Hungría, J.; El Bari, H.; Siles, J.; Chica, A.; Martín, M. Effects of C/N ratio on anaerobic co-digestion of cabbage, cauliflower, and restaurant food waste. Biomass Convers. Biorefinery 2021, 11, 2133–2145. [Google Scholar] [CrossRef]
- Kafle, G.K.; Bhattarai, S.; Kim, S.H.; Chen, L. Effect of feed to microbe ratios on anaerobic digestion of Chinese cabbage waste under mesophilic and thermophilic conditions: Biogas potential and kinetic study. J. Environ. Manag. 2014, 133, 293–301. [Google Scholar] [CrossRef]
- Pramanik, S.K. Anaerobic co-digestion of municipal organic solid waste: Achievements and perspective. Bioresour. Technol. Rep. 2022, 20, 101284. [Google Scholar] [CrossRef]
- Li, J.; Wan, D.; Jin, S.; Ren, H.; Wang, Y.; Huang, J.; Li, H.; Zhang, G. Fast treatment and recycling method of large-scale vegetable wastes. Sci. Total Environ. 2023, 892, 164308. [Google Scholar] [CrossRef] [PubMed]
- Beniche, I.; El Bari, H.; Siles, J.A.; Chica, A.F.; Martín, M.Á. Methane production by anaerobic co-digestion of mixed agricultural waste: Cabbage and cauliflower. Environ. Technol. 2021, 42, 4550–4558. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Lapa, N.; Moldão, M.; Duarte, E. Opportunities and challenges in the anaerobic co-digestion of municipal sewage sludge and fruit and vegetable wastes: A review. Energy Nexus 2023, 10, 100202. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Andrew Lin, K.Y.; Hong, E.; Kwon, E.E.; Lee, J. The valorization of food waste via pyrolysis. J. Clean. Prod. 2020, 259, 120816. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Li, J.; Yan, B.; Chen, G. Pyrolysis of food waste and food waste solid digestate: A comparative investigation. Bioresour. Technol. 2022, 354, 127191. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.H.; Mubashir, T.; Schulze, M.; Irfan, R.M. Thermochemical conversion of cabbage waste to bioenergy and bio-chemicals production. Int. J. Energy Res. 2022, 46, 20206–20215. [Google Scholar] [CrossRef]
- Pradhan, S.; Abdelaal, A.H.; Mroue, K.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Biochar from vegetable wastes: Agro-environmental characterization. Biochar 2020, 2, 439–453. [Google Scholar] [CrossRef]
- Pradhan, S.; Shahbaz, M.; Abdelaal, A.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Optimization of process and properties of biochar from cabbage waste by response surface methodology. Biomass Convers. Biorefinery 2020, 12, 5479–5491. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Sarsaiya, S.; Awasthi, M.K.; Liu, T.; Zhao, J.; Kumar, S.; Zhang, Z. Changes in global trends in food waste composting: Research challenges and opportunities. Bioresour. Technol. 2020, 299, 122555. [Google Scholar] [CrossRef]
- Mahmoud, Y. Using Broccoli Plant Wastes in Sheep Rations. Egypt. J. Nutr. Feed. 2016, 19, 277–287. [Google Scholar] [CrossRef]
- Abul-Fadl, M.M. Nutritional and chemical evaluation of white cauliflower by-products flour and the effect of its addition on beef sausage quality. J. Appl. Sci. Res. 2012, 8, 693–704. [Google Scholar]
- Liakou, V.; Pateraki, C.; Palaiogeorgou, A.M.; Kopsahelis, N.; Machado de Castro, A.; Guimarães Freire, D.M.; Nychas, G.J.E.; Papanikolaou, S.; Koutinas, A. Valorisation of fruit and vegetable waste from open markets for the production of 2,3-butanediol. Food Bioprod. Process. 2018, 108, 27–36. [Google Scholar] [CrossRef]
- Landin-Sandoval, V.J.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Reynel-Avila, H.E.; Gonzalez-Ponce, H.A. Valorization of agri-food industry wastes to prepare adsorbents for heavy metal removal from water. J. Environ. Chem. Eng. 2020, 8, 104067. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Extraction and bioconversion of kaempferol metabolites from cauliflower outer leaves through fungal fermentation. Biochem. Eng. J. 2016, 116, 27–33. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Impact of Fermentation Pretreatment on Drying Behaviour and Antioxidant Attributes of Broccoli Waste Powdered Ingredients. Foods 2023, 12, 3526. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Gómez, V.; González-Barrio, R.; Baenas, N.; Moreno, D.A.; Periago, M.J. Dietary-fibre-rich fractions isolated from broccoli stalks as a potential functional ingredient with phenolic compounds and glucosinolates. Int. J. Mol. Sci. 2022, 23, 13309. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shao, L.; Wang, Y.; Kou, W.; Cao, Y.; Zhang, D. Biogas by two-stage microbial anaerobic and semi-continuous digestion of Chinese cabbage waste. Chin. J. Chem. Eng. 2015, 23, 847–852. [Google Scholar] [CrossRef]
- Doria, E.; Buonocore, D.; Marra, A.; Bontà, V.; Gazzola, A.; Dossena, M.; Verri, M.; Calvio, C. Bacterial-Assisted Extraction of Bioactive Compounds from Cauliflower. Plants 2022, 11, 816. [Google Scholar] [CrossRef]
- Angiolillo, L.; Spinelli, S.; Conte, A.; Del Nobile, M.A. Extract from broccoli byproducts to increase fresh filled pasta shelf life. Foods 2019, 8, 621. [Google Scholar] [CrossRef]
- Thery, T.; Lynch, K.M.; Zannini, E.; Arendt, E.K. Isolation, characterisation and application of a new antifungal protein from broccoli seeds—New food preservative with great potential. Food Control 2020, 117, 107356. [Google Scholar] [CrossRef]
- Le, T.N.; Sakulsataporn, N.; Chiu, C.-H.; Hsieh, P.-C. Polyphenolic profile and varied bioactivities of processed Taiwanese grown broccoli: A comparative study of edible and non-edible parts. Pharmaceuticals 2020, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Cano, R.D.; Salcedo-Hernández, R.; Casados-Vázquez, L.E.; Wrobel, K.; Bideshi, D.K.; Barboza-Corona, J.E. Class I defensins (BraDef) from broccoli (Brassica oleracea var. italica) seeds and their antimicrobial activity. World J. Microbiol. Biotechnol. 2020, 36, 30. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT-Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Valverde, J.; Kehoe, K.; Reilly, K.; Rai, D.K.; Barry-Ryan, C. Development of a Novel Functional Soup Rich in Bioactive Sulforaphane Using Broccoli (Brassica oleracea L. ssp. italica) Florets and Byproducts. Food Bioprocess Technol. 2014, 7, 1310–1321. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Composition and antioxidant capacity of a novel beverage produced with green tea and minimally-processed byproducts of broccoli. Innov. Food Sci. Emerg. Technol. 2011, 12, 361–368. [Google Scholar] [CrossRef]
- Beukema, M.; Faas, M.M.; de Vos, P. The effects of different dietary fiber pectin structures on the gastrointestinal immune barrier: Impact via gut microbiota and direct effects on immune cells. Exp. Mol. Med. 2020, 52, 1364–1376. [Google Scholar] [CrossRef]
- Park, S.Y.; Yoon, K.Y. Enzymatic production of soluble dietary fiber from the cellulose fraction of Chinese cabbage waste and potential use as a functional food source. Food Sci. Biotechnol. 2015, 24, 529–535. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef]
- saleh, s. Quality Attributes of Shamy Bread Supplemented with Cauliflower Wastes. Egypt. J. Food Sci. 2022, 50, 73–82. [Google Scholar] [CrossRef]
- Nartea, A.; Fanesi, B.; Pacetti, D.; Lenti, L.; Fiorini, D.; Lucci, P.; Frega, N.G.; Falcone, P.M. Cauliflower by-products as functional ingredient in bakery foods: Fortification of pizza with glucosinolates, carotenoids and phytosterols. Curr. Res. Food Sci. 2023, 6, 100437. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.; Siddiqui, M.W.; Dávila-Aviña, J.; González-Aguilar, G. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Patras, A. Stability and colour evaluation of red cabbage waste hydroethanolic extract in presence of different food additives or ingredients. Food Chem. 2019, 275, 539–548. [Google Scholar] [CrossRef]
- Cicio, A.; Serio, R.; Zizzo, M.G. Anti-inflammatory potential of Brassicaceae-derived phytochemicals: In vitro and in vivo evidence for a putative role in the prevention and treatment of IBD. Nutrients 2022, 15, 31. [Google Scholar] [CrossRef]
- Nisar, A.; Jagtap, S.; Deshpande, M.; Harsulkar, A.; Ranjekar, P.; Prakash, O. Phytochemicals in the treatment of inflammation-associated diseases: The journey from preclinical trials to clinical practice. Front. Pharmacol. 2023, 14, 1177050. [Google Scholar] [CrossRef]
- Sturm, C.; Wagner, A.E. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Du, H.; Liu, D.; Ma, Z. The role of natural products in chronic inflammation. Front. Pharmacol. 2022, 13, 901538. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, S.-J.; Park, S.-J.; Eom, S.-H.; Gu, G.-J.; Kim, S.H.; Youn, H.-S. Phenethyl isothiocyanate regulates inflammation through suppression of the TRIF-dependent signaling pathway of Toll-like receptors. Life Sci. 2013, 92, 793–798. [Google Scholar] [CrossRef]
- Ernst, I.M.; Wagner, A.E.; Schuemann, C.; Storm, N.; Höppner, W.; Döring, F.; Stocker, A.; Rimbach, G. Allyl-, butyl- and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 2011, 63, 233–240. [Google Scholar] [CrossRef]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Seth, S.; Bhattacharyya, D.K.; Bhowal, J. Evaluation of Therapeutic Properties of Lignins Extracted from Cauliflower Wastes for Their Potent Valorization Through Sustainable Approach. Waste Biomass Valorization 2021, 12, 3849–3873. [Google Scholar] [CrossRef]
- Mandrich, L.; Caputo, E. Brassicaceae-derived anticancer agents: Towards a green approach to beat cancer. Nutrients 2020, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Orouji, N.; Asl, S.K.; Taghipour, Z.; Habtemariam, S.; Nabavi, S.M.; Rahimi, R. Glucosinolates in cancer prevention and treatment: Experimental and clinical evidence. Med. Oncol. 2023, 40, 344. [Google Scholar] [CrossRef] [PubMed]
- Satomi, S.; Takahashi, S.; Yoshida, K.; Shimizu, S.; Inoue, T.; Takara, T.; Suganuma, H. Effects of broccoli sprout supplements enriched in glucoraphanin on liver functions in healthy middle-aged adults with high-normal serum hepatic biomarkers: A randomized controlled trial. Front. Nutr. 2022, 9, 1077271. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Bao, J.; Lu, Y.; Lu, X.; Tian, P.; Zhang, X.; Yang, J.; Shi, X.; Pu, Z.; Li, S. Glucoraphanin and sulforaphane biosynthesis by melatonin mediating nitric oxide in hairy roots of broccoli (Brassica oleracea L. var. italica Planch): Insights from transcriptome data. BMC Plant Biol. 2022, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Vázquez, M.A.; García-Padilla, S.; García-Almendárez, B.E.; Whitaker, J.R.; Regalado, C. Broccoli processing wastes as a source of peroxidase. J. Agric. Food Chem. 2007, 55, 10396–10404. [Google Scholar] [CrossRef]
- Song, Y.; Nguyen, Q.A.; Wi, S.G.; Yang, J.; Bae, H.J. Strategy for dual production of bioethanol and D-psicose as value-added products from cruciferous vegetable residue. Bioresour. Technol. 2017, 223, 34–39. [Google Scholar] [CrossRef]
- Kim, H.M.; Park, J.H.; Choi, I.S.; Wi, S.G.; Ha, S.; Chun, H.H.; Hwang, I.M.; Chang, J.Y.; Choi, H.-J.; Kim, J.-C.; et al. Effective approach to organic acid production from agricultural kimchi cabbage waste and its potential application. PLoS ONE 2018, 13, e0207801. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Predescu, A.M.; Țurcanu, A.A.; Vidu, R.; Predescu, C.; Bobirica, C.; Bobirica, L.; Orbeci, C. Valorization of Agri-Food Wastes as Sustainable Eco-Materials for Wastewater Treatment: Current State and New Perspectives. Materials 2021, 14, 4581. [Google Scholar] [CrossRef] [PubMed]
| Cruciferous Vegetable | Dry Matter (DM) | Carbohydrates | Protein | Lipids | Fiber | Minerals | Reference |
|---|---|---|---|---|---|---|---|
| Broccoli (g/100 g, raw) | - | 6.27 | 2.57 | 0.34 | 2.4 | K, Ca, P: 0.303, 0.046, 0.067 | [20] |
| Cauliflower (g/100 g, raw) | - | 4.97 | 1.92 | 0.28 | 2 | K, Ca, P: 0.299, 0.022, 0.44 | |
| Cabbage (g/100 g, raw) | - | 5.8 | 1.28 | 0.1 | 2.5 | K, Ca, P: 0.170, 0.040, 0.020 | |
| Cabbage leaf (g/100 g DM) | 5.94 g/100 g | 43.94 | 22 | 42.5 | 23.36 | - | [21] |
| Chinese cabbage (g/100 g fresh weight) | - | 2.20 | 1.50 | 0.20 | 1.00 | K, Ca, Fe: 25.1, 10.5, 85.0 | [5] |
| Kale (g/100 g fresh weight) | - | 10.0 | 3.28 | 0.74 | 1.94 | K, Ca, Fe: 44.6, 13.5, 160.0 | |
| Arugula (g/100 g fresh weight) | - | 3.65 | 2.58 | 0.66 | 1.60 | K, Ca: 37.0, 16.0 | |
| Brussels sprouts (g/100 g fresh weight) | - | 8.67 | 2.55 | 0.51 | 26.94 | K, Ca, Fe: 38.9, 4.2, 140.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinali, T.S.; Zhang, Y.; Altaf, M.; Nsabiyeze, A.; Han, Z.; Shi, S.; Shang, N. The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods 2024, 13, 1163. https://doi.org/10.3390/foods13081163
Shinali TS, Zhang Y, Altaf M, Nsabiyeze A, Han Z, Shi S, Shang N. The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods. 2024; 13(8):1163. https://doi.org/10.3390/foods13081163
Chicago/Turabian StyleShinali, Tharushi S., Yiying Zhang, Moater Altaf, Assa Nsabiyeze, Zixin Han, Shuyuan Shi, and Nan Shang. 2024. "The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts" Foods 13, no. 8: 1163. https://doi.org/10.3390/foods13081163
APA StyleShinali, T. S., Zhang, Y., Altaf, M., Nsabiyeze, A., Han, Z., Shi, S., & Shang, N. (2024). The Valorization of Wastes and Byproducts from Cruciferous Vegetables: A Review on the Potential Utilization of Cabbage, Cauliflower, and Broccoli Byproducts. Foods, 13(8), 1163. https://doi.org/10.3390/foods13081163





