Assessing Seasonal Effects on Identification of Cultivation Methods of Short–Growth Cycle Brassica chinensis L. Using IRMS and NIRS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Stable Isotope Analysis
2.3. NIRS Analysis
2.4. Statistical Analysis and Chemometrics Methods
3. Results and Discussion
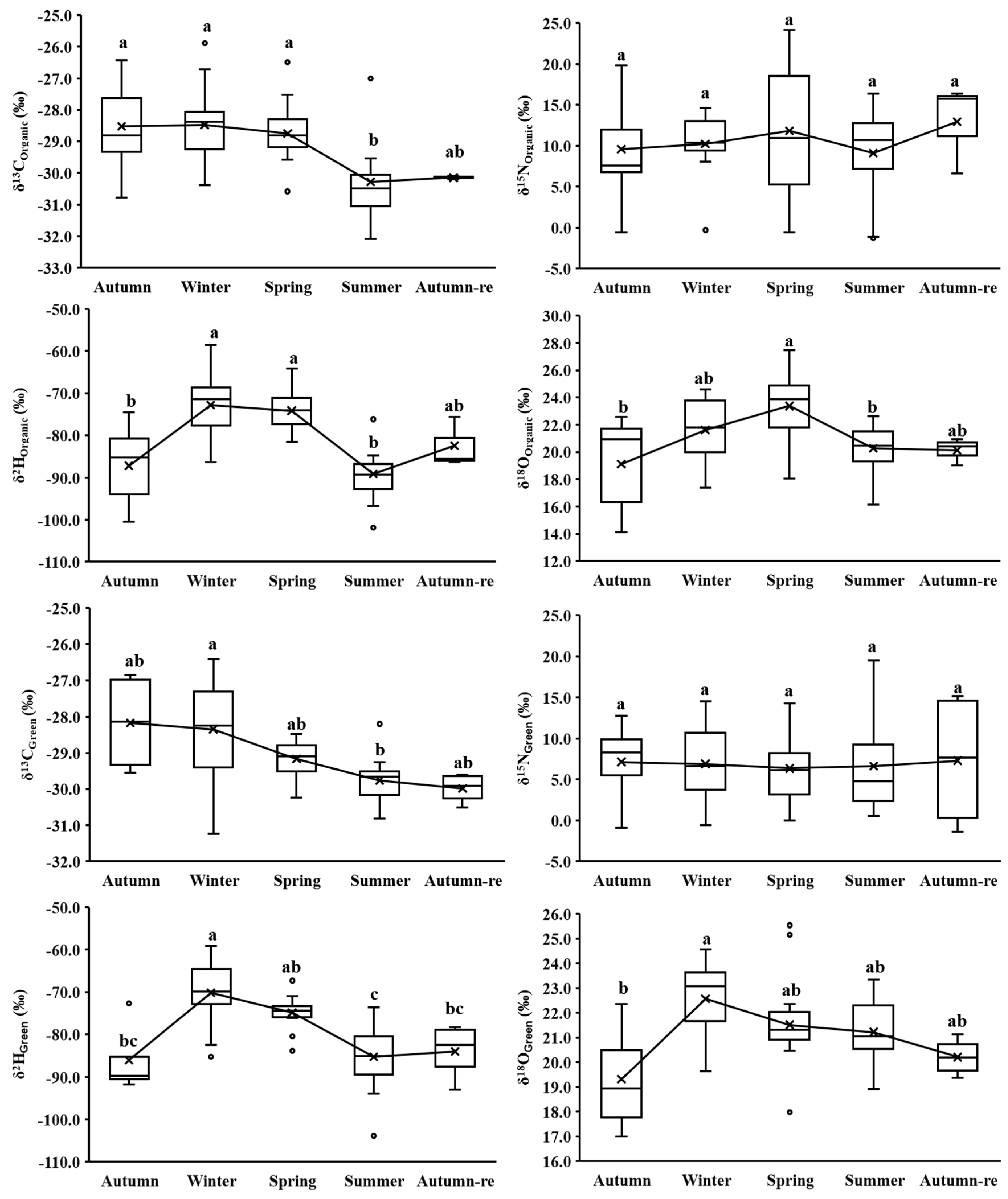
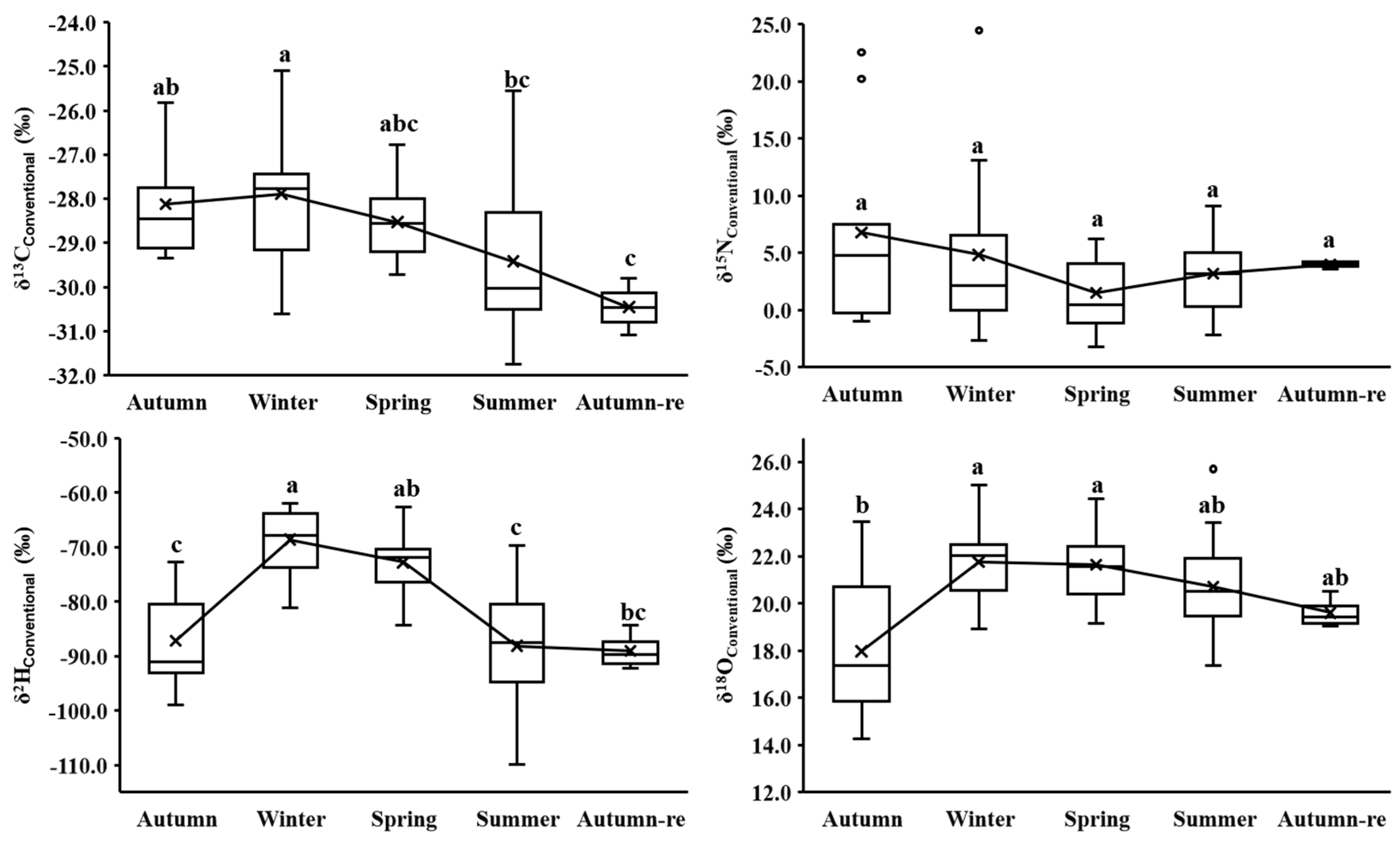
3.1. Overall and Seasonal Isotopes of Different BC Cultivation Methods
3.2. Seasonal Isotopes for Each BC Cultivation Method
3.3. PLS-DA Isotope Models to Identify BC Cultivation Methods
3.4. NIR Spectra to Identify BC Cultivation Methods
3.5. Combined IRMS and NIRS to Identify BC Cultivation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trávníček, J.; Schlatter, B.; Willer, H. The World of Organic Agriculture 2024: Summary. In The World of Organic Agriculture. Statistics and Emerging Trends 2024; Willer, H., Trávníček, J., Schlatter, S., Eds.; Druckerei Hachenburg PMS GmbH: Hachenburg, Germany, 2024; p. 30. [Google Scholar]
- Central People’s Government of the People’s Republic of China: Outline of the Fourteenth Five-Year Plan for National Economic and Social Development of the People’s Republic of China and the Long-Range Objectives Through the Year 2035. Available online: https://www.gov.cn/xinwen/2021-03/13/content_5592681.htm?eqid=e414542f0006c8bd00000003646dff6b (accessed on 13 March 2021).
- China National Standards for General Principles for Green Product Assessment (GB/T 33761-2017). Available online: http://down.foodmate.net/standard/sort/3/55288.html (accessed on 12 March 2017).
- China Agriculture Industry Standard for Green Food-Environmental Quality for Production Area (NY/T 391-2021). Available online: http://down.foodmate.net/standard/sort/5/101955.html (accessed on 1 November 2021).
- Spink, J.; Moyer, D.C.; Park, H.; Wu, Y.; Fersht, V.; Shao, B.; Hong, M.; Paek, S.Y.; Edelev, D. Introducing Food Fraud including translation and interpretation to Russian, Korean, and Chinese languages. Food Chem. 2015, 189, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Giannioti, Z.; Ogrinc, N.; Suman, M.; Camin, F.; Bontempo, L. Isotope ratio mass spectrometry methods for distinguishing organic from conventional food products: A review. TrAC Trend. Anal. Chem. 2024, 170, 117476. [Google Scholar] [CrossRef]
- de Andrade, J.C.; Galvan, D.; Effting, L.; Lelis, C.; Melquiades, F.L.; Bona, E.; Conte-Junior, C.A. An Easy-to-Use and Cheap Analytical Approach Based on NIR and Chemometrics for Tomato and Sweet Pepper Authentication by Non-volatile Profile. Food Anal. Methods 2023, 16, 567–580. [Google Scholar]
- Ruan, S.; Luo, H.; Wu, F.; He, L.; Lai, R.; Tang, X. Organic cultivation induced regulation in yield formation, grain quality attributes, and volatile organic compounds of fragrant rice. Food Chem. 2023, 405, 134845. [Google Scholar] [CrossRef]
- Kelly, S.; Mihailova, A. Organic food authenticity. In Food Forensics: Stable Isotopes as a Guide to Authenticity and Origin; Carter, J.F., Chesson, L.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 273–302. [Google Scholar]
- Liu, Z.; Yuan, Y.; Xie, T.; Zhang, Y.; Shao, S.; Nie, J.; Xia, W.; Rogers, K.M.; Zhang, W. Long-term agricultural effects on the authentication accuracy of organic, green, and conventional rice using isotopic and elemental chemometric analyses. J. Agric. Food Chem. 2020, 68, 1213–1225. [Google Scholar] [PubMed]
- Yuan, Y.; Hu, G.; Zhao, M.; Chen, T.; Zhang, Y.; Zhu, J.; Wang, Q. Variations in the natural 15N abundance of Brassica chinensis grown in uncultivated soil affected by different nitrogen fertilizers. J. Agric. Food Chem. 2014, 62, 11386–11392. [Google Scholar]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Sim, J.; McGoverin, C.; Oey, I.; Frew, R.; Kebede, B. Near-infrared reflectance spectroscopy accurately predicted isotope and elemental compositions for origin traceability of coffee. Food Chem. 2023, 427, 136695. [Google Scholar] [PubMed]
- Sánchez, M.T.; Garrido-Varo, A.; Guerrero, J.E.; Pérez-Marín, D. NIRS technology for fast authentication of green asparagus grown under organic and conventional production systems. Postharvest Biol. Technol. 2013, 85, 116–123. [Google Scholar]
- Bahar, B.; Schmidt, O.; Moloney, A.P.; Scrimgeour, C.M.; Begley, I.S.; Monahan, F.J. Seasonal variation in the C, N and S stable isotope composition of retail organic and conventional Irish beef. Food Chem. 2008, 106, 1299–1305. [Google Scholar]
- Pan, L.; Li, H.; Zhao, J. Improvement of the prediction of a visual apple ripeness index under seasonal variation by NIR spectral model correction. Spectrochim. Acta A 2023, 302, 123075. [Google Scholar] [CrossRef] [PubMed]
- Scheelbeek, P.F.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef]
- China National Standards for Organic Products-Requirements for Production, Processing, Labeling and Management System (GB/T 19630-2019). Available online: http://down.foodmate.net/standard/sort/3/62495.html (accessed on 1 January 2020).
- Liu, X.; Liu, Z.; Qian, Q.; Song, W.; Rogers, K.M.; Rao, Q.; Wang, S.; Zhang, Q.; Shao, S.; Tian, M.; et al. Isotope chemometrics determines farming methods and geographical origin of vegetables from Yangtze River Delta Region, China. Food Chem. 2021, 342, 128379. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.H. Applying Norris derivatives understanding and correcting the factors which affect diffuse transmittance spectra. NIR News 2001, 12, 6–9. [Google Scholar]
- Kennard, R.W.; Stone, L.A. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar]
- Li, H.D.; Xu, Q.S.; Liang, Y.Z. libPLS: An integrated library for partial least squares regression and discriminant analysis. Chemometr. Intell. Lab. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Cruz-Tirado, J.P.; Pierna, J.; Rogez, H.; Barbin, D.F.; Baeten, V. Authentication of cocoa (Theobroma cacao) bean hybrids by NIR-hyperspectral imaging and chemometrics. Food Control 2020, 118, 107445. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2017, 43, 237–247. [Google Scholar]
- Chalk, P.M.; Inácio, C.T.; Magalhães, A.M.T. From fertilizer to food: Tracing nitrogen dynamics in conventional and organic farming systems using 15N natural abundance. In Managing Soils for Food Security and Climate Change Adaptation and Mitigation; Heng, L.K., Sakadevan, K., Dercon, G., Nguyen, M.L., Eds.; FAO: Rome, Italy, 2014; pp. 339–348. [Google Scholar]
- Fageria, N.K. Role of soil organic matter in maintaining sustainability of cropping systems. Commun. Soil Sci. Plant Anal. 2012, 43, 2063–2113. [Google Scholar] [CrossRef]
- Hati, K.M.; Mandal, K.G.; Misra, A.K.; Ghosh, P.K.; Bandyopadhyay, K.K. Effect of inorganic fertilizer and farmyard manure on soil physical properties, root distribution, and water-use efficiency of soybean in Vertisols of central India. Bioresour. Technol. 2006, 97, 2182–2188. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Vilalta, J.; Poyatos, R.; Aguadé, D.; Retana, J.; Mencuccini, M. A new look at water transport regulation in plants. New Phytol. 2014, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Craig, H. Isotopic variations in meteoric waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lian, E.; Yang, C.; Deng, K.; Qian, P.; Xiao, S.; Liu, Z.; Yang, S. Seasonal variability of stable isotopes in the Changjiang (Yangtze) river water and its implications for natural climate and anthropogenic impacts. Environ. Sci. Eur. 2020, 32, 1–13. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, L.; Chai, X.; Yao, T. A model-based determination of spatial variation of precipitation δ18O over China. Chem. Geol. 2008, 249, 203–212. [Google Scholar]
- Liu, J.; Song, X.; Yuan, G.; Sun, X.; Yang, L. Stable isotopic compositions of precipitation in China. Tellus B 2014, 66, 22567. [Google Scholar] [CrossRef]
- Araguás-Araguás, L.; Froehlich, K.; Rozanski, K. Stable isotope composition of precipitation over southeast Asia. J. Geophys. Res. Atmos. 1998, 103, 28721–28742. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, K.; Yue, Y. Projecting global fertilizer consumption under shared socioeconomic pathway (SSP) scenarios using an approach of ensemble machine learning. Sci. Total Environ. 2024, 912, 169130. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide to Interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]



| Seasons | No. of Samples | ORG | GRE | CON |
|---|---|---|---|---|
| Autumn (September–November 2020) | 22 | 9 | 4 | 9 |
| Winter (December 2020–February 2021) | 35 | 11 | 11 | 13 |
| Spring (March–May 2021) | 52 | 23 | 12 | 18 |
| Summer (June–August 2021) | 55 | 18 | 13 | 24 |
| Autumn-re (September 2021) | 11 | 3 | 4 | 4 |
| Total | 175 | 63 | 44 | 68 |
| Seasons | Stable Isotopes | Cultivation Methods | ||
|---|---|---|---|---|
| ORG | GRE | CON | ||
| Autumn | δ13C | −28.53 ± 1.32 a | −28.17 ± 1.44 a | −28.12 ± 1.18 a |
| δ15N | 9.58 ± 6.05 a | 7.13 ± 5.78 a | 6.80 ± 8.79 a | |
| δ2H | −87.20 ± 9.04 a | −86.02 ± 8.91 a | −87.19 ± 9.78 a | |
| δ18O | 19.13 ± 3.27 a | 19.31 ± 2.35 a | 17.98 ± 3.20 a | |
| Winter | δ13C | −28.48 ± 1.34 a | −28.34 ± 1.54 a | −27.88 ± 1.60 a |
| δ15N | 10.24 ± 4.16 a | 6.90 ± 4.76 ab | 4.84 ± 7.46 b | |
| δ2H | −72.86 ± 8.01 a | −70.16 ± 8.12 a | −68.63 ± 5.73 a | |
| δ18O | 21.63 ± 2.49 a | 22.57 ± 1.62 a | 21.75 ± 1.72 a | |
| Spring | δ13C | −28.75 ± 0.81 a | −29.17 ± 0.54 a | −28.53 ± 0.86 a |
| δ15N | 11.82 ± 7.89 a | 6.35 ± 4.43 a | 1.50 ± 3.08 b | |
| δ2H | −74.17 ± 4.70 a | −74.87 ± 4.21 a | −72.80 ± 5.52 a | |
| δ18O | 23.37 ± 2.61 a | 21.51 ± 2.26 ab | 21.65 ± 1.50 b | |
| Summer | δ13C | −30.28 ± 1.35 a | −29.76 ± 0.65 a | −29.42 ± 1.54 a |
| δ15N | 9.11 ± 5.17 a | 6.64 ± 5.51 ab | 3.17 ± 3.33 b | |
| δ2H | −89.10 ± 6.47 a | −85.25 ± 8.51 a | −88.13 ± 9.71 a | |
| δ18O | 20.28 ± 1.74 a | 21.22 ± 1.33 a | 20.71 ± 2.02 a | |
| Autumn-re | δ13C | −30.14 ± 0.05 a | −29.98 ± 0.44 a | −30.45 ± 0.56 a |
| δ15N | 12.94 ± 5.46 a | 7.29 ± 8.75 a | 4.01 ± 0.31 a | |
| δ2H | −82.49 ± 6.02 a | −84.03 ± 6.86 a | −89.00 ± 3.55 a | |
| δ18O | 20.14 ± 0.99 a | 20.21 ± 0.79 a | 19.61 ± 0.66 a | |
| Instruments | Cultivation Methods | Models | Calibration Accuracy (%) | Validation Accuracy (%) |
|---|---|---|---|---|
| IRMS | ORG vs. CON | PLS-DA | 77.55 (76/98) | 75.76 (25/33) |
| ORG vs. GRE | PLS-DA | 71.25 (57/80) | 51.85 (14/27) | |
| GRE vs. CON | PLS-DA | 73.81 (62/84) | 53.57 (15/28) | |
| NIR | ORG vs. CON | PLS-DA | 87.76 (86/98) | 78.79 (26/33) |
| NSD(5,5,2) a-PLS-DA | 91.84 (90/98) | 81.82 (27/33) | ||
| ORG vs. GRE | PLS-DA | 100 (80/80) | 62.96 (17/27) | |
| NSD(9,9,2)-PLS-DA | 100 (80/80) | 70.37 (19/27) | ||
| GRE vs. CON | PLS-DA | 96.43 (81/84) | 71.43 (20/28) | |
| NSD(9,9,1)-PLS-DA | 100 (84/84) | 67.86 (19/28) | ||
| IRMS-NIR | ORG vs. CON | PLS-DA | 83.67 (82/98) | 87.88 (29/33) |
| NSD(5,5,2)-PLS-DA | 89.80 (88/98) | 87.88 (29/33) | ||
| ORG vs. GRE | PLS-DA | 98.75 (79/80) | 81.48 (22/27) | |
| NSD(3,3,2)-PLS-DA | 100 (80/80) | 88.89 (24/27) | ||
| GRE vs. CON | PLS-DA | 90.48 (76/84) | 75.00 (21/28) | |
| NSD(3,3,1)-PLS-DA | 100 (84/84) | 71.43 (20/28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Fan, K.; Lu, Y.; Zhao, H.; Rao, Q.; Geng, H.; Chen, Y.; Rogers, K.M.; Song, W. Assessing Seasonal Effects on Identification of Cultivation Methods of Short–Growth Cycle Brassica chinensis L. Using IRMS and NIRS. Foods 2024, 13, 1165. https://doi.org/10.3390/foods13081165
Liu X, Fan K, Lu Y, Zhao H, Rao Q, Geng H, Chen Y, Rogers KM, Song W. Assessing Seasonal Effects on Identification of Cultivation Methods of Short–Growth Cycle Brassica chinensis L. Using IRMS and NIRS. Foods. 2024; 13(8):1165. https://doi.org/10.3390/foods13081165
Chicago/Turabian StyleLiu, Xing, Kai Fan, Yangyang Lu, Hong Zhao, Qinxiong Rao, Hao Geng, Yijiao Chen, Karyne Maree Rogers, and Weiguo Song. 2024. "Assessing Seasonal Effects on Identification of Cultivation Methods of Short–Growth Cycle Brassica chinensis L. Using IRMS and NIRS" Foods 13, no. 8: 1165. https://doi.org/10.3390/foods13081165