Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Mash Treatment and Juice Pressing
2.3. Chemical Analysis
2.3.1. Soluble Solids Content
2.3.2. HPLC Analysis of Polyphenols
2.3.3. HPLC Analysis of L-Ascorbic, Malic and Citric Acid
2.3.4. Antioxidant Activity
2.4. Statistical Analysis
3. Results and Discussion
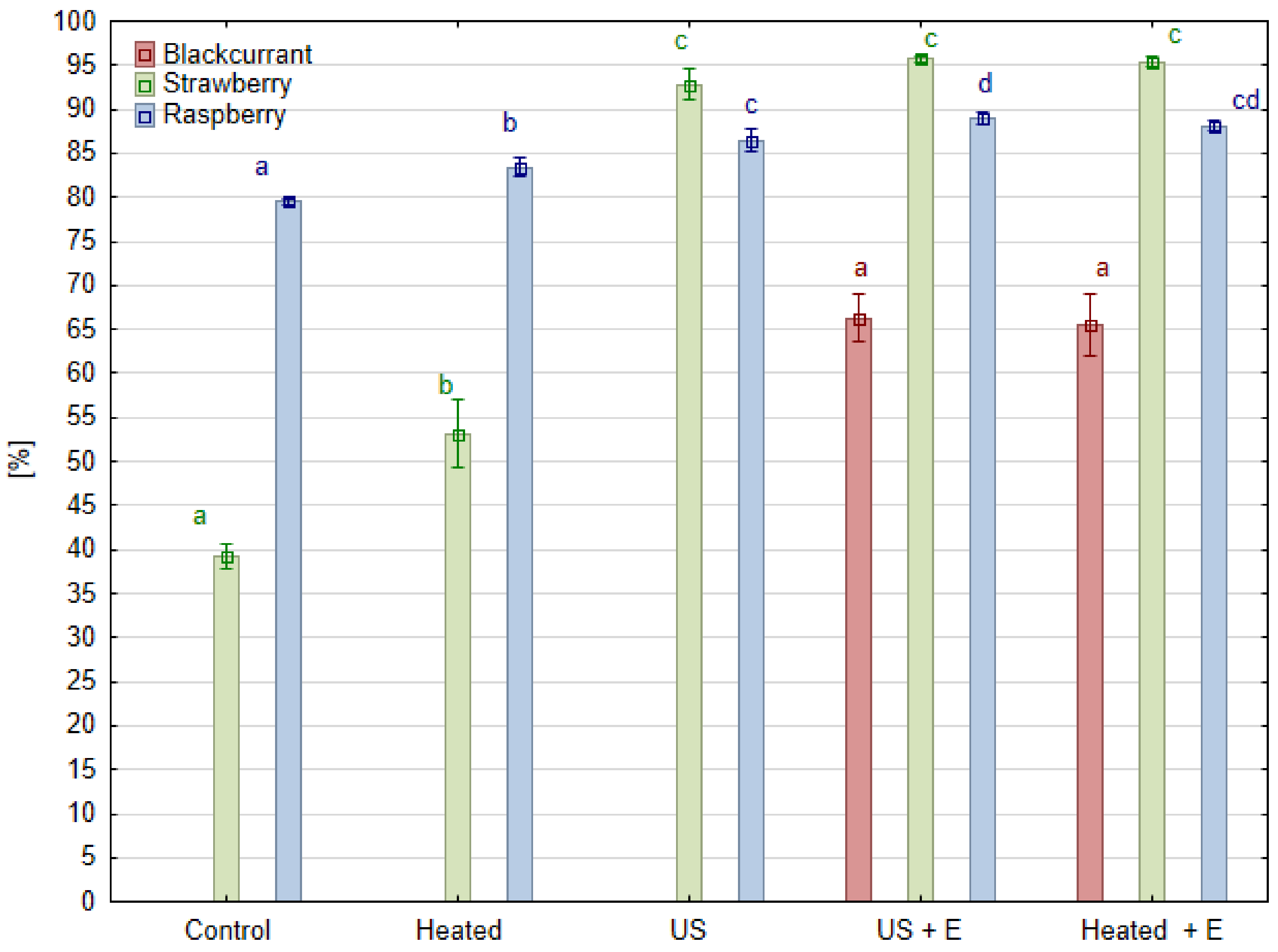
3.1. Pressing Yield
3.2. Soluble Solids
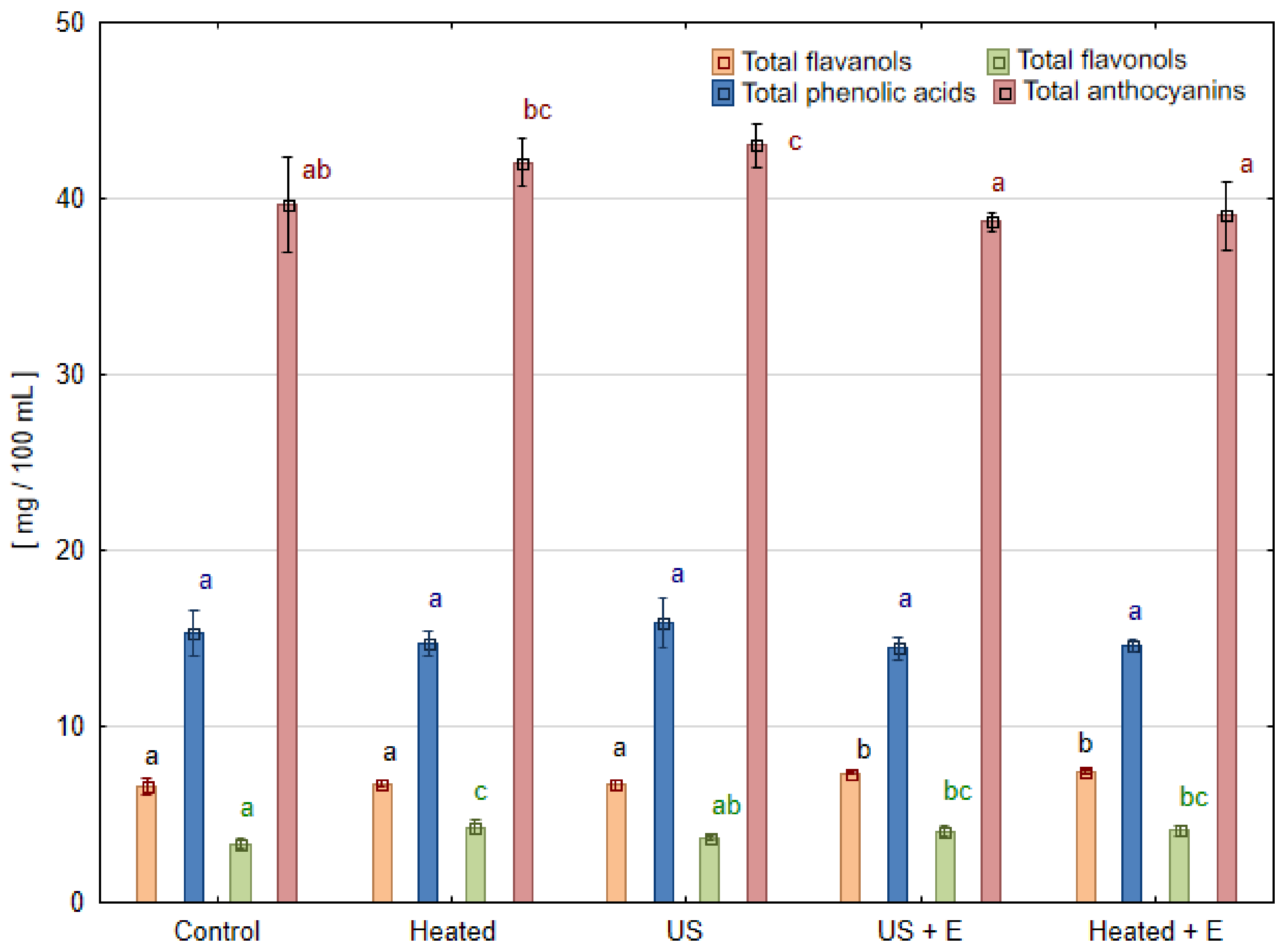
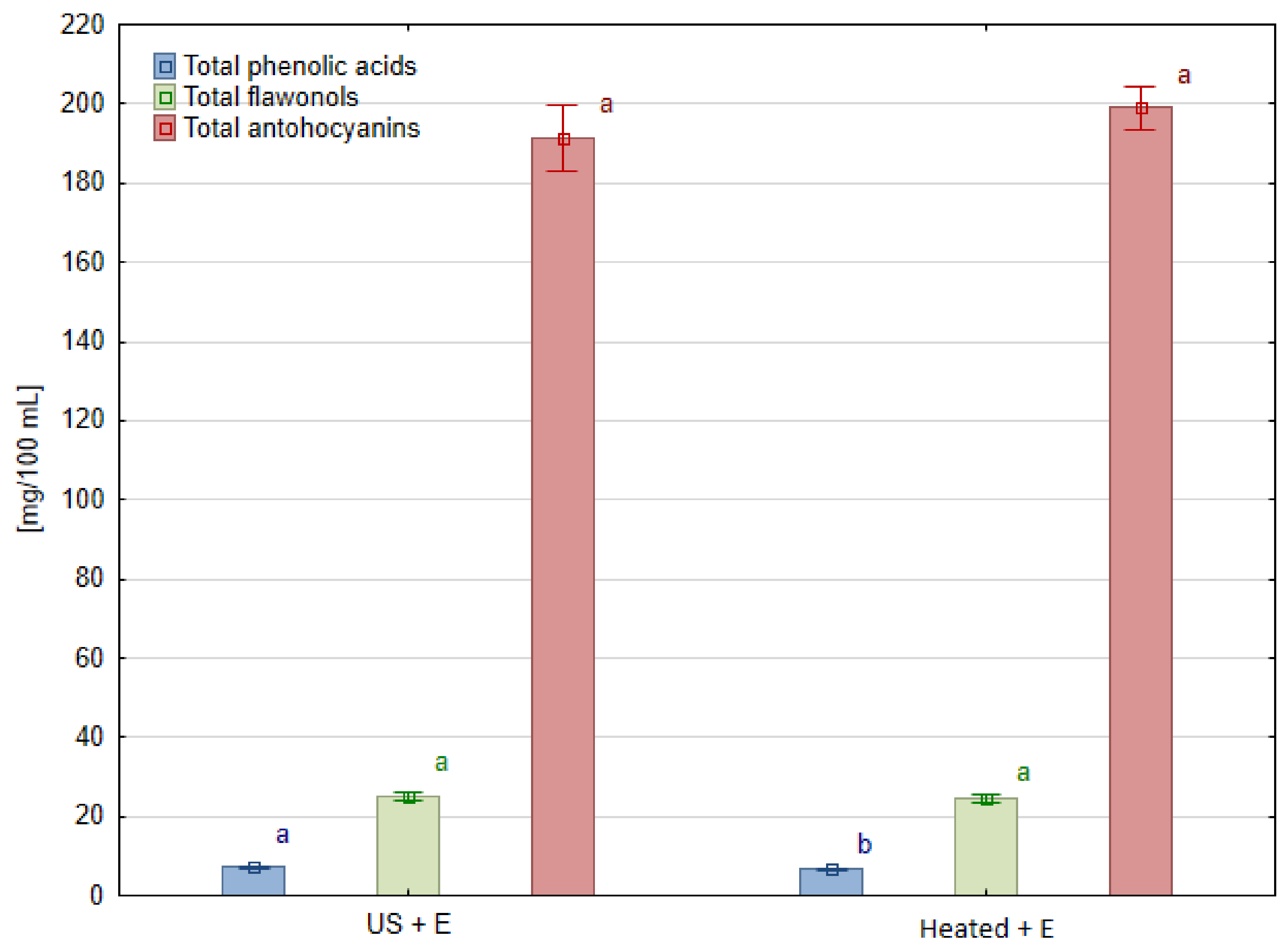
3.3. Phenolic Compounds
3.4. Ascorbic, Malic, and Citric Acid
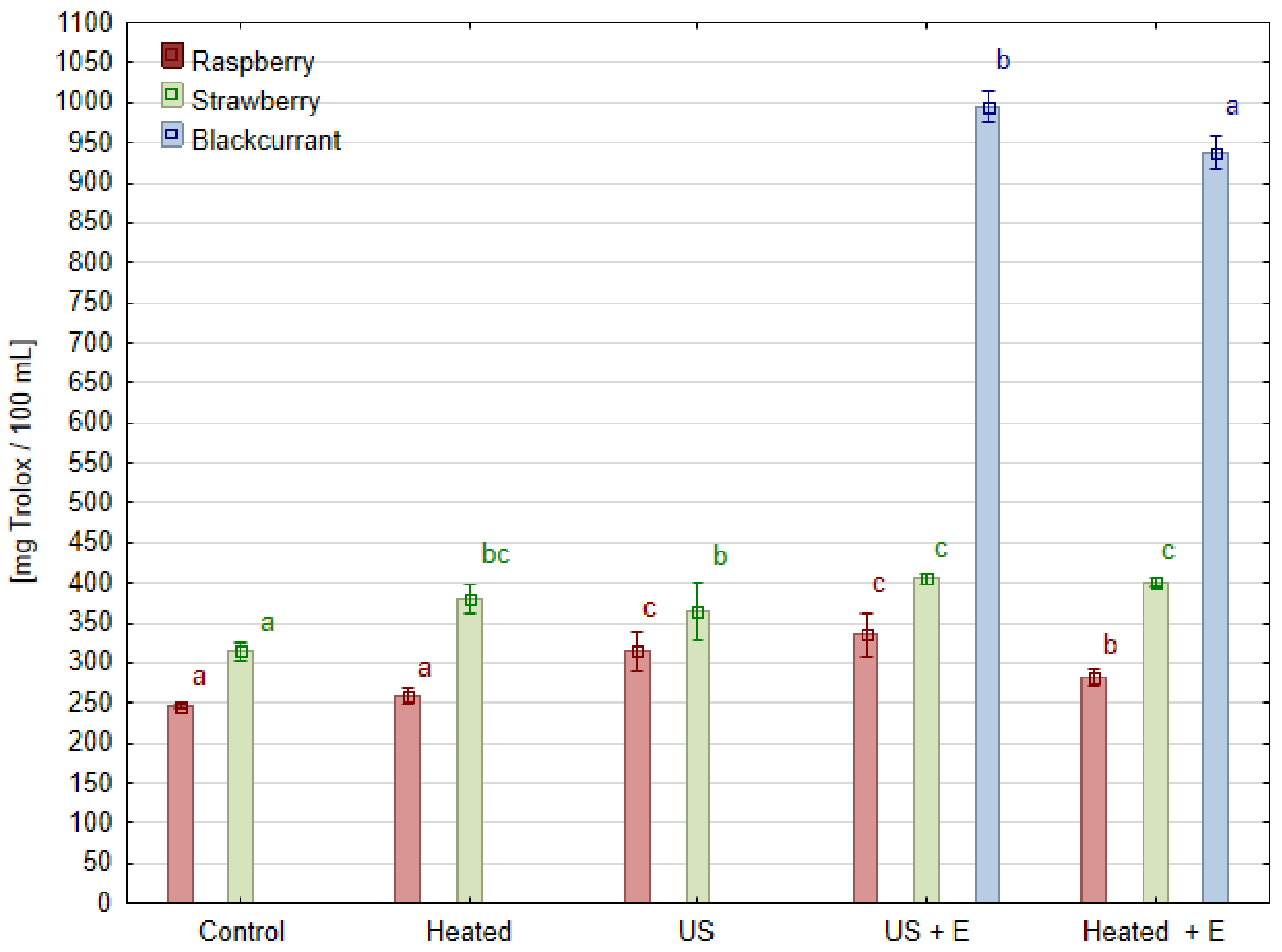
3.5. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemanowicz, M.; Krukowski, A. Comparisons of Qualitative and Quantitative Issues in the Fruit Supply Industries in The Netherlands, Poland, Greece, and Spain. J. Hortic. Sci. Biotechnol. 2009, 84, 13–17. [Google Scholar] [CrossRef]
- National Center for Agricultural Support. Krajowy Ośrodek Wsparcia Rolnictwa; National Center for Agricultural Support: Warsaw, Poland, 2018; ISBN 978-83-66255-00-5. [Google Scholar]
- Central Statistical Office in Poland. Available online: https://stat.gov.pl/en/ (accessed on 11 December 2023).
- Grużewska, A.; Gugała, M.; Zarzecka, K. Analiza rynku owoców jagodowych-wybrane elementy. Zesz. Nauk. SGGW W Warszawie-Probl. Rol. Świat. 2018, 18, 152–161. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Beltsville Human Nutrition Research Center. Food Data Central. Foundation Foods. Available online: https://fdc.nal.usda.gov/ (accessed on 3 April 2024).
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The Health Benefits of Blackcurrants. Food Funct. 2012, 3, 795. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising Health Benefits of the Strawberry: A Focus on Clinical Studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Snyder, D.M. Raspberries and Human Health: A Review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K. Opportunities and Challenges in Application of Ultrasound in Food Processing. Crit. Rev. Food Sci. Nutr. 2011, 51, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Guo, N.; Teh, C.Y.; Hay, J.X.W. Theory and Fundamentals of Ultrasound. In Advances in Ultrasound Technology for Environmental Remediation; SpringerBriefs in Molecular Science; Springer: Dordrecht, The Netherlands, 2013; pp. 5–12. ISBN 978-94-007-5532-1. [Google Scholar]
- Priyadarshini, A.; Rajauria, G.; O’Donnell, C.P.; Tiwari, B.K. Emerging Food Processing Technologies and Factors Impacting Their Industrial Adoption. Crit. Rev. Food Sci. Nutr. 2019, 59, 3082–3101. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of Ultrasound in Food Technology: Processing, Preservation and Extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Butz, P.; Tauscher, B. Emerging Technologies: Chemical Aspects. Food Res. Int. 2002, 35, 279–284. [Google Scholar] [CrossRef]
- Larsen, L.R.; Van Der Weem, J.; Caspers-Weiffenbach, R.; Schieber, A.; Weber, F. Effects of Ultrasound on the Enzymatic Degradation of Pectin. Ultrason. Sonochem. 2021, 72, 105465. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.-M.; Gouda, M.; Zhu, Y.-Y.; Ye, X.-Q.; Chen, J.-C. Ultrasound-Assisted Extraction Optimization of Proanthocyanidins from Kiwi (Actinidia chinensis) Leaves and Evaluation of Its Antioxidant Activity. Antioxidants 2021, 10, 1317. [Google Scholar] [CrossRef] [PubMed]
- Khouryieh, H.A. Novel and Emerging Technologies Used by the U.S. Food Processing Industry. Innov. Food Sci. Emerg. Technol. 2021, 67, 102559. [Google Scholar] [CrossRef]
- Nielsen, I.L.F.; Haren, G.R.; Magnussen, E.L.; Dragsted, L.O.; Rasmussen, S.E. Quantification of Anthocyanins in Commercial Black Currant Juices by Simple High-Performance Liquid Chromatography. Investigation of Their pH Stability and Antioxidative Potency. J. Agric. Food Chem. 2003, 51, 5861–5866. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Killermann, K.V.; Rehmann, D.; Weidlich, H.H. Effect of Mash Enzyme and Heat Treatments on the Cellular Antioxidant Activity of Black Currant (Ribes nigrum), Raspberry (Rubus idaeus), and Blueberry (Vaccinium myrtillus) Juices. CyTA-J. Food 2017, 15, 277–283. [Google Scholar] [CrossRef]
- Nguyen, C.; Nguyen, H. Ultrasonic Effects on the Quality of Mulberry Juice. Beverages 2018, 4, 56. [Google Scholar] [CrossRef]
- Cortez, R.E. Characterization of Blackcurrant Berries (Ribes nigrum) and the Evaluation of Their Bioactive Compounds after Ultrasound-Assisted Water Extractions, Enzymatic Treatments, and Fermentation. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2019. [Google Scholar]
- Laaksonen, O.A.; Mäkilä, L.; Sandell, M.A.; Salminen, J.-P.; Liu, P.; Kallio, H.P.; Yang, B. Chemical-Sensory Characteristics and Consumer Responses of Blackcurrant Juices Produced by Different Industrial Processes. Food Bioprocess Technol. 2014, 7, 2877–2888. [Google Scholar] [CrossRef]
- Kidoń, M.; Narasimhan, G. Effect of Ultrasound and Enzymatic Mash Treatment on Bioactive Compounds and Antioxidant Capacity of Black, Red and White Currant Juices. Molecules 2022, 27, 318. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska-Kubzdela, E.; Szwengiel, A.; Ratajkiewicz, H.; Nowak, K. Effect of Ultrasound, Heating and Enzymatic Pre-Treatment on Bioactive Compounds in Juice from Berberis amurensis Rupr. Ultrason. Sonochem. 2020, 63, 104971. [Google Scholar] [CrossRef]
- Bora, S.J.; Handique, J.; Sit, N. Effect of Ultrasound and Enzymatic Pre-Treatment on Yield and Properties of Banana Juice. Ultrason. Sonochem. 2017, 37, 445–451. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour Degradation and Quality Parameters of Sonicated Orange Juice Using Response Surface Methodology. LWT—Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Han, Z.; Sun, D.-W. Effects of Ultrasound Treatments on Quality of Grapefruit Juice. Food Chem. 2013, 141, 3201–3206. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Jiang, A. Effect of Ultrasound Treatment on Quality and Microbial Load of Carrot Juice. Food Sci. Technol. 2016, 36, 111–115. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and Biotransformation of Various Dietary Anthocyanins in Vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Sui, X. Literature Review. In Impact of Food Processing on Anthocyanins; Springer Theses; Springer: Singapore, 2017; pp. 5–14. ISBN 978-981-10-2611-9. [Google Scholar]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal Stability of Anthocyanins and Colourless Phenolics in Pomegranate (Punica granatum L.) Juices and Model Solutions. Food Chem. 2013, 138, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and Temperature Effects on Degradation Kinetics and Storage Stability of Total Anthocyanins in Blueberry Juice. J. Agric. Food Chem. 2010, 58, 10076–10084. [Google Scholar] [CrossRef] [PubMed]
- Romero Rodriguez, M.A.; Vazquez Oderiz, M.L.; Lopez Hernandez, J.; Lozano, J.S. Determination of Vitamin C and Organic Acids in Various Fruits by HPLC. J. Chromatogr. Sci. 1992, 30, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska-Kubzdela, E. Effect of Ultrasonic, Thermal and Enzymatic Treatment of Mash on Yield and Content of Bioactive Compounds in Strawberry Juice. Appl. Sci. 2023, 13, 4268. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in Global Vitamin C Recommendations: A Review of RDA Criteria and Underlying Health Perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]


| Species | Sample–Mash Treatment | ||||
|---|---|---|---|---|---|
| ‘Control’ | ‘Heated’ | ‘US’ | ‘US + E’ | ‘Heated + E’ | |
| Raspberry | 12.05 ± 0.26 a | 12.04 ± 0.14 a | 11.92 ± 0.37 a | 11.89 ± 0.27 a | 12.21 ± 0.24 a |
| Strawberry | 7.25 ± 0.16 a | 7.43 ± 0.05 ab | 7.27 ± 0.19 a | 7.54 ± 0.15 b | 7.47 ± 0.08 ab |
| Blackcurrant | no juice | no juice | no juice | 16.40 ± 0.09 a | 16.50 ± 0.17 a |
| Sample | Control | Heated | US | US + E | Heated + E | |
|---|---|---|---|---|---|---|
| PHENOLIC ACIDS | Chlorogenic acid derivative A | 0.72 ± 0.03 a | 0.73 ± 0.05 a | 0.72 ± 0.07 ab | 0.68 ± 0.04 a | 0.82 ± 0.09 b |
| Chlorogenic acid derivative B | 0.80 ± 0.23 a | 0.91 ± 0.07 a | 0.64 ± 0.29 a | 0.68 ± 0.14 a | 0.93 ± 0.11 a | |
| Ellagic acid | 11.05 ± 0.89 a | 9.98 ± 0.96 a | 14.32 ± 0.81 b | 13.92 ± 1.13 b | 12.72 ± 1.30 ab | |
| FLAVONOLS | Quercetin derivative | 0.32 ± 0.07 a | 0.39 ± 0.03 a | 0.32 ± 0.08 a | 0.33 ± 0.05 a | 0.41 ± 0.02 a |
| Quercetin-rutinoside | 0.47 ± 0.09 a | 0.54 ± 0.06 ab | 0.61 ± 0.07 b | 0.88 ± 0.07 c | 0.66 ± 0.02 b | |
| Quercetin-galactoside | 0.39 ± 0.30 a | 0.64 ± 0.05 a | 0.23 ± 0.36 a | 0.39 ± 0.48 a | 0.68 ± 0.12 a | |
| Quercetin-glucoside | 2.26 ± 0.25 a | 2.15 ± 0.07 a | 2.53 ± 0.33 a | 2.6 ± 0.29 a | 2.14 ± 0.11 a | |
| ANTHOCYANINS | Cyanidin 3-sophoroside | 51.06 ± 4.97 a | 50.58 ± 4.66 a | 103.58 ± 1.34 b | 97.66 ± 1.24 b | 43.24 ± 7.6 a |
| Cyanidin 3-glucosyl-rutinoside | 12.79 ± 0.94 b | 12.61 ± 1.14 b | 0.57 ± 0.21 a | 0.66 ± 0.18 a | 13.01 ± 1.43 b | |
| Cyanidin-3-glucoside | 11.92 ± 0.31 a | 12.52 ± 0.68 a | 16.80 ± 0.65 b | 16.09 ± 0.29 b | 11.73 ± 0.78 a | |
| Cyanidin 3-rutinoside | 8.41 ± 0.80 b | 8.45 ± 0.90 b | 0.26 ± 0.40 a | 0.57 ± 0.14 a | 8.82 ± 1.56 b 1 | |
| Sample | Control | Heated | US | US + E | Heated + E | |
|---|---|---|---|---|---|---|
| FLAVANOLS | Procyanidin dimer B1 | 1.37 ± 0.05 a | 1.64 ± 0.08 b | 1.66 ± 0.27 b | 1.80 ± 0.04 bc | 1.90 ± 0.05 c |
| Catechin | 5.19 ± 0.43 abc | 5.11 ± 0.17 ab | 5.02 ± 0.17 a | 5.52 ± 0.06 c | 5.49 ± 0.09 bc | |
| PHENOLIC ACIDS | Chlorogenic acid derivatives | 15.26 ± 1.28 a | 14.74 ± 0.71 a | 15.94 ± 1.40 a | 14.43 ± 0.69 a | 14.64 ± 0.36 a |
| FLAVONOLS | Quercetin derivative A | 0.17 ± 0.02 a | 0.26 ± 0.04 b | 0.27 ± 0.07 b | 0.30 ± 0.01 b | 0.28 ± 0.01 b |
| Quercetin-3-rutinoside | 1.61 ± 0.18 a | 2.05 ± 0.21 b | 1.60 ± 0.01 a | 1.90 ± 0.24 ab | 2.01 ± 0.20 b | |
| Quercetin-3-glucoside | 0.11 ± 0.01 a | 0.25 ± 0.32 a | 0.11 ± 0.01 a | 0.12 ± 0.01 a | 0.11 ± 0.01 a | |
| Quercetin derivative B | 0.27 ± 0.03 a | 0.36 ± 0.02 bc | 0.35 ± 0.05 b | 0.40 ± 0.00 c | 0.37 ± 0.02 bc | |
| Quercetin derivative C | 0.11 ± 0.01 a | 0.13 ± 0.01 b | 0.13 ± 0.00 b | 0.11 ± 0.00 a | 0.11 ± 0.00 a | |
| Kaempferol-3-rutinoside | 0.85 ± 0.05 a | 0.95 ± 0.05 b | 0.90 ± 0.03 ab | 0.95 ± 0.06 b | 0.96 ± 0.05 b | |
| Quercetin derivative D | 0.24 ± 0.02 a | 0.27 ± 0.01 b | 0.27 ± 0.01 b | 0.26 ± 0.01 ab | 0.26 ± 0.01 ab | |
| ANTHOCYANINS | Cyanidin-3-glucoside | 2.03 ± 0.21 a | 2.29 ± 0.06 ab | 2.43 ± 0.28 b | 2.35 ± 0.23 ab | 2.31 ± 0.14 ab |
| Pelargonidin-3-glucoside | 32.82 ± 2.03 ab | 34.34 ± 0.95 b | 35.04 ± 0.95 b | 31.62 ± 0.48 a | 31.93 ± 1.66 a | |
| Pelargonidin-3-rutinoside | 0.06 ± 0.05 a | 0.08 ± 0.06 a | 0.08 ± 0.01 a | 0.06 ± 0.04 a | 0.08 ± 0.01 a | |
| Cyanidin-3-malonylglucoside | 0.25 ± 0.03 a | 0.30 ± 0.01 bc | 0.31 ± 0.01 c | 0.28 ± 0.02 ab | 0.28 ± 0.02 ab | |
| Pelargonidin-3-(6″-malonylglucoside) | 4.51 ± 0.64 ab | 5.04 ± 0.33 bc | 5.18 ± 0.03 c | 4.37 ± 0.06 a | 4.42 ± 0.29 a 1 | |
| Sample | US + E | Heated + E | |
|---|---|---|---|
| PHENOLIC ACIDS | Chlorogenic acid derivatives A | 1.92 ± 0.06 b | 1.79 ± 0.07 a |
| Chlorogenic acid derivatives B | 5.18 ± 0.16 b | 4.90 ± 0.07 a | |
| FLAVONOLS | unidentified flavonol B | 10.09 ± 0.46 a | 10.30 ± 0.48 a |
| Quercetin-3-rutinoside | 3.10 ± 0.08 a | 3.03 ± 0.24 a | |
| Quercetin-3-galactoside | 0.43 ± 0.05 a | 0.41 ± 0.05 a | |
| Quercetin-3-glucoside | 6.94 ± 0.26 b | 6.41 ± 0.21 a | |
| Quercetin | 0.13 ± 0.01 a | 0.13 ± 0.02 a | |
| ANTHOCYANINS | Delphinidin-3-glucoside | 28.08 ± 1.28 a | 29.45 ± 0.71 b |
| Delphinidin-3-rutinoside | 97.56 ± 4.56 a | 101.19 ± 2.36 a | |
| Cyanidin-3-glucoside | 8.53 ± 0.32 a | 9.02 ± 0.49 a | |
| Cyanidin-3-rutinoside | 52.75 ± 2.20 a | 54.93 ± 1.83 a | |
| Peonidin-3-glucoside | 2.19 ± 0.11 a | 2.29 ± 0.04 a | |
| Peonidin-3-rutinoside | 0.75 ± 0.04 a | 0.77 ± 0.01 a 1 | |
| Sample | Ascorbic Acid | Malic Acid | Citric Acid | Total | |
|---|---|---|---|---|---|
| Raspberry | Control | 24.57 ± 0.41 c | 88.90 ± 5.00 b | 2050 ± 58.00 a | 2164 ± 56.00 a |
| Heated | 23.05 ± 1.07 bc | 88.60 ± 7.80 b | 2064 ± 59.00 a | 2176 ± 63.00 a | |
| US | 21.07 ± 1.47 ab | 91.78 ± 6.30 b | 1843 ± 201.00 a | 1956 ± 196.00 a | |
| US + E | 20.90 ± 0.86 ab | 84.40 ± 9.80 a | 1965 ± 95.00 a | 2071 ± 86.00 a | |
| Heated + E | 20.34 ± 0.71 a | 82.10 ± 6.90 a | 2054 ± 38.00 a | 2160 ± 41.00 a | |
| Strawberry | Control | 33.12 ± 2.19 a | 112.10 ± 27.70 ab | 785.0 ± 71.00 a | 931.0 ± 98.00 a |
| Heated | 29.86 ± 4.18 a | 100.10 ± 16.20 ab | 811.0 ± 39.00 a | 941.0 ± 55.00 ab | |
| US | 28.00 ± 3.34 a | 90.30 ± 8.00 a | 797.0 ± 12.00 a | 915.0 ± 8.00 a | |
| US + E | 29.58 ± 3.07 a | 121.00 ± 12.50 b | 888.0 ± 13.00 b | 1038 ± 25.00 b | |
| Heated + E | 28.47 ± 1.97 a | 118.70 ± 19.50 ab | 884.0 ± 33.00 b | 1031 ± 56.00 b | |
| Blackcurrant | Control | no juice | no juice | no juice | no juice |
| Heated | no juice | no juice | no juice | no juice | |
| US | no juice | no juice | no juice | no juice | |
| US + E | 129.00 ± 4.40 a | 370.30 ± 30.40 a | 4682 ± 97.00 a | 5181 ± 110.0 a | |
| Heated + E | 129.80 ± 1.90 a | 401.80 ± 36.40 a | 4718 ± 90.00 a | 5250 ± 115.0 a 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piecko, J.; Mieszczakowska-Frąc, M.; Celejewska, K.; Szwejda-Grzybowska, J. Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit. Foods 2024, 13, 1231. https://doi.org/10.3390/foods13081231
Piecko J, Mieszczakowska-Frąc M, Celejewska K, Szwejda-Grzybowska J. Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit. Foods. 2024; 13(8):1231. https://doi.org/10.3390/foods13081231
Chicago/Turabian StylePiecko, Jan, Monika Mieszczakowska-Frąc, Karolina Celejewska, and Justyna Szwejda-Grzybowska. 2024. "Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit" Foods 13, no. 8: 1231. https://doi.org/10.3390/foods13081231
APA StylePiecko, J., Mieszczakowska-Frąc, M., Celejewska, K., & Szwejda-Grzybowska, J. (2024). Impact of Ultrasound Pretreatment on Juice Yield and Bioactive Content in Juice Produced from Selected Berries Fruit. Foods, 13(8), 1231. https://doi.org/10.3390/foods13081231






