Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanicals
2.2. Chemical Analysis
2.2.1. Headspace—Solid Phase Micro Extraction (HS-SPME)
2.2.2. Gas Chromatography—Mass Spectrometry (GC-MS) Analysis
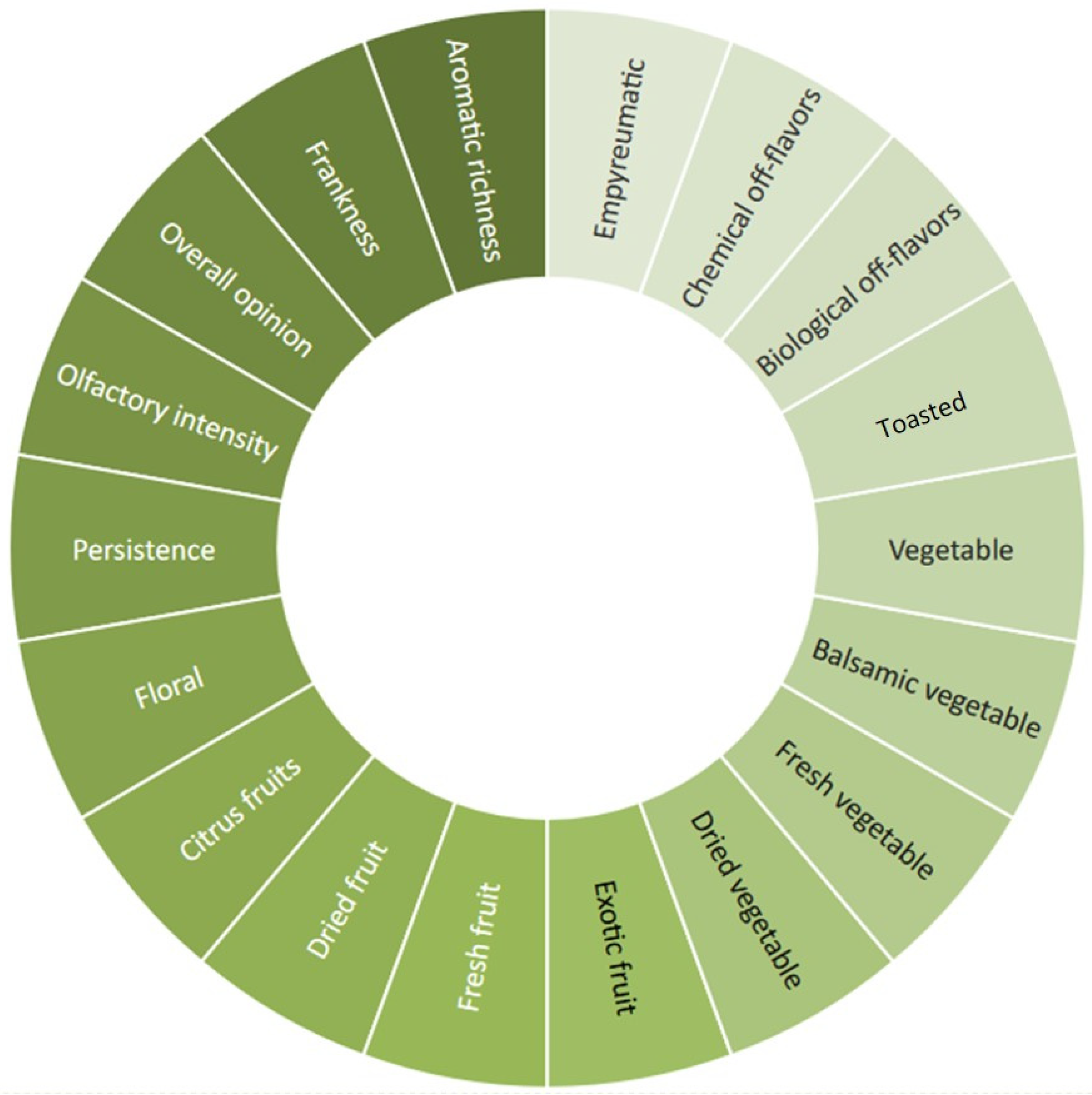
2.3. Sensory Analysis
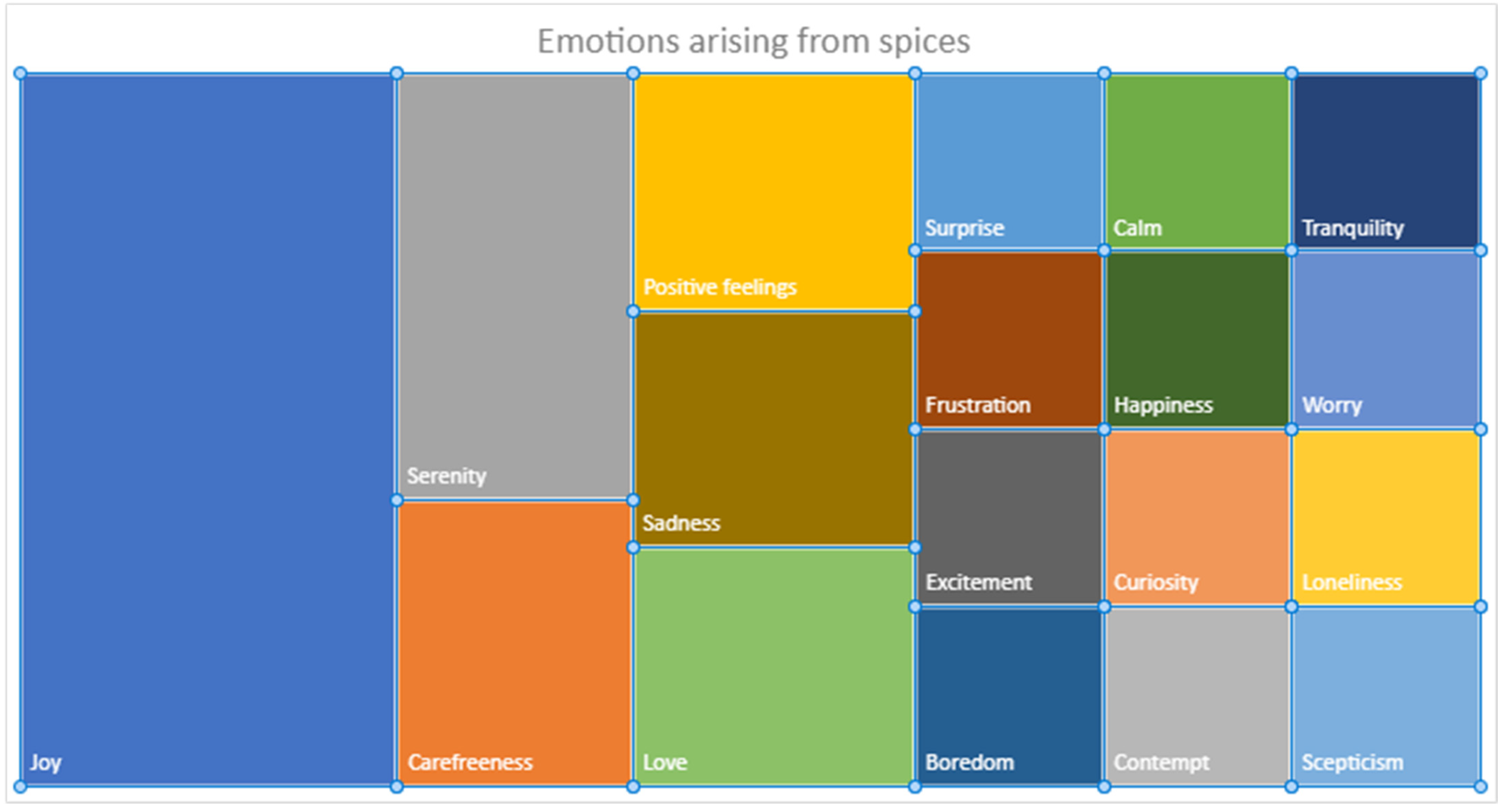
2.4. Emotional Evaluation (Explicit Method)
2.5. Emotional Evaluation (Implicit Method)
2.5.1. Instrumentation
2.5.2. Signal Processing and Features Extraction
ECG
- ○
- Heart rate (HR): the number of heartbeats recorded within a time unit. It is measured in beats per minute (bpm), and is usually associated with the sympathetic activity of the ANS.
- ○
- Standard deviation of the normal R–R intervals (SDNN): it is an estimate of the HRV influenced by both the sympathetic and para-sympathetic branches of the ANS. It is measured in ms.
- ○
- Root mean square of the successive differences (RMSSD): it represents the root mean square of the differences between R–R intervals close to each other. It is an estimate of the parasympathetic activity of the ANS and it is measured in ms.
- ○
- Number of normal R–R intervals differing for more than 50 ms (NN50): it is capable of estimating the number (or the percentage) of the normal R–R intervals differing for more than 50 ms from each other. Under certain experimental conditions, as can be assumed in the present study (i.e., resting state short-term recordings), it can be referred to the parasympathetic activity of the ANS.
- ○
- Variance of the R–R intervals (VAR): it refers to the variability of the R–R intervals.
- ○
- SD1: standard deviation of the projection of the Poincaré plot on the perpendicular line to the identity. It estimates the short-term HRV.
- ○
- SD2: standard deviation of the projection of the Poincaré plot on the parallel line to the identity. It estimates the long-term HRV.
- ○
- Cardiac sympathetic index (CSI): it is obtained by the Poincaré plot and calculated as the ratio between the standard deviation of the projection of the plot on the parallel line to the identity and that on the perpendicular line (SD2/SD1). It represents a reliable sympathetic indicator.
- ○
- Cardiac vagal index (CVI): it is obtained by the Poincaré plot and calculated as log10 (SD1 × SD2). It is assumed to be a good parasympathetic indicator.
- ○
- Low frequency (LF): power spectral density of the ECG signal at low frequencies (0.04–0.15 Hz). It is usually considered to estimate the sympathetic activity of the ANS.
- ○
- High frequency (HF): power spectral density of the ECG signal at high frequencies (0.15–0.4 Hz). It represents an estimator of both the sympathetic and parasympathetic activity of the ANS.
- ○
- Low-to-high frequency components ratio (LF/HF): it indicates the overall balance between low and high frequency components of the ECG signal. A ratio exceeding 1 is related to sympathetic dominance, whereas for values below 1, the parasympathetic nervous system appears to be prevalent. Despite being largely used, as it relies on frequency-domain features, the LF/HF can be affected more largely by artifacts than occurring for time-domain derived features.
GSR
EEG
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of the Spontaneous Volatile Emission Profiles of All the Studied Spices
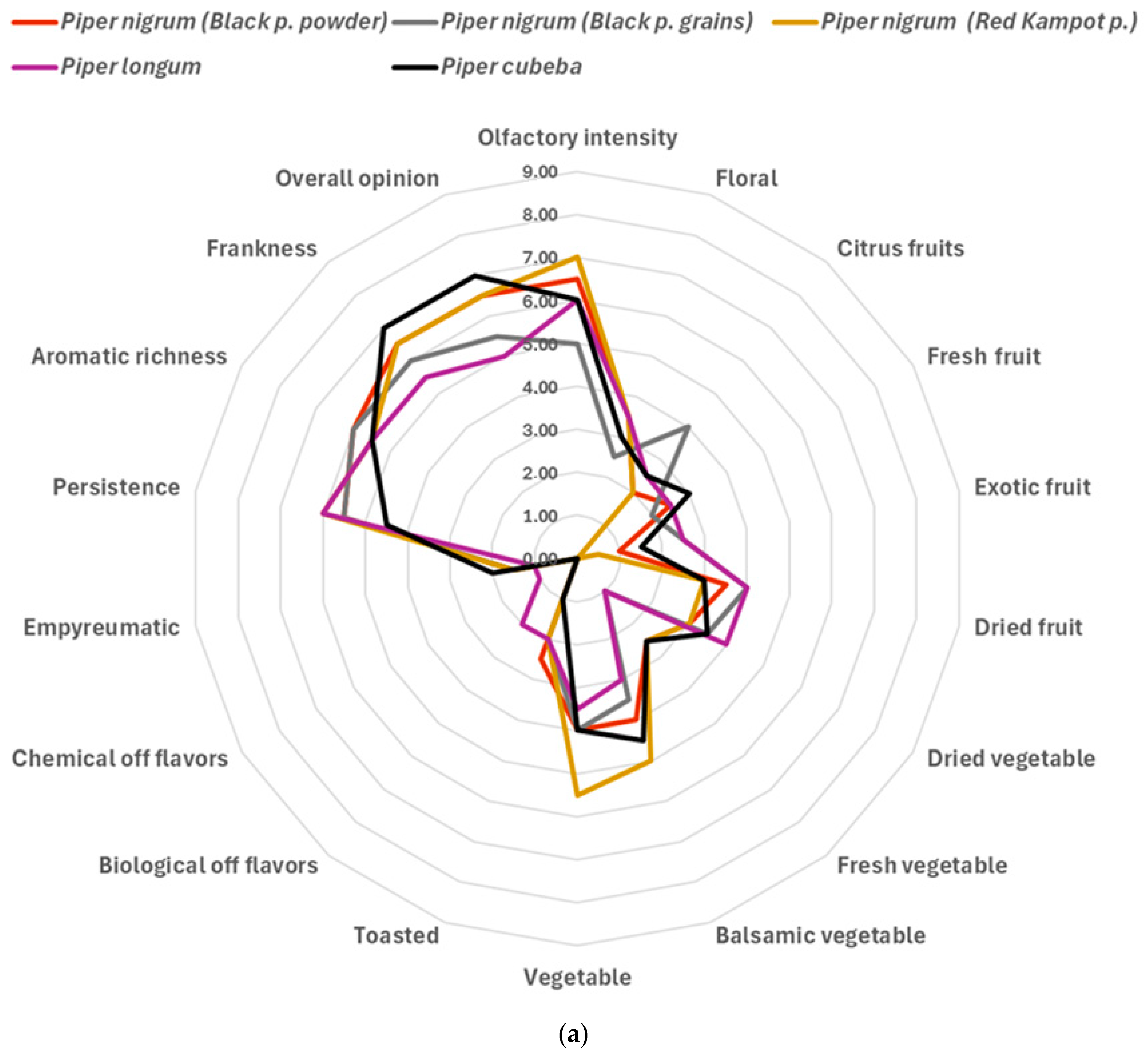
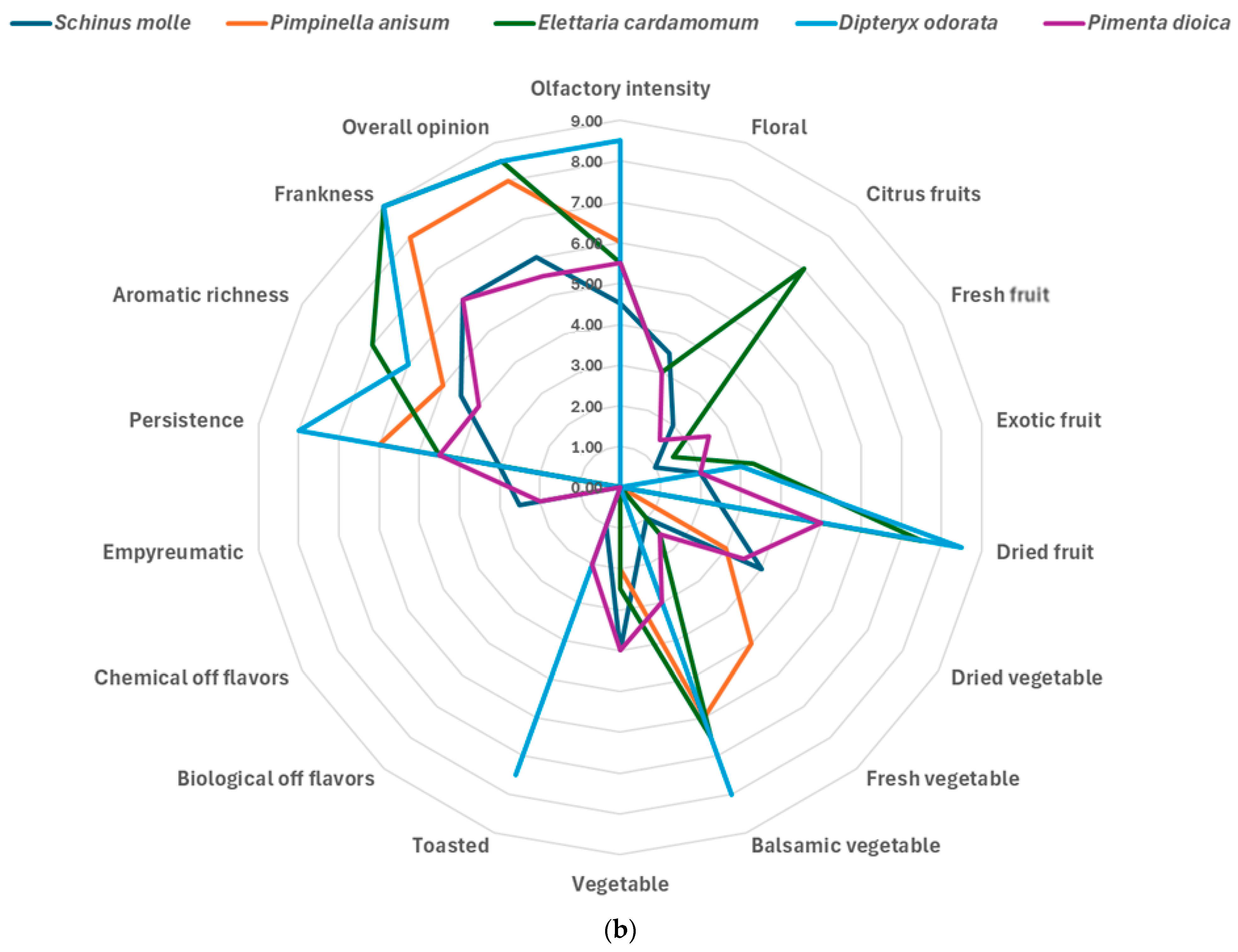
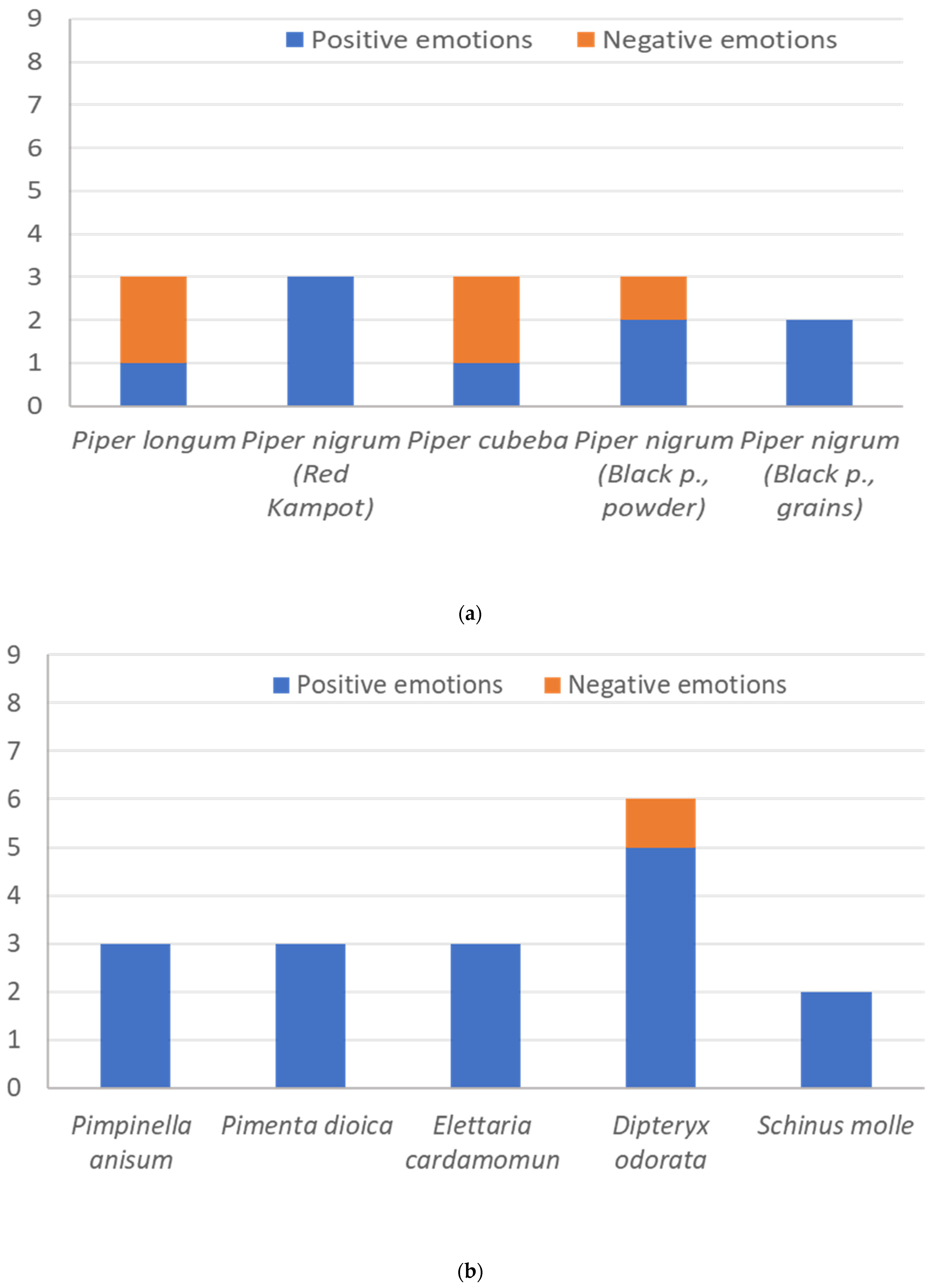
3.2. Sensory Analysis and Emotional Evaluation (Explicit Methods)
3.3. Emotional Evaluation by Implicit Methods
3.3.1. Relationships with Self-Reported Emotions
3.3.2. Relationships with Perceived Pleasantness
4. Conclusions and Future Works
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szűcs, V.; Szabó, E.; Lakner, Z.; Székács, A. National seasoning practices and factors affecting the herb and spice consumption habits in Europe. Food Control 2018, 83, 147–156. [Google Scholar] [CrossRef]
- Siddiq, M.; Uebersax, M.A. Handbook of Vegetables and Vegetable Processing; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and spices-biomarkers of intake based on human intervention studies—A systematic review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef] [PubMed]
- Ceto, X.; Sarma, M.; Valle, M. Analysis of spices & herbs and its phenolic content by means of an electronic tongue. LWT-Food Sci. Technol. 2024, 191, 115578. [Google Scholar]
- Cuvelier, M.E.; Berset, C.; Richard, H. Antioxidant constituents in sage (Salvia officinalis). J. Agric. Food Chem. 1994, 42, 665–669. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Uberegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S. fruticosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; Fisher, C.; Gorman, M.; Knowles, S.; LeBlanc, J.; Ritchie, C.; Schindell, K.; Ettinger, L.; McSweeney, M.B. Effect of piperine on saltiness perception. Foods 2023, 12, 296. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.; LeBlanc, J.; Ritchie, C.; Gorman, M.; Ettinger, L.; McSweeney, M.B. Effect of white pepper addition on the sensory perception of sodium-reduced soup with an emphasis on saltiness perception. J. Sens. Stud. 2023, 38, e12874. [Google Scholar] [CrossRef]
- McNamara, F.N.; Randall, A.; Gunthorpe, M.J. Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br. J. Pharmacol. 2005, 144, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Kilcast, D.; Angus, F. Reducing Salt in Foods; Igarss 2014; Woodhead Publishing Ltd.: Sawston, UK, 2014; ISBN 9781845693046. [Google Scholar]
- Sasikumar, B.; Swetha, V.P.; Parvathy, V.A.; Sheeja, T.E. 22—Advances in Adulteration and Authenticity Testing of Herbs and Spices. In Advances in Food Authenticity Testing; Woodhead Publishing Ltd.: Sawston, UK, 2016; pp. 585–624. [Google Scholar] [CrossRef]
- Kunová, S.; Taglieri, I.; Hašcík, P.; Ben Hsouna, A.; Mnif, W.; Venturi, F.; Kačániová, M. Dried Herbs as an Easy-to-Use and Cost-Effective Alternative to Essential Oils to Extend the Shelf Life of Sheep Lump Cheese. Foods 2023, 12, 4487. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Ban, Z.; Garzoli, S.; Elizondo-Luevano, J.H.; Ben Hsouna, A.; Ben Saad, R.; Bianchi, A.; Venturi, F.; Kluz, M.I.; et al. Enhancing the Shelf Life of Sous-Vide Red Deer Meat with Piper nigrum Essential Oil: A Study on Antimicrobial Efficacy against Listeria monocytogenes. Molecules 2024, 29, 4179. [Google Scholar] [CrossRef]
- Djebbi, T.; Ascrizzi, R.; Bedini, S.; Farina, P.; Sanmartin, C.; Jouda Mediouni, B.J.; Bozzini, M.F.; Flamini, G.; Conti, B. Physicochemical and repellent properties of chitosan films loaded with essential oils for producing an active packaging effective against the food pest Sitophilus oryzae. J. Stored Prod. Res. 2024, 106, 102297. [Google Scholar] [CrossRef]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Nguyen, L.; Duong, L.T.; Mentreddy, R.S. The US import demand for spices and herbs by differentiated sources. J. Appl. Res. Med. Aromat. Plants 2019, 12, 13–20. [Google Scholar] [CrossRef]
- Croy, I.; Olgun, S.; Joraschky, P. Basic emotions elicited by odors and pictures. Emotion 2011, 11, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Tonacci, A.; Taglieri, I.; Sanmartin, C.; Billeci, L.; Crifaci, G.; Ferroni, G.; Braceschi, G.P.; Odello, L.; Venturi, F. Taste the emotions: Pilot for a novel, sensors-based approach to emotional analysis during coffee tasting. J. Sci. Food Agric. 2023; in press. [Google Scholar] [CrossRef]
- Izard, C.E. Emotion theory and research: Highlights, unanswered questions, and emerging issues. Annu. Rev. Psychol. 2009, 60, 1–25. [Google Scholar] [CrossRef]
- Tonacci, A.; Billeci, L.; Di Mambro, I.; Marangoni, R.; Sanmartin, C.; Venturi, F. Wearable Sensors for Assessing the Role of Olfactory Training on the Autonomic Response to Olfactory Stimulation. Sensors 2021, 21, 770. [Google Scholar] [CrossRef]
- Brouwer, A.M.; Hogervorst, M.A.; Grootjen, M.; van Erp, J.B.F.; Zandstra, E.H. Neurophysiological responses during cooking food associated with different emotions. Food Qual. Prefer. 2017, 62, 307–316. [Google Scholar] [CrossRef]
- De Luca, R.; Botelho, D. The unconscious perception of smells as a driver of consumer responses: A framework integrating the emotion-cognition approach to scent marketing. Acad. Mark. Sci. 2021, 11, 145–161. [Google Scholar] [CrossRef]
- Meiselman, H.L. A review of the current state of emotion research in product development. Food Res. Int. 2015, 76, 192–199. [Google Scholar] [CrossRef]
- Billeci, L.; Sanmartin, C.; Tonacci, A.; Taglieri, I.; Ferroni, G.; Marangoni, R.; Venturi, F. Wearable sensors to measure the influence of sonic seasoning on wine consumers in a live context: A preliminary proof-of-concept study. J. Sci. Food Agric. 2024, in press. [CrossRef] [PubMed]
- Tonacci, A.; Scalzini, G.; Díaz-Guerrero, P.; Sanmartin, C.; Taglieri, I.; Ferroni, G.; Flamini, G.; Odello, L.; Billeci, L.; Venturi, F. Chemosensory analysis of emotional wines: Merging of explicit and implicit methods to measure emotions aroused by red wines. Food Res. Int. 2024, 190, 114611. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology, NIST. NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Pub. Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Billeci, L.; Sanmartin, C.; Tonacci, A.; Taglieri, I.; Bachi, L.; Ferroni, G.; Braceschi, G.P.; Odello, L.; Venturi, F. Wearable sensors to evaluate autonomic response to olfactory stimulation: The influence of short intensive sensory training. Biosensors 2023, 13, 478. [Google Scholar] [CrossRef]
- Parichanon, P.; Ascrizzi, R.; Tani, C.; Sanmartin, C.; Taglieri, I.; Macaluso, M.; Flamini, G.; Pieracci, Y.; Venturi, F.; Conti, B. The protective combined effect of chitosan and essential oil coatings on cheese and cured meat against the oviposition of Piophila casei. Food Bioscence 2023, 56, 10313. [Google Scholar] [CrossRef]
- Rossi, F. Assessing sensory panelist performance using repeatability and reproducibility measures. Food Qual. Prefer. 2001, 12, 467–479. [Google Scholar] [CrossRef]
- EN ISO 8589:2010; Sensory Analysis—General Guidance for the Design of Test Rooms. European Committee for Standardization: Brussels, Belgium, 2010.
- Ferrarini, R.; Carbognin, C.; Casarotti, E.M.; Nicolis, E.; Nencini, A.; Meneghini, A.M. The emotional response to wine consumption. Food Qual. Prefer. 2010, 21, 720–725. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Boucsein, W. Electrodermal activity. In Techniques in Psychophysiology; Springer: New York, NY, USA, 2012; Volume 3. [Google Scholar]
- Emotiv: Emotiv Insight Brainwear 5 Channel Wireless EEG Headset. 2019. Available online: https://www.emotiv.com/insight/ (accessed on 31 October 2024).
- Emotiv: Software. 2019. Available online: https://www.emotiv.com (accessed on 31 October 2024).
- Diaz-Maroto, M.C.; Perez-Coello, M.S.; Cabezucto, M.D. Headspace Solid-Phase Microextraction Analysis of Volatile Components of Spices Key Words Gas chromatography-mass spectrometry Solid-phase microextraction Volatile compounds in spices Basil, oregano, and bay leaves. Chromatographia 2002, 55, 729–735. [Google Scholar]
- The Good Scents Company (TGSC) Information System. Available online: https://www.thegoodscentscompany.com/index.html (accessed on 31 August 2024).
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Katerina, V.; Klara, U.; Samnang, N.; Ladislav, K. Chemical Composition of Essential Oils and Supercritical Carbon Dioxide Extracts from Amomum kravanh, Citrus hystrix and Piper nigrum ‘Kampot’ . Molecules 2023, 28, 7748. [Google Scholar] [CrossRef]
- Dash, M.; Singh, S.; Sahoo, B.C.; Sahoo, S.; Sahoo, R.K.; Nayak, S.; Kar, B. Potential role of Indian long pepper (Piper longum L.) volatiles against free radicals and multidrug resistant isolates. Nat. Prod. Res. 2021, 36, 4271–4275. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Kormod, L.; Labib, R.M.; Farag, M.A. Metabolome Based Volatiles Mapping of Roasted Umbelliferous Fruits Aroma via HS-SPME GC/MS and Peroxide Levels Analyses. J. Chromatogr. B 2018, 1099, 117–126. [Google Scholar] [CrossRef]
- Noumi, E.; Snoussi, M.; Alreshidi, M.M.; Rekha, P.D.; Saptami, K.; Caputo, L.; De Martino, L.; Souza, L.F.; Msaada, K.; Mancini, E.; et al. Chemical and Biological Evaluation of Essential Oils from Cardamom Species. Molecules 2018, 23, 2818. [Google Scholar] [CrossRef]
- Morsy, N.F.S. A short extraction time of high quality hydrodistilled cardamom (Elettaria cardamomum L. Maton) essential oil using ultrasound as a pretreatment. Ind. Crops Prod. 2015, 65, 287–292. [Google Scholar] [CrossRef]
- Morsy, N.F.S.; Hammad, K.S.M. Volatile Constituents, Radical Scavenging and Cytotoxic Activities of Mexican allspice (Pimenta dioica L. Merrill) Berries Essential Oil. J. Essent. Oil Bear. Plants 2018, 21, 859–868. [Google Scholar] [CrossRef]
- García-Fajardo, J.; Martínez-Sosa, M.; Estarrón-Espinosa, M.; Vilarem, G.; Gaset, A.; de Santos, J.M. Comparative Study of the Oil and Supercritical CO2 Extract of Mexican Pimento (Pimenta dioica Merrill). J. Essent. Oil Res. 1997, 9, 181–185. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus molle L. and Schinus terebinthifolius Raddi berries essential oils. J. Food Sci. 2010, 75, C466–C472. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.d.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Bajer, T.; Surmová, S.; Eisner, A.; Ventura, K.; Bajerová, P. Use of simultaneous distillation-extraction, supercritical fluid extraction and solid-phase microextraction for characterisation of the volatile profile of Dipteryx odorata (Aubl.) willd. Ind. Crop. Prod. 2018, 119, 313–321. [Google Scholar] [CrossRef]
- U.S. FDA. CFR—Code of Federal Regulations Title 21—Food and Drugs Chapter I—Food and Drug Administration Department of Health and Human Services Subchapter B—Food for Human Consumption. 2017. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101&showFR=1&subpartNode=21:2.0.1.1.2.2 (accessed on 28 September 2024).
- Modupalli, N.; Naik, M.; Sunil, C.K.; Natarajan, V. Emerging non-destructive methods for quality and safety monitoring of spices. Trends Food Sci. Technol. 2021, 108, 133–147. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Straszewski, T.; Siegel, J.T. Differential Effects of High- and Low-Arousal Positive Emotions on Help-Seeking for Depression. Appl. Psychol. Health Well Being 2020, 12, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Tian, Y.; Zhang, L.; Chen, Y.; Bai, Y.; Li, D.; Liu, J.; Gable, P.; Yin, H. The role of valence, arousal, stimulus type, and temporal paradigm in the effect of emotion on time perception: A meta-analysis. Psychon. Bull. Rev. 2023, 30, 1–21. [Google Scholar] [CrossRef]
- Martin, C.; Ravel, N. Beta and gamma oscillatory activities associated with olfactory memory tasks: Different rhythms for different functional networks? Front. Behav. Neurosci. 2014, 8, 218. [Google Scholar] [CrossRef]
- Chrea, C.; Grandjean, D.; Delplanque, S.; Cayeux, I.; Le Calvee, B.; Aymard, L.; Velazco, M.; Sander, D.; Scherer, K.R. Mapping the semantic space for the subjective experience of emotional responses to odors. Chem. Senses 2009, 34, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.; Adhikari, K.; Chambers, E., IV; Alavi, S.; King, S.; Haub, M. Spices in a product affect emotions: A study with an extruded snack product. Foods 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]

| Botanical Name | Family | Native | Common Name in English, French, Spanish, German and Italian | Organ or Part Used |
|---|---|---|---|---|
| Herbs | ||||
| Pimpinella anisum L. | Apiaceae | Southwest Asia, Greece, Egypt, and India | (eng) anise, (fra) anis, (esp) anís, (deu) anis, (ita) anice | Aniseed fruit |
| Elettaria cardamomum (L.) | Zingiberaceae | Southern India | (eng) cardamom, (fra) cardamome, (esp) cardamomo, (deu) kardamom, (ita) cardamomo | Seeds |
| Dipteryx odorata (Aubl.) Forsyth f. | Fabaceae | Central and South America | (eng) tonka bean, (fra) fève tonka, (esp) haba tonka, (deu) tonkabohne, (ita) fava tonka | Seeds and fruit |
| Schinus molle L. | Anacardiaceae | Brazil | (eng) pink pepper or false pepper, (fra) faux poivrier, (esp) pimienta de brasil o pimienta rosada, (deu) rosa pfeffer, (ita) pepe rosa | Pepper fruit |
| Pimenta dioica (L.) Merr. | Myrtaceae | West Indies and Central America especially Jamaica and Cuba | (eng) jamaica pepper or allspice, (fra) poivre de la jamaïque, (esp) pimienta de jamaica, (deu) jamaika pfeffer, (ita) pepe garofanato | Berries and leaf |
| Piperaceae | ||||
| Piper longum L. | Piperaceae | India | (eng) long pepper, (fra) poivre long, (esp) pimienta larga, (deu) langer pfeffer, (ita) pepe lungo | Pepper fruit and dried leaf |
| Piper cubeba L.f. | Piperaceae | India | (eng) cubeb pepper, (fra) poivre cubèbe, (esp) pimienta cubeba, (deu) kubeben pfeffer, (ita) pepe cubebe | Pepper fruit |
| Piper nigrum L. (black p. in powder) | Piperaceae | India | (eng) black pepper, (fra) poivre noir, (esp) pimienta negra, (deu) schwarzer pfeffer, (ita) pepe nero | Peppercorn |
| Piper nigrum L. (black p. in grains) | Piperaceae | India | (eng) black pepper, (fra) poivre noir, (esp) pimienta negra, (deu) schwarzer pfeffer, (ita) pepe nero | Peppercorn |
| Piper nigrum L. | Piperaceae | Cambodia | (eng) Kampot pepper, (fra) poivre de Kampot, (esp) pimienta roja Kampot, (deu) Kampot-pfeffer, (ita) pepe rosso di Kampot | Peppercorn |
| Compounds | l.r.i. a | Relative Abundance (%) ± SD b | ||||
|---|---|---|---|---|---|---|
| Piper cubeba | Piper nigrum (Black p. in Powder) | Piper nigrum (Black p. in Grains) | Piper Longum | Piper nigrum (Red Kampot) | ||
| α-thujene | 931 | 10.6 ± 0.28 | - c | 1.2 ± 0.04 | - | - |
| α-pinene | 941 | 7.3 ± 0.25 | 1.0 ± 0.21 | 4.9 ± 0.06 | 1.0 ± 0.16 | 4.0 ± 0.39 |
| sabinene | 976 | 34.6 ± 0.69 | 0.2 ± 0.02 | 11.0 ± 0.13 | 0.2 ± 0.04 | 0.2 ± 0.02 |
| β-pinene | 982 | 3.6 ± 1.17 | 5.8 ± 0.56 | 13.6 ± 0.01 | 0.9 ± 0.23 | 11.3 ± 0.47 |
| myrcene | 993 | 1.6 ± 0.20 | 3.7 ± 0.08 | 2.6 ± 0.04 | 0.1 ± 0.01 | 4.5 ± 0.20 |
| α-phellandrene | 1005 | 1.5 ± 0.03 | 7.4 ± 0.18 | 0.1 ± 0.17 | - | 9.3 ± 0.31 |
| δ-3-carene | 1011 | 0.5 ± 0.04 | 29.1 ± 0.77 | - | 0.4 ± 0.08 | 34.8 ± 0.08 |
| α-terpinene | 1018 | 0.7 ± 0.04 | 0.6 ± 0.66 | 0.1 ± 0.11 | - | 0.9 ± 0.36 |
| o-cymene | 1024 | - | 0.3 ± 0.21 | - | - | - |
| p-cymene | 1027 | 1.6 ± 0.06 | 2.2 ± 0.17 | 0.1 ± 0.19 | 0.1 ± 0.00 | 1.9 ± 0.09 |
| limonene | 1032 | 14.5 ± 1.17 | 31.5 ± 0.21 | 41.4 ± 0.38 | 1.5 ± 0.18 | 25.1 ± 0.24 |
| 1,8-cineole | 1034 | 0.9 ± 0.47 | - | - | 0.3 ± 0.03 | - |
| (Z)-β-ocimene | 1042 | - | - | - | 0.3 ± 0.06 | - |
| (E)-β-ocimene | 1052 | 0.1 ± 0.01 | - | - | 0.3 ± 0.02 | - |
| γ-terpinene | 1062 | 1.2 ± 0.00 | 0.6 ± 0.01 | 0.1 ± 0.20 | - | 0.5 ± 0.03 |
| acetophenone | 1068 | - | - | - | 1.1 ± 0.79 | - |
| cis-sabinene hydrate | 1070 | 0.4 ± 0.04 | - | 0.1 ± 0.11 | - | - |
| terpinolene | 1088 | 0.3 ± 0.02 | 3.1 ± 0.10 | 0.1 ± 0.17 | - | 2.1 ± 0.01 |
| linalool | 1101 | 2.9 ± 0.35 | 0.2 ± 0.03 | - | 1.8 ± 0.18 | 0.1 ± 0.07 |
| 4-terpineol | 1178 | 0.2 ± 0.00 | - | - | - | - |
| (E)-anethole | 1283 | - | - | 0.2 ± 0.00 | 0.7 ± 0.03 | - |
| n-tridecane | 1300 | - | - | - | 5.1 ± 0.80 | - |
| piperonal | 1333 | - | - | - | 0.2 ± 0.04 | - |
| δ-elemene | 1340 | 0.6 ± 0.00 | 1.1 ± 0.18 | - | 0.3 ± 0.06 | 0.1 ± 0.07 |
| α-cubebene | 1350 | 3.3 ± 0.04 | 0.1 ± 0.02 | 0.2 ± 0.01 | 0.4 ± 0.11 | - |
| α-ylangene | 1372 | - | - | - | 0.1 ± 0.01 | - |
| α-copaene | 1376 | 3.1 ± 0.02 | 2.1 ± 0.42 | 4.7 ± 0.05 | 1.2 ± 0.01 | - |
| β-cubebene | 1390 | 3.9 ± 0.08 | - | 0.2 ± 0.00 | 0.3 ± 0.06 | - |
| β-elemene | 1392 | 0.4 ± 0.04 | 0.1 ± 0.01 | 0.1 ± 0.00 | 3.6 ± 0.24 | 0.1 ± 0.08 |
| α-gurjunene | 1410 | 0.2 ± 0.01 | - | - | - | - |
| cis-α-bergamotene | 1416 | - | - | - | 1.1 ± 0.08 | - |
| β-caryophyllene | 1420 | 1.7 ± 0.06 | 10.3 ± 1.99 | 17.7 ± 0.21 | 22.4 ± 0.45 | 4.8 ± 0.54 |
| β-copaene | 1429 | 0.1 ± 0.08 | - | - | 0.5 ± 0.00 | - |
| trans-α-bergamotene | 1438 | - | 0.3 ± 0.15 | - | 1.6 ± 0.04 | - |
| α-humulene | 1456 | 0.7 ± 0.04 | 0.3 ± 0.07 | 0.5 ± 0.01 | 9.6 ± 0.25 | 0.2 ± 0.02 |
| (E)-β-farnesene | 1460 | - | - | - | 1.3 ± 0.21 | - |
| alloaromadendrene | 1461 | 0.5 ± 0.01 | - | - | - | - |
| γ-muurolene | 1477 | 0.5 ± 0.01 | - | - | 0.5 ± 0.05 | - |
| germacrene D | 1478 | 0.6 ± 0.04 | - | - | 9.1 ± 0.01 | - |
| ar-curcumene | 1483 | - | - | - | 1.9 ± 0.05 | - |
| β-selinene | 1485 | 0.1 ± 0.07 | - | - | 3.1 ± 0.24 | 0.1 ± 0.09 |
| 1-pentadecene | 1488 | - | - | - | 3.4 ± 0.16 | - |
| α-selinene | 1494 | 0.4 ± 0.01 | - | - | 1.6 ± 0.01 | - |
| bicyclogermacrene | 1496 | 0.3 ± 0.05 | - | - | - | - |
| α-zingiberene | 1496 | - | - | - | 1.6 ± 0.71 | - |
| α-muurolene | 1498 | 0.2 ± 0.01 | - | - | - | - |
| n-pentadecane | 1500 | - | - | - | 9.3 ± 0.63 | - |
| (Z)-α-bisabolene | 1504 | - | - | - | 1.1 ± 0.08 | - |
| β-bisabolene | 1509 | - | - | 0.4 ± 0.01 | 5.8 ± 0.43 | - |
| cubebol | 1516 | 0.7 ± 0.04 | - | - | - | - |
| 7-epi-α-selinene | 1517 | - | - | - | 2.0 ± 0.18 | - |
| δ-cadinene | 1525 | 0.3 ± 0.01 | 0.1 ± 0.09 | 0.3 ± 0.01 | - | - |
| β-sesquiphellandrene | 1525 | - | - | - | 0.7 ± 0.15 | - |
| (E)-γ-bisabolene | 1535 | - | - | - | 0.5 ± 0.08 | - |
| germacrene B | 1554 | - | - | - | 1.4 ± 0.15 | - |
| caryophyllene oxide | 1581 | - | - | - | 0.5 ± 0.01 | - |
| humulene epoxide II | 1608 | - | - | - | 0.1 ± 0.11 | - |
| Monoterpene hydrocarbons | 78.1 ± 0.08 | 85.4 ± 2.96 | 75.3 ± 0.18 | 4.8 ± 0.52 | 94.7 ± 0.81 | |
| Oxygenated monoterpenes | 4.4 ± 0.08 | 0.2 ± 0.03 | 0.1 ± 0.11 | 2.0 ± 0.16 | 0.1 ± 0.07 | |
| Sesquiterpene hydrocarbons | 16.8 ± 0.03 | 14.4 ± 2.93 | 24.2 ± 0.29 | 71.8 ± 1.9 | 5.1 ± 0.81 | |
| Oxygenated sesquiterpenes | 0.7 ± 0.04 | - | - | 0.6 ± 0.09 | - | |
| Phenylpropanoids | - | - | 0.2 ± 0.00 | 0.7 ± 0.03 | - | |
| Non-terpene derivatives | - | - | - | 19.2 ± 0.85 | - | |
| Total identified (%): | 100 ± 0.01 | 100 ± 0.00 | 99.7 ± 0.01 | 99.1 ± 0.26 | 99.8 ± 0.06 | |
| Compounds | l.r.i. a | Relative Abundance (%) ± SD b | ||||
|---|---|---|---|---|---|---|
| Pimpinella anisum (Anise) | Elettaria cardamomum (Cardamom) | Pimenta dioica (Jamaica Pepper) | Schinus molle (Pink Pepper) | Dipteryx odorata (Tonka Bean) | ||
| butyrolactone | 918 | - c | - | - | - | 0.6 ± 0.08 |
| α-thujene | 931 | - | 0.4 ± 0.09 | 1.1 ± 0.05 | 1.0 ± 0.23 | - |
| α-pinene | 941 | - | 0.8 ± 0.16 | 6.0 ± 0.52 | 22.4 ± 4.11 | - |
| camphene | 955 | - | - | 0.2 ± 0.06 | 1.0 ± 0.37 | - |
| benzaldehyde | 959 | 0.2 ± 0.05 | - | - | - | - |
| sabinene | 976 | 0.1 ± 0.10 | 3.4 ± 0.33 | 1.0 ± 0.08 | 20.3 ± 2.32 | - |
| β-pinene | 982 | 0.1 ± 0.10 | 0.3 ± 0.02 | 5.7 ± 0.55 | 2.0 ± 0.54 | - |
| myrcene | 993 | - | 2.2 ± 0.01 | 2.8 ± 0.04 | 2.6 ± 0.31 | - |
| α-phellandrene | 1005 | - | - | 0.5 ± 0.01 | 20.3 ± 3.37 | - |
| δ-3-carene | 1011 | 0.2 ± 0.11 | - | 9.5 ± 0.64 | 8.0 ± 8.10 | - |
| α-terpinene | 1018 | - | 0.1 ± 0.11 | - | 0.8 ± 0.72 | - |
| p-cymene | 1027 | 0.1 ± 0.06 | 0.3 ± 0.04 | 3.1 ± 0.25 | 4.8 ± 1.96 | - |
| limonene | 1032 | 1.7 ± 1.15 | 4.1 ± 0.59 | 9.3 ± 0.13 | 15.6 ± 3.4 | 0.2 ± 0.10 |
| 1,8-cineole | 1034 | 0.3 ± 0.16 | 43.2 ± 3.13 | 29.4 ± 1.13 | - | - |
| (E)-β-ocimene | 1052 | - | 0.1 ± 0.12 | - | - | - |
| γ-terpinene | 1062 | 0.2 ± 0.06 | 0.4 ± 0.10 | 0.5 ± 0.01 | 0.4 ± 0.30 | - |
| cis-sabinene hydrate | 1070 | - | 0.7 ± 0.04 | - | - | - |
| 1-octanol | 1071 | - | - | 0.3 ± 0.01 | - | - |
| terpinolene | 1088 | - | 0.3 ± 0.16 | - | 0.6 ± 0.01 | - |
| p-cymenene | 1089 | - | - | 0.4 ± 0.08 | - | - |
| linalool | 1101 | 0.1 ± 0.02 | 4.8 ± 0.36 | 1.0 ± 0.06 | - | 0.1 ± 0.04 |
| nonanal | 1104 | 0.5 ± 0.49 | - | - | - | 0.2 ± 0.14 |
| 4-terpineol | 1178 | - | 0.6 ± 0.01 | 1.0 ± 0.01 | - | - |
| α-terpineol | 1189 | - | 1.6 ± 0.16 | 0.5 ± 0.02 | - | - |
| methyl chavicol | 1197 | 2.1 ± 0.08 | - | 0.9 ± 0.03 | - | 0.3 ± 0.08 |
| decanal | 1204 | 0.3 ± 0.17 | - | - | - | - |
| p-anisaldehyde | 1256 | 0.7 ± 0.09 | - | - | - | - |
| linalyl acetate | 1259 | - | 5.1 ± 0.25 | - | - | - |
| (E)-anethole | 1283 | 91.1 ± 2.37 | 0.2 ± 0.01 | - | - | 2.1 ± 0.42 |
| carvacrol | 1298 | - | - | - | - | 0.2 ± 0.02 |
| α-terpinyl acetate | 1352 | - | 30.7 ± 1.77 | - | - | - |
| eugenol | 1358 | - | - | 3.8 ± 0.91 | - | - |
| hydrocoumarin | 1376 | - | - | - | - | 0.6 ± 0.06 |
| α-copaene | 1376 | - | - | 1.5 ± 0.07 | - | - |
| geranyl acetate | 1385 | - | 0.1 ± 0.14 | - | - | - |
| β-elemene | 1392 | - | - | 0.5 ± 0.06 | - | - |
| methyl eugenol | 1403 | - | - | 16.8 ± 1.66 | - | - |
| β-caryophyllene | 1420 | - | - | 3.2 ± 0.21 | - | 0.1 ± 0.08 |
| coumarin | 1432 | - | - | - | - | 95.6 ± 1.02 |
| α-himachalene | 1448 | 0.2 ± 0.00 | - | - | - | - |
| α-humulene | 1456 | - | - | 0.2 ± 0.03 | - | - |
| germacrene D | 1478 | - | - | - | 0.2 ± 0.04 | - |
| β-chamigrene | 1485 | 2.1 ± 0.08 | - | - | - | - |
| caryophyllene oxide | 1581 | - | - | 0.8 ± 0.11 | - | - |
| Monoterpene hydrocarbons | 2.3 ± 1.57 | 12.4 ± 0.63 | 39.9 ± 1.99 | 99.8 ± 0.04 | 0.2 ± 0.10 | |
| Oxygenated monoterpenes | 0.5 ± 0.18 | 86.8 ± 0.40 | 31.9 ± 1.05 | - | 0.4 ± 0.06 | |
| Sesquiterpene hydrocarbons | 2.3 ± 0.08 | - | 5.5 ± 0.37 | 0.2 ± 0.04 | 0.1 ± 0.08 | |
| Oxygenated sesquiterpenes | - | - | 0.8 ± 0.11 | - | - | |
| Phenylpropanoids | 93.2 ± 2.28 | 0.2 ± 0.01 | 21.4 ± 2.54 | - | 2.4 ± 0.50 | |
| Non-terpene derivatives | 1.6 ± 0.62 | - | 0.3 ± 0.01 | - | 97.0 ± 0.74 | |
| Total identified (%): | 100 ± 0.00 | 99.3 ± 0.25 | 99.8 ± 0.01 | 100 ± 0.01 | 100 ± 0.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taglieri, I.; Tonacci, A.; Flamini, G.; Díaz-Guerrero, P.; Ascrizzi, R.; Bachi, L.; Procissi, G.; Billeci, L.; Venturi, F. Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study. Foods 2025, 14, 110. https://doi.org/10.3390/foods14010110
Taglieri I, Tonacci A, Flamini G, Díaz-Guerrero P, Ascrizzi R, Bachi L, Procissi G, Billeci L, Venturi F. Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study. Foods. 2025; 14(1):110. https://doi.org/10.3390/foods14010110
Chicago/Turabian StyleTaglieri, Isabella, Alessandro Tonacci, Guido Flamini, Pierina Díaz-Guerrero, Roberta Ascrizzi, Lorenzo Bachi, Giorgia Procissi, Lucia Billeci, and Francesca Venturi. 2025. "Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study" Foods 14, no. 1: 110. https://doi.org/10.3390/foods14010110
APA StyleTaglieri, I., Tonacci, A., Flamini, G., Díaz-Guerrero, P., Ascrizzi, R., Bachi, L., Procissi, G., Billeci, L., & Venturi, F. (2025). Novel Perspectives for Sensory Analysis Applied to Piperaceae and Aromatic Herbs: A Pilot Study. Foods, 14(1), 110. https://doi.org/10.3390/foods14010110










