Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities
Abstract
:1. Introduction
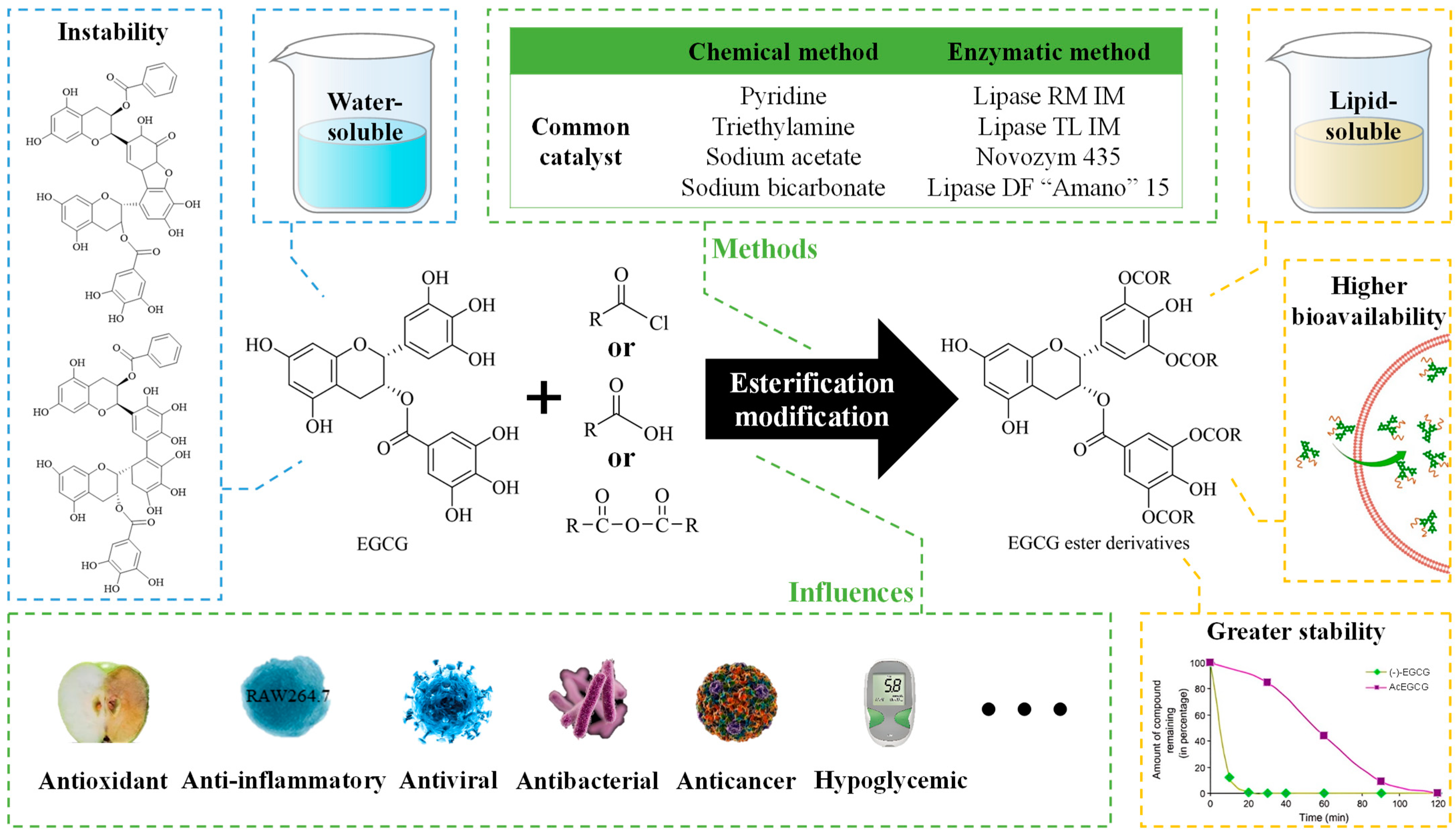
2. Methods for Esterification and Analysis
2.1. Methods for Esterification
2.1.1. Chemical Modifications
2.1.2. Enzymatic Modifications
2.2. Methods for the Analysis of EGCG Esterification Products
3. Biological Activities of EGCG Esterification Products
3.1. Antioxidant Activity
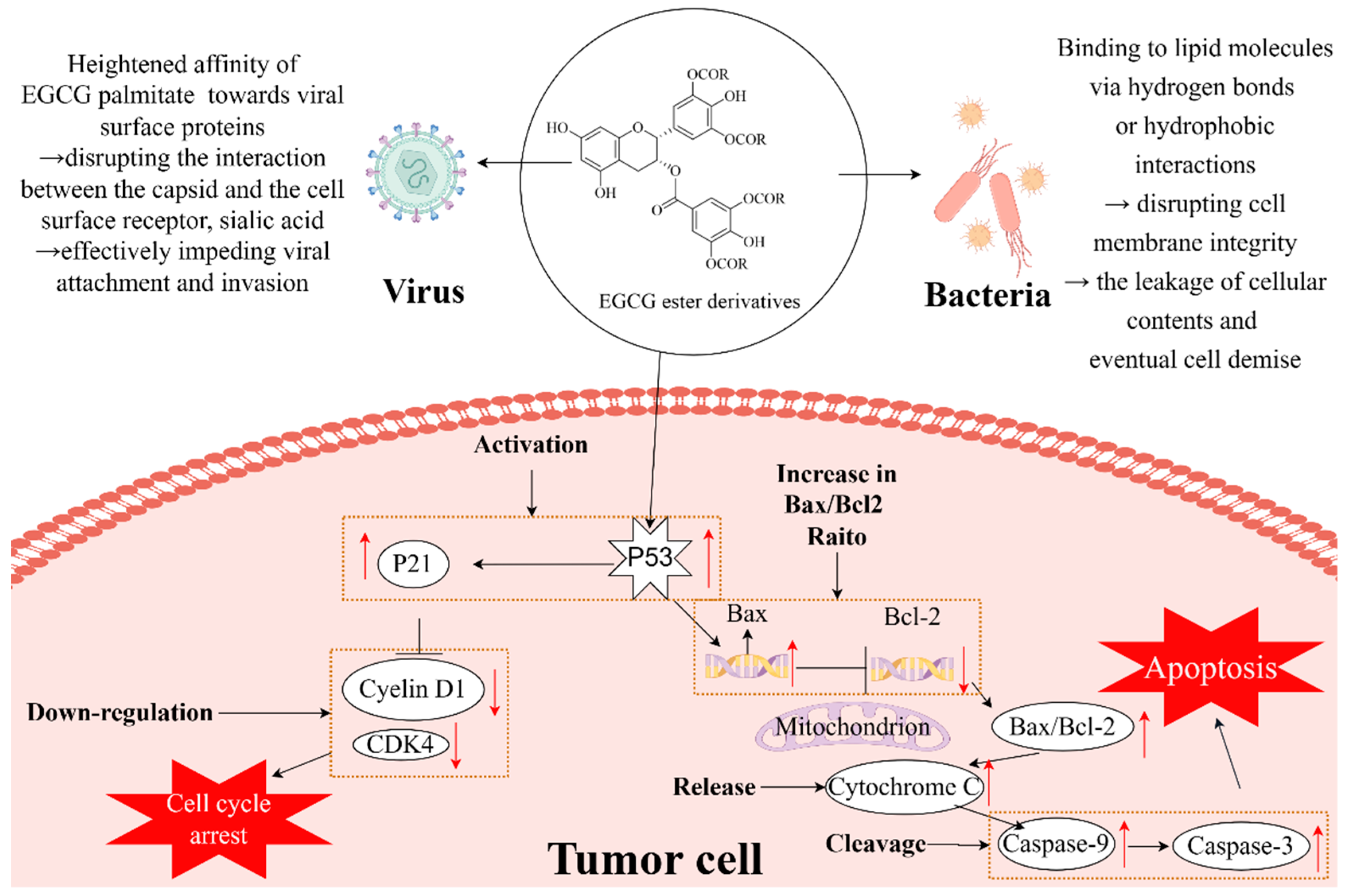
3.2. Antibacterial Activity
3.3. Antiviral Activity
3.4. Anticancer Activity
3.5. Other Biological Activities
4. Application of EGCG Derivatives
4.1. Antioxidants
4.2. Preserving Agents
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Zhu, Z.; Bassey, A.P.; Khan, I.A.; Huang, M.; Zhang, X. Inhibitory mechanism of catechins against advanced glycation end products of glycated myofibrillar protein through anti-aggregation and anti-oxidation. LWT 2021, 147, 111550. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ma, R.; Sun, W.; Ji, Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella flexneri. Int. J. Environ. Res. Public Health 2023, 20, 4676. [Google Scholar] [CrossRef]
- Ferrari, E.; Bettuzzi, S.; Naponelli, V. The Potential of Epigallocatechin Gallate (EGCG) in Targeting Autophagy for Cancer Treatment: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6075. [Google Scholar] [CrossRef]
- Nikoo, M.; Regenstein, J.M.; Gavlighi, H.A. Antioxidant and Antimicrobial Activities of (-)-Epigallocatechin-3-gallate (EGCG) and its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, M.; Wan, J.; Zheng, X.; Teng, C.; Xie, X.; Pan, W.; Hu, B.; Huang, J.; Liu, Z.; et al. Research progress of epigallocatechin-3-gallate (EGCG) on anti-pathogenic microbes and immune regulation activities. Food Funct. 2021, 12, 9607–9619. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Liu, S. Targeted acylation for all the hydroxyls of (+)-catechin and evaluation of their individual contribution to radical scavenging activity. Food Chem. 2016, 197, 415–421. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Udenigwe, C.C. Recent advances in protein–polyphenol interactions focusing on structural properties related to antioxidant activities. Curr. Opin. Food Sci. 2022, 45, 100840. [Google Scholar] [CrossRef]
- Sahadevan, R.; Singh, S.; Binoy, A.; Sadhukhan, S. Chemico-biological aspects of (−)-epigallocatechin-3-gallate (EGCG) to improve its stability, bioavailability and membrane permeability: Current status and future prospects. Crit. Rev. Food Sci. Nutr. 2023, 63, 10382–10411. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Yang, C.S. Bioavailability and stability issues in understanding the cancer preventive effects of tea polyphenols. J. Sci. Food Agric. 2006, 86, 2256–2265. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, N.; Li, Y.; Chen, S.; Xia, Y. Antioxidant activities of lipophilic (−)-epigallocatechin gallate derivatives in vitro and in lipid-based food systems. Food Biosci. 2021, 42, 101055. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Li, Z.; Ma, C.; Lou, Z.; Yokoyama, W.; Wang, H. Lipase-catalyzed synthesis of acetylated EGCG and antioxidant properties of the acetylated derivatives. Food Res. Int. 2014, 56, 279–286. [Google Scholar] [CrossRef]
- Van den Berg, M.; Cattley, R.; Cherrie, J.W.; Dorman, D.C.; Dunnick, J.K.; Gohlke, J.M.; Jinot, J.; Käfferlein, H.U.; Kopylev, L.; Matsumoto, M.; et al. Carcinogenicity of some nitrobenzenes and other industrial chemicals. Lancet Oncol. 2018, 19, e681–e682. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef]
- Lam, W.H.; Kazi, A.; Kuhn, D.J.; Chow, L.M.C.; Chan, A.S.C.; Dou, Q.P.; Chan, T.H. A potential prodrug for a green tea polyphenol proteasome inhibitor: Evaluation of the peracetate ester of (−)-epigallocatechin gallate [(−)-EGCG]. Bioorganic Med. Chem. 2004, 12, 5587–5593. [Google Scholar] [CrossRef]
- Liu, L.; Jin, C.; Zhang, Y. Lipophilic phenolic compounds (Lipo-PCs): Emerging antioxidants applied in lipid systems. RSC Adv. 2014, 4, 2879–2891. [Google Scholar] [CrossRef]
- Faggiano, A.; Ricciardi, M.; Proto, A. Catalytic Routes to Produce Polyphenolic Esters (PEs) from Biomass Feedstocks. Catalysts 2022, 12, 447. [Google Scholar] [CrossRef]
- Liu, B.; Kang, Z.; Yan, W. Synthesis, Stability, and Antidiabetic Activity Evaluation of (−)-Epigallocatechin Gallate (EGCG) Palmitate Derived from Natural Tea Polyphenols. Molecules 2021, 26, 393. [Google Scholar] [CrossRef]
- Qian, H.; Mathiowitz, E. Acyl chloride-facilitated condensation polymerization for the synthesis of heat-sensitive poly(anhydride-ester)s. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5899–5915. [Google Scholar] [CrossRef]
- Kohri, T.; Nanjo, F.; Suzuki, M.; Seto, R.; Matsumoto, N.; Yamakawa, M.; Hojo, H.; Hara, Y. Synthesis of (-)-[4-3H]Epigallocatechin Gallate and Its Metabolic Fate in Rats after Intravenous Administration. J. Agric. Food Chem. 2001, 49, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Shahidi, F. Lipophilized Epigallocatechin Gallate (EGCG) Derivatives as Novel Antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; Du, Q.; Ito, Y. Purification of long-chain fatty acid ester of epigallocatechin-3-Ogallate by high-speed counter-current chromatography. J. Chromatogr. A 2002, 982, 163–165. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.; Wang, T.; Chang, M.; Jin, Q.; Wang, X. Microwave-assisted synthesis and antioxidant activity of palmitoyl-epigallocatechin gallate. LWT 2019, 101, 663–669. [Google Scholar] [CrossRef]
- Mustafa, J.; Khan, S.I.; Ferreira, D.; Khan, I.A. Synthesis, spectroscopic and anti-tumor studies of polyphenol-linoleates derived from natural polyphenols. Eur. J. Lipid Sci. Technol. 2007, 109, 552–559. [Google Scholar] [CrossRef]
- Mori, S.; Miyake, S.; Kobe, T.; Nakaya, T.; Fuller, S.D.; Kato, N.; Kaihatsu, K. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: Effect of alkyl chain length. Bioorganic Med. Chem. Lett. 2008, 18, 4249–4252. [Google Scholar] [CrossRef]
- Yan, W.; Shao, Y.; Liu, B. A Novel Synthesis Method of Lipophilic Egcg Palmitate and Evaluation for Its Alpha Amylase and Alpha Glucosidase Inhibtory Potetial. J. Food Sci. Technol. 2019, 4, 830–839. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Oh, W.Y.; Shahidi, F. Epigallocatechin (EGC) esters as potential sources of antioxidants. Food Chem. 2020, 309, 125609. [Google Scholar] [CrossRef]
- Lei, C.; Tang, X.; Li, H.; Chen, H.; Yu, S. Molecular hybridization of grape seed extract: Synthesis, structural characterization and anti-proliferative activity in vitro. Food Res. Int. 2020, 131, 109005. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, H.; Yin, J.; Wang, Y.; Zhao, Y.; Yu, J.; Engelhardt, U.H. Separation and antioxidant activities of new acetylated EGCG compounds. Sci. Rep. 2023, 13, 20964. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.F.; Lin, Y.-H.; Lin, M.; Kao, Y.-F.; Wang, R.-W.; Teng, L.-W.; Chuang, S.-H.; Chang, J.-M.; Yuan, T.-T.; Fu, K.C.; et al. Synthesis and structure–activity relationship of 3-O-acylated (–)-epigallocatechins as 5α-reductase inhibitors. Eur. J. Med. Chem. 2010, 45, 6068–6076. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakne, K.; Farmani, J.; Shahidi, F. Enzymatic lipophilization of bioactive compounds with high antioxidant activity: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–18, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, L.; Huang, X.; Zhu, S.; Ma, C.; Wang, H. Identification and Antioxidant Abilities of Enzymatic-Transesterification (-)-Epigallocatechin-3-O-gallate Stearyl Derivatives in Non-Aqueous Systems. Antioxidants 2021, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Salihu, A.; Alam, M.Z. Solvent tolerant lipases: A review. Process Biochem. 2015, 50, 86–96. [Google Scholar] [CrossRef]
- Cannazza, P.; Donzella, S.; Pellis, A.; Contente, M.L. Mycobacterium smegmatis acyltransferase: The big new player in biocatalysis. Biotechnol. Adv. 2022, 59, 107985. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zheng, L.; Cui, X.; Huang, H.; Geng, X.; Xie, X. Regioselective acylation of resveratrol catalyzed by lipase under microwave. Green Chem. Lett. Rev. 2018, 11, 312–317. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ma, C.; Huang, D.; Chen, S.; Zhu, S.; Wang, H. Enzymatic molecular modification of water-soluble polyphenols: Synthesis, structure, bioactivity and application. Crit. Rev. Food Sci. Nutr. 2022, 63, 12637–12651. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Poveda, A.; Jimenez-Barbero, J.; Ballesteros, A.; Plou, F.J. Regioselective Lipase-Catalyzed Synthesis of 3-O-Acyl Derivatives of Resveratrol and Study of Their Antioxidant Properties. J. Agric. Food Chem. 2010, 58, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, N.; Chen, S.; Li, Y. Study of acetylated EGCG synthesis by enzymatic transesterification in organic media. Arab. J. Chem. 2020, 13, 8824–8834. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, N.; Li, Y.; Chen, S.-W. Efficient enzymatic modification of epigallocatechin gallate in ionic liquids. Green Chem. Lett. Rev. 2021, 14, 415–424. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, L.; Huang, X.; Zhu, S.; Ma, C.; Wang, H. Structural characterization and antioxidant property of enzymatic-transesterification derivatives of (-)-epigallocatechin-3-O-gallate and vinyl laurate. J. Food Sci. 2021, 86, 4717–4729. [Google Scholar] [CrossRef]
- Peng, H.; Shahidi, F. Enzymatic Synthesis and Antioxidant Activity of Mono- and Diacylated Epigallocatechin Gallate and Related By-Products. J. Agric. Food Chem. 2022, 70, 9227–9242. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, L.; Li, C.; Chen, R.; Liu, C.; Chen, M. Lipophilized Epigallocatechin Gallate Derivative Exerts Anti-Proliferation Efficacy through Induction of Cell Cycle Arrest and Apoptosis on DU145 Human Prostate Cancer Cells. Nutrients 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Milivojević, A.; Ćorović, M.; Carević, M.; Banjanac, K.; Vujisić, L.; Veličković, D.; Bezbradica, D. Highly efficient enzymatic acetylation of flavonoids: Development of solvent-free process and kinetic evaluation. Biochem. Eng. J. 2017, 128, 106–115. [Google Scholar] [CrossRef]
- Zhu, S.; Li, Y.; Ma, C.-Y.; Lou, Z.-X.; Chen, S.-W.; Dai, J.; Wang, H.-X. Optimization of lipase-catalyzed synthesis of acetylated EGCG by response surface methodology. J. Mol. Catal. B Enzym. 2013, 97, 87–94. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, M.E.M.B.; Franco, Y.E.M.; Messias, M.C.F.; Longato, G.B.; Pamphile, J.A.; Carvalho, P.d.O. Biocatalytic Synthesis of Flavonoid Esters by Lipases and Their Biological Benefits. Planta Medica 2017, 83, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mo, L.; Wu, B.; Ma, C.; Wang, H. Effect of structural stability of lipase in acetonitrile on its catalytic activity in EGCG esterification reaction: FTIR and MD simulation. Int. J. Biol. Macromol. 2024, 255, 128266. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Gopinath, K.P.; Vo, D.-V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, W. Ionic liquids: Green solvents for nonaqueous biocatalysis. Enzym. Microb. Technol. 2005, 37, 19–28. [Google Scholar] [CrossRef]
- Chen, P.; Du, Q.-Z. Isolation and Purification of a Novel Long-chain Acyl Catechin from Lipophilic Tea Polyphenols. Chin. J. Chem. 2003, 21, 979–981. [Google Scholar] [CrossRef]
- Wojtanowski, K.K.; Mroczek, T. Detection, identification and structural elucidation of flavonoids using liquid chromatography coupled to mass spectrometry. Curr. Org. Chem. 2020, 24, 104–112. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- de Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Kaihatsu, K.; Nishino, K.; Ogawa, M.; Kato, N.; Yamaguchi, A. Antibacterial and Antifungal Activities of New Acylated Derivatives of Epigallocatechin Gallate. Front. Microbiol. 2012, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xia, C.; Luo, Q.; Zhu, Y.; Tang, X.; Chen, J. Active components, antioxidant capacity and quality evaluation of Sichuan black tea. Food mach. 2021, 37, 24–32. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilised epigallocatechin gallate (EGCG) derivatives and their antioxidant potential in food and biological systems. Food Chem. 2012, 131, 22–30. [Google Scholar] [CrossRef]
- Ignasimuthu, K.; Prakash, R.; Murthy, P.S.; Subban, N. Enhanced bioaccessibility of green tea polyphenols and lipophilic activity of EGCG octaacetate on gram-negative bacteria. LWT 2019, 105, 103–109. [Google Scholar] [CrossRef]
- Ali, B.; Lee, L.H.; Laskar, N.; Shaikh, N.; Tahir, H.; Hsu, S.D.; Newby, R., Jr.; Valsechi-Diaz, J.; Chu, T. Modified Green Tea Polyphenols, EGCG-S and LTP, Inhibit Endospore in Three Bacillus spp. Adv. Microbiol. 2017, 07, 175–187. [Google Scholar] [CrossRef]
- Melok, A.L.; Lee, L.H.; Mohamed Yussof, S.A.; Chu, T. Green Tea Polyphenol Epigallocatechin-3-Gallate-Stearate Inhibits the Growth of Streptococcus mutans: A Promising New Approach in Caries Prevention. Dent. J. 2018, 6, 38. [Google Scholar] [CrossRef]
- Kaihatsu, K.; Mori, S.; Matsumura, H.; Daidoji, T.; Kawakami, C.; Kurata, H.; Nakaya, T.; Kato, A. Broad and potent anti-influenza virus spectrum of epigallocatechin-3-O-gallate-monopalmitate. J. Mol. Genet. Med. 2009, 3, 195–197. [Google Scholar] [CrossRef]
- de Oliveira, A.; Adams, S.D.; Lee, L.H.; Murray, S.R.; Hsu, S.D.; Hammond, J.R.; Dickinson, D.; Chen, P.; Chu, T.-C. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem. Toxicol. 2013, 52, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Lee, L.H.; Adams, S.D. EGCG-S Impacts Oxidative Stress and Infection of Enterovirus 69 in Lung Cells. Adv. Biosci. Biotechnol. 2021, 12, 109–124. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, C.-M.; Shahidi, F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. J. Funct. Foods 2012, 4, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Man, G.C.W.; Wang, J.; Song, Y.; Wong, J.H.; Zhao, Y.; Lau, T.S.; Leung, K.T.; Chan, T.H.; Wang, H.; Kwong, J.; et al. Therapeutic potential of a novel prodrug of green tea extract in induction of apoptosis via ERK/JNK and Akt signaling pathway in human endometrial cancer. BMC Cancer 2020, 20, 964. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (-)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Sang, S.; Hong, J.; Kwon, S.-J.; Lee, M.-J.; Ho, C.-T.; Yang, C.S. Peracetylation as a Means of Enhancing in Vitro Bioactivity and Bioavailability of Epigallocatechin-3-Gallate. Drug Metab. Dispos. 2006, 34, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.-S.; Pan, M.-H.; Ho, C.-T.; Shahidi, F. Protective effects of epigallocatechin gallate (EGCG) derivatives on azoxymethane-induced colonic carcinogenesis in mice. J. Funct. Foods 2012, 4, 323–330. [Google Scholar] [CrossRef]
- Minnelli, C.; Galeazzi, R.; Laudadio, E.; Amici, A.; Rusciano, D.; Armeni, T.; Cantarini, M.; Stipa, P.; Mobbili, G. Monoalkylated Epigallocatechin-3-gallate (C18-EGCG) as Novel Lipophilic EGCG Derivative: Characterization and Antioxidant Evaluation. Antioxidants 2020, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Chia (Salvia hispanica L.) oil stability: Study of the effect of natural antioxidants. LWT 2017, 75, 107–113. [Google Scholar] [CrossRef]
- Kusunoki, C.; Yang, L.; Yoshizaki, T.; Nakagawa, F.; Ishikado, A.; Kondo, M.; Morino, K.; Sekine, O.; Ugi, S.; Nishio, Y.; et al. Omega-3 polyunsaturated fatty acid has an anti-oxidant effect via the Nrf-2/HO-1 pathway in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2013, 430, 225–230. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, S.M.; Dykes, G.A. Potential mechanisms for the effects of tea extracts on the attachment, biofilm formation and cell size of Streptococcus mutans. Biofouling 2013, 29, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Maeda, M.; Okubo, S.; Shimamura, T. Role of Hydrogen Peroxide in Bactericidal Action of Catechin. Biol. Pharm. Bull. 2004, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Anita, P.; Sivasamy, S.; Madan Kumar, P.D.; Balan, I.N.; Ethiraj, S. In vitro antibacterial activity of Camellia sinensis extract against cariogenic microorganisms. J. Basic Clin. Pharm. 2014, 6, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Oh, Y.J.; Lim, J.; Youn, M.; Lee, I.; Pak, H.K.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, K.; Hojo, H.; Suzuki, M.; Nanjo, F.; Kumazawa, S.; Nakayama, T. Relationship between Antibacterial Activity of (+)-Catechin Derivatives and Their Interaction with a Model Membrane. J. Agric. Food Chem. 2004, 52, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Lee, L.H.; Aponte, T.; Lopez, S.; Lalata, G.; Herrera, G.; Yussof, A.; Dickinson, D.; Hsu, S. Sporicidal Activity of Novel Formulations Containing Lipophilic Epigallocatechin-3-Gallate and Natural Ingredients. Microbiol. Infect. Dis. 2019, 3, 1–6. [Google Scholar] [CrossRef]
- Chu, T.; Lee, L.H.; Yussof, A.; Lopez, S.; Herrera, G.; Luna, P.; Uddin, M.; Wu, L.; Murzaku, J.A.; Dickinson, D.; et al. Enhanced Sporicidal Activity of Alcohol and Epigallocatechin-Palmitate-Based Hand Hygiene Formulations Comprised of Plant-Derived Compounds. J. Biosci. Med. 2020, 8, 89–99. [Google Scholar] [CrossRef]
- Pradhan, P.; Nguyen, M.L. Herpes simplex virus virucidal activity of MST-312 and epigallocatechin gallate. Virus Res. 2018, 249, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-y.; Li, Y.-q.; Guo, Z.-w.; Zhou, X.-h.; Lu, M.-d.; Xue, T.-c.; Gao, B. ERK1/2-HNF4α axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. Acta Pharmacol. Sin. 2020, 41, 278–285. [Google Scholar] [CrossRef]
- Shahid, F.; Noreen; Ali, R.; Badshah, S.L.; Jamal, S.B.; Ullah, R.; Bari, A.; Majid Mahmood, H.; Sohaib, M.; Akber Ansari, S. Identification of Potential HCV Inhibitors Based on the Interaction of Epigallocatechin-3-Gallate with Viral Envelope Proteins. Molecules 2021, 26, 1257. [Google Scholar] [CrossRef]
- Ge, M.; Xiao, Y.; Chen, H.; Luo, F.; Du, G.; Zeng, F. Multiple antiviral approaches of (–)-epigallocatechin-3-gallate (EGCG) against porcine reproductive and respiratory syndrome virus infection in vitro. Antivir. Res. 2018, 158, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Da Silva-Junior, E.F.; Silva, L.R. Multi-target Approaches of Epigallocatechin-3-O-gallate (EGCG) and its Derivatives against Influenza Viruses. Curr. Top. Med. Chem. 2022, 22, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, R.; Park, Y.-I.; Cha, Y.-E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (−)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Li, Q.-S.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Downard, K.M. Catechin inhibition of influenza neuraminidase and its molecular basis with mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 222–230. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, E.V.; Colpitts, C.C. The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus attachment inhibitor. Sci. Rep. 2022, 12, 12899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-Q.; Wei, X.-L.; Fang, Y.-P.; Gan, R.-Y.; Wang, M.; Ge, Y.-Y.; Zhang, D.; Cheng, L.-Z.; Corke, H. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 2022, 60, 1243–1264. [Google Scholar] [CrossRef] [PubMed]
- Stamos, J.D.; Lee, L.H.; Taylor, C.; Elias, T.; Adams, S.D. In Vitro and In Silico Analysis of the Inhibitory Activity of EGCG-Stearate against Herpes Simplex Virus-2. Microorganisms 2022, 10, 1462. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, J.; Zheng, J.-Y.; Pearl, H.; Dickinson, D.; Fu, B.; Hsu, S. A Proprietary Topical Preparation Containing EGCG-Stearate and Glycerin with Inhibitory Effects on Herpes Simplex Virus: Case Study. Inflamm. Allergy Drug Targets 2012, 11, 364–368. [Google Scholar] [CrossRef]
- McCauley, J.A.; Rudd, M.T. Hepatitis C virus NS3/4a protease inhibitors. Curr. Opin. Pharmacol. 2016, 30, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Bae, J.W.; Kim, J.M.; Lee, S.G.; Han, M. Antiproliferative and apoptotic effect of epigallocatechin-3-gallate on Ishikawa cells is accompanied by sex steroid receptor downregulation. Int. J. Mol. Med. 2012, 30, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, Q.; Wang, S.; Shi, C. Epigallocatechin-3-gallate inhibits the formation of neutrophil extracellular traps and suppresses the migration and invasion of colon cancer cells by regulating STAT3/CXCL8 pathway. Mol. Cell. Biochem. 2023, 478, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Martel, F. The Role of EGCG in Breast Cancer Prevention and Therapy. Mini Rev. Med. Chem. 2021, 21, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.-W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar] [CrossRef]
- Dhatwalia, S.K.; Kumar, M.; Dhawan, D.K. Role of EGCG in Containing the Progression of Lung Tumorigenesis—A Multistage Targeting Approach. Nutr. Cancer 2018, 70, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wan, S.B.; Yang, H.; Yuan, J.; Chan, T.H.; Dou, Q.P. Chapter 7—EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment. Adv. Clin. Chem. 2011, 53, 155–177. [Google Scholar] [CrossRef]
- Matsumura, K.; Kaihatsu, K.; Mori, S.; Cho, H.H.; Kato, N.; Hyon, S.H. Enhanced antitumor activities of (-)-epigallocatechin-3-O-gallate fatty acid monoester derivatives in vitro and in vivo. Biochem. Biophys. Res. Commun. 2008, 377, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Agarwal, R.; Mukhtar, H.; Nieminen, A.-L. Green Tea Constituent Epigallocatechin-3-Gallate and Induction of Apoptosis and Cell Cycle Arrest in Human Carcinoma Cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef]
- Landis-Piwowar, K.R.; Huo, C.; Chen, D.; Milacic, V.; Shi, G.; Chan, T.H.; Dou, Q.P. A Novel Prodrug of the Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate as a Potential Anticancer Agent. Cancer Res. 2007, 67, 4303–4310. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Lam, W.H.; Kazi, A.; Daniel, E.; Song, S.; Chow, L.M.C.; Chan, A.S.C.; Dou, Q.P. Synthetic peracetate tea polyphenols as potent proteasome inhibitors and apoptosis inducers in human cancer cells. Front. Biosci. -Landmark 2005, 10, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chiou, Y.-S.; Pan, M.-H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, R.; Wang, R.; Hu, Y.; Dong, X.; Xu, A.e. The design, synthesis and biological evaluation of pro-EGCG derivatives as novel anti-vitiligo agents. RSC Adv. 2016, 6, 106308–106315. [Google Scholar] [CrossRef]
- Du, J.; Wang, Y.; Tu, Y.; Guo, Y.; Sun, X.; Xu, X.; Liu, X.; Wang, L.; Qin, X.; Zhu, M.; et al. A prodrug of epigallocatechin-3-gallate alleviates high glucose-induced pro-angiogenic factor production by inhibiting the ROS/TXNIP/NLRP3 inflammasome axis in retinal Müller cells. Exp. Eye Res. 2020, 196, 108065. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.; Zhong, Y.J.; Perera, N.; Shahidi, F. Antiglycation activity of lipophilized epigallocatechin gallate (EGCG) derivatives. Food Chem. 2016, 190, 1022–1026. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Bouwens, M.; van de Rest, O.; Dellschaft, N.; Bromhaar, M.G.; de Groot, L.C.; Geleijnse, J.M.; Müller, M.; Afman, L.A. Fish-oil supplementation induces antiinflammatory gene expression profiles in human blood mononuclear cells1. Am. J. Clin. Nutr. 2009, 90, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Huang, Y.; Kong, A.-N. Synergistic anti-inflammatory effects of low doses of curcumin in combination with polyunsaturated fatty acids: Docosahexaenoic acid or eicosapentaenoic acid. Biochem. Pharmacol. 2010, 79, 421–430. [Google Scholar] [CrossRef]
- Choi, J.I.; Kim, W.M.; Lee, H.G.; Kim, Y.O.; Yoon, M.H. Role of neuronal nitric oxide synthase in the antiallodynic effects of intrathecal EGCG in a neuropathic pain rat model. Neurosci. Lett. 2012, 510, 53–57. [Google Scholar] [CrossRef]
- Ning, W.; Wang, S.; Dong, X.; Liu, D.; Fu, L.; Jin, R.; Xu, A. Epigallocatechin-3-gallate (EGCG) suppresses the trafficking of lymphocytes to epidermal melanocytes via inhibition of JAK2: Its implication for vitiligo treatment. Biol. Pharm. Bull. 2015, 38, 1700–1706. [Google Scholar] [CrossRef]
- Ning, W.; Wang, S.; Liu, D.; Fu, L.; Jin, R.; Xu, A. Potent effects of peracetylated (-)-epigallocatechin-3-gallate against hydrogen peroxide-induced damage in human epidermal melanocytes via attenuation of oxidative stress and apoptosis. Clin. Exp. Dermatol. 2016, 41, 616–624. [Google Scholar] [CrossRef]
- Kamiyama, O.; Sanae, F.; Ikeda, K.; Higashi, Y.; Minami, Y.; Asano, N.; Adachi, I.; Kato, A. In vitro inhibition of α-glucosidases and glycogen phosphorylase by catechin gallates in green tea. Food Chem. 2010, 122, 1061–1066. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Spilsbury, K.; Garrett, K.L.; Shen, W.-Y.; Constable, I.J.; Rakoczy, P.E. Overexpression of Vascular Endothelial Growth Factor (VEGF) in the Retinal Pigment Epithelium Leads to the Development of Choroidal Neovascularization. Am. J. Pathol. 2000, 157, 135–144. [Google Scholar] [CrossRef]
- Kumar, A.; Shamsuddin, N. Retinal Muller Glia Initiate Innate Response to Infectious Stimuli via Toll-Like Receptor Signaling. PLoS ONE 2012, 7, e29830. [Google Scholar] [CrossRef]
- Jaffe, G.J.; Ying, G.-S.; Toth, C.A.; Daniel, E.; Grunwald, J.E.; Martin, D.F.; Maguire, M.G. Macular Morphology and Visual Acuity in Year Five of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 252–260. [Google Scholar] [CrossRef]
- Xu, J.; Tu, Y.; Wang, Y.; Xu, X.; Sun, X.; Xie, L.; Zhao, Q.; Guo, Y.; Gu, Y.; Du, J.; et al. Prodrug of epigallocatechin-3-gallate alleviates choroidal neovascularization via down-regulating HIF-1alpha/VEGF/VEGFR2 pathway and M1 type macrophage/microglia polarization. Biomed. Pharmacother. 2020, 121, 109606. [Google Scholar] [CrossRef]
- Mazumder, K.; Biswas, B.; Al Mamun, A.; Billah, H.; Abid, A.; Sarkar, K.K.; Saha, B.; Azom, S.; Kerr, P.G. Investigations of AGEs’ inhibitory and nephroprotective potential of ursolic acid towards reduction of diabetic complications. J. Nat. Med. 2022, 76, 490–503. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Hu, X.; Pan, J.; Liao, Y.; Ding, H. Inhibitory effect of epicatechin gallate on protein glycation. Food Res. Int. 2019, 122, 230–240. [Google Scholar] [CrossRef]
- Kanner, J.; Rosenthal, I. An assessment of lipid oxidation in foods (Technical Report). Pure Appl. Chem. 1992, 64, 1959–1964. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Romsted, L.S.; Bravo-Diaz, C. Modeling chemical reactivity in emulsions. Curr. Opin. Colloid Interface Sci. 2013, 18, 3–14. [Google Scholar] [CrossRef]
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Cao, L.-L.; Pang, M.; Hou, Z.-G.; Jiang, S.-T. Preparation of camellia oil-based W/O emulsions stabilized by tea polyphenol palmitate: Structuring camellia oil as a potential solid fat replacer. Food Chem. 2019, 276, 209–217. [Google Scholar] [CrossRef]
- Burenjargal, M.; Narangerel, T.; Batmunkh, T.; Dong, A.; Idesh, S. A review of the bioactive properties of Mongolian plants, with a focus on their potential as natural food preservatives. Food Sci. Nutr. 2023, 11, 5736–5752. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Zhou, Q.; Mo, M.; Wang, A.; Tang, B.; He, Q. Changes in N-nitrosamines, Residual Nitrites, Lipid Oxidation, Biogenic Amines, and Microbiota in Chinese Sausages Following Treatment with Tea Polyphenols and Their Palmitic Acid-modified Derivatives. J. Food Prot. 2023, 86, 100072. [Google Scholar] [CrossRef]
- Shi, D. Green Tea Polyphenols as Potential Food Additives; Montclair State University: Montclair, NJ, USA, 2018; Available online: https://digitalcommons.montclair.edu/etd/112 (accessed on 23 January 2024).

| Acyl Donor | Medium | Catalyst | Reaction Conditions | Yield (%) | Reference | |
|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | |||||
| Acetic anhydride | - | Pyridine | 45 | 20 | 98.3 | [20] |
| Acetic anhydride | - | Pyridine | Room temperature | 24 | 82 | [15] |
| Stearyl chloride | Ethyl acetate | Pyridine | 50 | 6 | 56.9 | [21] |
| Eicosapentaenoyl chloride | Ethyl acetate | Pyridine | 50 | 6 | 42.7 | |
| Docosahexaenoyl chloride | Ethyl acetate | Pyridine | 50 | 6 | 30.7 | |
| Palmitoyl chloride | Ethyl acetate | - | 40 | 3 | 46.3 | [22] |
| Palmitoyl chloride | Ethyl acetate | Sodium bicarbonate | 60 | 8 | 53.5 | [23] |
| Palmitoyl chloride | Acetone | Sodium acetate | 40 | 6 | 63.3 | [14] |
| Oleyl chloride | Acetone | DMAP, DCC | Room temperature | 12 | 98 | [24] |
| Palmitoyl chloride | Tetrahydrofuran | Triethylamine | 25 | 24 | 23 | [25] |
| Acyl Donor | Medium | Lipase | Source of Lipase | Reaction Conditions | Yield (%) | Reference | |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | ||||||
| Vinyl acetate | Acetonitrile | Lipase RM IM | Rhizomucor miehei | 40 | 8 | 84.5 | [12] |
| Vinyl acetate | Acetonitrile/isopropyl alcohol | Lipase RM IM | Rhizomucor miehei | 50 | 10 | 83.2 | [38] |
| Vinyl acetate | [Bmim][BF4] | Novozym 435 | Candida antarctica lipase B | 70 | 10 | 98.7 | [39] |
| Vinyl laurate | Acetone | Lipase DF “Amano” 15 | Rhizopus delemar | 50 | 96 | 80.1 | [40] |
| Vinyl stearate | Acetonitrile | Lipase DF “Amano” 15 | Rhizopus delemar | 50 | 96 | 65.2 | [32] |
| Vinyl fatty acids with different chain lengths (C2-C18) | N, N-Dimethylformamide | Lipase PS | Burkholderia cepacia | 50 | 8 | 13.9~35.7 | [41] |
| Vinyl fatty acids with different chain lengths (C4-C20) | N, N-Dimethylformamide | Lipase PL | Pancreas | 57 | 1.5 | 35~39 | [25] |
| Vinyl fatty acids with different chain lengths (C2-C12) | [Bmim][BF4] | Novozym 435 | Candida antarctica lipase B | 70 | 12 | - | [11] |
| Lauric acid | Ethyl alcohol | Lipozyme TL IM | Thermomyces lanuginosus | 45 | 12 | 63.2 | [42] |
| Bioactivity | Acyl Donor | Action Site | Model System | Effect | Reference |
|---|---|---|---|---|---|
| Antioxidant | C2 | 5′,3″,5″ | DPPH; Sunflower oil | ↑ | [12] |
| C12 | 5″; 3″,5″; 5′,3″,5″ | Hydroxide; DPPH; ABTS Soybean oil | ↓ ↑ | [40] | |
| C16 | 4′ | ABTS Edible lard | ↓ ↑ | [14] | |
| C16 | 4′ | Sunflower oil | ↑ | [23] | |
| C2-C12 | 3″,5″ | ABTS; DPPH; Hydroxide Sunflower oil; Oil-in-water emulsion | ↓ ↑ | [11] | |
| C2-C18 | 4′; 5′; 4″; 5″; 3′,5′; 3″,5″; 5′,5″; 4′,5″; 4″,5′; 4′,4″ | DPPH; ABTS; FRAP Iron chelates | ↓ ↑ | [41] | |
| C18; C20; C22 | 3′,5′,3″,5″ | DPPH | ↑ | [21] | |
| C18, C20, C22 | 3′,5′,3″,5″ | Corn oil; β-carotene/linoleic acid; Fresh pork; LDL cholesterol | ↑ | [56] | |
| Antimicrobial | C2 | 5,7,3′,4′,5″,3″,4″,5″ | Staphylococcus aureus, Bacillus subtilis (GPB); Escherichia coli, Yersinia enterocolitis (GNB) | ↑ | [57] |
| C16 | 3′,4′,4″,5″ | Staphylococcus aureus, Bacillus subtilis (GPB); Escherichia coli, Pseudomonas aeruginosa (GNB) | ↑ | [54] | |
| C18 | 5′ | Bacillus cereus, Bacillus subtilis | ↑ | [58] | |
| C18 | 5′ | Streptococcus mutans | ↑ | [59] | |
| Antiviral | C16 | 4′ | Chicken eggs (influenza virus) | ↑ | [60] |
| C16 | 4′ | Vero cell (HSV-1) | ↑ | [61] | |
| C18 | 4′ | Lung cell lines A549 and MRC-5 (Enterovirus 69) | ↑ | [62] | |
| C4-C20 | 3′; 4′; 4″; 5″ | MDCK cells (influenza A/PR8/34) | ↑ | [25] | |
| C18; C20; C22 | 3′,5′,3″,5″ | HCV (protease) | ↑ | [63] | |
| Anticancer | C2 | 5,7,3′,4′,5″,3″,4″,5″ | Endometrial cells | ↑ | [64] |
| C2 | 5,7,3′,4′,5″,3″,4″,5″ | AN3CA and RL95-2 (Human endometrial Cancer cells) | ↑ | [65] | |
| C2 | 5,7,3′,4′,5″,3″,4″,5″ | KYSE150 (Human esophageal squamous cell) and HCT116 (Human colon cancer cell) | ↑ | [66] | |
| C12 | 3′,5′,3″,5″ | DU145 cells and RWPE-1 cells (human prostate cells) | ↑ | [42] | |
| C22 | 3′,5′,3″,5″ | Mice | ↑ | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Quan, W.; Wang, X.; Cheng, Y.; Jiao, Y. Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities. Foods 2024, 13, 1232. https://doi.org/10.3390/foods13081232
Zhuang Y, Quan W, Wang X, Cheng Y, Jiao Y. Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities. Foods. 2024; 13(8):1232. https://doi.org/10.3390/foods13081232
Chicago/Turabian StyleZhuang, Yingjun, Wei Quan, Xufeng Wang, Yunhui Cheng, and Ye Jiao. 2024. "Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities" Foods 13, no. 8: 1232. https://doi.org/10.3390/foods13081232
APA StyleZhuang, Y., Quan, W., Wang, X., Cheng, Y., & Jiao, Y. (2024). Comprehensive Review of EGCG Modification: Esterification Methods and Their Impacts on Biological Activities. Foods, 13(8), 1232. https://doi.org/10.3390/foods13081232





