Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Colostrum Samples
2.3. Pretreatment of Colostrum Samples
2.4. UHPLC-ESI-MS/MS
2.5. Data Processing and Statistical Analyses
3. Results and Discussion
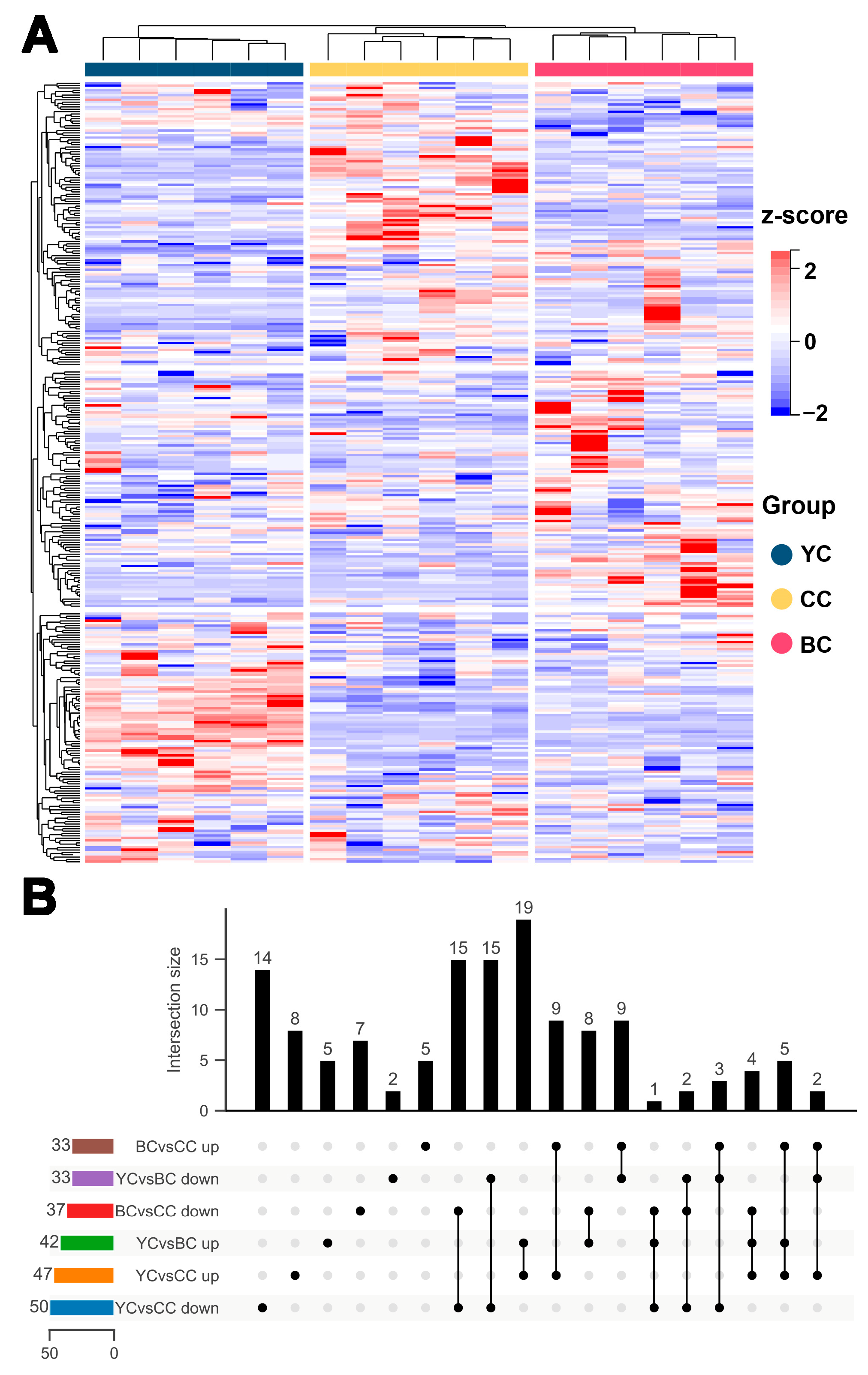
3.1. Identification and Multivariate Statistical Comparison of Metabolites Among YC, BC, and CC
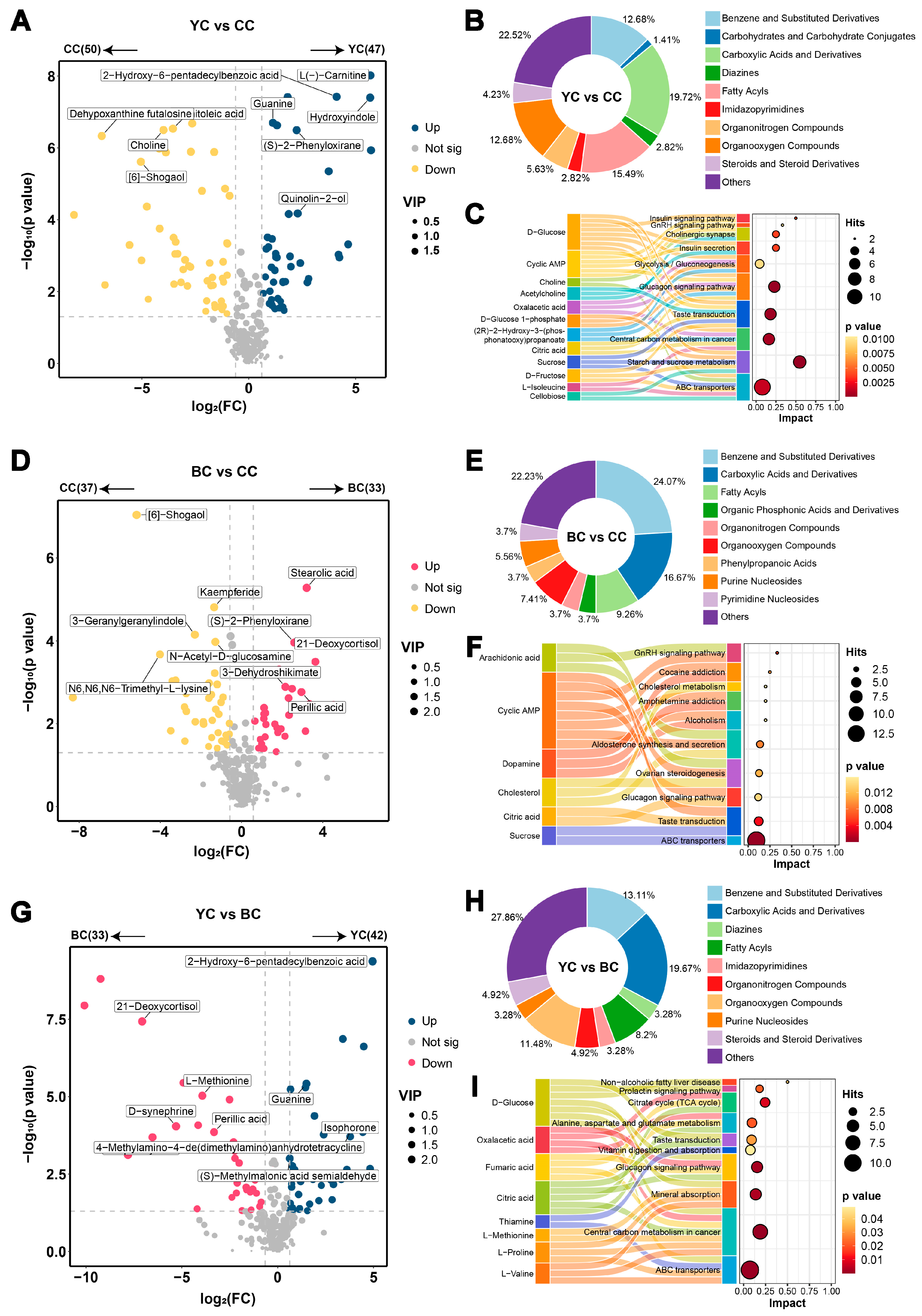
3.2. Metabolic Profile Difference Between YC and CC
3.3. Metabolic Profile Difference Between BC and CC
3.4. Metabolic Profile Difference Between YC and BC
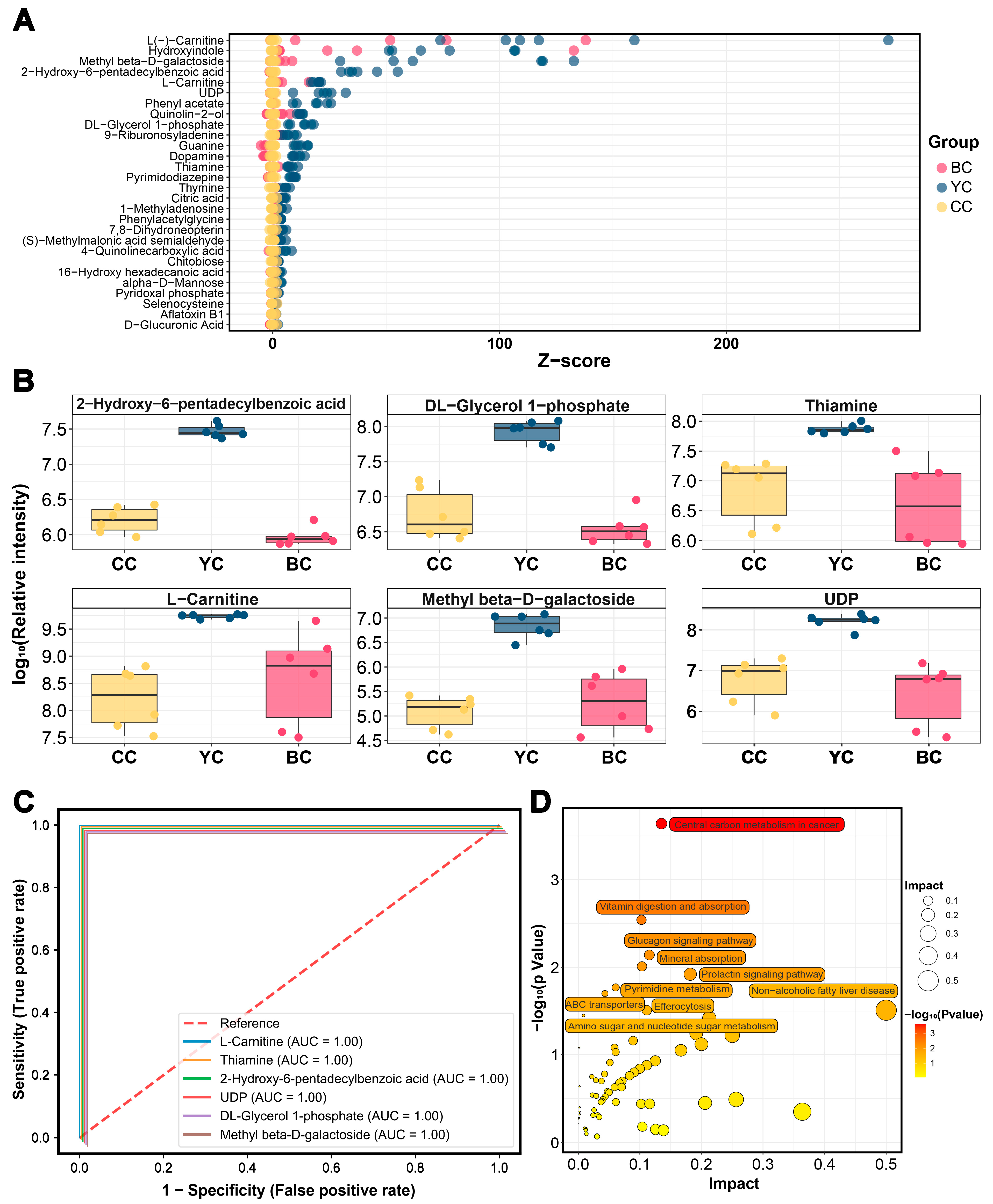
3.5. Characteristic Metabolic Biomarkers of YC
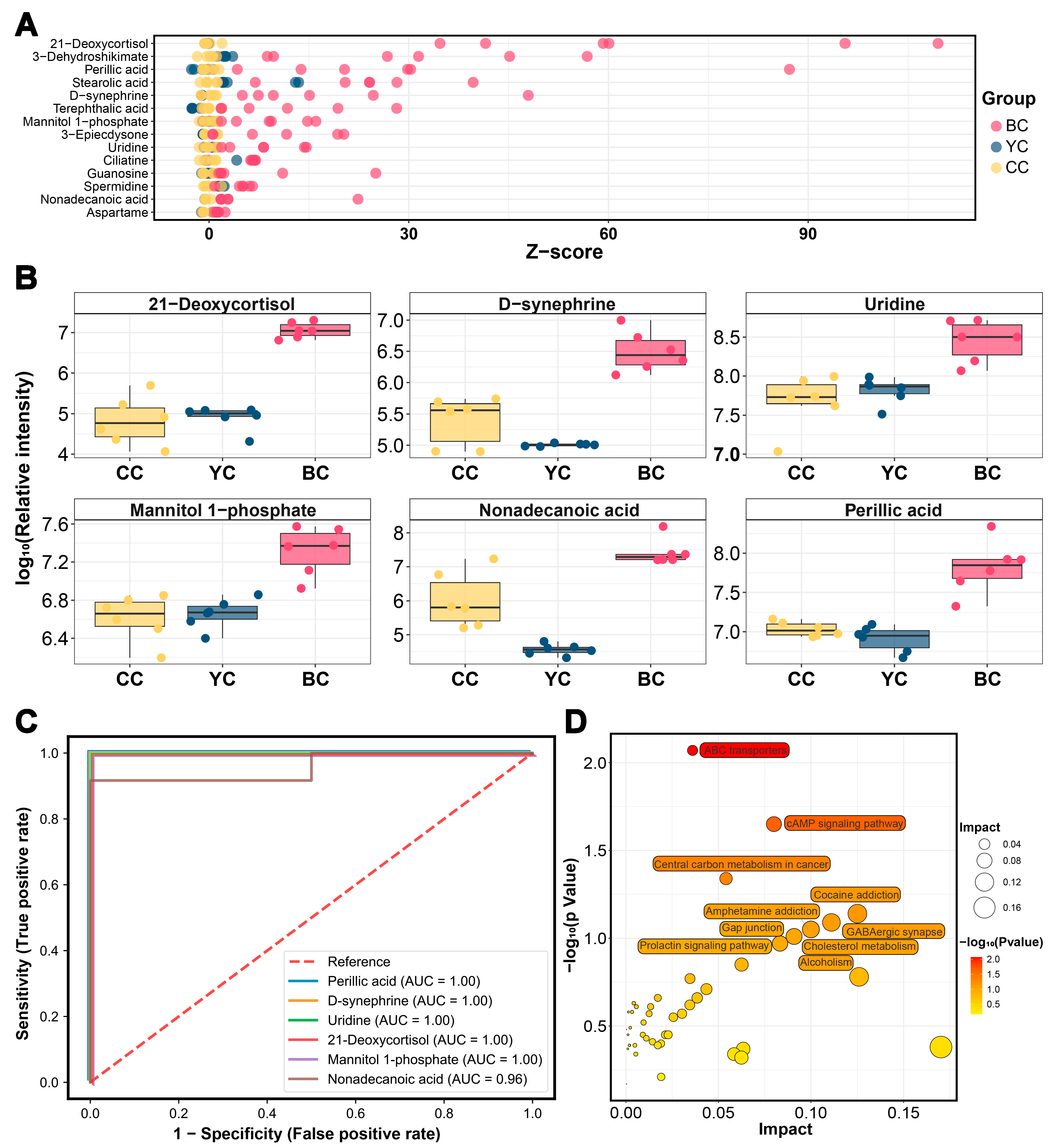
3.6. Characteristic Metabolic Biomarkers of BC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| YC | yak colostrum |
| BC | buffalo colostrum |
| CC | cow colostrum |
| UHPLC-MS/MS | ultra-high-performance liquid chromatogre-mass spectrometry |
| UHPLC-HRMS | ultra-high-performance liquid chromatogre-high resolution mass spectrometry |
| 1H NMR | 1H nuclear magnetic resonance |
| QC | quality control |
| LC-MS | liquid chromatography-mass spectrometry |
| UHPLC-ESI-MS/MS | ultra-high-performance liquid chromatography-electrospray ionization tandem mass spectrometry |
| CV | coefficient of variance |
| RT | retention time |
| HMDB | Human Metabolome Database |
| PCA | principal component analysis |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| DEMs | differential expression metabolites |
| VIP | variable importance for the projection |
| FC | fold-change |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TIC | total ion chromatogram |
| QA | quality assurance |
| R2X | goodness of fit (R2) X cumulative |
| R2Y | goodness of fit (R2) Y cumulative |
| Q2 | goodness of prediction |
| TCA | tricarboxylic acid |
| ATP | adenosine triphosphate |
| HPA | hypothalamic–pituitary–adrenal |
| HAT | histone acetyltransferases |
| UDP | uridine diphosphate |
References
- Feng, X.; Ma, R.; Tian, J.; Wang, Y.; Tong, L.; Wen, W.; Mu, T.; Yu, B.; Gu, Y.; Zhang, J. Non-targeted metabolomics identifies biomarkers in milk with high and low milk fat percentage. Food Res. Int. 2024, 179, 113989. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.S.; Akram, N.; Faisal, Z.; Saeed, F.; Rasheed, A.; Ahmed, F.; Afzaal, M. Bovine colostrum: Therapeutic potential and clinical evidence. Int. Dairy J. 2024, 157, 105996. [Google Scholar] [CrossRef]
- Sangild, P.T.; Vonderohe, C.; Melendez Hebib, V.; Burrin, D.G. Potential benefits of bovine colostrum in pediatric nutrition and health. Nutrients. 2021, 13, 105637. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Pei, J.; Bao, P.; Wang, X.; Guo, S.; Cao, M.; Kang, Y.; Yan, P.; Guo, X. The study of yak colostrum nutritional content based on foodomics. Foods 2023, 12, 17078. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, N.; Zhao, X.; Zhang, Y.; Han, R.; Yang, J.; Zhao, S.; Li, S.; Guo, T.; Zang, C.; et al. Metabolomic biomarkers identify differences in milk produced by Holstein cows and other minor dairy animals. J. Proteomics. 2016, 136, 174–182. [Google Scholar] [CrossRef]
- Li, A.; Liu, C.; Han, X.; Zheng, J.; Zhang, G.; Qi, X.; Du, P.; Liu, L. Tibetan Plateau yak milk: A comprehensive review of nutritional values, health benefits, and processing technology. Food Chem. X. 2023, 20, 100919. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Huang, L.; Huang, Z.; Romeih, E.; Yang, P.; Zeng, Q.; Li, L. Effect of buffalo breed on the detailed milk composition in Guangxi, China. Foods 2023, 12, 1603. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, X.; Yu, S.; Cao, S. Quantitative proteomic analysis of whey proteins in the colostrum and mature milk of yak (Bos grunniens). J. Sci. Food Agr. 2015, 95, 592–597. [Google Scholar] [CrossRef]
- An, Z.; Wei, K.; Yao, Z.; Yang, L.; Wang, C. Fatty acid composition comparison between colostrum and mature milk in buffaloes. Int. J. Dairy Technol. 2024, 77, 1244–1249. [Google Scholar] [CrossRef]
- Windarsih, A.; Arifah, M.F.; Suratno; Rohman, A. The application of untargeted metabolomics using UHPLC-HRMS and chemometrics for authentication of horse milk Fdulterated with cow milk. Food Anal. Methods 2023, 16, 401–412. [Google Scholar] [CrossRef]
- Liu, H.; Tayyari, F.; Khoo, C.; Gu, L. A 1H NMR-based approach to investigate metabolomic differences in the plasma and urine of young women after cranberry juice or apple juice consumption. J. Funct. Foods. 2015, 14, 76–86. [Google Scholar] [CrossRef]
- Khamis, M.M.; Adamko, D.J.; El-Aneed, A. Mass spectrometric based approaches in urine metabolomics and biomarker discovery. Mass Spectrom. Rev. 2017, 36, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Zelena, E.; Dunn, W.B.; Broadhurst, D.; Francis-McIntyre, S.; Carroll, K.M.; Begley, P.; O’Hagan, S.; Knowles, J.D.; Halsall, A.; Wilson, I.D.; et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 2009, 81, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Reig, M.; Jaumot, J.; Garcia-Reiriz, A.; Tauler, R. Evaluation of changes induced in rice metabolome by Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis strategies. Anal. Bioanal. Chem. 2015, 407, 8835–8847. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 351, D521–D526. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 351, D527–D532. [Google Scholar] [CrossRef]
- Abdelrazig, S.; Safo, L.; Rance, G.A.; Fay, M.W.; Theodosiou, E.; Topham, P.D.; Kim, D.; Fernandez-Castane, A. Metabolic characterisation of Magnetospirillum gryphiswaldense MSR-1 using LC-MS-based metabolite profiling. Rsc Adv. 2020, 10, 32548–32560. [Google Scholar] [CrossRef]
- Bai, U.; Su, X.; Zheng, Z.; Zhang, L.; Ma, Y.; Dou, Y.; Zhang, X.; Su, G.; Li, G.; Zhang, L. Comparative metabolomics analysis of Small-Tailed Han and DairyMeade ovine milk. Eur. Food Res. Technol. 2021, 247, 2869–2876. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, M. Metabolomic profiles in yak mammary gland tissue during the lactation cycle. PLoS ONE. 2019, 14, e02192207. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Arora, S.; Sarkar, M. Yak milk and milk products: Functional, bioactive constituents and therapeutic potential. Int. Dairy J. 2023, 142, 105637. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Zhou, M.; Zhang, Z.; Zhan, Y.; Liu, G.; Zhu, M.; Wang, C. A metabolomics comparison in milk from two Dezhou donkey strains. Eur. Food Res. Technol. 2022, 248, 1267–1275. [Google Scholar] [CrossRef]
- Yuan, X.; Shi, W.; Jiang, J.; Li, Z.; Fu, P.; Yang, C.; Rehman, S.U.; Pauciullo, A.; Liu, Q.; Shi, D. Comparative metabolomics analysis of milk components between Italian Mediterranean buffaloes and Chinese Holstein cows based on LC-MS/MS technology. PLoS ONE 2022, 17, e02628781. [Google Scholar] [CrossRef]
- McCann, M.R.; George De La Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-carnitine and acylcarnitines: Mitochondrial biomarkers for precision medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Dettmer, A.M.; Murphy, A.M.; Guitarra, D.; Slonecker, E.; Suomi, S.J.; Rosenberg, K.L.; Novak, M.A.; Meyer, J.S.; Hinde, K. Cortisol in neonatal mother’s milk predicts later infant social and cognitive functioning in rhesus monkeys. Child Dev. 2018, 89, 525–538. [Google Scholar] [CrossRef]
- Beery, A.K.; Jackson, B.; Halstead, E.; Windorski, S.M.; Nnodim-Amadi, C.; Upin, E. Acute decrease in mothers’ cortisol following nursing and milk expression. Horm. Behav. 2023, 153, 105387. [Google Scholar] [CrossRef]
- Han, B.; Meng, Y.; Li, M.; Yang, Y.; Ren, F.; Zeng, Q.; Nout, M.J.R. A survey on the micro biological and chemical composition of buffalo milk in China. Food Control. 2007, 18, 742–746. [Google Scholar] [CrossRef]
- Peng, J.; Ma, L.; Kwok, L.; Zhang, W.; Sun, T. Untargeted metabolic footprinting reveals key differences between fermented brown milk and fermented milk metabolomes. J. Dairy Sci. 2022, 105, 2771–2790. [Google Scholar] [CrossRef]
- Xu, W.; Dong, Q.; Zhao, G.; Han, B. Analysis of metabolites of bactrain camel milk in Alxa of China before and after fermentation with fermenting agent TR1 based on untargeted LC-MS/MS based metabolomics. Heliyon 2023, 9, e185228. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; Iwouno, J.O.; Ofoedu, E.O.; Ogueke, C.C.; Igwe, V.S.; Agunwah, I.M.; Ofoedum, A.F.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Revisiting food-sourced vitamins for consumer diet and health needs: A perspective review, from vitamin classification, metabolic functions, absorption, utilization, to balancing nutritional requirements. PeerJ. 2021, 9, e11940. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Bermudez, R.; Fouz, R.; Rico, M.; Camino, F.; Souza, T.K.; Miranda, M.; Dieguez, F.J. Factors affecting fatty acid composition of Holstein cow’s milk. Animals 2023, 13, 5744. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanyam, K.; Swaminathan, V.; Ranganathan, A.; Kundu, T.K. Small molecule modulators of histone acetyltransferase p300. J. Biol. Chem. 2003, 278, 19134–19140. [Google Scholar] [CrossRef] [PubMed]
- Nallamilli, S.R.; Kumar, V.R.; Himabindu, V.; Ram, B.; Aalapati, S.R. Synthesis and antibacterial activity of anacardic acid derivatives. Lett. Drug Des. Discov. 2011, 8, 626–632. [Google Scholar] [CrossRef]
- Kubo, I.; Masuoka, N.; Ha, T.J.; Tsujimoto, K. Antioxidant activity of anacardic acids. Food Chem. 2006, 99, 555–562. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, C.; Wang, N.; Meng, Y.; Wang, Y. Anacardic acid suppresses fibroblast-like synoviocyte proliferation and invasion and ameliorates collagen-induced arthritis in a mouse model. Cytokine 2018, 111, 350–356. [Google Scholar] [CrossRef]
- Tasnadi, G.; Stasko, M.; Ditrich, K.; Hall, M.; Faber, K. Preparative-scale enzymatic synthesis of rac-glycerol-1-phosphate from crude glycerol using acid phosphatases and phosphate. ChemSusChem 2020, 13, 1759–1763. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Li, Q.; Wang, J.; Cheng, J.; Xue, J.; Shi, J. The chemical composition and nitrogen distribution of Chinese yak (Maiwa) milk. Int. J. Mol. Sci. 2011, 12, 4885–4895. [Google Scholar] [CrossRef]
- Caboni, P.; Murgia, A.; Porcu, A.; Manis, C.; Ibba, I.; Contu, M.; Scano, P. A metabolomics comparison between sheep’s and goat’s milk. Food Res. Int. 2019, 119, 869–875. [Google Scholar] [CrossRef]
- Mineguchi, Y.; Goto, K.; Sudo, Y.; Hirayama, K.; Kashiwagi, H.; Sasagase, I.; Kitazawa, H.; Asakuma, S.; Fukuda, K.; Urashima, T. Characterisation of sugar nucleotides in colostrum of dairy domestic farms animals. Int. Dairy J. 2021, 113, 104897. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Wang, Y.; Liang, T.; Jiang, T.; Liu, P.; Li, X.; Lang, D.; Liu, Q.; Shen, C. p-Synephrine ameliorates alloxan-induced diabetes mellitus through inhibiting oxidative stress and inflammation via suppressing the NF-kappa B and MAPK pathways. Food Funct. 2023, 14, 1971–1988. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Tan, X.; Tang, S.; Liu, X.; Shao, L.; Wang, C.; Huang, L. Citrus p-synephrine improves energy homeostasis by regulating amino acid metabolism in HFD-induced mice. Nutrients 2024, 16, 2482. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Mannitol: Physiological functionalities, determination methods, biotechnological production, and applications. Appl. Microbiol. Biot. 2020, 104, 6941–6951. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Raphael, T.J.; Kuttan, G. Protective role of perillic acid against radiation-induced oxidative stress, cytokine profile, DNA damage, and intestinal toxicity in mice. J. Environ. Pathol. Tox. 2010, 29, 199–212. [Google Scholar] [CrossRef]
- Raphael, T.J.; Kuttan, G. Immunomodulatory activity of naturally occurring monoterpenes carvone, limonene, and perillic acid. Immunopharm. Immunot. 2003, 25, 285–294. [Google Scholar] [CrossRef]
- Hanssen, R.; Rigoux, L.; Albus, K.; Kretschmer, A.C.; Thanarajah, S.E.; Chen, W.; Hinze, Y.; Giavalisco, P.; Steculorum, S.M.; Cornely, O.A.; et al. Circulating uridine dynamically and adaptively regulates food intake in humans. Cell Rep. Med. 2023, 4, 1008971. [Google Scholar] [CrossRef]
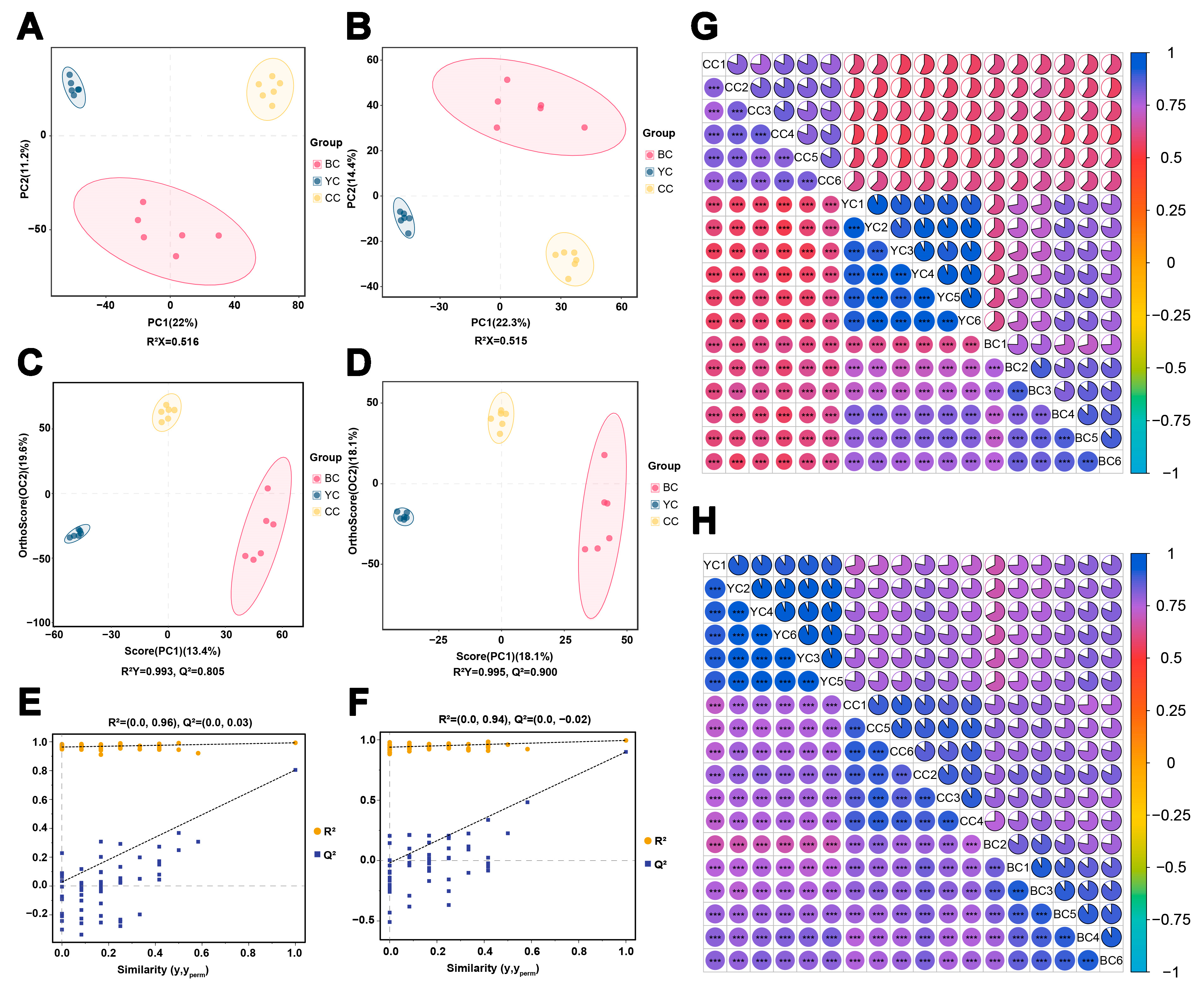
| Comparison | R2X | R2Y | Q2 | |||
|---|---|---|---|---|---|---|
| Positive Mode | Negative Mode | Positive Mode | Negative Mode | Positive Mode | Negative Mode | |
| YC vs. CC | 0.389 | 0.413 | 1.000 | 1.000 | 0.950 | 0.949 |
| BC vs. CC | 0.334 | 0.364 | 1.000 | 0.997 | 0.903 | 0.880 |
| YC vs. BC | 0.342 | 0.427 | 0.999 | 0.999 | 0.902 | 0.960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, C.; Huang, J.; Zeng, Q.; Li, L.; Yang, P.; Wang, P.; Chu, M.; Luo, J.; Ren, F.; et al. Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS. Foods 2025, 14, 232. https://doi.org/10.3390/foods14020232
Wang Y, Li C, Huang J, Zeng Q, Li L, Yang P, Wang P, Chu M, Luo J, Ren F, et al. Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS. Foods. 2025; 14(2):232. https://doi.org/10.3390/foods14020232
Chicago/Turabian StyleWang, Yuzhuo, Changhui Li, Jiaxiang Huang, Qingkun Zeng, Ling Li, Pan Yang, Pengjie Wang, Min Chu, Jie Luo, Fazheng Ren, and et al. 2025. "Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS" Foods 14, no. 2: 232. https://doi.org/10.3390/foods14020232
APA StyleWang, Y., Li, C., Huang, J., Zeng, Q., Li, L., Yang, P., Wang, P., Chu, M., Luo, J., Ren, F., & Zhang, H. (2025). Untargeted Metabolomics Reveals Key Differences Between Yak, Buffalo, and Cow Colostrum Based on UHPLC-ESI-MS/MS. Foods, 14(2), 232. https://doi.org/10.3390/foods14020232









