Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Spectral Data Acquisition with a Portable Vis/NIR Spectrophotometer
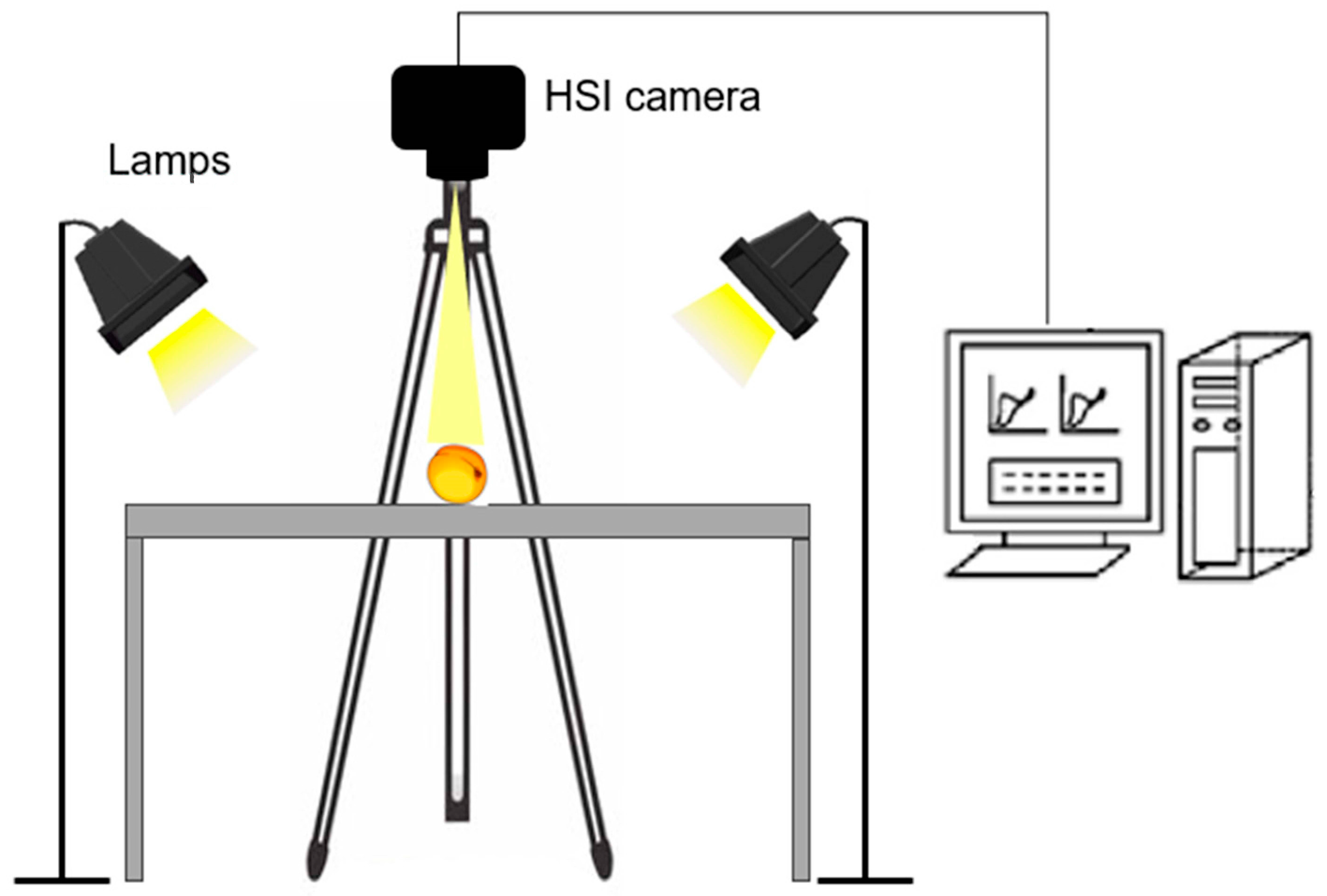
2.3. Spectral Data Acquisition with a Portable HSI Device
2.4. Fruit Quality Traits
2.5. Chemometric Methods
2.5.1. Data Analysis
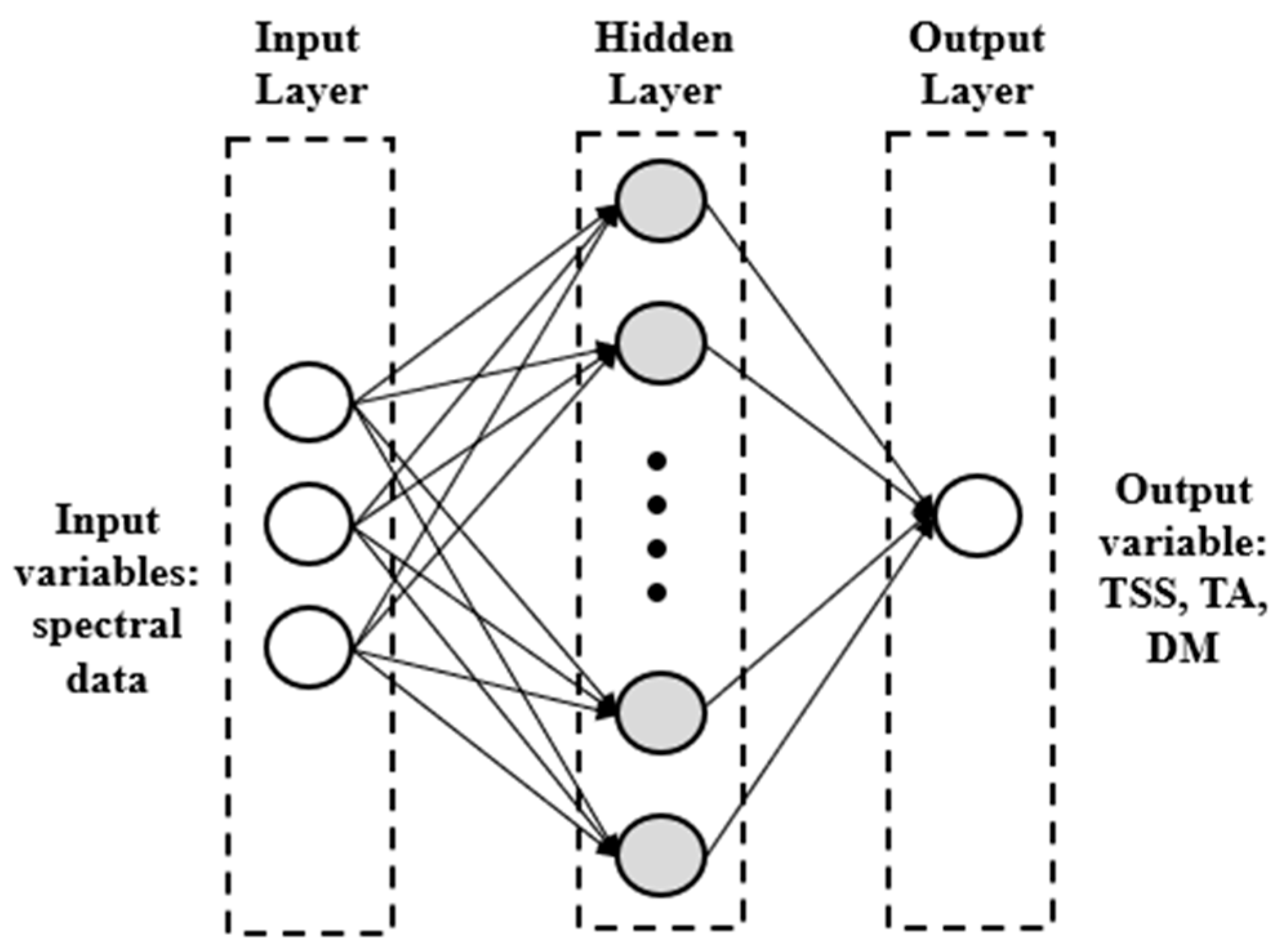
2.5.2. Artificial Neural Network Models
3. Results
3.1. General Aspects of Apricot Fruit
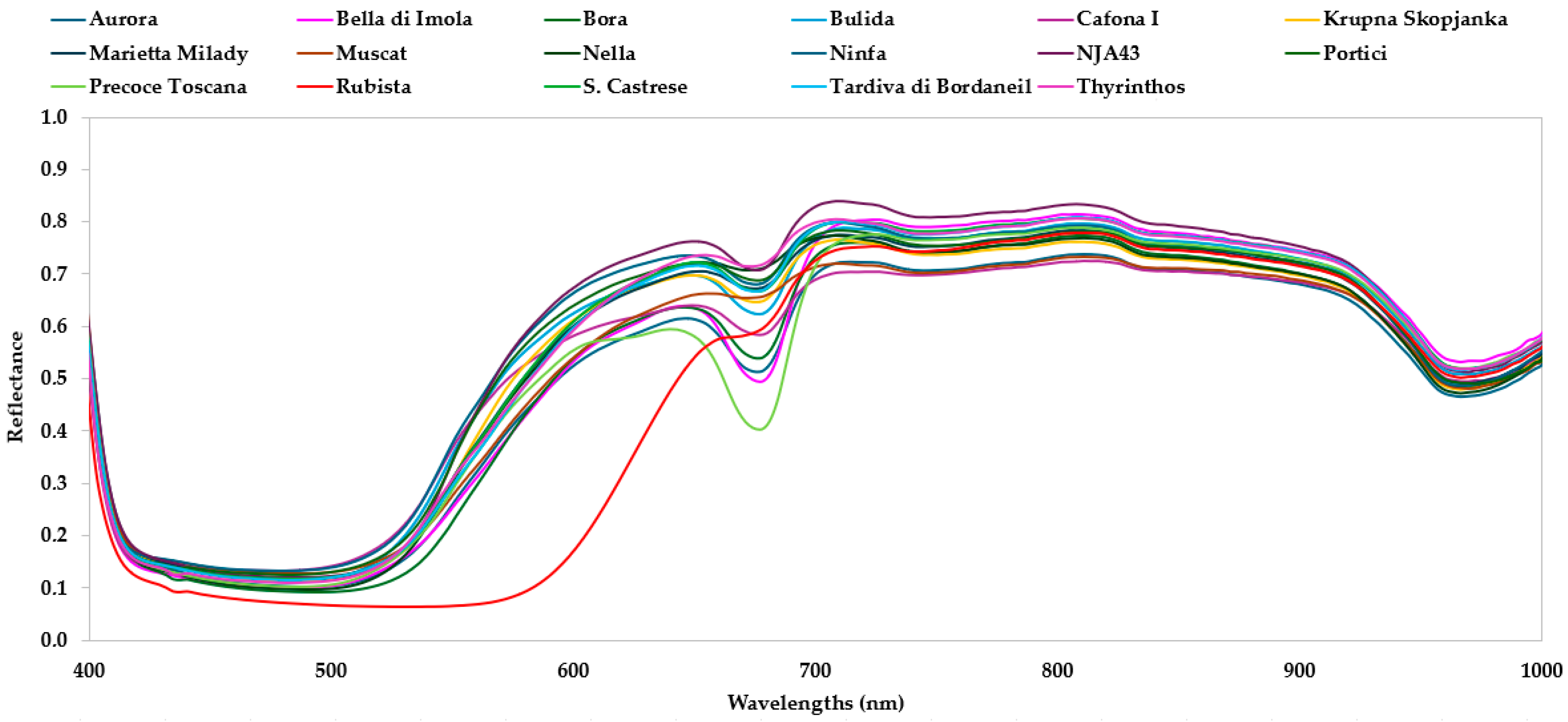
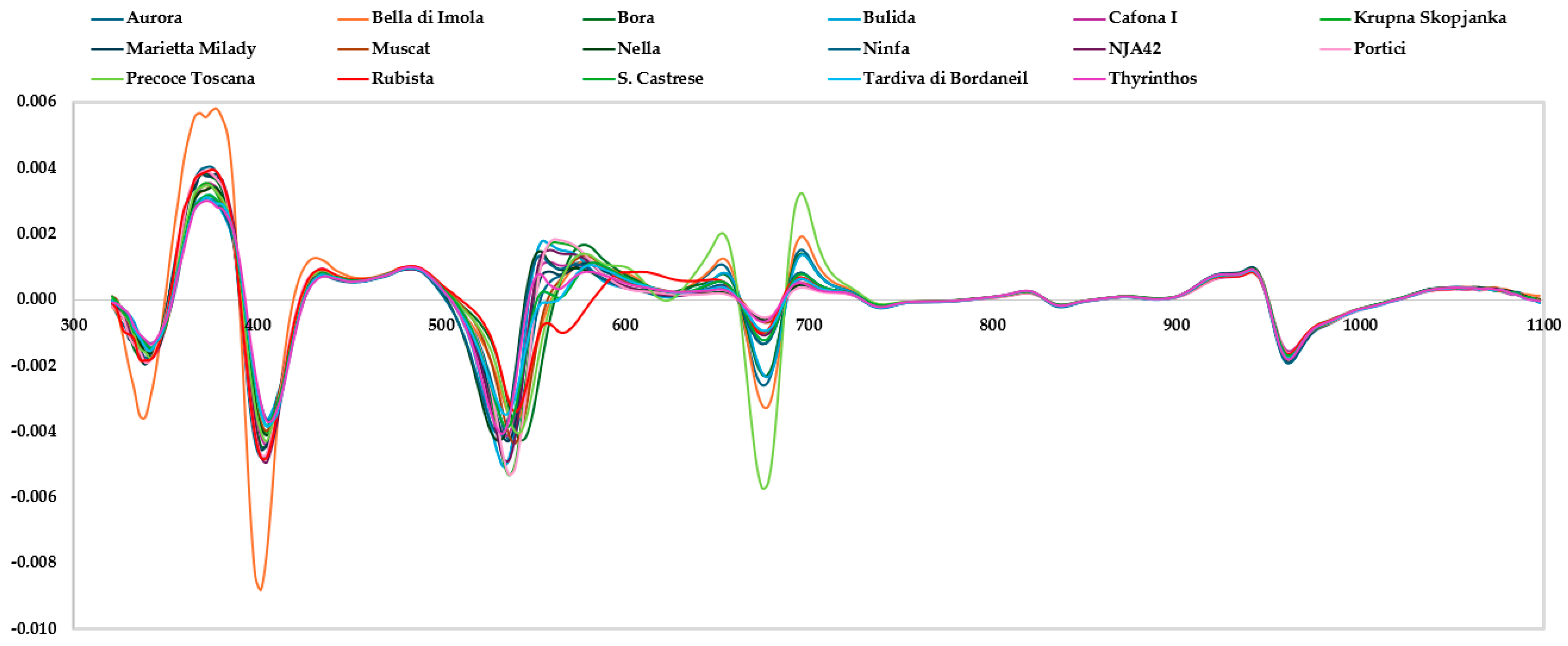
3.2. Spectrum Analysis
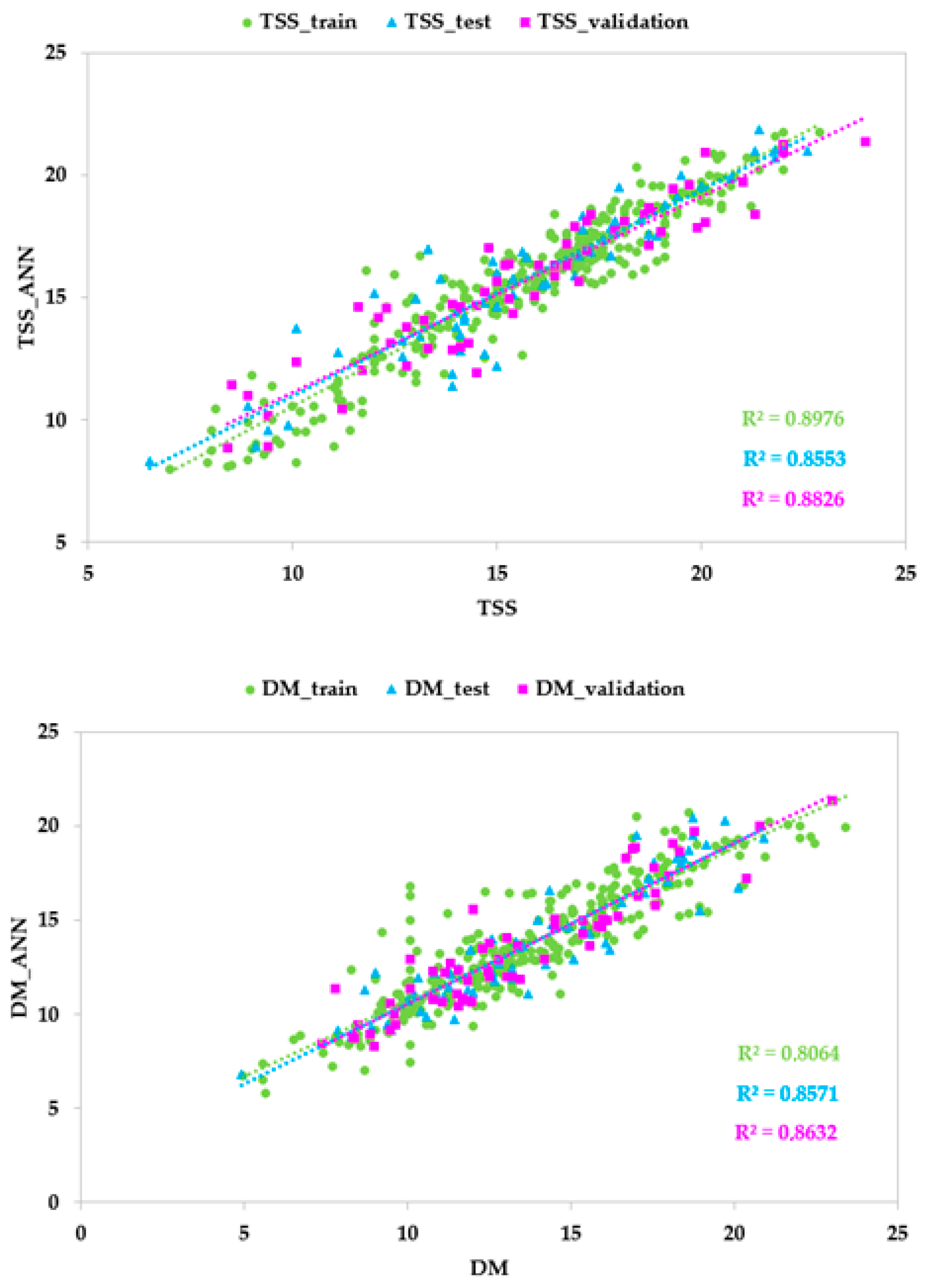
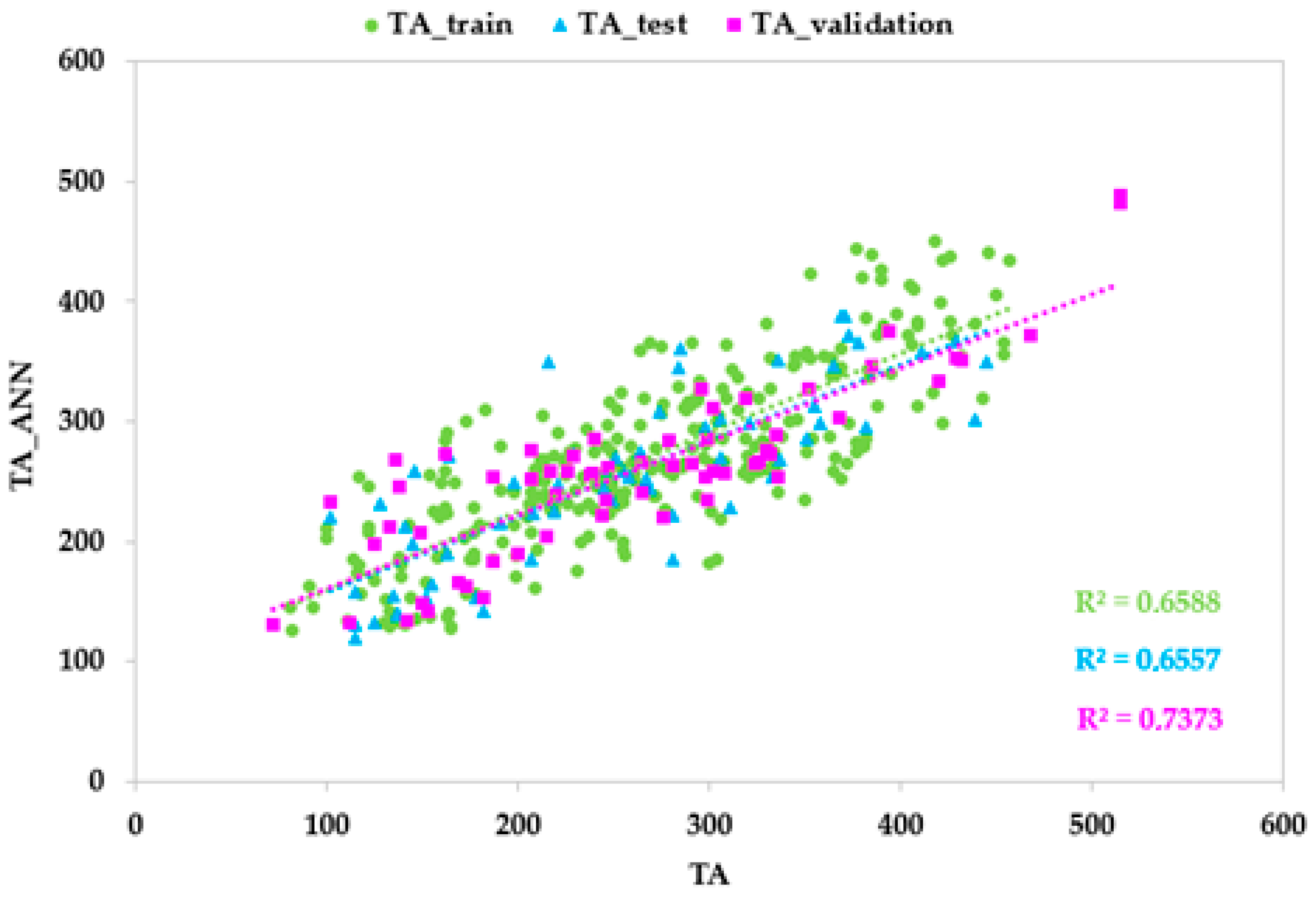
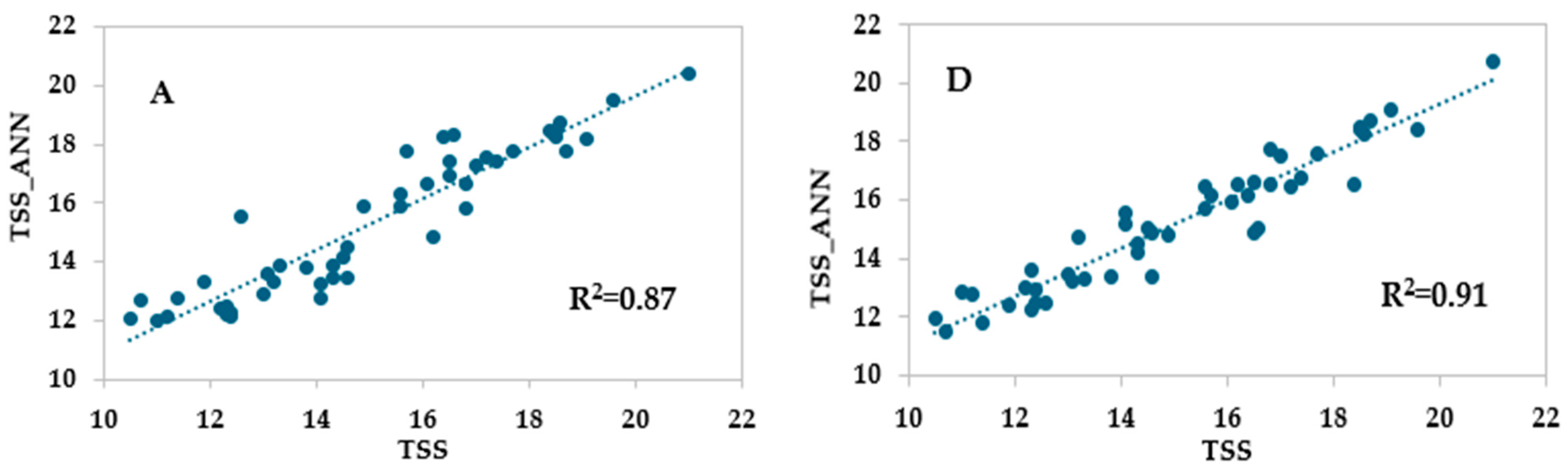
3.3. ANN Models Based on Vis/NIR Spectrophotometer Data
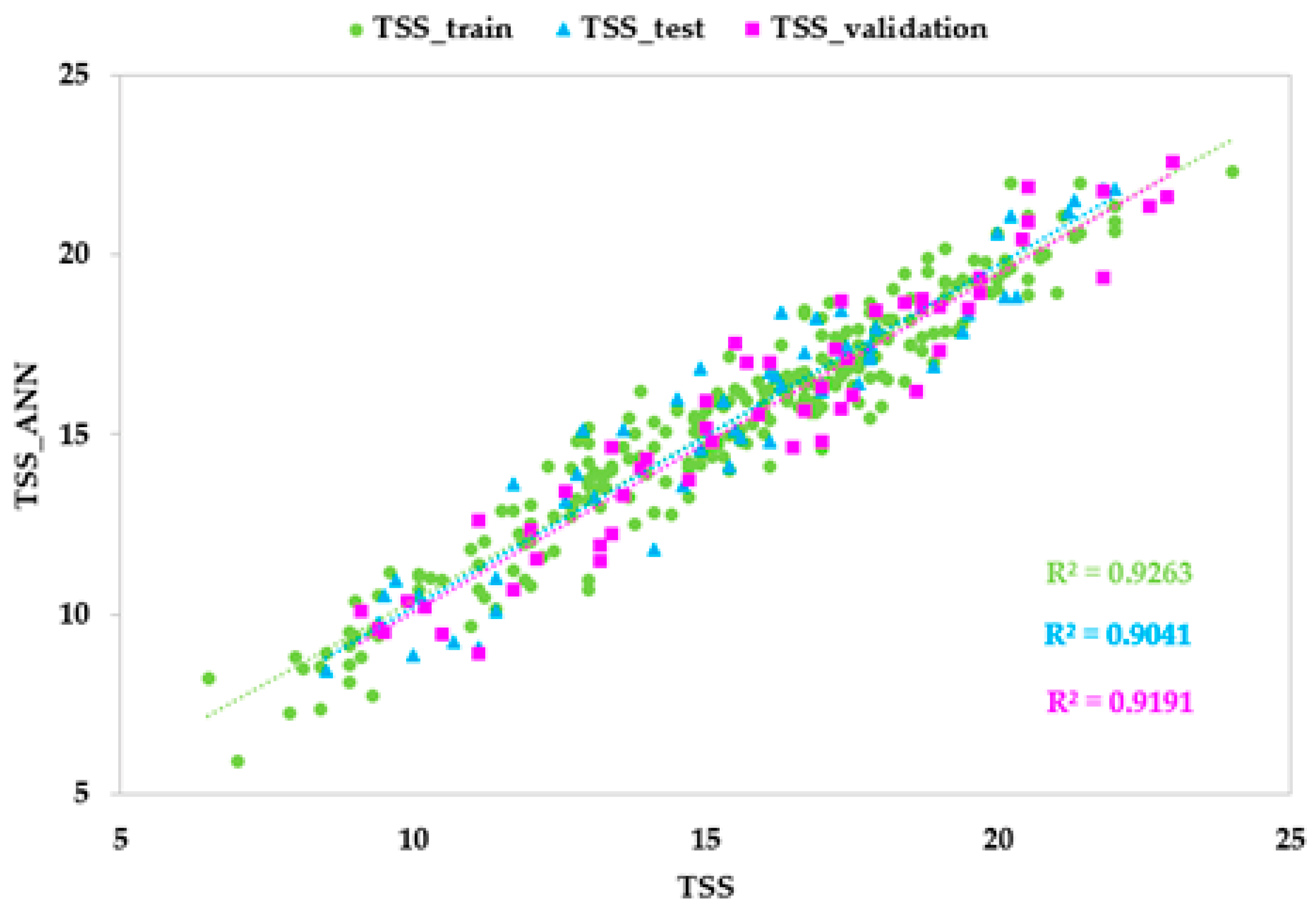
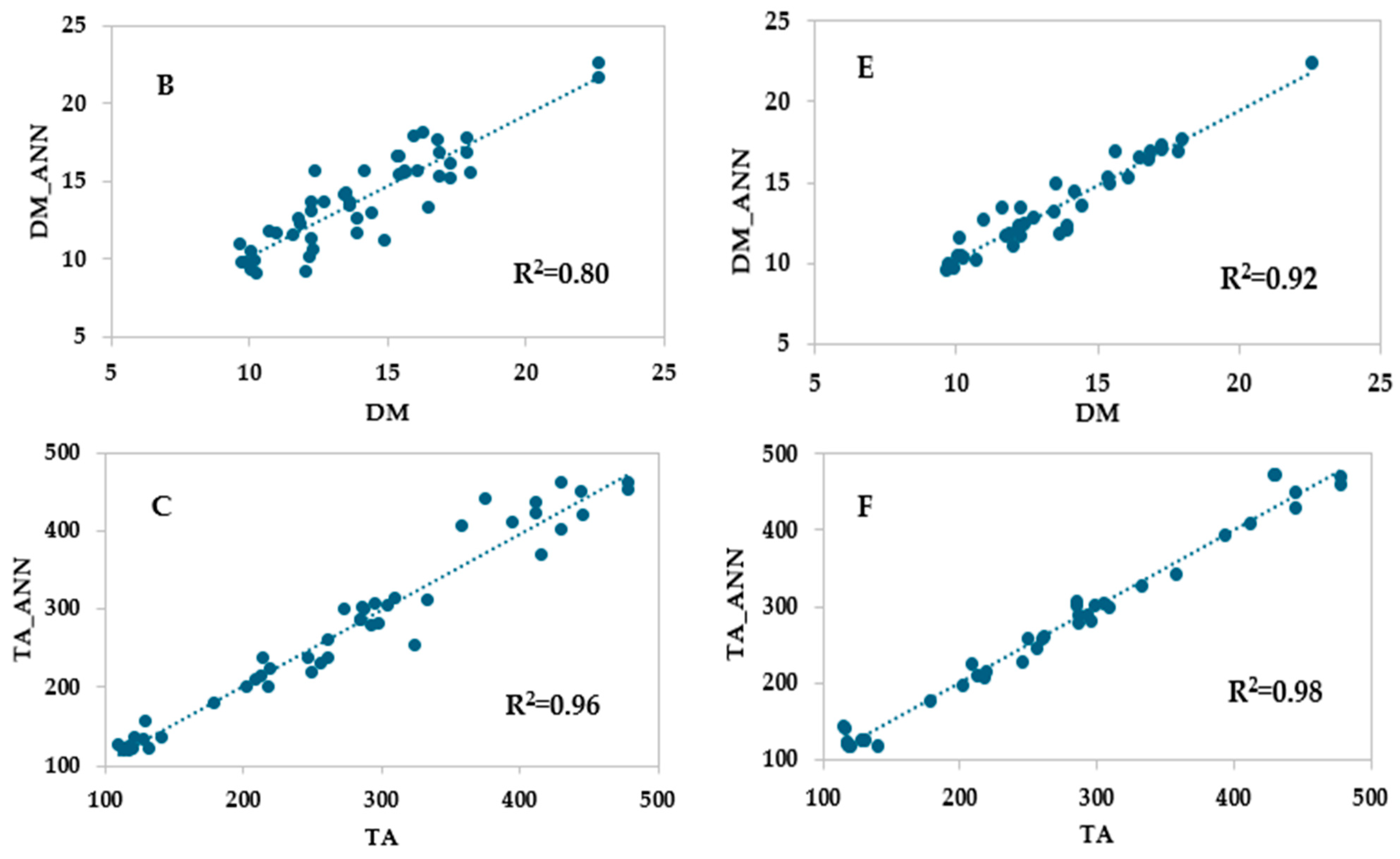
3.4. ANN Models Based on HSI Data
3.5. Sensitivity Analysis
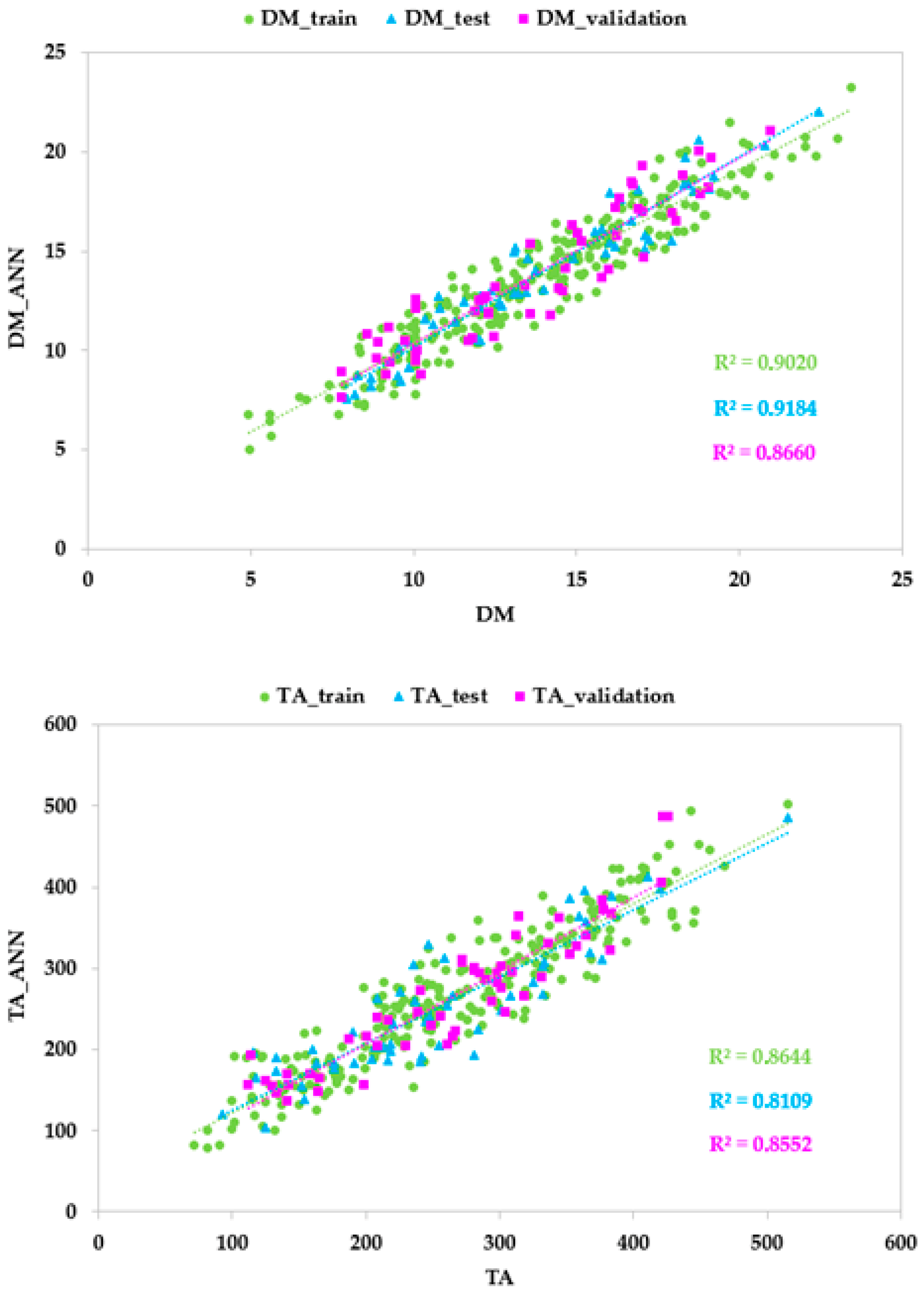
3.6. Validation of Developed Models with External Datasets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization Statistical Databases. 2024. Available online: http://faostat.fao.org (accessed on 30 January 2024).
- Hegedüs, A.; Pfeiffer, P.; Papp, N.; Abrankó, L.; Blázovics, A.; Pedryc, A.; Stefanovits-Bányai, E. Accumulation of antioxidants in apricot fruit through ripening: Characterization of a genotype with enhanced functional properties. Biol. Res. 2011, 44, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Leccese, A.; Bartolini, S.; Viti, R. From genotype to apricot fruit quality: The antioxidant properties contribution. Plant Foods Hum. Nutr. 2012, 67, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.M.; Renard, C.M.G.C. Rapid and non-destructive analysis of apricot fruit quality using FT-near-infrared spectroscopy. Food Chem. 2009, 113, 1323–1328. [Google Scholar] [CrossRef]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.M.; Renard, C.M.G.C. Application of ATR-FTIR for a rapid and simultaneous determination of sugars and organic acids in apricot fruit. Food Chem. 2009, 115, 1133–1140. [Google Scholar] [CrossRef]
- Camps, C.; Christen, D. Non-destructive assessment of apricot fruit quality by portable visible-near infrared spectroscopy. LWT-Food Sci. Technol. 2009, 42, 1125–1131. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciccoritti, R.; Paliotta, M.; Carbone, K. Classification and prediction of early-to-late ripening apricot quality using spectroscopic techniques combined with chemometric tools. Sci. Hortic. 2018, 240, 310–317. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciccoritti, R.; Carbone, K. Vibrational spectroscopy as a green technology for predicting nutraceutical properties and antiradical potential of early-to-late apricot genotypes. Postharvest Biol. Technol. 2019, 155, 156–166. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Yang, Y.; Mu, B.; Nikitina, M.A.; Xiao, X. Vis/Nir optical biosensors applications for fruit monitoring. Biosens. Bioelectron. 2022, 11, 100197. [Google Scholar] [CrossRef]
- Ozdogan, G.; Lin, X.; Sun, D.W. Rapid and noninvasive sensory analyses of food products by hyperspectral imaging: Recent application developments. Trends Food Sci. Technol. 2021, 111, 151–165. [Google Scholar] [CrossRef]
- Amoriello, T.; Ciorba, R.; Ruggiero, G.; Amoriello, M.; Ciccoritti, R. A Performance Evaluation of Two Hyperspectral Imaging Systems for the Prediction of Strawberries’ Pomological Traits. Sensors 2024, 24, 174. [Google Scholar] [CrossRef] [PubMed]
- Fatchurrahman, D.; Nosrati, M.; Amodio, M.L.; Chaudhry, M.M.A.; de Chiara, M.L.V.; Mastrandrea, L.; Colelli, G. Comparison Performance of Visible-NIR and Near-Infrared Hyperspectral Imaging for Prediction of Nutritional Quality of Goji Berry (Lycium barbarum L.). Foods 2021, 10, 1676. [Google Scholar] [CrossRef] [PubMed]
- Munera, S.; Rodríguez-Ortega, A.; Aleixos, N.; Cubero, S.; Gómez-Sanchis, J.; Blasco, J. Detection of Invisible Damages in ‘Rojo Brillante’ Persimmon Fruit at Different Stages Using Hyperspectral Imaging and Chemometrics. Foods 2021, 10, 2170. [Google Scholar] [CrossRef]
- Goyal, S. Artificial Neural Networks in Fruits: A Comprehensive Review. Int. J. Image Graph. Signal Process. 2014, 5, 53–63. [Google Scholar] [CrossRef]
- Naroui Rad, M.R.; Koohkan, S.; Fanaei, H.R.; Pahlavan Rad, M.R. Application of Artificial Neural Networks to predict the final fruit weight and random forest to select important variables in native population of melon (Cucumis melo L.). Sci. Hortic. 2015, 181, 108–112. [Google Scholar] [CrossRef]
- Torkashvand, A.M.; Ahmadi, A.; Nikravesh, N.L. Prediction of kiwifruit firmness using fruit mineral nutrient concentration by artificial neural network (ANN) and multiple linear regressions (MLR). J. Integr. Agric. 2017, 16, 1634–1644. [Google Scholar] [CrossRef]
- Huang, X.; Wang, H.; Qu, S.; Luo, W.; Gao, Z. Using artificial neural network in predicting the key fruit quality of loquat. Food Sci. Nutr. 2021, 9, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Wang, Z.; Niu, H.; Zhang, H.; Zhang, Y.; Tang, Y.; Liu, Y. A non-destructive testing method for soluble solid content in Korla fragrant pears based on electrical properties and artificial neural network. Food Sci. Nutr. 2020, 8, 5172–5181. [Google Scholar] [CrossRef] [PubMed]
- Della Strada, G.; Pennone, F.; Fideghelli, C.; Monastra, F.; Cobianchi, D. Monografia di Cultivar di Albicocco; Ministero delle Politiche Agricole e Forestali: Roma, Italy, 1989. [Google Scholar]
- Sartori, A.; Vitellozzi, F.; Grassi, F.; Della Strada, G.; Fideghelli, C. Drupacee. In Il Germoplasma Frutticolo in Italia; Istituto Sperimentale per la Frutticoltura: Roma, Italy, 2004; Volume I. [Google Scholar]
- Giovannoni, J.; Nguyen, C.; Ampofo, B.; Zhong, S.; Fei, Z. The epigenome and transcriptional dynamics of fruit ripening. Annu. Rev. Plant Biol. 2017, 68, 61–84. [Google Scholar] [CrossRef]
- Minas, I.S.; Anthony, B.M.; Pieper, J.R.; Sterle, D.G. Large-scale and accurate non-destructive visual to near infrared spectroscopy-based assessment of the effect of rootstock on peach fruit internal quality. Eur. J. Agron. 2023, 143, 126706. [Google Scholar] [CrossRef]
- Escribano, S.; Biasi, W.V.; Lerud, R.; Slaughter, D.C.; Mitcham, E.J. Non-destructive prediction of soluble solids and dry matter content using NIR spectroscopy and its relationship with sensory quality in sweet cherries. Postharvest Biol. Technol. 2017, 128, 112–120. [Google Scholar] [CrossRef]
- Amoriello, T.; Mellara, F.; Amoriello, M.; Ciccoritti, R. Evaluation of Nutritional Values of Edible Algal Species Using a Shortwave Infrared Hyperspectral Imaging and Machine Learning Technique. Foods 2024, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Amoriello, T.; Ciccoritti, R.; Ferrante, P. Prediction of Strawberries’ Quality Parameters Using Artificial Neural Networks. Agronomy 2022, 12, 963. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 851/2000 of 27 April 2000 Laying down the Marketing Standard for Apricots. 2000. Available online: https://www.fao.org/faolex/results/details/es/c/LEX-FAOC036585/ (accessed on 26 October 2024).
- Ruiz, D.; Egea, J.; Tomás-Barberán, F.A.; Gil, M.I. Carotenoids from new apricot (Prunus armenica L.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef]
- Manley, M.; Joubert, E.; Myburgh, L.; Kidd, M. Prediction of Soluble Solids Content and Post-Storage Internal Quality of Bulida Apricots Using near Infrared Spectroscopy. J. Near Infrared Spectrosc. 2007, 15, 179–188. [Google Scholar] [CrossRef]
- Benelli, A.; Cevoli, C.; Fabbri, A.; Ragni, L. Hyperspectral imaging to measure apricot attributes during storage. J. Agric. Eng. 2022, 53, 1311. [Google Scholar] [CrossRef]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 2003, 106, 135–141. [Google Scholar] [CrossRef]
- Toledo-Martín, E.M.; García-García, M.C.; Font, R.; Moreno-Rojas, J.M.; Gomez, P.; Salinas-Navarro, M.; Del Río-Celestino, M. Application of visible/near-infrared spectroscopy for predicting internal and external quality in pepper. J. Sci. Food Agric. 2016, 96, 3114–3125. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Pu, H.; Liu, D.; Wang, L.; Sun, D.W. Soluble solids content and pH prediction and maturity discrimination of Lychee fruits using visible and near infrared hyperspectral imaging. Food Anal. Methods 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Pizarroso, J.; Portela, J.; Munoz, A. NeuralSens: Sensitivity Analysis of Neural Networks. J. Stat. Softw. 2022, 10, 1–36. [Google Scholar] [CrossRef]
- Ho, S.Y.; Phua, K.; Wong, L.; Goh, W.W.B. Extensions of the External Validation for Checking Learned Model Interpretability and Generalizability. Patter 2020, 1, 100129. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Ciorba, R.; Mitrano, F.; Cutuli, M.; Amoriello, T.; Ciaccia, C.; Testani, E.; Ceccarelli, D. Diversification and Soil Management Effects on the Quality of Organic Apricots. Agronomy 2021, 11, 1791. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Garner, D.; Crisosto, G.M.; Bowerman, E. Increasing ‘Blackamber’ plum (Prunus salicina Lindell) consumer acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Costa, G.; Noferini, M.; Fiori, G.; Ziosi, V.; Berthod, N.; Rossier, J. Establishment of the optimal harvest time in apricot (‘Orangered’and ‘Bergarouge’) by means of a new index based on vis spectroscopy. Acta Hortic. 2010, 862, 533–538. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.; Neri, F. Understanding tree fruit quality based on consumer acceptance. Acta Hortic. 2006, 712, 183–189. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Antonucci, F.; Costa, C.; Talento, C.; Ciccoritti, R. An artificial class modelling approach to identify the most largely diffused cultivars of sweet cherry (Prunus avium L.) in Italy. Food Chem. 2020, 333, 127515. [Google Scholar] [CrossRef] [PubMed]
- Ferik, F.; Ates, D.; Ercisli, S.; Erdogan, A.; Orhan, E.; Tanyolac, M.B. Genome-wide association links candidate genes to fruit firmness, fruit flesh color, flowering time, and soluble solid content in apricot (Prunus armeniaca L.). Mol. Biol. Rep. 2022, 49, 5283–5291. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Schmitz-Eiberger, M. Significance of skin flavonoids for UV-B protection in apple fruits. J. Exp. Bot. 2003, 54, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Intrigliolo, D.S.; Castel, J.R. Response of plum trees to deficit irrigation under two crop levels: Tree growth, yield and fruit quality. Irrig. Sci. 2010, 28, 525–534. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Ruiz, D.; Salazar, J.A.; Rubio, M.; Martínez-García, P.J.; Martínez-Gómez, P. Analysis of metabolites and gene expression changes relative to apricot (Prunus armeniaca L.) fruit quality during development and ripening. Front. Plant Sci. 2020, 11, 1269. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, O.; Bayazit, S.; Sumbul, A. Fruit quality and phytochemical attributes of some apricot (Prunus armeniaca L.) cultivars as affected by genotypes and seasons. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 284–294. [Google Scholar] [CrossRef]
- Pennone, F.; Berra, L.; Viti, R.; Guerriero, R. Albicocco. Terra E Vita 2005, 46, 6–14. [Google Scholar]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Akin, E.B.; Karabulut, I.; Topcu, A. Some compositional properties of main Malatya apricot (Prunus armeniaca L.) varieties. Food Chem. 2008, 107, 939–948. [Google Scholar] [CrossRef]
- Milosevic, T.; Milosevic, N.; Glisic, I.; Mladenovic, J. Fruit quality, phenolics content and antioxidant capacity of new apricot cultivars from Serbia. Acta Sci. Polonorum. Hortorum Cultus 2012, 11, 3–15. [Google Scholar]
- Drogoudi, P.D.; Vemmos, S.; Pantelidis, G.; Petri, E.; Tzoutzoukou, C.; Karayiannis, I. Physical characters and antioxidant, sugar, and mineral nutrient contents in fruit from 29 apricot (Prunus armeniaca L.) cultivars and hybrids. J. Agric. Food Chem. 2008, 56, 10754–10760. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Escárate, P.; Farias, G.; Naranjo, P.; Zoffoli, J.P. Estimation of Soluble Solids for Stone Fruit Varieties Based on Near-Infrared Spectra Using Machine Learning Techniques. Sensors 2022, 22, 6081. [Google Scholar] [CrossRef] [PubMed]
- Grabska, J.; Beć, K.B.; Ueno, N.; Huck, C.W. Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods 2023, 12, 1946. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Huang, Y.; Yan, H.; Xiong, Y.; Min, S. A novel algorithm for spectral interval combination optimization. Anal. Chim. Acta 2016, 948, 19–29. [Google Scholar] [CrossRef]
| Genotype | Pedigree | Origin | Ripening Time * |
|---|---|---|---|
| Aurora | Goldrich × Ouardi | Italy (Emilia Romagna) | Early (−21) |
| Bella d’Imola | Open pollination | Italy (Emilia Romagna) | Early-Medium (−7) |
| Bora | Early Blush × PA7005-2 (Rival × PA 63-265) | Italy (Emilia Romagna) | Early-Medium (−14) |
| Bulida | Unknown | Spain | Medium-Late (9) |
| Cafona I | Unknown | Italy (Campania) | Medium (0) |
| Krupna Skopjanka | Unknown | Macedonia | Medium (0) |
| Marietta Milady | S. Francesco × Polonais | Italy (Tuscany) | Medium (6) |
| Muscat | Unknown | France | Medium-Late (9) |
| Nella | Unknown | Italy (Campania) | Early (−21) |
| Ninfa | Ouardi × Thyrinthos | Italia (Emilia Romagna) | Early (−21) |
| NJA42 | Unknown | Italy (Tuscany) | Medium-Late (10) |
| Portici | Unknown | Italy (Campania) | Medium-Late (9) |
| Precoce Toscana | Unknown | Italy (Tuscany) | Medium (0) |
| Rubista | Unknown | Unknown | Early-Medium (−14) |
| S. Castrense | Unknown | Italy (Campania) | Medium (6) |
| Tardiva di Bordaneil | Unknown | France | Early (−18) |
| Thyrinthos | Unknown | Greece | Early-Medium (−14) |
| CV | Weight (g) | Longitudinal Diameter (mm) | Lateral Diameter (mm) | Equatorial Diameter (mm) |
|---|---|---|---|---|
| Aurora | 45 ± 23 bcd | 40 ± 3 cd | 38 ± 4 cd | 40 ± 2 cd |
| Bella di Imola | 69 ± 12 a | 51 ± 4 a | 49 ± 5 a | 47 ± 2 a |
| Bora | 70 ± 11 a | 50 ± 4 a | 48 ± 3 a | 47 ± 3 a |
| Bulida | 59 ± 11 b | 47 ± 4 b | 48 ± 4 a | 46 ± 4 ab |
| Cafona I | 39 ± 5 d | 38 ± 3 d | 39 ± 3 cd | 38 ± 3 d |
| Krupna Skopjanka | 46 ± 8 c | 42 ± 3 c | 45 ± 3 ab | 40 ± 2 cd |
| Marietta Milady | 54 ± 6 b | 44 ± 4 b | 43 ± 3 bc | 44 ± 2 b |
| Muscat | 30 ± 4 e | 38 ± 2 d | 36 ± 3 d | 34 ± 3 e |
| Nella | 56 ± 7 b | 44 ± 2 bc | 46 ± 2 ab | 46 ± 3 ab |
| Ninfa | 43 ± 10 cd | 41 ± 3 cd | 41 ± 4 bc | 41 ± 3 c |
| NJA42 | 57 ± 15 b | 48 ± 6 ab | 43 ± 4 bc | 44 ± 4 b |
| Portici | 50 ± 9 bc | 48 ± 9 ab | 42 ± 5 bc | 41 ± 2 c |
| Precoce Toscana | 47 ± 4 c | 46 ± 4 b | 43 ± 3 bc | 39 ± 2 d |
| Rubista | 53 ± 11 b | 42 ± 4 c | 43 ± 3 bc | 41 ± 3 c |
| S. Castrense | 39 ± 5 d | 39 ± 2 d | 39 ± 3 cd | 38 ± 2 d |
| Tardiva di Bordaneil | 66 ± 10 ab | 47 ± 3 b | 48 ± 3 a | 48 ± 2 a |
| Thyrinthos | 51 ± 8 bc | 45 ± 4 b | 44 ± 2 b | 43 ± 2 bc |
| CV | L* | a* | b* |
|---|---|---|---|
| Aurora | 55.99 ± 6.54 a | 12.82 ± 6.18 bcde | 36.38 ± 6.63 cd |
| Bella di Imola | 59.87 ± 4.11 a | 18.75 ± 3.88 b | 44.24 ± 4.39 a |
| Bora | 57.70 ± 4.12 a | 19.39 ± 4.18 b | 41.08 ± 5.12 ab |
| Bulida | 59.44 ± 4.93 a | 11.28 ± 3.31 de | 43.88 ± 6.28 a |
| Cafona I | 59.70 ± 4.77 a | 10.88 ± 3.96 e | 33.07 ± 6.25 d |
| Krupna Skopjanka | 59.13 ± 4.88 a | 14.60 ± 2.97 cd | 40.92 ± 6.20 ab |
| Marietta Milady | 60.67 ± 4.66 a | 14.80 ± 2.40 cd | 41.14 ± 7.09 ab |
| Muscat | 57.30 ± 6.77 a | 14.24 ± 3.11 cd | 34.40 ± 7.23 d |
| Nella | 57.49 ± 5.60 a | 15.39 ± 5.79 c | 40.81 ± 6.34 ab |
| Ninfa | 61.78 ± 6.35 a | 12.15 ± 4.02 cde | 39.13 ± 7.50 bc |
| NJA42 | 60.80 ± 4.21 a | 14.67 ± 2.47 cd | 42.85 ± 3.92 ab |
| Portici | 61.50 ± 7.90 a | 12.76 ± 3.76 bcde | 43.10 ± 9.60 ab |
| Precoce Toscana | 58.89 ± 4.04 a | 13.84 ± 4.12 bcd | 44.44 ± 3.51 a |
| Rubista | 36.42 ± 4.49 b | 29.25 ± 5.33 a | 13.65 ± 4.99 e |
| S. Castrense | 56.99 ± 4.21 a | 15.35 ± 2.66 c | 32.97 ± 6.60 d |
| Tardiva di Bordaneil | 62.30 ± 9.07 a | 19.12 ± 5.99 b | 41.70 ± 6.39 ab |
| Thyrinthos | 59.81 ± 9.08 a | 18.86 ± 8.71 b | 41.85 ± 9.48 ab |
| CV | FF (N) | TSS (g 100 g−1 FW) | DM (g 100 g−1 FW) | TA (mEq 100 L−1) |
|---|---|---|---|---|
| Aurora | 10 ± 4 ef | 14.9 ± 2.3 cd | 14.2 ± 1.9 d | 391 ± 61 a |
| Bella di Imola | 46 ± 29 a | 15.5 ± 2.6 c | 12.4 ± 1.8 e | 271 ± 37 d |
| Bora | 47 ± 36 a | 14.1 ± 3.0 d | 12.0 ± 2.7 ef | 358 ± 50 b |
| Bulida | 13 ± 7 ef | 17.3 ± 1.0 b | 17.5 ± 1.7 b | 203 ± 79 e |
| Cafona I | 10 ± 8 f | 19.2 ± 1.6 a | 17.7 ± 1.6 b | 154 ± 22 fg |
| Krupna Skopjanka | 8 ± 4 f | 17.3 ± 0.7 b | 15.4 ± 1.7 c | 320 ± 30 c |
| Marietta Milady | 31 ± 28 bc | 15.2 ± 1.6 cd | 12.4 ± 1.9 e | 264 ± 37 d |
| Muscat | 11 ± 8 ef | 19.6 ± 2.1 a | 19.3 ± 2.5 a | 173 ± 27 f |
| Nella | 17 ± 9 def | 12.3 ± 2.7 e | 10.5 ± 2.4 gh | 260 ± 26 d |
| Ninfa | 22 ± 9 cde | 11.4 ± 3.3 e | 9.4 ± 1.9 i | 206 ± 76 e |
| NJA42 | 38 ± 34 ab | 15.1 ± 1.2 cd | 12.0 ± 1.4 ef | 202 ± 59 e |
| Portici | 19 ± 15 def | 19.5 ± 1.8 a | 17.0 ± 2.5 b | 130 ± 30 g |
| Precoce Toscana | 15 ± 9 ef | 15.1 ± 1.7 cd | 12.1 ± 0.9 ef | 272 ± 53 d |
| Rubista | 14 ± 15 ef | 15.9 ± 1.3 c | 12.9 ± 1.8 e | 417 ± 43 a |
| S. Castrense | 19 ± 9 def | 19.0 ± 1.9 a | 15.9 ± 2.2 c | 275 ± 53 d |
| Tardiva di Bordaneil | 26 ± 13 cd | 12.4 ± 2.6 e | 11.0 ± 1.9 fg | 285 ± 64 d |
| Thyrinthos | 26 ± 7 cd | 11.2 ± 2.1 e | 9.8 ± 1.6 hi | 315 ± 30 c |
| TSS | DM | TA | ||
|---|---|---|---|---|
| Topology (input–hidden–output) | MLP (260–17–1) | MLP (260–27–1) | MLP (260–17–1) | |
| Activation Function | Hidden Neurons Output Neurons | Logistic Logistic | Exp Tahn | Logistic Logistic |
| Training set | r | 0.947 | 0.898 | 0.819 |
| R2 | 0.898 | 0.806 | 0.671 | |
| RMSE | 1.048 | 1.535 | 51.814 | |
| MAE | 0.049 | 0.045 | 1.110 | |
| Test set | r | 0.925 | 0.926 | 0.825 |
| R2 | 0.855 | 0.857 | 0.681 | |
| RMSE | 1.319 | 1.379 | 55.073 | |
| MAE | 0.080 | 0.047 | 0.376 | |
| Validation set | r | 0.939 | 0.929 | 0.810 |
| R2 | 0.883 | 0.863 | 0.656 | |
| RMSE | 1.286 | 1.310 | 53.588 | |
| MAE | 0.031 | 0.032 | 2.374 |
| TSS | DM | TA | ||
|---|---|---|---|---|
| Topology (input–hidden–output) | MLP (260–31–1) | MLP (260–20–1) | MLP (260–50–1) | |
| Activation Function | Hidden Neurons Output Neurons | Identity Identity | Identity Identity | Logistic Logistic |
| Training set | r | 0.962 | 0.950 | 0.930 |
| R2 | 0.926 | 0.902 | 0.864 | |
| RMSE | 0.935 | 1.187 | 34.32 | |
| MAE | 0.084 | 0.159 | 0.655 | |
| Test set | r | 0.951 | 0.958 | 0.901 |
| R2 | 0.904 | 0.918 | 0.811 | |
| RMSE | 1.103 | 1.039 | 38.974 | |
| MAE | 0.055 | 0.009 | 2.237 | |
| Validation set | r | 0.959 | 0.931 | 0.925 |
| R2 | 0.919 | 0.866 | 0.855 | |
| RMSE | 1.112 | 1.288 | 32.741 | |
| MAE | 0.300 | 0.090 | 0.953 |
| TSS | DM | TA | |
|---|---|---|---|
| Vis/NIR spectrophotometer (310–1100 nm) | 410–420 740–760 850–920 950–970 | 380–420 470–490 740–770 840–1000 | 320–350 410–500 530–550 655–665 720–920 990–1060 |
| HSI device (400–1000 nm) | 460–480 730–790 800–920 940–990 | 400–440 730–780 850–890 900–980 | 400–430 480–520 540–570 630–700 730–770 870–950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoriello, T.; Ciorba, R.; Ruggiero, G.; Masciola, F.; Scutaru, D.; Ciccoritti, R. Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning. Foods 2025, 14, 196. https://doi.org/10.3390/foods14020196
Amoriello T, Ciorba R, Ruggiero G, Masciola F, Scutaru D, Ciccoritti R. Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning. Foods. 2025; 14(2):196. https://doi.org/10.3390/foods14020196
Chicago/Turabian StyleAmoriello, Tiziana, Roberto Ciorba, Gaia Ruggiero, Francesca Masciola, Daniela Scutaru, and Roberto Ciccoritti. 2025. "Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning" Foods 14, no. 2: 196. https://doi.org/10.3390/foods14020196
APA StyleAmoriello, T., Ciorba, R., Ruggiero, G., Masciola, F., Scutaru, D., & Ciccoritti, R. (2025). Vis/NIR Spectroscopy and Vis/NIR Hyperspectral Imaging for Non-Destructive Monitoring of Apricot Fruit Internal Quality with Machine Learning. Foods, 14(2), 196. https://doi.org/10.3390/foods14020196







