Curcumin Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid Phase Transition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Expression and Purification of α-Syn Protein
2.3. Liquid–Liquid Phase Separation Assay
2.4. DIC and Fluorescence Microscopy
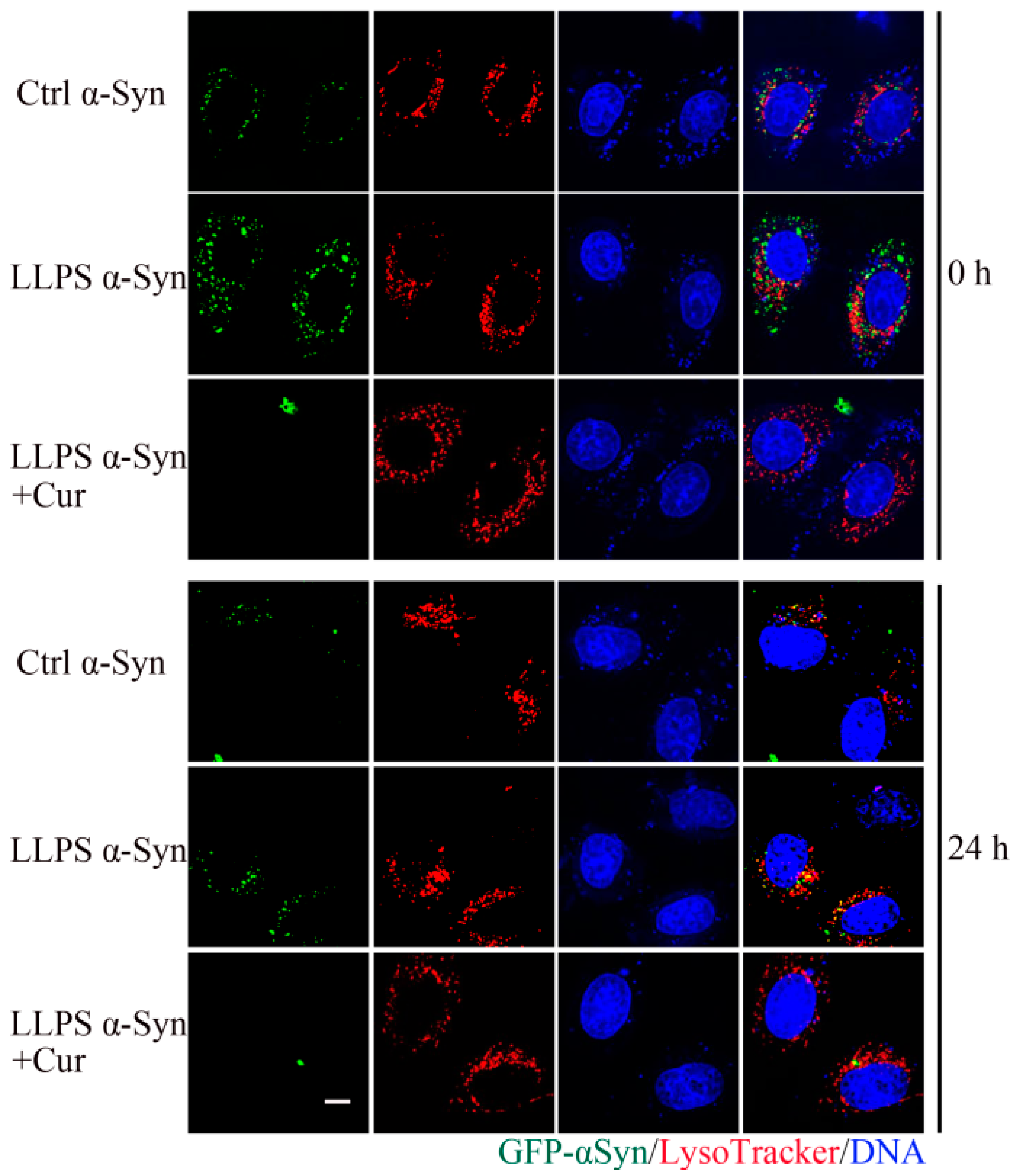
2.5. Co-Localization of Curcumin and α-Syn during Droplets Formation
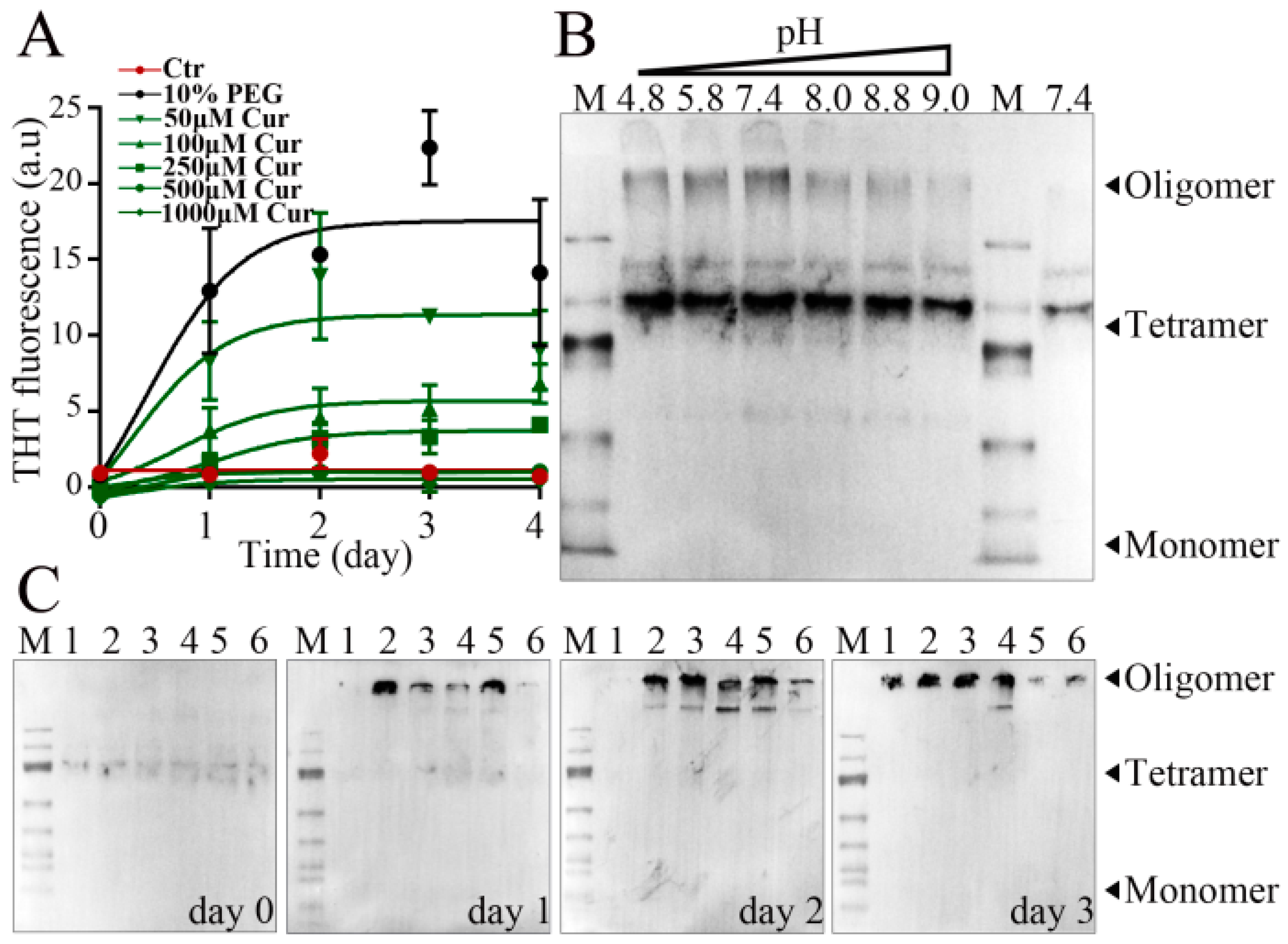
2.6. Thioflavin-T Fluorescence Assay
2.7. Native PAGE
2.8. Cell Viability Assay
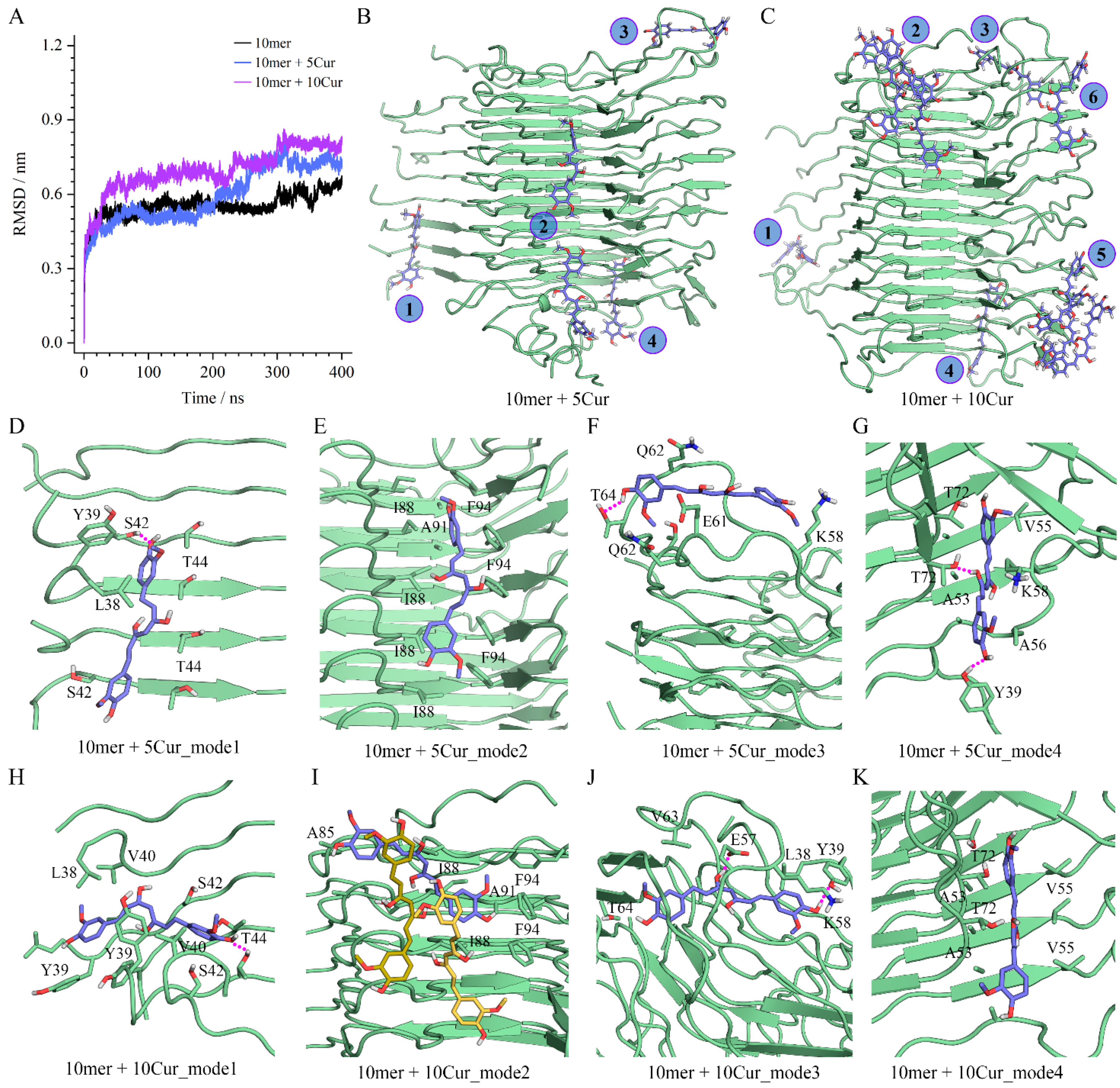
2.9. Molecular Simulations
2.10. Statistical Analysis
3. Results and Discussion
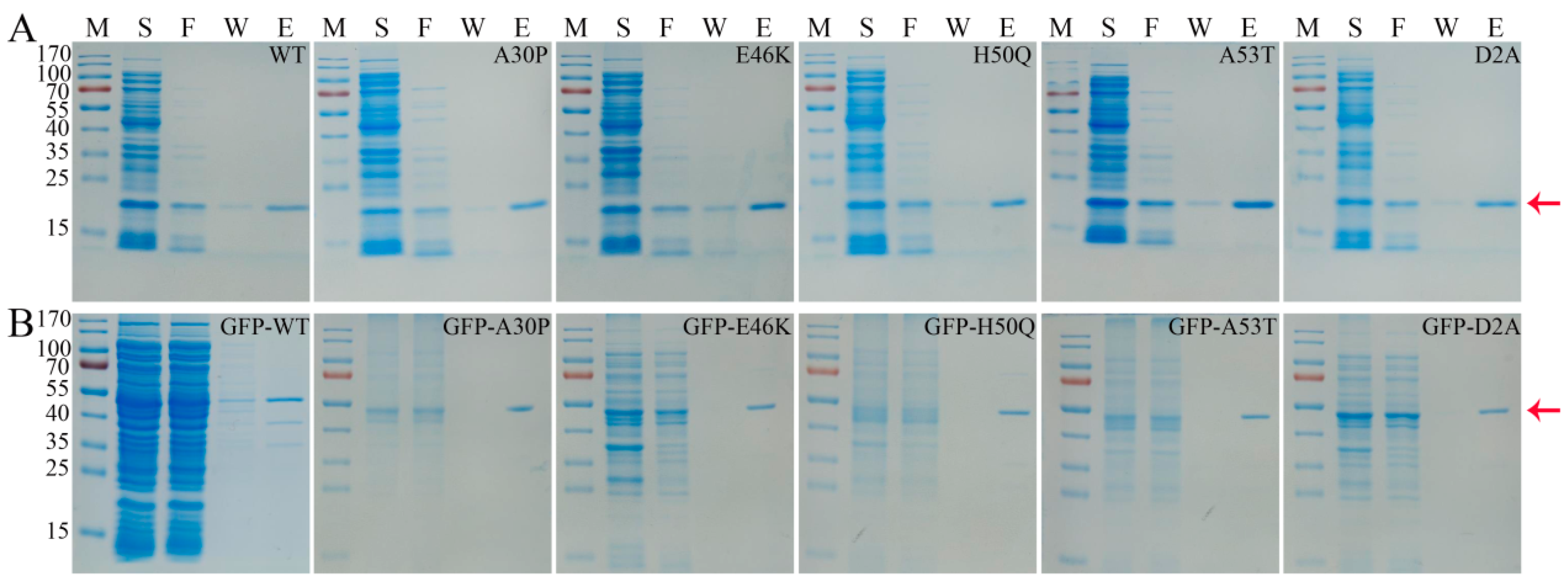
3.1. Purification of α-Syn Protein
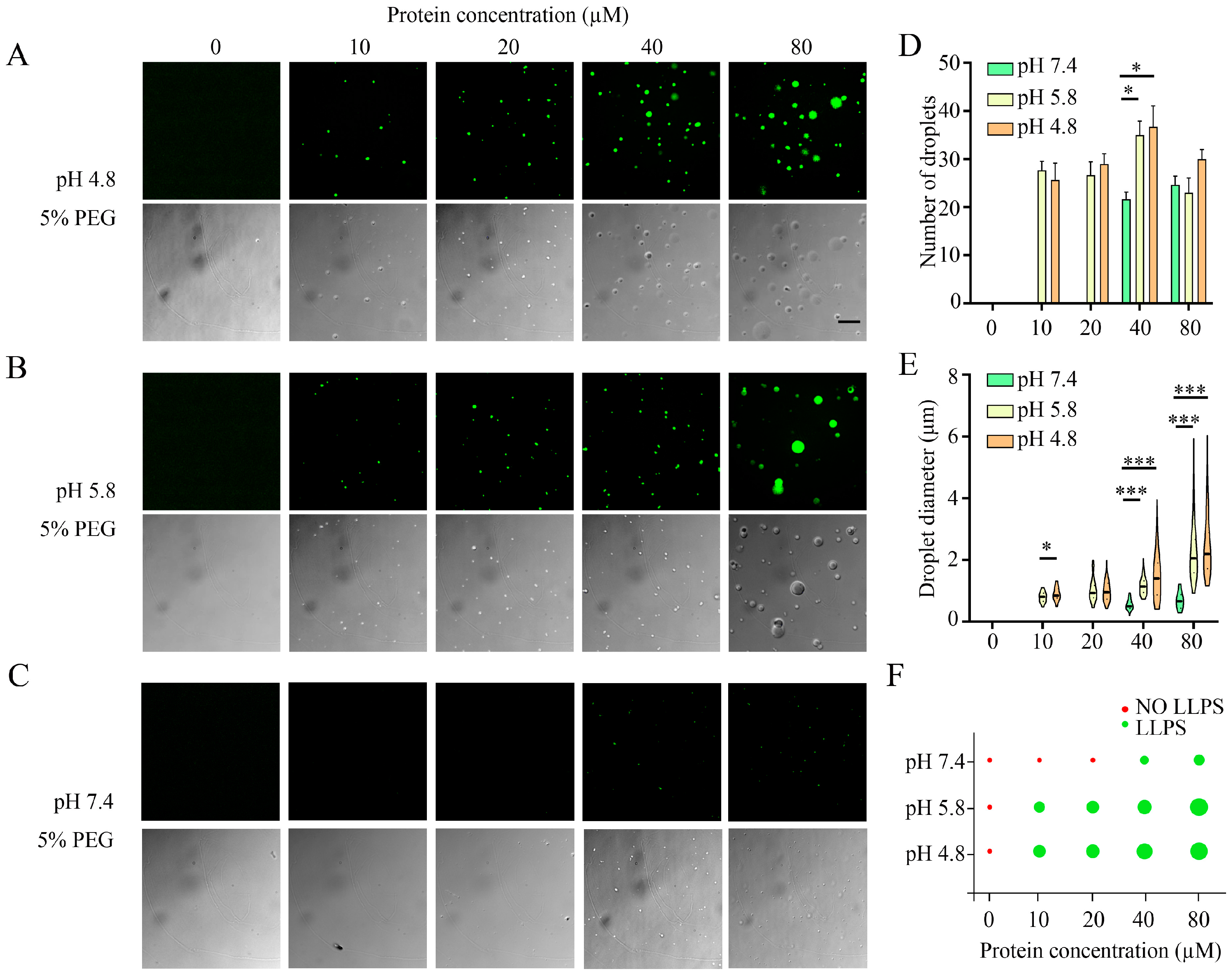
3.2. Investigation of Liquid–Liquid Phase Separation in the Aggregation of α-Syn
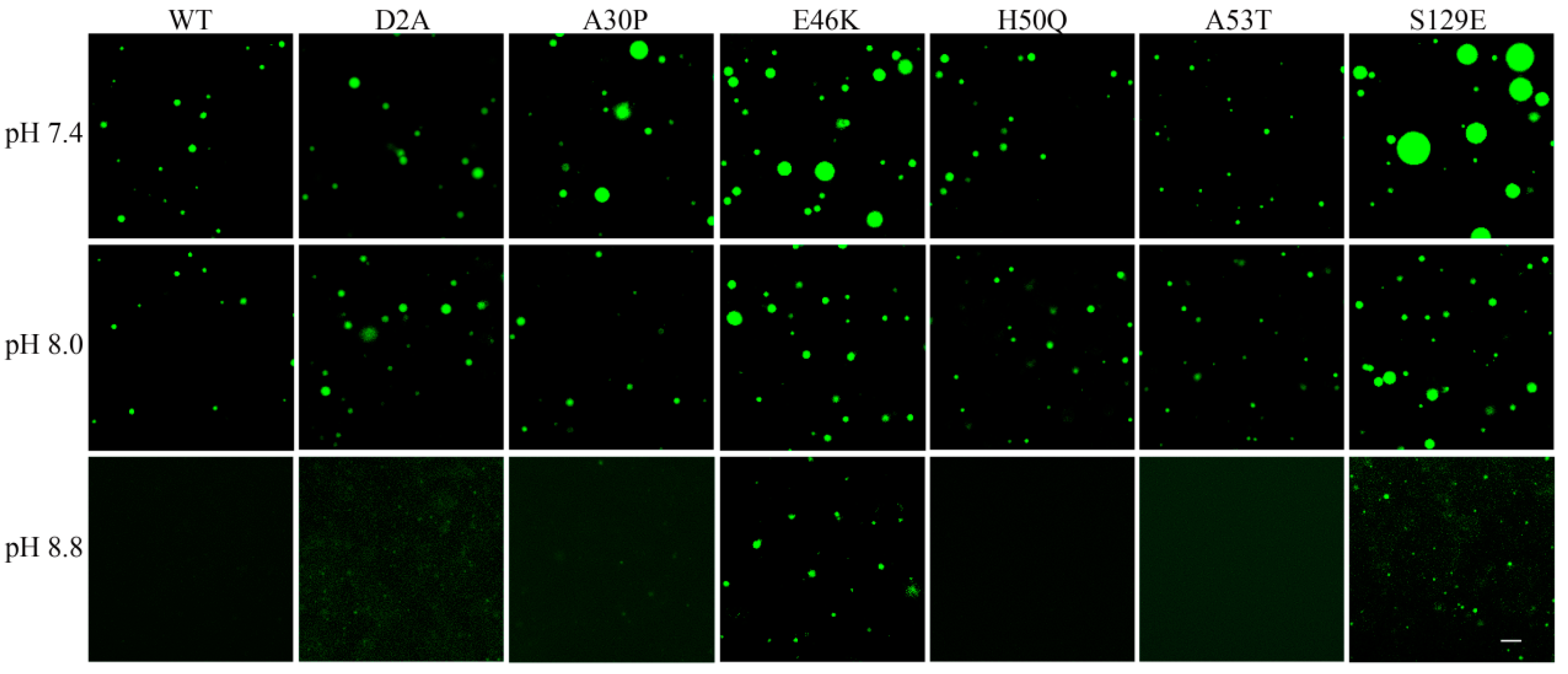
3.3. Effects of Parkinson’s Disease-Relevant Mutations on Droplet Formation
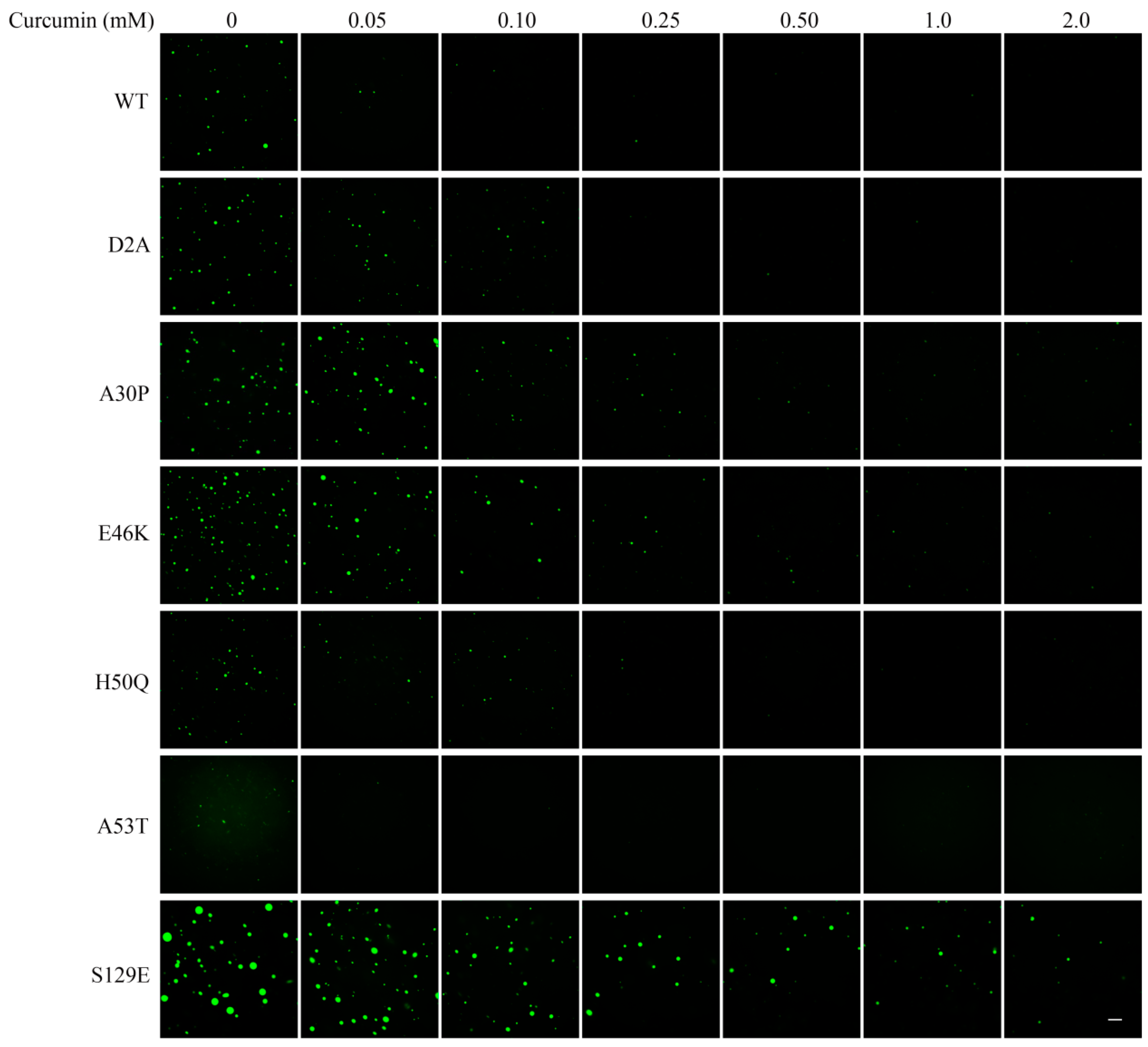
3.4. Effect of Curcumin on Droplet Formation
3.5. Co-Localization of Curcumin and α-Syn during Droplets Formation
3.6. Effect of Curcumin on the Aggregation of α-Syn during LLPS
3.7. Effect of Curcumin on the Cytotoxicity of α-Syn Aggregates
3.8. Insights into Molecular Interactions Derived from Molecular Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fusco, G.; Chen, S.W.; Williamson, P.T.F.; Cascella, R.; Perni, M.; Jarvis, J.A.; Cecchi, C.; Vendruscolo, M.; Chiti, F.; Cremades, N.; et al. Structural basis of membrane disruption and cellular toxicity by α-synuclein oligomers. Science 2017, 358, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Singh, N.; Kumar, R.; Patel, K.; Pandey, S.; Datta, D.; Mahato, J.; Panigrahi, R.; Navalkar, A.; Mehra, S.; et al. α-Synuclein aggregation nucleates through liquid-liquid phase separation. Nat. Chem. 2020, 12, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Hardenberg, M.C.; Sinnige, T.; Casford, S.; Dada, S.T.; Poudel, C.; Robinson, E.A.; Fuxreiter, M.; Kaminksi, C.F.; Schierle, G.S.K.; Nollen, E.A.A.; et al. Observation of an α-synuclein liquid droplet state and its maturation into Lewy body-like assemblies. J. Mol. Cell Biol. 2021, 13, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.I.; Cheng, M.H.; Font, J.; Schwartz, A.C.; Ledwitch, K.; Duran, A.; Mabry, S.J.; Belovich, A.N.; Zhu, Y.; Carter, A.M.; et al. Psychomotor impairments and therapeutic implications revealed by a mutation associated with infantile Parkinsonism-Dystonia. Elife 2021, 10, e68039. [Google Scholar] [CrossRef] [PubMed]
- Mahoney-Sanchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef] [PubMed]
- Schweighauser, M.; Shi, Y.; Tarutani, A.; Kametani, F.; Murzin, A.G.; Ghetti, B.; Matsubara, T.; Tomita, T.; Ando, T.; Hasegawa, K.; et al. Structures of α-synuclein filaments from multiple system atrophy. Nature 2020, 585, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Ferreira, R.; Kovacik, L.; Ni, D.; Stahlberg, H. New insights on the structure of α-synuclein fibrils using cryo-electron microscopy. Curr. Opin. Neurobiol. 2020, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Dhavale, D.D.; Tsai, C.; Bagchi, D.P.; Engel, L.A.; Sarezky, J.; Kotzbauer, P.T. A sensitive assay reveals structural requirements for α-synuclein fibril growth. J. Biol. Chem. 2017, 292, 9034–9050. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Roeters, S.J.; Schilderink, N.; Hommersom, B.; Heeren, R.M.; Woutersen, S.; Claessens, M.M.; Subramaniam, V. The Impact of N-terminal Acetylation of α-Synuclein on Phospholipid Membrane Binding and Fibril Structure. J. Biol. Chem. 2016, 291, 21110–21122. [Google Scholar] [CrossRef] [PubMed]
- Dahmene, M.; Berard, M.; Oueslati, A. Dissecting the Molecular Pathway Involved in PLK2 Kinase-mediated α-Synuclein-selective Autophagic Degradation. J. Biol. Chem. 2017, 292, 3919–3928. [Google Scholar] [CrossRef]
- Tsigelny, I.F.; Sharikov, Y.; Kouznetsova, V.L.; Greenberg, J.P.; Wrasidlo, W.; Overk, C.; Gonzalez, T.; Trejo, M.; Spencer, B.; Kosberg, K.; et al. Molecular determinants of α-synuclein mutants’ oligomerization and membrane interactions. ACS Chem. Neurosci. 2015, 6, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Huang, J.F.; Akimoto, M.; Shi, T.Y.; Melacini, G. Atomic Resolution Map of Hierarchical Self-Assembly for an Amyloidogenic Protein Probed through Thermal N-15-R-2 Correlation Matrices. J. Am. Chem. Soc. 2021, 143, 4668–4679. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Ivanova, M.I.; Sawaya, M.R.; Cascio, D.; Reyes, F.E.; Shi, D.; Sangwan, S.; Guenther, E.L.; Johnson, L.M.; Zhang, M.; et al. Structure of the toxic core of α-synuclein from invisible crystals. Nature 2015, 525, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Mo, X.L.; Wang, J.Y.; Ye, X.Y.; Yu, H.J.; Liu, Y.H. α-Synuclein phase separation and amyloid aggregation are modulated by C-terminal truncations. FEBS Lett. 2022, 596, 1388–1400. [Google Scholar] [CrossRef]
- Soldner, F.; Stelzer, Y.; Shivalila, C.S.; Abraham, B.J.; Latourelle, J.C.; Barrasa, M.I.; Goldmann, J.; Myers, R.H.; Young, R.A.; Jaenisch, R. Parkinson-associated risk variant in distal enhancer of α-synuclein modulates target gene expression. Nature 2016, 533, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Theillet, F.X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gegg, M.E.; Verona, G.; Schapira, A.H.V. Glucocerebrosidase deficiency promotes release of α-synuclein fibrils from cultured neurons. Hum. Mol. Genet. 2020, 29, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Trinkaus, V.A.; Riera-Tur, I.; Martinez-Sanchez, A.; Bauerlein, F.J.B.; Guo, Q.; Arzberger, T.; Baumeister, W.; Dudanova, I.; Hipp, M.S.; Hartl, F.U.; et al. In situ architecture of neuronal α-Synuclein inclusions. Nat. Commun. 2021, 12, 2110. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, A.; Perez-Berlanga, M.; De Rossi, P.; Polymenidou, M. Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force. Dev. Cell 2020, 55, 45–68. [Google Scholar] [CrossRef]
- Zhao, L.; Chang, Q.; Cong, Z.Z.; Zhang, Y.L.; Liu, Z.X.; Zhao, Y.H. Effects of dietary polyphenols on maternal and fetal outcomes in maternal diabetes. Food Funct. 2023, 14, 8692–8710. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, G.R.; Zhang, Y.H.; Sun, P.C.; Du, Q.M.; Zhou, P. Influence of curcumin on the Al(III)-induced conformation transition of silk fibroin and resulting potential therapy for neurodegenerative diseases. RSC Adv. 2012, 2, 9106–9113. [Google Scholar] [CrossRef]
- Raghu, S.V.; Kudva, A.K.; Krishnamurthy, R.G.; Mudgal, J.; George, T.; Baliga, M.S. Neuroprotective effects of dietary plants and phytochemicals against radiation-induced cognitive and behavioral deficits: A comprehensive review of evidence and prospects for future research. Food Funct. 2023, 14, 5921–5935. [Google Scholar] [CrossRef]
- Bozkurt, O.; Kocaadam-Bozkurt, B.; Yildiran, H. Effects of curcumin, a bioactive component of turmeric, on type 2 diabetes mellitus and its complications: An updated review. Food Funct. 2022, 13, 11999–120100. [Google Scholar] [CrossRef]
- Rodriguez, L.C.; Foressi, N.N.; Celej, M.S. Modulation of α-synuclein phase separation by biomolecules. Biochim. Biophys. Acta Proteins Proteom. 2023, 1871, 140885. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, N.; Nielsen, S.B.; Buell, A.K.; Kaspersen, J.D.; Arosio, P.; Vad, B.S.; Paslawski, W.; Christiansen, G.; Valnickova-Hansen, Z.; Andreasen, M.; et al. The role of stable α-synuclein oligomers in the molecular events underlying amyloid formation. J. Am. Chem. Soc. 2014, 136, 3859–3868. [Google Scholar] [CrossRef]
- Marotta, N.P.; Ara, J.; Uemura, N.; Lougee, M.G.; Meymand, E.S.; Zhang, B.; Petersson, E.J.; Trojanowski, J.Q.; Lee, V.M.Y. A-synuclein from patient Lewy bodies exhibits distinct pathological activity that can be propagated in vitro. Acta Neuropathol. Commun. 2021, 9, 188. [Google Scholar] [CrossRef]
- Tuttle, M.D.; Comellas, G.; Nieuwkoop, A.J.; Covell, D.J.; Berthold, D.A.; Kloepper, K.D.; Courtney, J.M.; Kim, J.K.; Barclay, A.M.; Kendall, A.; et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat. Struct. Mol. Biol. 2016, 23, 409–415. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, J.X.; Wang, Z.G.; Yao, Y.J.; Han, X.; Zhao, Y.L.; Liu, J.P.; Zhang, S.Q. Identification of a cyclodextrin inclusion complex of antimicrobial peptide CM4 and its antimicrobial activity. Food Chem. 2017, 221, 296–301. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, J.X.; Li, G.; Xu, Y.Y.; Lu, K.; Wang, Z.G.; Liu, J.P. Antimicrobial activity and mechanism of peptide CM4 against. Food Funct. 2020, 11, 7245–7254. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Sang, M.; Zhang, J.X.; Li, B.; Chen, Y.Q. TRAIL-CM4 fusion protein shows antibacterial activity and a stronger antitumor activity than solo TRAIL protein. Protein Expr. Purif. 2016, 122, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cieplak, P.; Kollman, P.A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 2015, 21, 1049–1074. [Google Scholar] [CrossRef]
- Vassetti, D.; Pagliai, M.; Procacci, P. Assessment of GAFF2 and OPLS-AA General Force Fields in Combination with the Water Models TIP3P, SPCE, and OPC3 for the Solvation Free Energy of Druglike Organic Molecules. J. Chem. Theory Comput. 2019, 15, 1983–1995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xin, J.Y.; Wang, Z.G.; Wu, W.P.; Liu, Y.T.; Min, Z.Z.; Xin, Y.Y.; Liu, B.; He, J.; Zhang, X.W.; et al. Structural basis of a bi-functional malonyl-CoA reductase (MCR) from the photosynthetic green non-sulfur bacterium. Mbio 2023, 14, e03233-22. [Google Scholar] [CrossRef] [PubMed]
- Ubbiali, D.; Fratini, M.; Piersimoni, L.; Ihling, C.H.; Kipping, M.; Heilmann, I.; Iacobucci, C.; Sinz, A. Direct Observation of “Elongated” Conformational States in α-Synuclein upon Liquid-Liquid Phase Separation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202205726. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chen, J.; Liu, Y. Curcumin Interacts with α-Synuclein Condensates To Inhibit Amyloid Aggregation under Phase Separation. ACS Omega 2022, 7, 30281–30290. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin Modulates α-Synuclein Aggregation and Toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.N.; Ghosh, D.; Das, S.; Anoop, A.; Jacob, R.S.; Singh, P.K.; Ayyagari, N.; Namboothiri, I.N.N.; Maji, S.K. Effect of curcumin analogs on α-synuclein aggregation and cytotoxicity. Sci. Rep. 2016, 6, 28511. [Google Scholar] [CrossRef]
- Pandey, N.; Strider, J.; Nolan, W.C.; Yan, S.X.; Galvin, J.E. Curcumin inhibits aggregation of α-synuclein. Acta Neuropathol. 2008, 115, 479–489. [Google Scholar] [CrossRef]
- Ahmad, B.; Lapidus, L.J. Curcumin Prevents Aggregation in α-Synuclein by Increasing Reconfiguration Rate. J. Biol. Chem. 2012, 287, 9193–9199. [Google Scholar] [CrossRef]
- Ahsan, N.; Mishra, S.; Jain, M.K.; Surolia, A.; Gupta, S. Curcumin Pyrazole and its derivative (N-(3-Nitrophenylpyrazole) Curcumin inhibit aggregation, disrupt fibrils and modulate toxicity of Wild type and Mutant α-Synuclein. Sci. Rep. 2015, 5, 9862. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Karmakar, S.; Bose, A.; Chowdhury, P.K. β-Cyclodextrin and Curcumin, a Potent Cocktail for Disaggregating and/or Inhibiting Amyloids: A Case Study with α-Synuclein. Biochemistry 2014, 53, 4081–4083. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-F.; Jiang, Z.-Q.; Cao, S.; Zhang, M.-X.; Wang, L.-H.; Liu, J.; Lu, Y.-H.; Wang, H.-Y.; Hong, X.-J.; Wang, Z.-G.; et al. Curcumin Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid Phase Transition. Foods 2024, 13, 1287. https://doi.org/10.3390/foods13091287
Li J-F, Jiang Z-Q, Cao S, Zhang M-X, Wang L-H, Liu J, Lu Y-H, Wang H-Y, Hong X-J, Wang Z-G, et al. Curcumin Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid Phase Transition. Foods. 2024; 13(9):1287. https://doi.org/10.3390/foods13091287
Chicago/Turabian StyleLi, Jian-Feng, Zi-Qun Jiang, Sen Cao, Meng-Xin Zhang, Li-Hui Wang, Jun Liu, Yan-Hua Lu, Hong-Yan Wang, Xiao-Jing Hong, Zhi-Guo Wang, and et al. 2024. "Curcumin Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid Phase Transition" Foods 13, no. 9: 1287. https://doi.org/10.3390/foods13091287
APA StyleLi, J.-F., Jiang, Z.-Q., Cao, S., Zhang, M.-X., Wang, L.-H., Liu, J., Lu, Y.-H., Wang, H.-Y., Hong, X.-J., Wang, Z.-G., & Liu, J.-P. (2024). Curcumin Inhibits α-Synuclein Aggregation by Acting on Liquid–Liquid Phase Transition. Foods, 13(9), 1287. https://doi.org/10.3390/foods13091287






