Optimization of Ultrasound-Assisted Extraction of Verbascum sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity
Abstract
1. Introduction
2. Material and Methods
2.1. Raw Material
2.2. Chemicals and Reagents
2.3. Extraction Method
2.3.1. Maceration Extraction
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.3.3. Response Surface Methodology and Optimization of UAE
2.3.4. Total Polyphenol Content
2.3.5. Total Flavonoids Content
2.4. Antioxidant Capacity of V. sinaiticum Leaf Extract
2.4.1. DPPH Radical Scavenger
2.4.2. ABTS•+ Radical Scavenging Test
2.5. Phytochemical Profiling by UHPLC-ESI-QTOF-MS/MS
2.6. ATR-FTIR
2.7. Scanning Electron Microscopy (SEM) Analysis
2.8. X-ray Diffraction (XRD) Analysis
2.9. Statistical Analysis
3. Result and Discussion
3.1. Effects of Ultrasonic-Assisted Extraction Parameters on Extraction Yield, Bioactive Compounds and Antioxidant Capacity
3.2. Optimization of UAE for Phenolic Compounds from V. sinaiticum
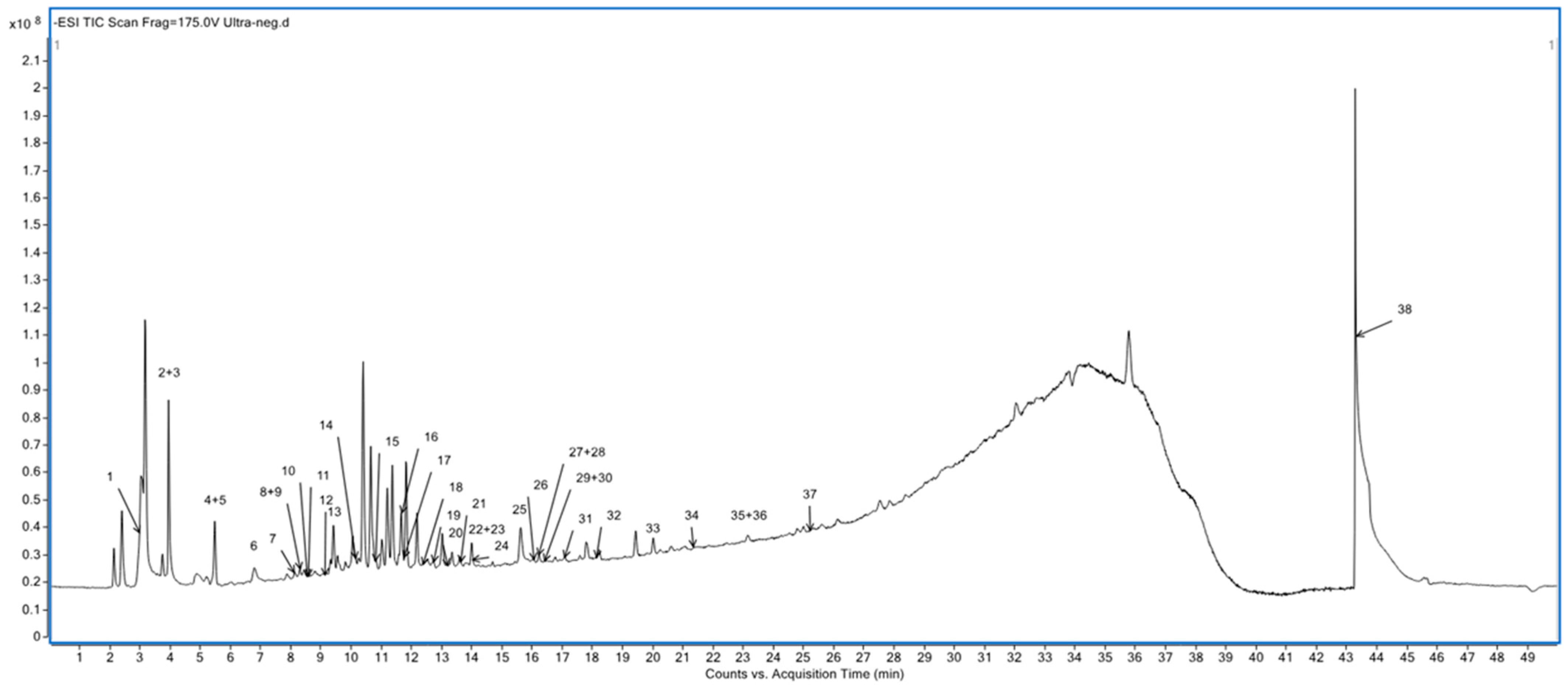
3.3. Characterization of Phenolic Compounds Using UHPLC-ESI-QTOF-MS/MS
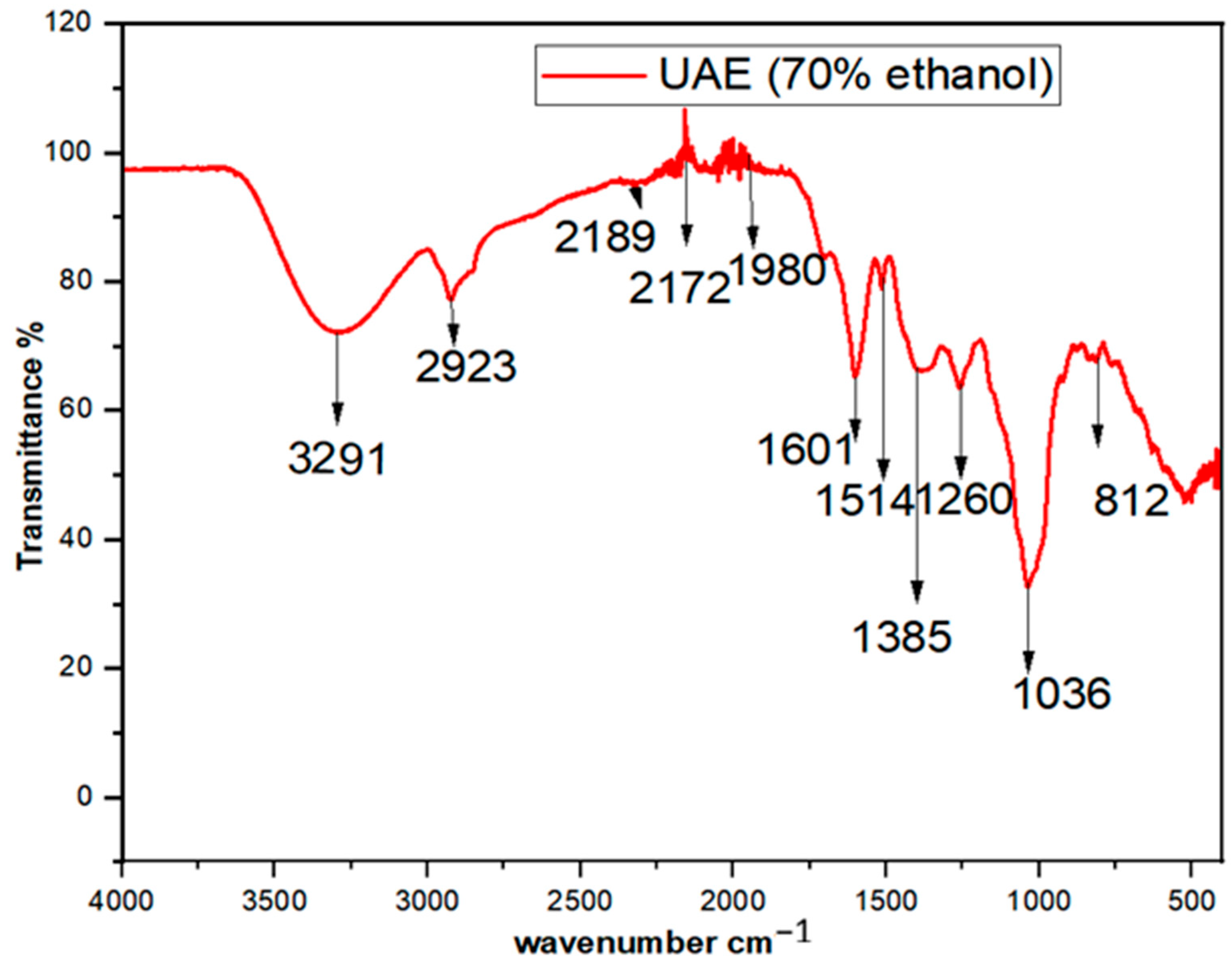
3.4. FTIR
3.5. XRD
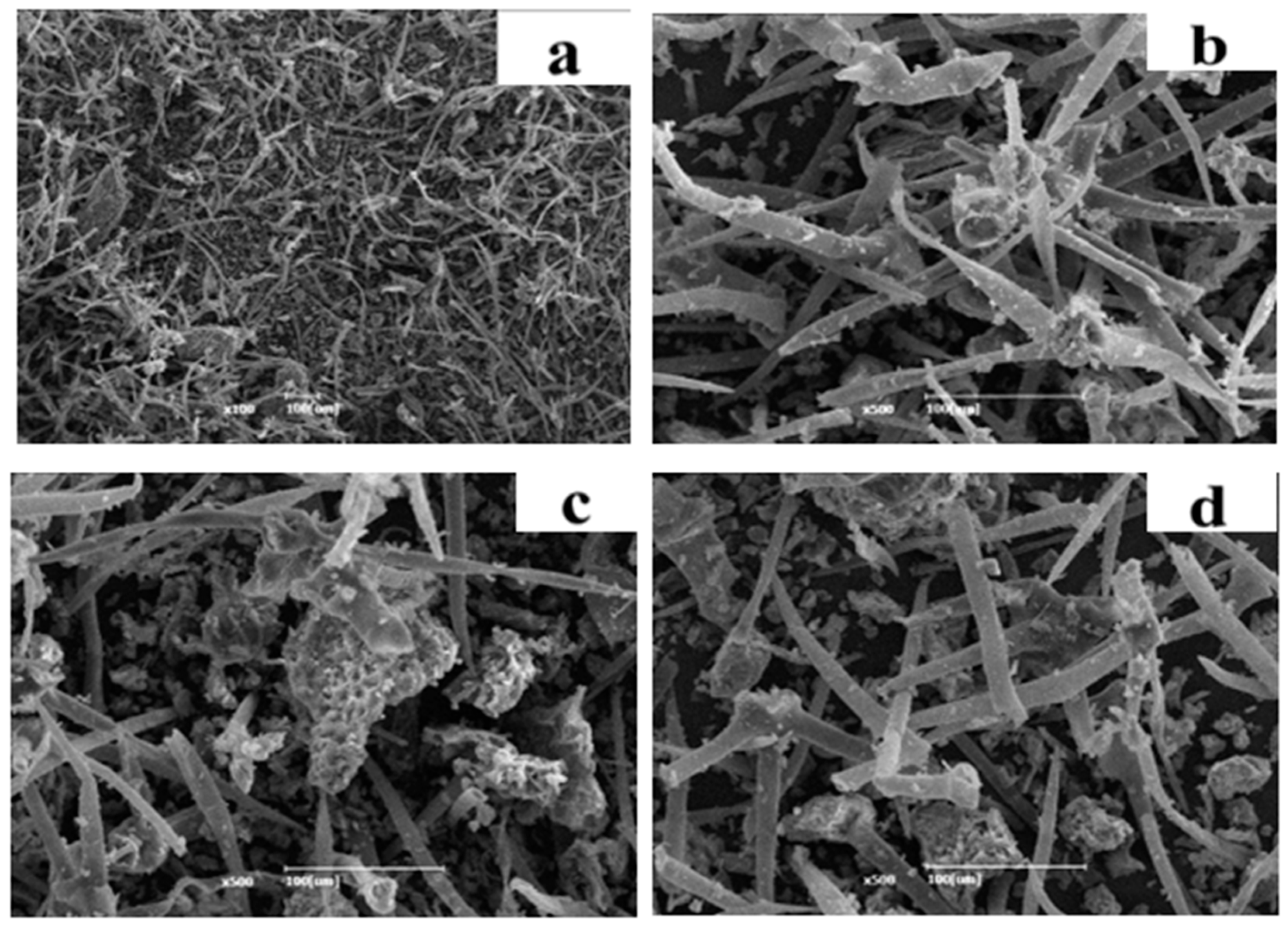
3.6. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ABTS | 2-azino-bis 3-ethylbenzeothiazoline-6-sulfonic acid diammonium salt |
| ACN | acetonitrile |
| CE | catechin Equivalent |
| CCD | central composite design |
| DPPH | 2,2,-diphenyl-1-picrylhydrazyl |
| Equ | equation |
| FBD | fluidized bed dryer, |
| FTIR | Fourier transform infrared |
| GAE | gallic acid equivalent |
| h | hour |
| ME | maceration |
| Mg CE/g D | milligram cathechin equivalent per gram dry extract |
| Min | minutes |
| Ov | oven dryer |
| SEM | scanning electron microscopy |
| TFC | total flavonoid content |
| TIC | total ionic component |
| TPC | total phenolic content |
| UAE | ultrasound-assisted extraction |
| UPHLC-QTOF MS/MS | ultra-high-performance liquid chromatography quadrupole time of flight mass spectroscopy |
| V. sinaiticum | Verbascum sinaiticum |
| XRD | X-ray diffractometer |
References
- Mergia, E.; Shibeshi, W.; Terefe, G.; Teklehaymanot, T. Antitrypanosomal activity of Verbascum sinaiticum Benth. (Scrophulariaceae) against Trypanosoma congolense isolates. BMC Complement. Altern. Med. 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asefa, M.; Teshome, N.; Degu, A. Anti-Inflammatory and Analgesic Activity of Methanolic Root Extract of Verbascum sinaiticum Benth. J. Inflamm. Res. 2022, 15, 6381–6392. [Google Scholar] [CrossRef] [PubMed]
- Lulseged, K.; Akele, M.Z.; Abiye, A.A.; Abebe, B.; Huluka, S.A. Wound Healing and Antioxidant Properties of 80% Methanol Leaf Extract of Verbascum sinaiticum (Scrophulariaceae): An Ethiopian Medicinal Plant. Evid.-Based Complement. Altern. Med. 2022, 2022, 9836773. [Google Scholar] [CrossRef]
- Afifi, M.S.A.; Ahmed, M.M.; Pezzuto, J.M.; Kinghornt, A.D. Cytotoxic flavonolignans and flavones from Verbascum sinaiticum leaves. Phytochemistry 1993, 34, 839–841. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Abdel-Azim, N.S.; Shahat, A.A.; Ismail, S.I.; Hammouda, F.M. Phytochemical and biological studies on Verbascum sinaiticum growing in Egypt. Nat. Prod. Sci. 2007, 13, 186–189. [Google Scholar]
- Aliero, A.A.; Afolayan, A.J. Antimicrobial activity of Solanum tomentosum. Afr. J. Biotechnol. 2006, 5, 369–372. Available online: http://www.academicjournals.org/AJB (accessed on 5 January 2024).
- Tadeg, H.; Mohammed, E.; Asres, K.; Gebre-Mariam, T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J. Ethnopharmacol. 2005, 100, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Kashyap, P.; Gadhave, R.K.; Jindal, N.; Kumar, S.; Guiné, R.P.F.; Mehra, R.; Kumar, H. Fluidized Bed Drying of Wheatgrass: Effect of Temperature on Drying Kinetics, Proximate Composition, Functional Properties, and Antioxidant Activity. Foods 2023, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Salarbashi, D.; Mortazavi, S.A.; Rezaei, K.; Rajaei, A.; Karimkhani, M.M. Optimization of ultrasound-assisted extraction of phenolic compounds from yarrow (Achillea beibrestinii) by response surface methodology. Food Sci. Biotechnol. 2012, 21, 1005–1011. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Identification and Quantification of Polyphenolic Compounds in Ten Pear Cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Mihailović, V.; Kreft, S.; Benković, E.T.; Ivanović, N.; Stanković, M.S. Chemical profile, antioxidant activity and stability in stimulated gastrointestinal tract model system of three Verbascum species. Ind. Crops Prod. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Workineh Shibeshi, E.M. Phytochemical Screening and In Vitro Antitrypanosomal Activity of Aqueous and Methanol Leaf Extract of Verbascum sinaiticum (Scrophulariaceae) against Trypanosoma congolense Field Isolate. J. Clin. Exp. Pathol. 2014, 45, 46. [Google Scholar] [CrossRef]
- Geyesa, J.M.; Esho, T.B.; Legesse, B.A.; Wotango, A.S. Antibacterial and antioxidant potential analysis of Verbascum sinaiticum leaf extract and its synthesized silver nanoparticles. Heliyon 2024, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, H.; Sadrameli, S.M.; Eslami, F.; Asoodeh, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar] [CrossRef]
- Shahbaz, F.; Akhter, N.; Shahid, M.; Riaz, M.; Anjum, F.; Hussain, F. Ultrasound Assisted Extraction and Characterization of Bioactives from Verbascum thapsus Roots to Evaluate Their Antioxidant and Medicinal Potential. Dose-Response 2022, 20, 15593258221097665. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar] [CrossRef]
- Shekhar, S.; Prakash, P.; Singha, P.; Prasad, K.; Singh, S.K. Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm. Foods 2023, 12, 1925. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, M.; Mai, X. Optimisation of ultrasonic-assisted extraction and biological activity of total flavonoids from leaves of Murrayae exotica using response surface methodology. Folia Hortic. 2023, 35, 135–148. [Google Scholar] [CrossRef]
- Babamoradi, N.; Yousefi, S.; Ziarati, P. Optimization of ultrasound-assisted extraction of functional polysaccharides from common mullein (Verbascum thapsus L.) flowers. J. Food Process Eng. 2018, 41, e12851. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Chen, G.; Yue, W.; Liang, Q.; Wu, Q. Optimisation of ultrasound assisted extraction of phenolic compounds from Sparganii rhizoma with response surface methodology. Ultrason. Sonochem. 2013, 20, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-H.; Fu, M.-L.; Liu, J.; Zhang, H.-F.; He, G.-Q.; Ruan, H. Optimization of ultrasonic-assisted extraction (UAE) of betulin from white birch bark using response surface methodology. Ultrason. Sonochem. 2009, 16, 599–604. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Şamli, R. Optimization of olive leaf extract obtained by ultrasound-assisted extraction with response surface methodology. Ultrason. Sonochem. 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Tsiaka, T.; Stavropoulou, N.A.; Giannakourou, M.C.; Strati, I.F.; Sinanoglou, V.J. Optimization of Ultrasound-Assisted Extraction and Characterization of the Phenolic Compounds in Rose Distillation Side Streams Using Spectrophotometric Assays and High-Throughput Analytical Techniques. Molecules 2023, 28, 7403. [Google Scholar] [CrossRef]
- Elshamy, S.; Handoussa, H.; El-Shazly, M.; Mohammed, E.D.; Kuhnert, N. Metabolomic profiling and quantification of polyphenols from leaves of seven Acacia species by UHPLC-QTOF-ESI-MS. Fitoterapia 2024, 172, 105741. [Google Scholar] [CrossRef]
- Idris, O.A.; Kerebba, N.; Horn, S.; Maboeta, M.S.; Pieters, R. Comparative phytochemistry using UPLC-ESI-QTOF-MS phenolic compounds profile of the water and aqueous ethanol extracts of Tagetes minuta and their cytotoxicity. S. Afr. J. Bot. 2024, 164, 50–65. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crops Prod. 2013, 49, 419–421. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Elnour, A.A.M.; Mirghani, M.E.S.; Kabbashi, N.A.; Alam, M.Z.; Musa, K.H. Active Fractions of Methanol Crude Obtained from Acacia seyal gum: Antioxidant Capacity, using FTIR Analysis. Borneo J. Pharm. 2019, 2, 94–107. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.A.; Dorantes, A.L.; Gallndez, M.J.; Cárdenas, S.E. Effect of a Novel Oil Extraction Method on Avocado (Persea americana Mill) Pulp Microstructure. Plant Foods Hum. Nutr. 2004, 59, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Vellur, S.; Pavadai, P.; Pandian, S.R.K.; Palanichamy, C.; Kabilan, S.J.; Sundar, K.; Kannan, S.; Kunjiappan, S. Optimization of ultrasound-assisted extraction of bioactive chemicals from Hemidesmus indicus (L.) R. Br. using response surface methodology and adaptive neuro-fuzzy inference system. Food Sci. Biotechnol. 2024, 33, 327–341. [Google Scholar] [CrossRef]
- Aybastier, Ö.; Işik, E.; Şahin, S.; Demir, C. Optimization of ultrasonic-assisted extraction of antioxidant compounds from blackberry leaves using response surface methodology. Ind. Crops Prod. 2013, 44, 558–565. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Yolmeh, M.; Najafi, M.B.H.; Farhoosh, R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM). Food Chem. 2014, 155, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Dadi, D.W.; Emire, S.A.; Hagos, A.D.; Assamo, F.T. Influences of Different Drying Methods and Extraction Solvents on Total Phenolic and Flavonoids, and Antioxidant Capacity of Moringa stenopetala Leaves. J. Pharmacogn. Phytochem. 2018, 7, 962–967. [Google Scholar]
- Gondal, H.Y.; Zamir, R.; Nisar, M.; Choudhary, M.I. Verbascum sinaiticum: A Rich Source of Antioxidant Phenylethanoid Glycosides. Nat. Prod. J. 2019, 10, 158–162. [Google Scholar] [CrossRef]
- Mzoughi, M.; Demircan, E.; Turan, O.Y.; Firatligil, E.; Ozcelik, B. Valorization of plum (Prunus domestica) peels: Microwave-assisted extraction, encapsulation and storage stability of its phenolic extract. J. Food Meas. Charact. 2023, 17, 3753–3773. [Google Scholar] [CrossRef]


| Symbols | Independent Variables | Factor Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| X1 | Temperature (°C) | 30 | 40 | 50 |
| X2 | Time (min) | 20 | 30 | 40 |
| X3 | Solvent-to-solute ratio (mL/g) | 20 | 30 | 40 |
| Run | X1 (°C) | X2 (Min) | X3 (mL/g) | Yield (%) | TPC (mg GAE/g) | TFC (mg CE/g) | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 30 | 20 | 20 | 19.97 | 156.69 | 37.55 | 45.88 | 28.24 |
| 2 | 50 | 20 | 20 | 20.33 | 157.14 | 38.06 | 45.93 | 29.45 |
| 3 | 30 | 40 | 20 | 20.48 | 159.8 | 41.55 | 46.5 | 29.48 |
| 4 | 50 | 40 | 20 | 20.77 | 169.28 | 51.1 | 47.13 | 29.45 |
| 5 | 30 | 20 | 40 | 20.5 | 164.92 | 44.61 | 46.8 | 29.26 |
| 6 | 50 | 20 | 40 | 20.57 | 165.71 | 46.27 | 46.88 | 29.94 |
| 7 | 30 | 40 | 40 | 20.65 | 167.78 | 50.78 | 46.98 | 27.99 |
| 8 | 50 | 40 | 40 | 20.84 | 169.31 | 51.31 | 47.23 | 28.76 |
| 9 | 23.2 | 30 | 30 | 20.69 | 168.7 | 50.99 | 47.1 | 29.48 |
| 10 | 56.8 | 30 | 30 | 20.89 | 169.88 | 51.41 | 49.52 | 30.71 |
| 11 | 40 | 13.2 | 30 | 19.35 | 151.03 | 32.45 | 44.23 | 28.88 |
| 12 | 40 | 46.8 | 30 | 21.58 | 171.78 | 61.55 | 56.88 | 35.24 |
| 13 | 40 | 30 | 13.2 | 19.95 | 156.03 | 35.67 | 44.93 | 28.62 |
| 14 | 40 | 30 | 46.8 | 21.59 | 179.09 | 59.47 | 57.08 | 35.42 |
| 15 | 40 | 30 | 30 | 21.6 | 179.8 | 64.49 | 52.45 | 35.88 |
| 16 | 40 | 30 | 30 | 21.53 | 178.61 | 58.73 | 56.63 | 32.57 |
| 17 | 40 | 30 | 30 | 21.53 | 177.99 | 53.98 | 54.3 | 32.31 |
| 18 | 40 | 30 | 30 | 21.53 | 175.65 | 53.57 | 54.1 | 30.1 |
| 19 | 40 | 30 | 30 | 21.5 | 174.7 | 52.96 | 61.85 | 38.89 |
| 20 | 40 | 30 | 30 | 21.15 | 172.76 | 51.41 | 49.67 | 29.49 |
| ME | 72 h | 30 mL/g | 20.85 ± 0.2 | 156.85 ± 0.09 | 34.14 ± 0.04 | 52.15 ± 0.01 | 31.34 ± 0.05 |
| Independent Variable | Dependent Variable (Response) | ||||
|---|---|---|---|---|---|
| Factors | Yield (%) | TPC (mg GAE/g) | TFC (mg CE/g) | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) |
| Intercept | 21.46 | 176.51 | 80.37 | 51.79 | 41.01 |
| Linear | |||||
| X1-temperature | 0.0755 | 1.03 | +2.99 | +0.0907 | +0.0907 |
| X2-sonication time | 0.9688 | 4.15 | +9.11 | +0.3745 | +0.3745 |
| X3-solvent-to-solute ratio | 0.5943 | 4.74 | +4.76 | +0.2769 | +0.2769 |
| Interaction | |||||
| X1∗X2 | 0.0375 | 1.22 | −2.69 | +0.0063 | +0.0063 |
| X2∗X3 | −0.0125 | −0.9500 | −4.18 | −0.0487 | −0.0487 |
| X1∗X3 | −0.6375 | −1.10 | −3.25 | −0.0662 | −0.0663 |
| quadratic | |||||
| X12 | −0.2847 | −2.88 | −1.77 | −0.2629 | −0.2629 |
| X22 | −0.7864 | −5.62 | −6.97 | −0.3753 | −0.3753 |
| X32 | −0.3020 | −3.37 | −1.67 | −0.2681 | −0.2681 |
| Temperature (°C) | Solvent-to-Solute Ratio (mL/g) | Time (min) | |
|---|---|---|---|
| Optimized parameters | 41.4261 | 36.3171 | 33.2215 |
| Predicted values | 178.741 mg GAE/g dw | ||
| Experimental value | 179.800 mg GAE/g dw | ||
| Peak | RT/min | [M-H]− and Other (m/z) | Diff (DB, mDa) | Molecular Weight | Formula | Identified Compound Name |
|---|---|---|---|---|---|---|
| 1 | 2.992 | 827.267 | −0.61 | 828.2742 | C30H52O26 | Verbascose |
| 2 | 3.958 | 393.1395 | −1.56 | 348.1415 | C15H24O9 | Leonuridine |
| 3 | 3.959 | 290.088 | −0.31 | 291.0953 | C11H17NO8 | Sarmentosin epoxide |
| 4 | 5.487 | 373.1138 | −0.75 | 374.121 | C16H22O10 | Gardoside * |
| 5 | 5.488 | 831.1854 | 2.3% | 786.1855 | C33H38O22 | Quercetin 3-glucuronide-7-rutinoside *** |
| 6 | 6.812 | 101.0604 | −4.16 | 102.0677 | C5H10O2 | Pivalic acid |
| 7 | 8.141 | 475.1814 | −1.29 | 476.1888 | C21H32O12 | Kanokoside A |
| 8 | 8.3 | 669.2031 | 0.05 | 669.2026 | C30H37O17 | Hirsutin *** |
| 9 | 8.317 | 785.2497 | −2.04 | 786.2582 | C35H46O20 | Magnoloside B |
| 10 | 8.551 | 403.1607 | 0.66 | 344.1471 | C16H24O8 | Iridotrial glucoside * |
| 11 | 8.582 | 435.1497 | −1.06 | 390.1515 | C17H26O10 | Loganin * |
| 12 | 9.146 | 655.1881 | −0.2 | 656.1953 | C29H36O17 | Hellicoside **** |
| 13 | 9.416 | 653.2081 | −1.05 | 608.2099 | C29H36O14 | Miconioside A *** |
| 14 | 10.161 | 593.1506 | −1.03 | 594.1579 | C27H30O15 | Saponarin *** |
| 15 | 10.81 | 463.0873 | −2.07 | 464.0945 | C21H20O12 | Isoaffinetin *** |
| 16 | 11.665 | 608.1736 | −0.79 | 607.1665 | C28H32O15 | Diosmin *** |
| 17 | 11.77 | 665.2074 | −0.66 | 666.216 | C31H38O16 | Quercetin 5,7,3′,4′-tetramethyl ether 3-rutinoside *** |
| 18 | 12.409 | 593.0923 | −2.06 | 594.101 | C29H22O14 | Catechin 7,4′-di-O-gallate *** |
| 19 | 12.75 | 401.1445 | −2.37 | 342.1307 | C16H22O8 | Coniferin |
| 20 | 13.217 | 697.2334 | −2.23 | 638.2211 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside *** |
| 21 | 13.649 | 354.2394 | −0.5 | 309.2411 | C17H31N3O2 | Palustrine |
| 22 | 13.998 | 827.1898 | −2.03 | 828.1974 | C35H40O23 | Luteolin 7-O-(2-apiofuranosyl-4-glucopyranosyl-6-malonyl)glucopyranoside *** |
| 23 | 14.00 | 697.2342 | 1.6 | 638.2201 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside *** |
| 24 | 14.051 | 841.457 | −2.44 | 796.4609 | C42H68O14 | Soyasaponin III |
| 25 | 15.625 | 285.0404 | −0.11 | 286.0477 | C15H10O6 | Luteolin *** |
| 26 | 16.052 | 987.5151 | −2.04 | 942.5169 | C48H78O18 | Soyasaponin I |
| 27 | 16.227 | 725.2279 | −2.27 | 726.2371 | C33H42O18 | Naringenin 7-O-(2″,6″-di-O-alpha-rhamnopyranosyl)-beta-glucopyranoside *** |
| 28 | 16.231 | 755.2395 | −1.66 | 756.2477 | C34H44O19 | Myricoside |
| 29 | 16.415 | 463.1031 | −1.16 | 418.1053 | C24H18O7 | 8-Caffeoyl-3,4-dihydro-5,7-dihydroxy-4-phenylcoumarin **** |
| 30 | 16.417 | 369.1183 | −2.53 | 310.1053 | C15H18O7 | Mellitoxin |
| 31 | 17.095 | 327.2172 | −1.67 | 328.2244 | C18H32O5 | 9-hydroperoxy-12,13-epoxy-10-octadecenoic acid |
| 32 | 18.125 | 299.0554 | −0.67 | 300.0627 | C16H12O6 | Mopachalcone *** |
| 33 | 20.00 | 433.092 | −1.80 | 434.0994 | C24H18O8 | Knipholone ** |
| 34 | 21.371 | 193.0867 | −1.65 | 194.0943 | C11H14O3 | Zingerone **** |
| 35 | 23.15 | 221.1539 | −3.38 | 222.162 | C14H22O2 | Rishitin |
| 36 | 23.151 | 293.1754 | −1.69 | 294.1831 | C17H26O4 | Embelin ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legesse, A.B.; Emire, S.A.; Tadesse, M.G.; Dadi, D.W.; Kassa, S.K.; Oyinloye, T.M.; Yoon, W.B. Optimization of Ultrasound-Assisted Extraction of Verbascum sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity. Foods 2024, 13, 1255. https://doi.org/10.3390/foods13081255
Legesse AB, Emire SA, Tadesse MG, Dadi DW, Kassa SK, Oyinloye TM, Yoon WB. Optimization of Ultrasound-Assisted Extraction of Verbascum sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity. Foods. 2024; 13(8):1255. https://doi.org/10.3390/foods13081255
Chicago/Turabian StyleLegesse, Alemu Belay, Shimelis Admassu Emire, Minbale Gashu Tadesse, Debebe Worku Dadi, Shimelis Kebede Kassa, Timilehin Martins Oyinloye, and Won Byong Yoon. 2024. "Optimization of Ultrasound-Assisted Extraction of Verbascum sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity" Foods 13, no. 8: 1255. https://doi.org/10.3390/foods13081255
APA StyleLegesse, A. B., Emire, S. A., Tadesse, M. G., Dadi, D. W., Kassa, S. K., Oyinloye, T. M., & Yoon, W. B. (2024). Optimization of Ultrasound-Assisted Extraction of Verbascum sinaiticum Leaves: Maximal Phenolic Yield and Antioxidant Capacity. Foods, 13(8), 1255. https://doi.org/10.3390/foods13081255






