Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats
Abstract
1. Introduction
2. Materials and Methods
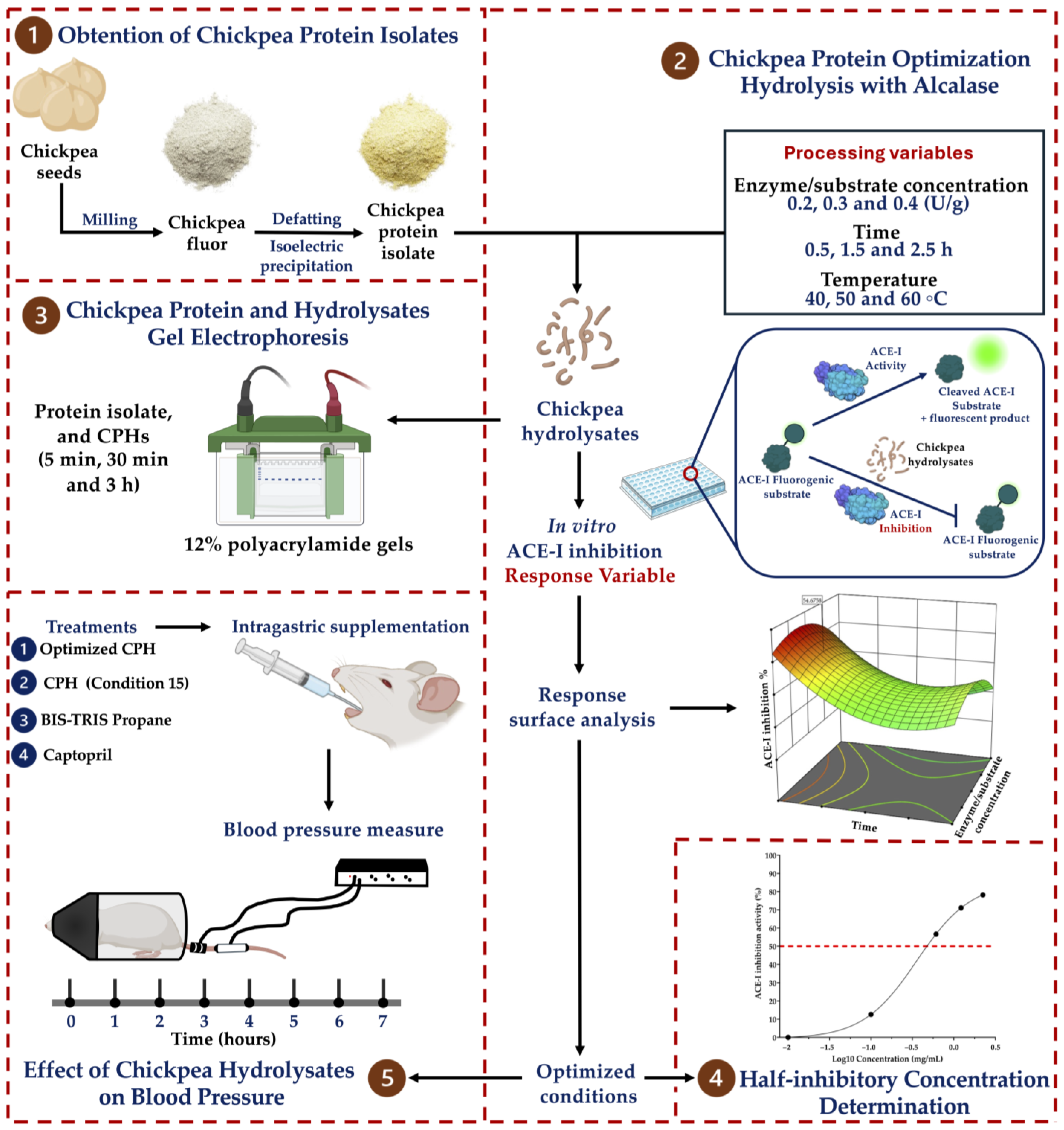
2.1. Obtention of the Chickpea Protein Isolate
2.2. Chickpea Protein Optimization Hydrolysis with Alcalase
2.3. Determination of the ACE-I Inhibition of the Chickpea Hydrolysates
2.4. Experimental Validation of the Response Surface Analysis
2.5. Half-Inhibitory Concentration
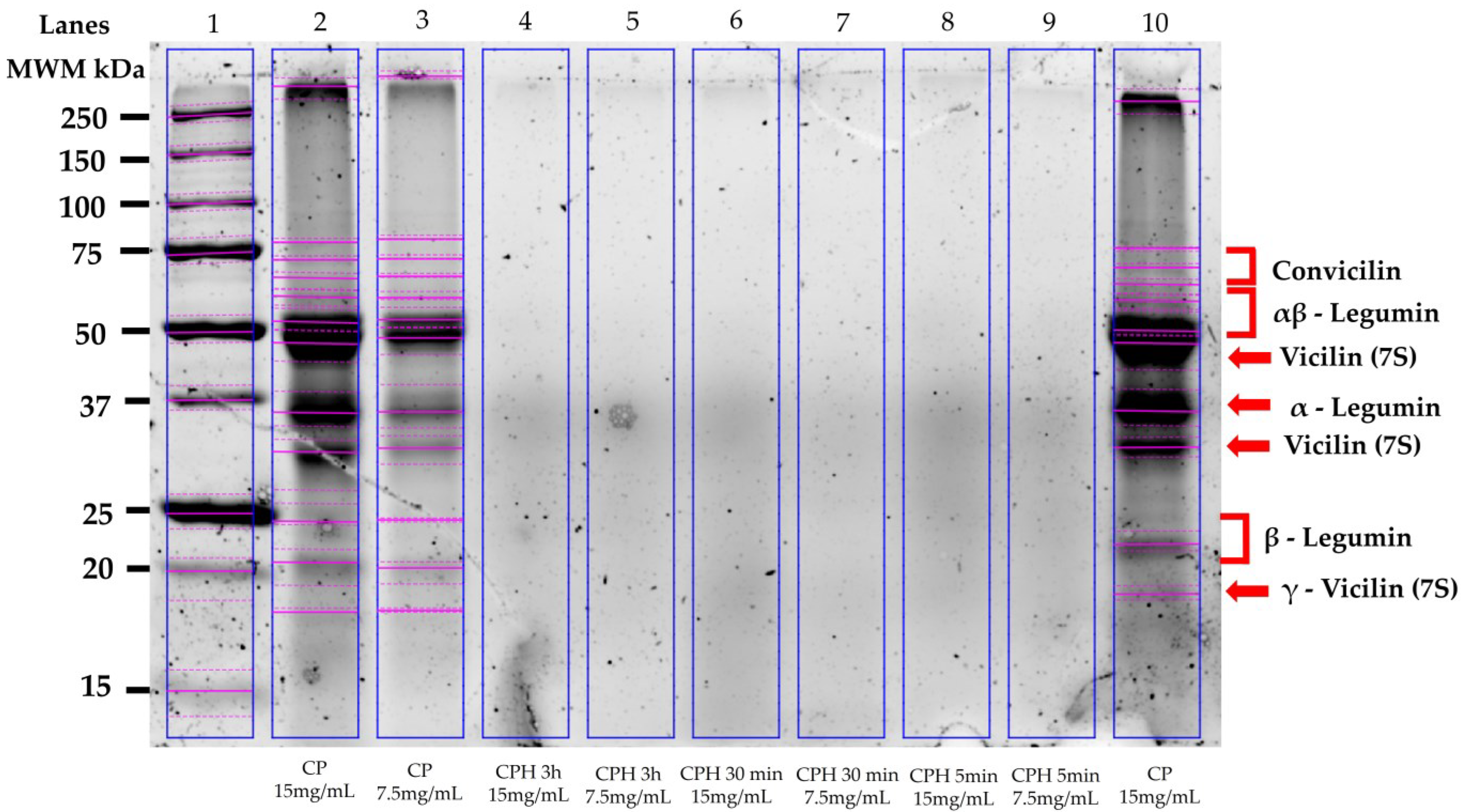
2.6. Chickpea Protein and Hydrolysate Gel Electrophoresis
2.7. Animals
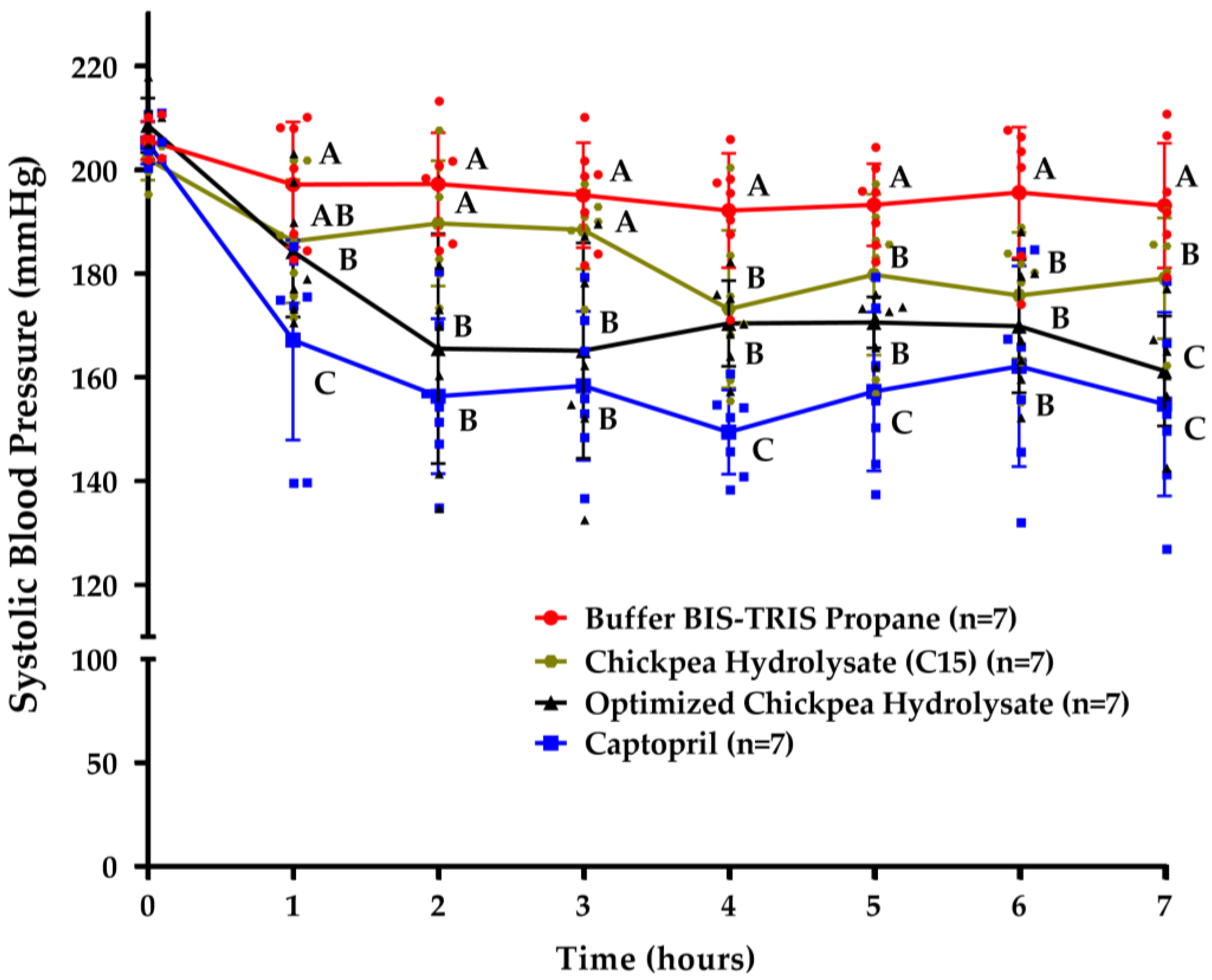
2.8. Effect of the Chickpea Hydrolysates on Blood Pressure
2.9. Statistical Analysis and Ethical Aspects
3. Results
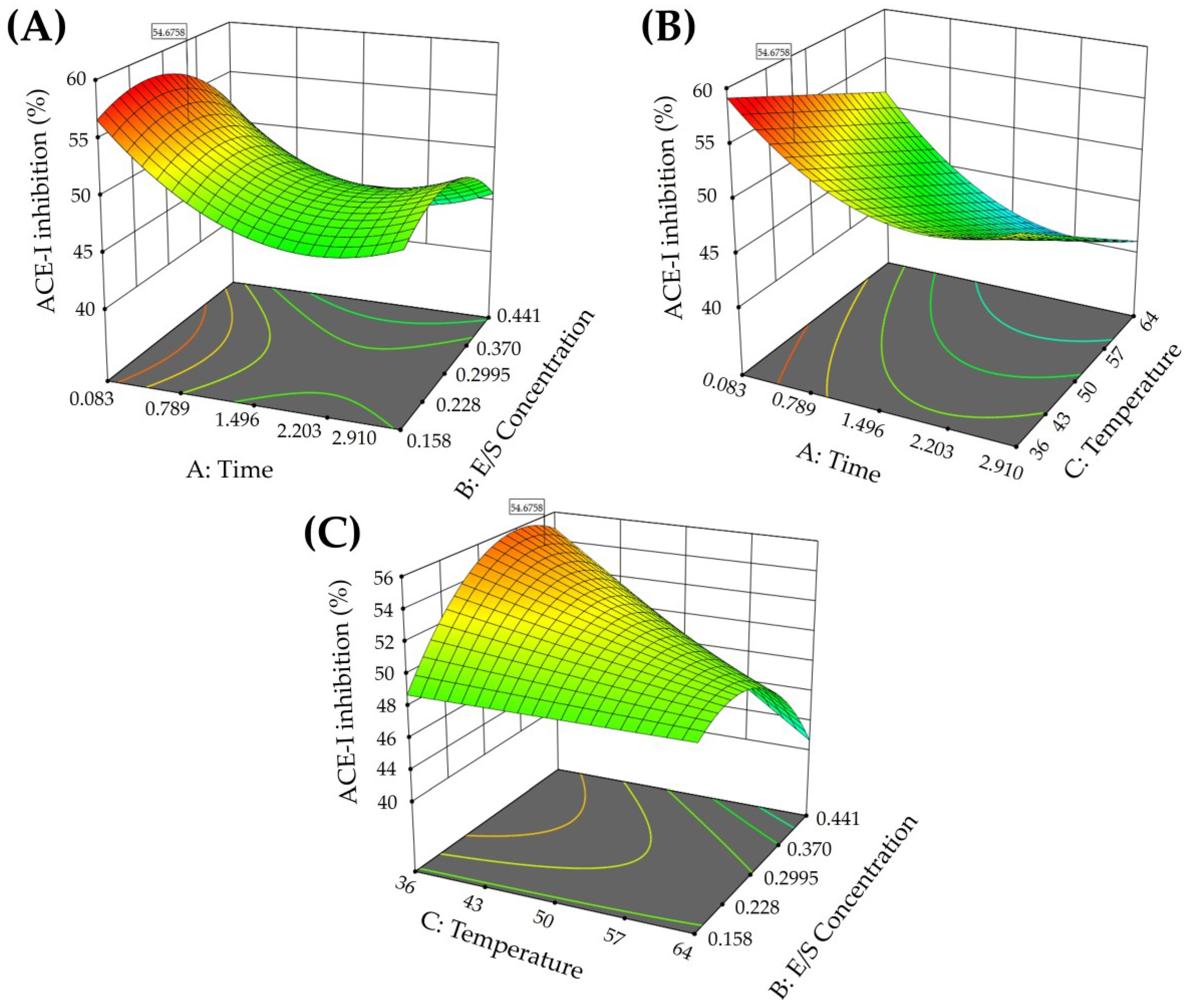
3.1. Protein Content and Optimization of the Chickpea Hydrolysate
3.2. ACE-I Inhibition of the Chickpea Hydrolysates and IC50
3.3. Electrophoretic Characterization of the Chickpea Proteins and Hydrolysates
3.4. Chickpea Hydrolysates Efficiently Reduce Systolic Blood Pressure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Su, C.; Xue, J.; Ye, C.; Chen, A. Role of the Central Renin-Angiotensin System in Hypertension. Int. J. Mol. Med. 2021, 47, 95. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Moran, A.E.; Whelton, P.K. Treatment of Hypertension: A Review. JAMA 2022, 328, 1849–1861. [Google Scholar] [CrossRef] [PubMed]
- Na Takuathung, M.; Sakuludomkan, W.; Khatsri, R.; Dukaew, N.; Kraivisitkul, N.; Ahmadmusa, B.; Mahakkanukrauh, C.; Wangthaweesap, K.; Onin, J.; Srichai, S.; et al. Adverse Effects of Angiotensin-Converting Enzyme Inhibitors in Humans: A Systematic Review and Meta-Analysis of 378 Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2022, 19, 8373. [Google Scholar] [CrossRef] [PubMed]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Kiersnowska, K.; Jakubczyk, A. Bioactive Peptides Obtained from Legume Seeds as New Compounds in Metabolic Syndrome Prevention and Diet Therapy. Foods 2022, 11, 3300. [Google Scholar] [CrossRef]
- Chávez-Ontiveros, J.; Reyes-Moreno, C.; Ramírez-Torres, G.I.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Montoya-Rodríguez, A.; Ontiveros, N.; Cuevas-Rodríguez, E.O. Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods 2022, 11, 2562. [Google Scholar] [CrossRef]
- Gupta, N.; Quazi, S.; Jha, S.K.; Siddiqi, M.K.; Verma, K.; Sharma, S.; Khan, R.H.; Bhagyawant, S.S. Chickpea Peptide: A Nutraceutical Molecule Corroborating Neurodegenerative and ACE-I Inhibition. Nutrients 2022, 14, 4824. [Google Scholar] [CrossRef]
- Arámburo-Gálvez, J.G.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Ramírez-Torres, G.I.; Flores-Mendoza, L.K.; Gastelum-Acosta, P.E.; Figueroa-Salcido, O.G.; Ontiveros, N. Prediction of ACE-I Inhibitory Peptides Derived from Chickpea (Cicer arietinum L.): In Silico Assessments Using Simulated Enzymatic Hydrolysis, Molecular Docking and ADMET Evaluation. Foods 2022, 11, 1576. [Google Scholar] [CrossRef]
- Real Hernandez, L.M.; Gonzalez de Mejia, E. Enzymatic Production, Bioactivity, and Bitterness of Chickpea (Cicer arietinum) Peptides. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1913–1946. [Google Scholar] [CrossRef] [PubMed]
- Khushairay, E.S.I.; Ghani, M.A.; Babji, A.S.; Yusop, S.M. The Nutritional and Functional Properties of Protein Isolates from Defatted Chia Flour Using Different Extraction pH. Foods 2023, 12, 3046. [Google Scholar] [CrossRef] [PubMed]
- Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Utilisation of Chickpea Protein Isolates for Production of Peptides with Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activity. J. Sci. Food Agric. 2002, 82, 960–965. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-Converting Enzyme Inhibitory Activity of Chickpea and Pea Protein Hydrolysates. Food Res. Int. 2010, 43, 1642–1649. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Giron-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of Ace Inhibitory Peptides by Digestion of Chickpea Legumin with Alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.-H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, R.J.; Cermeño, M.; Khalesi, M.; Kleekayai, T.; Amigo-Benavent, M. Application of in Silico Approaches for the Generation of Milk Protein-Derived Bioactive Peptides. J. Funct. Foods 2020, 64, 103636. [Google Scholar] [CrossRef]
- Ramírez-Torres, G.; Ontiveros, N.; Lopez-Teros, V.; Ibarra-Diarte, J.A.; Reyes-Moreno, C.; Cuevas-Rodríguez, E.O.; Cabrera-Chávez, F. Amaranth Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Molecules 2017, 22, 1905. [Google Scholar] [CrossRef]
- Chiang, W.-D.; Tsou, M.-J.; Tsai, Z.-Y.; Tsai, T.-C. Angiotensin I-Converting Enzyme Inhibitor Derived from Soy Protein Hydrolysate and Produced by Using Membrane Reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- Uluko, H.; Li, H.; Cui, W.; Zhang, S.; Liu, L.; Chen, J.; Sun, Y.; Su, Y.; Lv, J. Response Surface Optimization of Angiotensin Converting Enzyme Inhibition of Milk Protein Concentrate Hydrolysates in Vitro after Ultrasound Pretreatment. Innov. Food Sci. Emerg. Technol. 2013, 20, 133–139. [Google Scholar] [CrossRef]
- Wali, A.; Ma, H.; Rashid, M.T.; Liang, Q.F. Preparation of Rapeseed Protein Hydrolysates with ACE Inhibitory Activity by Optimization and Molecular Weight Distribution of Hydrolysates. Turk. J. Biochem. 2017, 42, 469–479. [Google Scholar] [CrossRef]
- Chang, L.; Lan, Y.; Bandillo, N.; Ohm, J.-B.; Chen, B.; Rao, J. Plant Proteins from Green Pea and Chickpea: Extraction, Fractionation, Structural Characterization and Functional Properties. Food Hydrocoll. 2022, 123, 107165. [Google Scholar] [CrossRef]
- Dullius, A.; Goettert, M.I.; de Souza, C.F.V. Whey Protein Hydrolysates as a Source of Bioactive Peptides for Functional Foods–Biotechnological Facilitation of Industrial Scale-Up. J. Funct. Foods 2018, 42, 58–74. [Google Scholar] [CrossRef]
- Jogi, N.; Yathisha, U.G.; Bhat, I.; Mamatha, B.S. Antihypertensive Activity of Orally Consumed ACE-I Inhibitory Peptides. Crit. Rev. Food Sci. Nutr. 2022, 62, 8986–8999. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Zhang, F.; Ma, Z.; Chen, S.; Ding, G.; Tian, X.; Feng, R. Isolation and Identification of the Angiotensin-I Converting Enzyme (ACE) Inhibitory Peptides Derived from Cottonseed Protein: Optimization of Hydrolysis Conditions. Int. J. Food Prop. 2019, 22, 1296–1309. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Abdul-Hamid, A.; Saari, N. Optimization of Bromelain-Aided Production of Angiotensin I-Converting Enzyme Inhibitory Hydrolysates from Stone Fish Using Response Surface Methodology. Mar. Drugs 2017, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Kheeree, N.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Choowongkomon, K.; Karnchanatat, A. ACE Inhibitory Peptides Derived from De-Fatted Lemon Basil Seeds: Optimization, Purification, Identification, Structure–Activity Relationship and Molecular Docking Analysis. Food Funct. 2020, 11, 8161–8178. [Google Scholar] [CrossRef]
- Mat Amin, A.; Harun, Z.; Liyana Muhamad Roslan, I. Optimization of Enzymatic Protein Hydrolysis Conditions to Obtain Maximum Angiotensin-I Converting Enzyme (ACE) Inhibitory Activity from Flower Crab (Portunis pelagicus) Meat. Asian J. Agric. Biol. 2019, 7, 146–155. [Google Scholar]
- Li, G.-H.; Shi, Y.-H.; Liu, H.; Le, G.-W. Antihypertensive Effect of Alcalase Generated Mung Bean Protein Hydrolysates in Spontaneously Hypertensive Rats. Eur. Food Res. Technol. 2006, 222, 733–736. [Google Scholar] [CrossRef]
- Da Costa, E.L.; da Rocha Gontijo, J.A.; Netto, F.M. Effect of Heat and Enzymatic Treatment on the Antihypertensive Activity of Whey Protein Hydrolysates. Int. Dairy J. 2007, 17, 632–640. [Google Scholar] [CrossRef]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of Antihypertensive Peptides from Mung Bean Protein Hydrolysate and Their Effects in Spontaneously Hypertensive Rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Shi, A.; Liu, H.; Liu, L.; Hu, H.; Wang, Q.; Adhikari, B. Isolation, Purification and Molecular Mechanism of a Peanut Protein-Derived ACE-Inhibitory Peptide. PLoS ONE 2014, 9, e111188. [Google Scholar] [CrossRef] [PubMed]
- Jamdar, S.N.; Deshpande, R.; Marathe, S.A. Effect of Processing Conditions and in Vitro Protein Digestion on Bioactive Potentials of Commonly Consumed Legumes. Food Biosci. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- Majumder, K.; Wu, J. Molecular Targets of Antihypertensive Peptides: Understanding the Mechanisms of Action Based on the Pathophysiology of Hypertension. Int. J. Mol. Sci. 2014, 16, 256–283. [Google Scholar] [CrossRef] [PubMed]
- Okagu, I.U.; Ezeorba, T.P.; Aham, E.C.; Aguchem, R.N.; Nechi, R.N. Recent Findings on the Cellular and Molecular Mechanisms of Action of Novel Food-Derived Antihypertensive Peptides. Food Chem. Mol. Sci. 2022, 4, 100078. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Limon, A.; Aguilar-Toalá, J.E.; Liceaga, A.M. Integration of Molecular Docking Analysis and Molecular Dynamics Simulations for Studying Food Proteins and Bioactive Peptides. J. Agric. Food Chem. 2022, 70, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, G.P. AHTPDB: A Comprehensive Platform for Analysis and Presentation of Antihypertensive Peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef]
- Mora-Melgem, J.A.; Arámburo-Gálvez, J.G.; Cárdenas-Torres, F.I.; Gonzalez-Santamaria, J.; Ramírez-Torres, G.I.; Arvizu-Flores, A.A.; Figueroa-Salcido, O.G.; Ontiveros, N. Dipeptidyl Peptidase IV Inhibitory Peptides from Chickpea Proteins (Cicer arietinum L.): Pharmacokinetics, Molecular Interactions, and Multi-Bioactivities. Pharmaceuticals 2023, 16, 1109. [Google Scholar] [CrossRef]
- López-Moreno, M.; Jiménez-Moreno, E.; Márquez Gallego, A.; Vera Pasamontes, G.; Uranga Ocio, J.A.; Garcés-Rimón, M.; Miguel-Castro, M. Red Quinoa Hydrolysates with Antioxidant Properties Improve Cardiovascular Health in Spontaneously Hypertensive Rats. Antioxidants 2023, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Piotrowicz, I.B.B.; Garcés-Rimón, M.; Moreno-Fernández, S.; Aleixandre, A.; Salas-Mellado, M.; Miguel-Castro, M. Antioxidant, Angiotensin-Converting Enzyme Inhibitory Properties and Blood-Pressure-Lowering Effect of Rice Bran Protein Hydrolysates. Foods 2020, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Antioxidant Properties, ACE/Renin Inhibitory Activities of Pigeon Pea Hydrolysates and Effects on Systolic Blood Pressure of Spontaneously Hypertensive Rats. Food Sci. Nutr. 2018, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Añón, M.C. Amaranth Seed Protein Hydrolysates Have in Vivo and in Vitro Antihypertensive Activity. Food Chem. 2011, 126, 878–884. [Google Scholar] [CrossRef]
- Janhavi, P.; Divyashree, S.; Sanjailal, K.; Muthukumar, S. DoseCal: A Virtual Calculator for Dosage Conversion between Human and Different Animal Species. Arch. Physiol. Biochem. 2022, 128, 426–430. [Google Scholar] [CrossRef]
- Liao, W.; Sun, G.; Xu, D.; Wang, Y.; Lu, Y.; Sun, J.; Xia, H.; Wang, S. The Blood-Pressure-Lowering Effect of Food-Protein-Derived Peptides: A Meta-Analysis of Recent Clinical Trials. Foods 2021, 10, 2316. [Google Scholar] [CrossRef]

| Factors | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Enzyme/substrate concentration (U/g) | 0.2 | 0.3 | 0.4 |
| Time (h) | 0.5 | 1.5 | 2.5 |
| Temperature (°C) | 40 | 50 | 60 |
| Conditions * | Time (h) | Enzyme/Substrate Concentration (U/g) | Temperature (°C) | ACE-I Inhibition (%) ** |
|---|---|---|---|---|
| C2 | 2.5 | 0.2 | 60 | 39.03 ± 3.31 a |
| C5 | 2.5 | 0.4 | 60 | 42.54 ± 4.97 abc |
| C1 | 1.5 | 0.1583 | 50 | 44.28 ± 2.26 ab |
| C3 | 1.5 | 0.441 | 50 | 44.47 ± 2.79 ab |
| C4 | 0.5 | 0.2 | 60 | 44.77 ± 2.16 ab |
| C8 (Central) | 1.5 | 0.3 | 50 | 46.03 ± 2.0 b |
| C7 | 1.5 | 0.3 | 64 | 46.60 ± 3.46 bc |
| C6 | 2.91 | 0.3 | 50 | 46.87 ± 1.12 b |
| C10 | 2.5 | 0.4 | 40 | 48.27 ± 8.50 abcde |
| C11 | 2.5 | 0.2 | 40 | 49.06 ± 5.67 abcde |
| C13 | 1.5 | 0.3 | 36 | 49.31 ± 3.64 bcde |
| C9 | 0.5 | 0.4 | 60 | 49.40 ± 2.53 bc |
| C12 | 0.5 | 0.4 | 40 | 51.31 ± 1.81 cd |
| C14 | 0.5 | 0.2 | 40 | 54.79 ± 2.10 de |
| C15 | 0.08 | 0.3 | 50 | 55.69 ± 3.14 e |
| Optimized | 0.5 | 0.2543 | 40 | 56.26 ± 0.85 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Mora-Melgem, J.A.; Camacho-Cervantes, D.L.; Gracia-Valenzuela, M.H.; Cuevas-Rodríguez, E.O.; Ontiveros, N. Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Foods 2024, 13, 1216. https://doi.org/10.3390/foods13081216
Figueroa-Salcido OG, Arámburo-Gálvez JG, Mora-Melgem JA, Camacho-Cervantes DL, Gracia-Valenzuela MH, Cuevas-Rodríguez EO, Ontiveros N. Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Foods. 2024; 13(8):1216. https://doi.org/10.3390/foods13081216
Chicago/Turabian StyleFigueroa-Salcido, Oscar Gerardo, Jesús Gilberto Arámburo-Gálvez, José Antonio Mora-Melgem, Diana Laura Camacho-Cervantes, Martina Hilda Gracia-Valenzuela, Edith Oliva Cuevas-Rodríguez, and Noé Ontiveros. 2024. "Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats" Foods 13, no. 8: 1216. https://doi.org/10.3390/foods13081216
APA StyleFigueroa-Salcido, O. G., Arámburo-Gálvez, J. G., Mora-Melgem, J. A., Camacho-Cervantes, D. L., Gracia-Valenzuela, M. H., Cuevas-Rodríguez, E. O., & Ontiveros, N. (2024). Alcalase-Based Chickpea (Cicer arietinum L.) Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Foods, 13(8), 1216. https://doi.org/10.3390/foods13081216








