Structure Characterization and Antioxidant Activity of a Novel Polysaccharide from Bacillus natto Fermented Millet Bran
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Extraction, Isolation, and Purification of Polysaccharides from Millet Bran
2.3. Structural Characterization
2.3.1. Homogeneity and Molecular Weight Determination
2.3.2. Monosaccharide Composition
2.3.3. Spectrometric Analysis
2.3.4. Triple Helix Structure Analysis
2.3.5. Methylation Detection
2.3.6. Nuclear Magnetic Resonance (NMR) Spectroscopic Analysis
2.3.7. Scanning Electron Microscopy (SEM) Analysis
2.3.8. Particle Size Measurement
2.3.9. Thermal Stability Analysis
2.4. Determination of Antioxidant Activity In Vitro
2.4.1. DPPH Radical Scavenging Activity
2.4.2. ABTS Radical Scavenging Activity
2.4.3. Hydroxyl Radical Scavenging Activity
2.4.4. Determination of Reducing Power
2.5. Statistical Analysis
3. Results and Discussion
3.1. Separation and Purification of FMBP-1
3.2. Structural Characterization of FMBP-1
3.2.1. Molecular Weight and Monosaccharide Composition Analysis
3.2.2. Analysis of UV Spectrum
3.2.3. FT-IR Analysis
3.2.4. Triple Helix Structural Analysis
3.2.5. Methylation Analysis
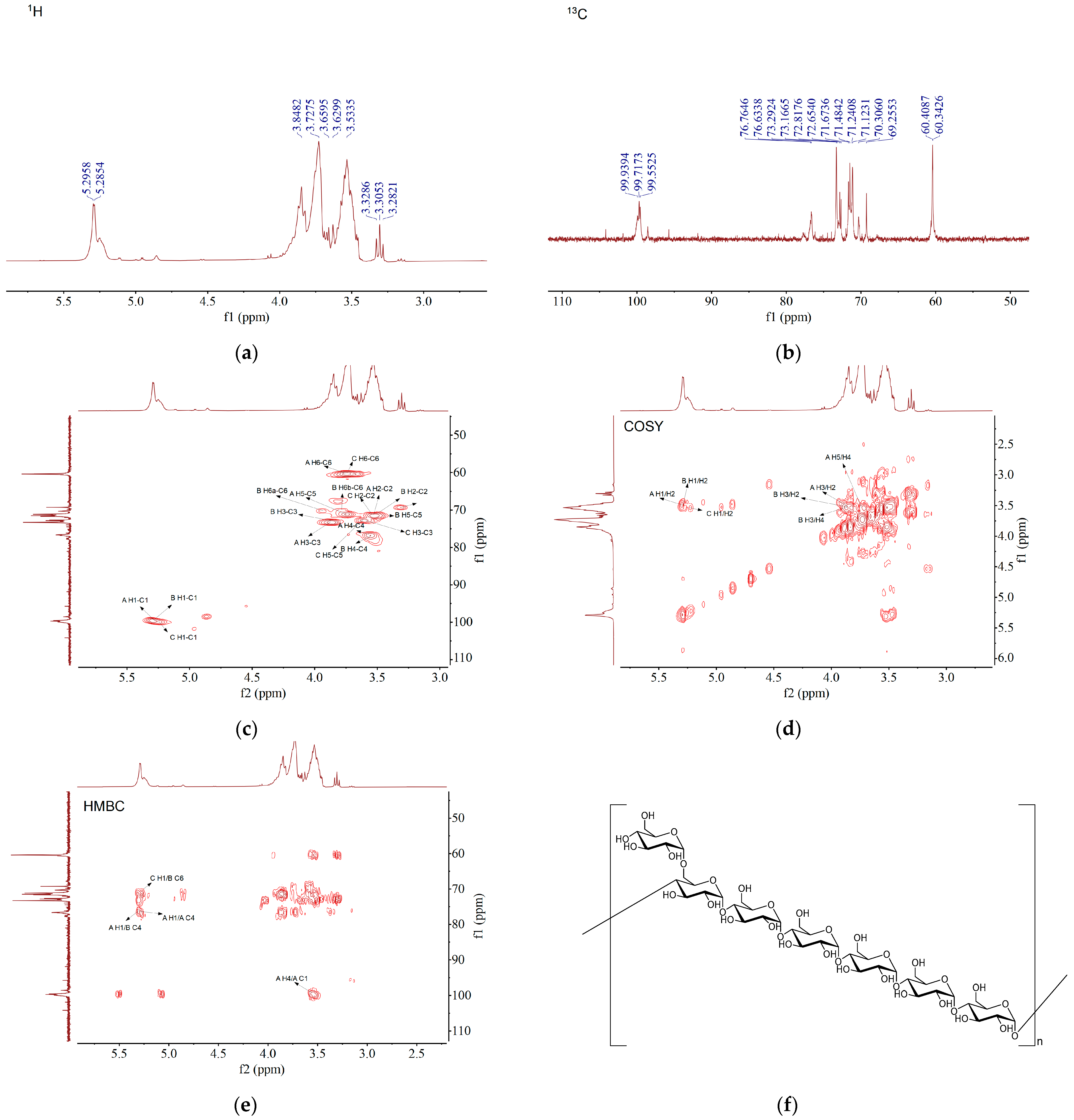
3.2.6. NMR Analysis
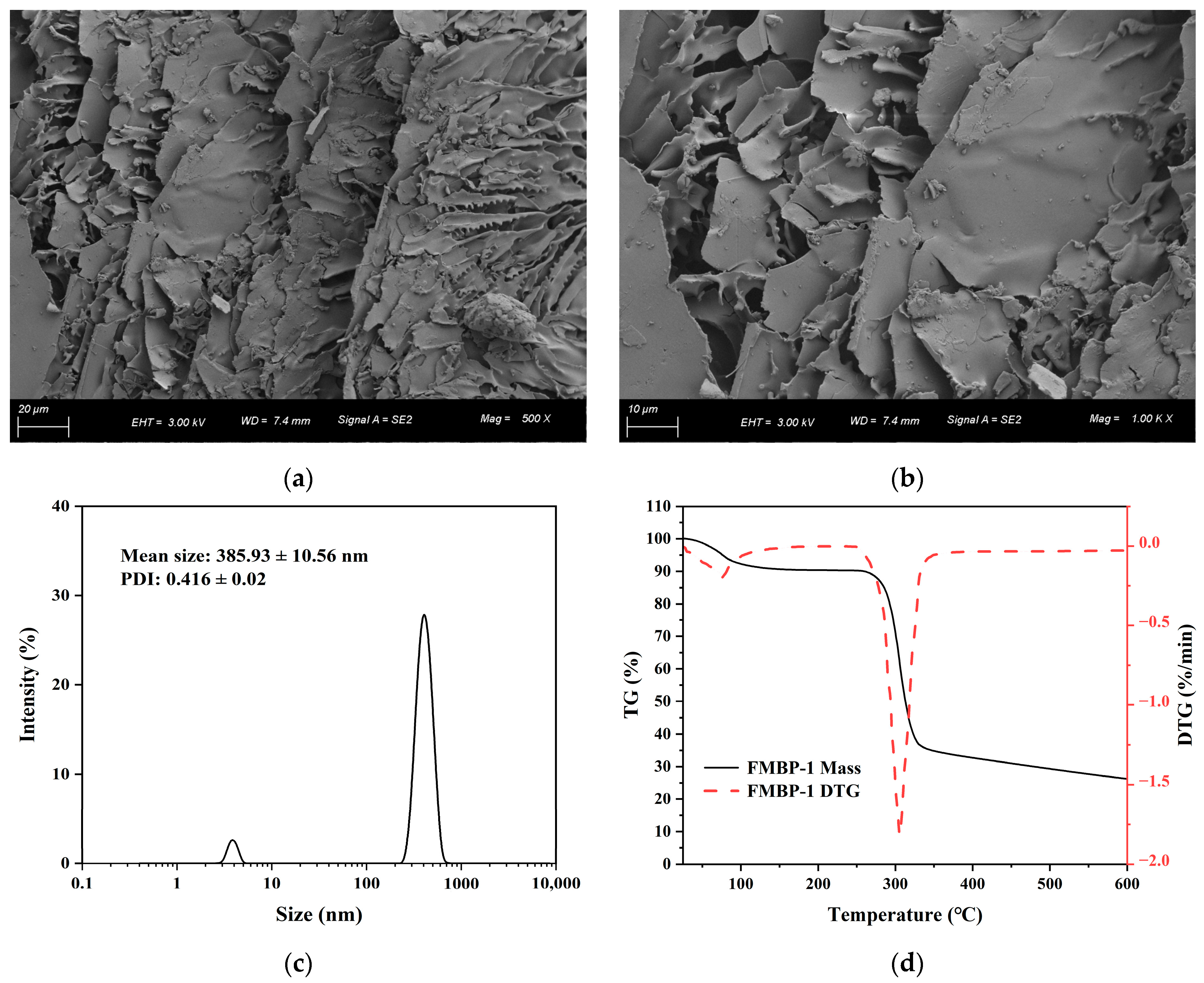
3.2.7. SEM Analysis
3.2.8. Particle Size Analysis
3.2.9. Thermal Property Analysis
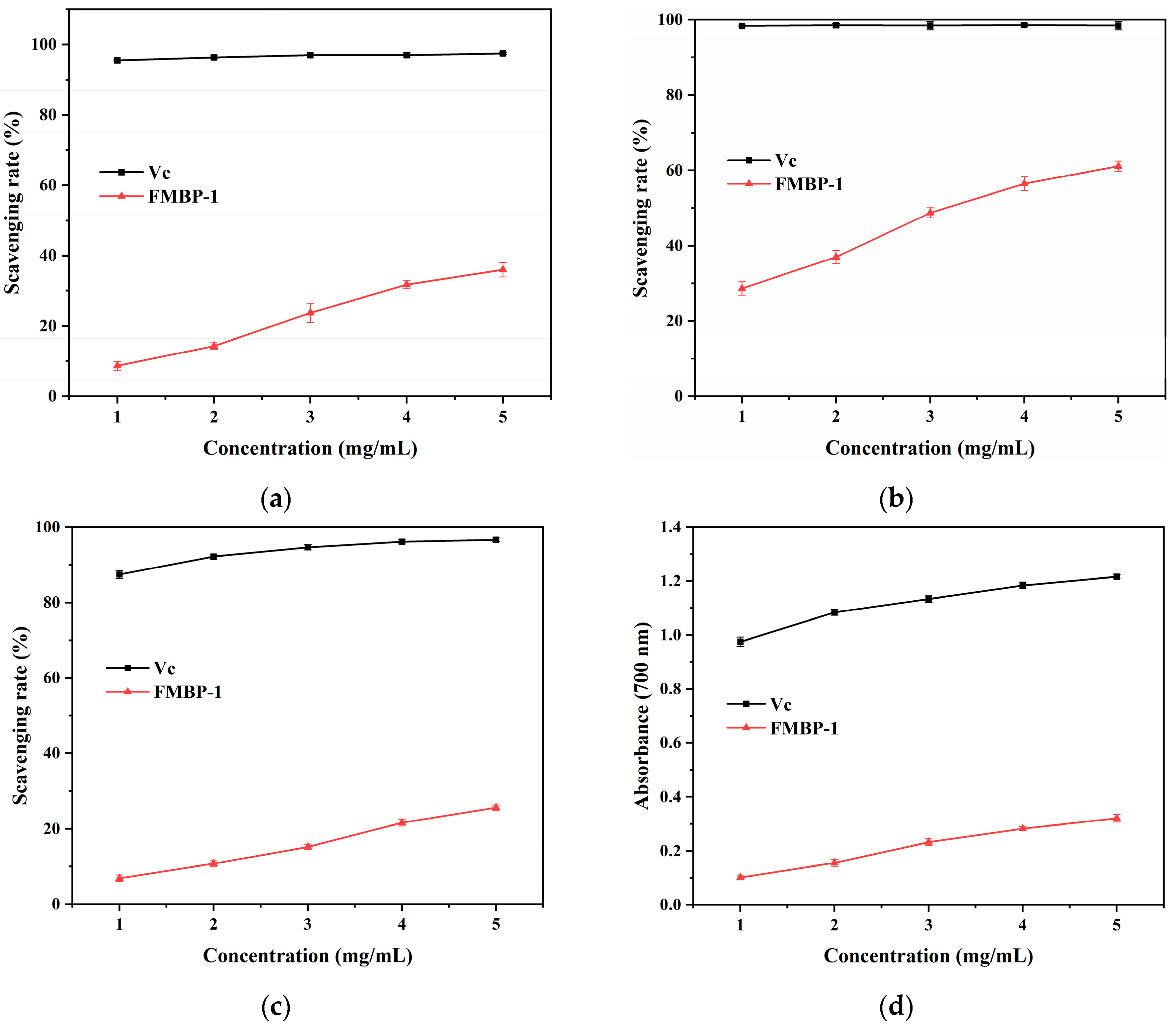
3.3. Antioxidant Activity Analysis of FMBP-1 In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, R.A.; Palanisamy, S.; Ma, N.; Talapphet, N.; Zhang, J.; Wang, C.; You, S. Extraction, structural characterization, and immunostimulatory activity of soluble non-starch polysaccharides of finger millet. Process Biochem. 2021, 111, 40–50. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiang, J.; Zheng, B.; Yuan, Y.; Luo, D.; Fan, J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. J. Cereal Sci. 2021, 99, 103217. [Google Scholar] [CrossRef]
- Li, P.; Cai, X.; Li, S.; Zhao, W.; Liu, J.; Zhang, X.; Zhang, A.; Guo, L.; Li, Z.; Liu, J. Nutrient and metabolite characteristics of the husk, bran and millet isolated from the foxtail millet (Setaria italica L.) during polishing. Food Chem. X 2024, 23, 101541. [Google Scholar] [CrossRef]
- Liang, K.; Liang, S.; Zhu, H. The physicochemical characteristics and phenolic bioaccessibility of defatted millet bran powder prepared using superfine grinding. LWT 2024, 201, 116173. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Bioactivities and antiradical properties of millet grains and hulls. J. Agric. Food. Chem. 2011, 59, 9563–9571. [Google Scholar] [CrossRef]
- Shan, S.; Li, Z.; Newton, I.P.; Zhao, C.; Li, Z.; Guo, M. A novel protein extracted from foxtail millet bran displays anti-carcinogenic effects in human colon cancer cells. Toxicol. Lett. 2014, 227, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sha, X.; Rahman, E.; Wang, Y.; Ji, B.; Wu, W.; Zhou, F. Antioxidant capacity and amino acid profile of millet bran wine and the synergistic interaction between major polyphenols. J. Food Sci. Technol.-Mysore 2018, 55, 1010–1020. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Gao, F.; Chen, W.; Zong, Y.; Li, J.; He, Z.; Du, R. Extraction, purification, and structural characterization of polysaccharides from Sanghuangporus vaninii with anti-inflammatory activity. Molecules 2023, 28, 6081. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. Extraction, structural analysis, and activities of rice bran polysaccharide. Chem. Biol. Drug Des. 2021, 98, 631–638. [Google Scholar] [CrossRef]
- Yang, W.; Wu, J.; Liu, W.; Ai, Z.; Cheng, Y.; Wei, Z.; Zhang, H.; Ma, H.; Cui, F.; Zhou, C.; et al. Structural characterization, antioxidant and hypolipidemic activity of Grifola frondosa polysaccharides in novel submerged cultivation. Food Biosci. 2021, 42, 101187. [Google Scholar] [CrossRef]
- Chen, B.; Qiao, Y.; Wang, X.; Zhang, Y.; Fu, L. Extraction, structural characterization, biological functions, and application of rice bran polysaccharides: A review. Foods 2023, 12, 639. [Google Scholar] [CrossRef]
- Miao, J.; Shi, W.; Zhang, J.; Zhang, X.; Zhang, H.; Wang, Z.; Qiu, J. Response surface methodology for the fermentation of polysaccharides from Auricularia auricula using Trichoderma viride and their antioxidant activities. Int. J. Biol. Macromol. 2020, 155, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.F.; Shen, S.Y.; Wang, Y.; Wang, Y.P.; Yi, Y.; Ying, G.Q. Fermentation-assisted extraction of polysaccharides from the roots of Codonopsis pilosula using a selected Rhizopus arrhizus strain. Food Bioprod. Process. 2024, 147, 27–33. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, L.; Zhang, A.; Zhao, H.; Zhang, B. Extraction and characterization of polysaccharides from Schisandra sphenanthera fruit by Lactobacillus plantarum CICC 23121-assisted fermentation. Int. J. Biol. Macromol. 2024, 259, 129135. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Pan, C.; Han, S.; Ma, L.; Qiao, Y.; Shi, B.; Peng, Q. Antioxidant and immunomodulatory activities of polysaccharides from fermented wheat products of Grifola frondosa: In vitro methods. Int. J. Food Sci. 2023, 2023, 3820276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chang, L.; Lin, Y. Changes in functionality of germinated and non-germinated brown rice fermented by Bacillus natto. Foods 2021, 10, 2779. [Google Scholar] [CrossRef]
- Lin, D.; Long, X.; Xiao, L.; Wu, Z.; Chen, H.; Zhang, Q.; Wu, D.; Qin, W.; Xing, B. Study on the functional properties and structural characteristics of soybean soluble polysaccharides by mixed bacteria fermentation and microwave treatment. Int. J. Biol. Macromol. 2020, 157, 561–568. [Google Scholar] [CrossRef]
- Chu, J.; Zhao, H.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C. Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem. 2019, 294, 79–86. [Google Scholar] [CrossRef]
- Yang, D.; Wang, A.; Lu, Z.; Lü, F.; Bie, X.; Meng, F.; Zhao, H. Effects of Bacillus natto fermentation on the structure and physicochemical properties of millet bran dietary fiber. J. Nanjing Agric. Univ. 2023, 46, 179–188. [Google Scholar]
- Dhahri, M.; Sioud, S.; Alsuhaymi, S.; Almulhim, F.; Haneef, A.; Saoudi, A.; Jaremko, M.; Emwas, A.-H.M. Extraction, Characterization, and Antioxidant Activity of Polysaccharides from Ajwa Seed and Flesh. Separations 2023, 10, 103. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, H.; Zhang, J.; Wen, C.; Zhou, J.; Yao, H.; He, Y.; Ma, H.; Duan, Y. Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 246, 116595. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Hu, X.; Zhang, Y.; Xu, H.; Tang, J.; Zhang, G.; Deng, J.; Kan, H.; Zhao, P.; Liu, Y. Extraction, characterization, antioxidant and anti-tumor activities of polysaccharides from Camellia fascicularis leaves. Int. J. Biol. Macromol. 2022, 222, 373–384. [Google Scholar] [CrossRef]
- Liu, H.M.; Cheng, J.; Wang, X.Y.; Jiang, Y.; Ni, J.; Zhang, Y.; Wang, W. Structure identification of Ganoderma lucidum spore polysaccharides and their antitumor activity in vivo. Molecules 2024, 29, 2348. [Google Scholar] [CrossRef]
- Mtetwa, M.D.; Qian, L.; Zhu, H.; Cui, F.; Zan, X.; Sun, W.; Wu, D.; Yang, Y. Ultrasound-assisted extraction and antioxidant activity of polysaccharides from Acanthus ilicifolius. J. Food Meas. Charact. 2020, 14, 1223–1235. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Extraction, purification and antioxidant activity of Juglans regia shell polysaccharide. Chem. Biol. Technol. Agric. 2023, 10, 75. [Google Scholar] [CrossRef]
- Jing, Y.; Cui, X.; Chen, Z.; Huang, L.; Song, L.; Liu, T.; Lv, W.; Yu, R. Elucidation and biological activities of a new polysaccharide from cultured Cordyceps militaris. Carbohydr. Polym. 2014, 102, 288–296. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Isolation, purification and physicochemical properties of polysaccharide from fruiting body of Hericium erinaceus and its effect on colonic health of mice. Int. J. Biol. Macromol. 2018, 107, 1310–1319. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, J.; Wang, G.; Li, X.; Liu, Y.; Xu, E.; Luo, A. Ultrasonic extraction, structural modification and gastric mucosal cells protective activity of a polysaccharide from Dendrobium denneanum. Arabian J. Chem. 2023, 16, 105033. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef]
- Jia, W.; Wang, W.; Yu, D.; Yu, Y.; Feng, Z.; Li, H.; Zhang, J.; Zhang, H. Structural elucidation of a polysaccharide from Flammulina velutipes and its lipid-lowering and immunomodulation activities. Polymers 2024, 16, 598. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, H.; Li, H.; Luo, Y.; Zhang, M.; Yu, R.; Huang, W.; Song, L.; Zhu, J. Structural elucidation and immunoregulatory activity of a new polysaccharide obtained from the edible part of Scapharca subcrenata. Process Biochem. 2023, 128, 76–93. [Google Scholar] [CrossRef]
- Niu, J.; Wang, S.; Wang, B.; Chen, L.; Zhao, G.; Liu, S.; Wang, S.; Wang, Z. Structure and anti-tumor activity of a polysaccharide from Bletilla ochracea Schltr. Int. J. Biol. Macromol. 2020, 154, 1548–1555. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Qiao, Z.R.; Cai, W.D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Yue, Y.; Wu, Y.; Zhang, W.; Xia, W.; Wu, M. Purification, structure identification and immune activity of a neutral polysaccharide from Cynanchum Auriculatum. Int. J. Biol. Macromol. 2023, 237, 124142. [Google Scholar] [CrossRef]
- Yang, X.; Wei, S.; Lu, X.; Qiao, X.; Simal-Gandara, J.; Capanoglu, E.; Wozniak, L.; Zou, L.; Cao, H.; Xiao, J.; et al. A neutral polysaccharide with a triple helix structure from ginger: Characterization and immunomodulatory activity. Food Chem. 2021, 350, 129261. [Google Scholar] [CrossRef]
- Pasha, I.; Ahmad, F. Monosaccharide composition and carbohydrates linkage identification in cereal brans using UHPLC/QqQ-DMRM-MS. J. Food Compos. Anal. 2021, 96, 103732. [Google Scholar] [CrossRef]
- Liu, H.; Zhuang, S.; Liang, C.; He, J.; Brennan, C.S.; Brennan, M.A.; Ma, L.; Xiao, G.; Chen, H.; Wan, S. Effects of a polysaccharide extract from Amomum villosum Lour. on gastric mucosal injury and its potential underlying mechanism. Carbohydr. Polym. 2022, 294, 119822. [Google Scholar] [CrossRef]
- Zhi, F.; Yang, T.L.; Wang, Q.; Jiang, B.; Wang, Z.P.; Zhang, J.; Chen, Y.Z. Isolation, structure and activity of a novel water-soluble polysaccharide from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2019, 133, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shang, F.; Yang, Z.; Wu, M.; Zhao, J. Structural analysis of a homogeneous polysaccharide from Achatina fulica. Int. J. Biol. Macromol. 2017, 98, 786–792. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef]
- Ren, A.; Chen, L.; Zhao, W.; Shan, S.; Li, Z.; Tang, Z. Extraction optimization and structural characterization of soluble dietary fiber in foxtail millet and its inhibitory activities on colon cancer. J. Funct. Foods 2023, 107, 105659. [Google Scholar] [CrossRef]
- Liu, Y.; Kan, Y.; Huang, Y.; Jiang, C.; Zhao, L.; Hu, J.; Pang, W. Physicochemical characteristics and antidiabetic properties of the polysaccharides from Pseudostellaria heterophylla. Molecules 2022, 27, 3719. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, J.; Yao, L.; You, F.; Yuan, J.; Wang, D.; Gu, S. Synthesis, characterization and antioxidant activity of selenium nanoparticle decorated with polysaccharide from hawthorn. J. Food Meas. Charact. 2023, 17, 6125–6134. [Google Scholar] [CrossRef]
- Ding, M.; Liu, Y.; Ye, Y.F.; Zhang, J.C.; Wang, J.H. Polysaccharides from the lignified okra: Physicochemical properties and rheological properties. Bioact. Carbohydr. Dietary Fibre 2021, 26, 100274. [Google Scholar] [CrossRef]
- Bagchi, S.; Kumar, K.J. Studies on water soluble polysaccharides from Pithecellobium dulce (Roxb.) Benth. seeds. Carbohydr. Polym. 2016, 138, 215–221. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Liu, X.; Chen, M.; Bai, B.; Yang, Y.; Bo, T.; Fan, S. The separation, purification, structure identification, and antioxidant activity of Elaeagnus umbellata polysaccharides. Molecules 2023, 28, 6468. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Shafie, M.H. A review of in vitro antioxidant and antidiabetic polysaccharides: Extraction methods, physicochemical and structure-activity relationships. Int. J. Biol. Macromol. 2024, 282, 137143. [Google Scholar] [CrossRef]
- Chen, N.; Jiang, T.Y.; Xu, J.X.; Xi, W.J.; Shang, E.; Xiao, P.; Duan, J.A. The relationship between polysaccharide structure and its antioxidant activity needs to be systematically elucidated. Int. J. Biol. Macromol. 2024, 270, 132391. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jin, Y.; Wang, J.; Xu, H. Structure identification of two polysaccharides from Morchella sextelata with antioxidant activity. Foods 2022, 11, 982. [Google Scholar] [CrossRef]
- Bo, S.; Dan, M.; Han, W.; Ochir, S.; Bao, L.; Liu, L.; Muschin, T.; Baigude, H. Physicochemical properties, immunostimulatory and antioxidant activities of a novel polysaccharide isolated from Mirabilis himalaica (Edgew) Heim. RSC Adv. 2022, 12, 17264–17275. [Google Scholar] [CrossRef]
- Chen, J.Q.; Miao, W.; Liu, Y.; Zhou, J.; Zhang, L.; Bian, X.Q.; Zhong, T.; Wu, J.L.; Li, N. Structural characterization, molecular dynamic simulation, and conformational visualization of a water-soluble glucan with high molecular weight from Gastrodia elata Blume. Int. J. Biol. Macromol. 2024, 263, 130207. [Google Scholar] [CrossRef]
- Cao, R.A.; Ji, R.; Tabarsa, M.; Palanisamy, S.; Talapphet, N.; Yelithao, K.; Wang, C.; You, S. Extraction, structural elucidation and immunostimulating properties of water-soluble polysaccharides from wheat bran. J. Food Biochem. 2020, 44, e13364. [Google Scholar] [CrossRef] [PubMed]

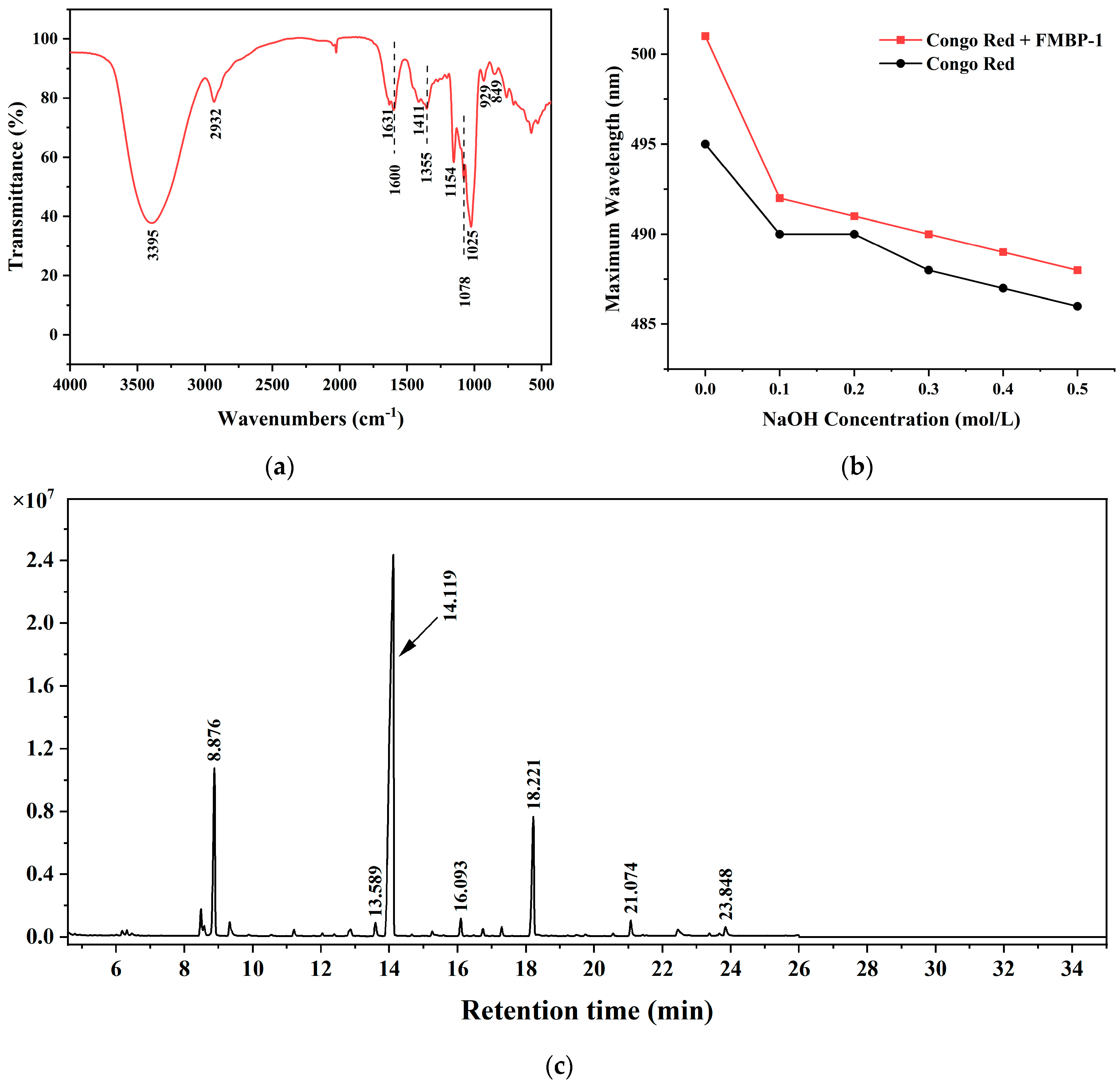
| Linkage Type | Methylated Sugar | Retention Time (min) | Mass Fragments (m/z) | Molar Ratio (%) |
|---|---|---|---|---|
| t-Glcp | 2,3,4,6-Me4-Glcp | 8.876 | 87, 102, 118, 129, 145, 161, 162, 205 | 14.88 |
| 6-Glcp | 2,3,4-Me3-Glcp | 13.598 | 87, 99, 102, 118, 129, 162, 189, 233 | 1.32 |
| 4-Glcp | 2,3,6-Me3-Glcp | 14.119 | 87, 102, 113, 118, 129, 162, 233 | 70.21 |
| 3,4-Glcp | 2,6-Me2-Glcp | 16.093 | 87, 118, 129, 143, 185, 203, 305 | 1.32 |
| 4,6-Glcp | 2,3-Me2-Glcp | 18.221 | 85, 102, 118, 127, 159, 162, 201, 261 | 12.28 |
| Code | Glycosyl Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A | →4)-α-D-Glcp-(1→ | 5.29 | 3.52 | 3.86 | 3.56 | 3.74 | 3.75–3.65 |
| 99.72 | 71.48 | 73.29 | 76.63 | 71.12 | 60.41 | ||
| B | →4,6)-α-D-Glcp-(1→ | 5.28 | 3.51 | 3.85 | 3.55 | 3.45 | 3.94, 3.80 |
| 99.55 | 71.67 | 73.16 | 76.76 | 71.24 | 70.31 | ||
| C | α-D-Glcp-(1→ | 5.24 | 3.55 | 3.60 | 3.31 | 3.60 | 3.75–3.65 |
| 99.94 | 71.67 | 72.82 | 69.26 | 72.65 | 60.34 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Fan, X.; Zhao, W.; Meng, F.; Lu, F.; Lu, Z.; Zhao, H. Structure Characterization and Antioxidant Activity of a Novel Polysaccharide from Bacillus natto Fermented Millet Bran. Foods 2025, 14, 278. https://doi.org/10.3390/foods14020278
Zhang H, Fan X, Zhao W, Meng F, Lu F, Lu Z, Zhao H. Structure Characterization and Antioxidant Activity of a Novel Polysaccharide from Bacillus natto Fermented Millet Bran. Foods. 2025; 14(2):278. https://doi.org/10.3390/foods14020278
Chicago/Turabian StyleZhang, Hanzhuo, Xia Fan, Wenjie Zhao, Fanqiang Meng, Fengxia Lu, Zhaoxin Lu, and Haizhen Zhao. 2025. "Structure Characterization and Antioxidant Activity of a Novel Polysaccharide from Bacillus natto Fermented Millet Bran" Foods 14, no. 2: 278. https://doi.org/10.3390/foods14020278
APA StyleZhang, H., Fan, X., Zhao, W., Meng, F., Lu, F., Lu, Z., & Zhao, H. (2025). Structure Characterization and Antioxidant Activity of a Novel Polysaccharide from Bacillus natto Fermented Millet Bran. Foods, 14(2), 278. https://doi.org/10.3390/foods14020278








