Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes
Abstract
:1. Introduction
1.1. Tomatoes
1.2. Nondestructive Detection Technology and Deep Learning
2. Bibliographic Search
3. Quality Assessment
4. Principle and Application
4.1. Mechanical Characteristic Technology
4.2. Electromagnetic Technology
4.2.1. Machine Vision Technique
4.2.2. Vis/NIR Spectroscopy
4.2.3. Hyperspectral Imaging Technique
4.2.4. Optical Property Measurement Technique
4.2.5. Raman Spectroscopy
4.2.6. X-Ray Technique
4.2.7. Nuclear Magnetic Resonance Technique
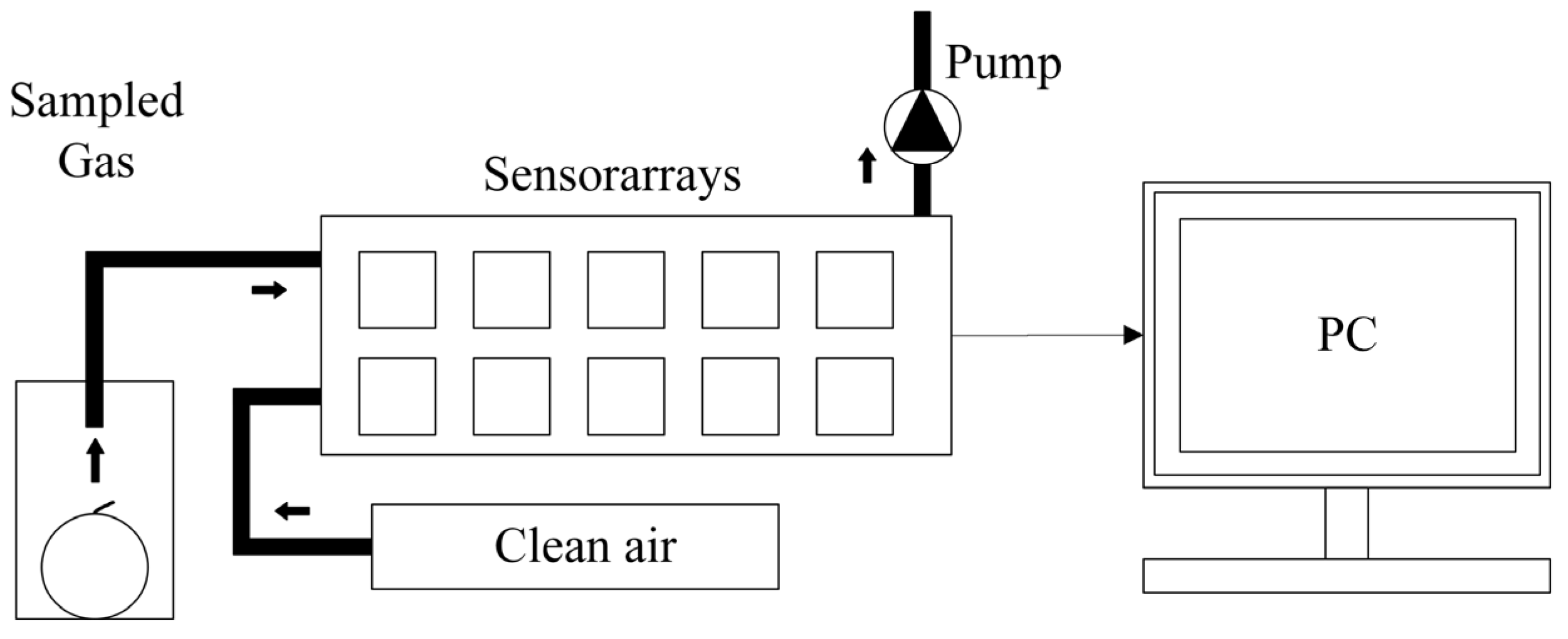
4.3. Electrochemical Sensor Technology
4.4. Technique Comparison
5. Deep Learning with Nondestructive Detection Technology in Application of Tomato Quality Assessment
5.1. Deep Learning in Electromagnetic Technology
5.1.1. Deep Learning in Machine Vision
5.1.2. Deep Learning in Spectral Technique
5.1.3. Deep Learning in X-Ray Technique
6. Limitations and Future Developments
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Qiang, Q.; Xiang, L.; Fernie, A.R.; Yang, J. Targeted Approaches to Improve Tomato Fruit Taste. Hortic. Res. 2023, 10, uhac229. [Google Scholar] [CrossRef] [PubMed]
- Fibiani, M. Influence of Year, Genotype and Cultivation System on Nutritional Values and Bioactive Compounds in Tomato (Solanum lycopersicum L.). Food Chem. 2022, 389, 133090. [Google Scholar] [CrossRef] [PubMed]
- Tilesi, F.; Lombardi, A.; Mazzucato, A. Scientometric and Methodological Analysis of the Recent Literature on the Health-Related Effects of Tomato and Tomato Products. Foods 2021, 10, 1905. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef]
- FAO Homepage|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/home/zh (accessed on 12 June 2023).
- Bai, L.; Zhu, Z.; Zhang, T. How to Improve Food Quality in the Domestic Market: The Role of “Same Line Same Standard Same Quality”—Evidence from a Consumer Choice Experiment in China. Sustainability 2021, 13, 5709. [Google Scholar] [CrossRef]
- De Ketelaere, B.; Lammertyn, J.; Molenberghs, G.; Desmet, M.; Nicolaї, B.; De Baerdemaeker, J. Tomato Cultivar Grouping Based on Firmness Change, Shelf Life and Variance during Postharvest Storage. Postharvest Biol. Technol. 2004, 34, 187–201. [Google Scholar] [CrossRef]
- Sibomana, M.S.; Workneh, T.S.; Audain, K. A Review of Postharvest Handling and Losses in the Fresh Tomato Supply Chain: A Focus on Sub-Saharan Africa. Food Secur. 2016, 8, 389–404. [Google Scholar] [CrossRef]
- Manivel-Chávez, R.A.; Garnica-Romo, M.G.; Arroyo-Correa, G.; Aranda-Sánchez, J.I. Optical and Mechanical Nondestructive Tests for Measuring Tomato Fruit Firmness. In Proceedings of the 22nd Congress of the International Commission for Optics: Light for the Development of the World, Puebla, Mexico, 15–19 August 2011; Rodríguez-Vera, R., Díaz-Uribe, R., Eds.; SPIE: Bellingham, WA, USA, 2011; p. 801176. [Google Scholar]
- Shi, Y.; Wang, Y.; Hu, X.; Li, Z.; Huang, X.; Liang, J.; Zhang, X.; Zheng, K.; Zou, X.; Shi, J. Nondestructive Discrimination of Analogous Density Foreign Matter inside Soy Protein Meat Semi-Finished Products Based on Transmission Hyperspectral Imaging. Food Chem. 2023, 411, 135431. [Google Scholar] [CrossRef]
- Mengyu, H.; Zhihua, L.; Xiaowei, H.; Yuan, W.; Xiaoou, W.; Xiaobo, Z.; Jiyong, S.; Zhangqi, H.; Litao, Y.; Liying, G.; et al. A Cell-Based Electrochemical Taste Sensor for Detection of Hydroxy-α-Sanshool. Food Chem. 2023, 418, 135941. [Google Scholar]
- Han, E.; Li, L.; Gao, T.; Pan, Y.; Cai, J. Nitrite Determination in Food Using Electrochemical Sensor Based on Self-Assembled MWCNTs/AuNPs/Poly-Melamine Nanocomposite. Food Chem. 2024, 437, 137773. [Google Scholar] [CrossRef]
- Huang, X.; Pan, S.; Sun, Z.; Ye, W.; Aheto, J.H. Evaluating Quality of Tomato during Storage Using Fusion Information of Computer Vision and Electronic Nose. J. Food Process Eng. 2018, 41, e12832. [Google Scholar] [CrossRef]
- Huang, X.; Lv, R.; Wang, S.; Aheto, J.H.; Dai, C. Integration of Computer Vision and Colorimetric Sensor Array for Nondestructive Detection of Mango Quality. J. Food Process Eng. 2018, 41, e12873. [Google Scholar] [CrossRef]
- Nturambirwe, J.F.I.; Opara, U.L. Machine Learning Applications to Non-Destructive Defect Detection in Horticultural Products. Biosyst. Eng. 2020, 189, 60–83. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Y.; Wei, S.; Chen, G.; Li, H.; Li, Y.; Tian, H.; Zhang, T.; Cui, H. A Comprehensive Review of Conventional and Deep Learning Approaches for Ground-Penetrating Radar Detection of Raw Data. Appl. Sci. 2023, 13, 7992. [Google Scholar] [CrossRef]
- Zhu, L.; Spachos, P.; Pensini, E.; Plataniotis, K.N. Deep Learning and Machine Vision for Food Processing: A Survey. Curr. Res. Food Sci. 2021, 4, 233–249. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, C.; Sun, J.; Cao, Y.; Yao, K.; Xu, M. A Deep Learning Method for Predicting Lead Content in Oilseed Rape Leaves Using Fluorescence Hyperspectral Imaging. Food Chem. 2023, 409, 135251. [Google Scholar] [CrossRef]
- Ji, W.; Wang, J.; Xu, B.; Zhang, T. Apple Grading Based on Multi-Dimensional View Processing and Deep Learning. Foods 2023, 12, 2117. [Google Scholar] [CrossRef]
- Hong, S.-J.; Park, S.; Lee, C.-H.; Kim, S.; Roh, S.-W.; Nurhisna, N.I.; Kim, G. Application of X-Ray Imaging and Convolutional Neural Networks in the Prediction of Tomato Seed Viability. IEEE Access 2023, 11, 38061–38071. [Google Scholar] [CrossRef]
- Rangarajan, A.K.; Purushothaman, R.; Ramesh, A. Tomato Crop Disease Classification Using Pre-Trained Deep Learning Algorithm. Procedia Comput. Sci. 2018, 133, 1040–1047. [Google Scholar] [CrossRef]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Zhang, S.; Griffiths, J.S.; Marchand, G.; Bernards, M.A.; Wang, A. Tomato Brown Rugose Fruit. Virus: An Emerging and Rapidly Spreading Plant RNA Virus That Threatens Tomato Production Worldwide. Mol. Plant Pathol. 2022, 23, 1262–1277. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, R.; Anandamurugan, S.; Pandiyan, P.; Kaliappan, V.K. Artificial Intelligence in Tomato Leaf Disease Detection: A Comprehensive Review and Discussion. J. Plant Dis. Prot. 2022, 129, 469–488. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato Responses to Salinity Stress: From Morphological Traits to Genetic Changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Chaudhary, P.; Sharma, A.; Singh, B.; Nagpal, A.K. Bioactivities of Phytochemicals Present in Tomato. J. Food Sci. Technol. 2018, 55, 2833–2849. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, J.; Yao, Z.; Huang, Q.; Tang, K.; Jiang, D.; Yang, Z. A Fluorescence Detection Method for Postharvest Tomato Epidermal Defects Based on Improved YOLOv5m. J. Sci. Food Agric. 2024, 104, 6615–6625. [Google Scholar] [CrossRef]
- Fekadu, A. Analysis of the Pre-Harvest Factors That Influence on the Postharvest Quality Attributes of Tomatoes (Lycopersicon esculentum Mill.): A Systematic Review. Sci. Hortic. 2024, 337, 113460. [Google Scholar] [CrossRef]
- Azzi, L.; Deluche, C.; Gévaudant, F.; Frangne, N.; Delmas, F.; Hernould, M.; Chevalier, C. Fruit Growth-Related Genes in Tomato. J. Exp. Bot. 2015, 66, 1075–1086. [Google Scholar] [CrossRef]
- Chattopadhyay, T.; Hazra, P.; Akhtar, S.; Maurya, D.; Mukherjee, A.; Roy, S. Skin Colour, Carotenogenesis and Chlorophyll Degradation Mutant Alleles: Genetic Orchestration behind the Fruit Colour Variation in Tomato. Plant Cell Rep. 2021, 40, 767–782. [Google Scholar] [CrossRef]
- Shahriari, Z.; Su, X.; Zheng, K.; Zhang, Z. Advances and Prospects of Virus-Resistant Breeding in Tomatoes. Int. J. Mol. Sci. 2023, 24, 15448. [Google Scholar] [CrossRef] [PubMed]
- Mohd Ali, M.; Hashim, N.; Bejo, S.K.; Shamsudin, R. Rapid and Nondestructive Techniques for Internal and External Quality Evaluation of Watermelons: A Review. Sci. Hortic. 2017, 225, 689–699. [Google Scholar] [CrossRef]
- Helyes, L.; Pék, Z.; Lugasi, A. Tomato Fruit Quality and Content Depend on Stage of Maturity. HortScience 2006, 41, 1400–1401. [Google Scholar] [CrossRef]
- Pék, Z.; Helyes, L.; Lugasi, A. Color Changes and Antioxidant Content of Vine and Postharvest-Ripened Tomato Fruits. HortScience 2010, 45, 466–468. [Google Scholar] [CrossRef]
- Ayuso-Yuste, M.C.; González-Cebrino, F.; Lozano-Ruiz, M.; Fernández-León, A.M.; Bernalte-García, M.J. Influence of Ripening Stage on Quality Parameters of Five Traditional Tomato Varieties Grown under Organic Conditions. Horticulturae 2022, 8, 313. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bioactive Compounds, Antioxidant Activity and Color of Hydroponic Tomato Fruits at Different Stages of Ripening. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 404–412. [Google Scholar] [CrossRef]
- Iraji, M.S. Comparison between Soft Computing Methods for Tomato Quality Grading Using Machine Vision. J. Food Meas. Charact. 2019, 13, 1–15. [Google Scholar] [CrossRef]
- Ziogas, V.; Michailidis, M.; Karagiannis, E.; Tanou, G.; Molassiotis, A. Manipulating Fruit Quality through Foliar Nutrition. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–417. ISBN 978-0-12-818732-6. [Google Scholar]
- Zhang, B.; Gu, B.; Tian, G.; Zhou, J.; Huang, J.; Xiong, Y. Challenges and Solutions of Optical-Based Nondestructive Quality Inspection for Robotic Fruit and Vegetable Grading Systems: A Technical Review. Trends Food Sci. Technol. 2018, 81, 213–231. [Google Scholar] [CrossRef]
- Lu, R. Principles of Solid Food Texture Analysis. In Instrumental Assessment of Food Sensory Quality; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–128. ISBN 978-0-85709-439-1. [Google Scholar]
- Xu, M.; Sun, J.; Cheng, J.; Yao, K.; Wu, X.; Zhou, X. Non-destructive Prediction of Total Soluble Solids and Titratable Acidity in Kyoho Grape Using Hyperspectral Imaging and Deep Learning Algorithm. Int. J. Food Sci. Technol. 2023, 58, 9–21. [Google Scholar] [CrossRef]
- Rahman, A.; Kandpal, L.; Lohumi, S.; Kim, M.; Lee, H.; Mo, C.; Cho, B.-K. Nondestructive Estimation of Moisture Content, pH and Soluble Solid Contents in Intact Tomatoes Using Hyperspectral Imaging. Appl. Sci. 2017, 7, 109. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Hu, D.; Chen, K. Quality Assessment of Tomato Fruit by Optical Absorption and Scattering Properties. Postharvest Biol. Technol. 2018, 143, 78–85. [Google Scholar] [CrossRef]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Maurotti, S.; Ferro, Y.; Galluccio, A.; Arturi, F.; Romeo, S.; Procopio, A.; Musolino, V.; Mollace, V.; Montalcini, T.; et al. A Rapid and Cheap Method for Extracting and Quantifying Lycopene Content in Tomato Sauces: Effects of Lycopene Micellar Delivery on Human Osteoblast-Like Cells. Nutrients 2022, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Lesage, P.; Destain, M.-F. Measurement of Tomato Firmness by Using a Non-Destructive Mechanical Sensor. Postharvest Biol. Technol. 1996, 8, 45–55. [Google Scholar] [CrossRef]
- Lien, C.-C.; Ay, C.; Ting, C.-H. Non-Destructive Impact Test for Assessment of Tomato Maturity. J. Food Eng. 2009, 91, 402–407. [Google Scholar] [CrossRef]
- Baltazar, A.; Aranda, J.I.; González-Aguilar, G. Bayesian Classification of Ripening Stages of Tomato Fruit Using Acoustic Impact and Colorimeter Sensor Data. Comput. Electron. Agric. 2008, 60, 113–121. [Google Scholar] [CrossRef]
- Mizrach, A. Nondestructive Ultrasonic Monitoring of Tomato Quality during Shelf-Life Storage. Postharvest Biol. Technol. 2007, 46, 271–274. [Google Scholar] [CrossRef]
- Ropelewska, E.; Szwejda-Grzybowska, J. Relationship of Textures from Tomato Fruit Images Acquired Using a Digital Camera and Lycopene Content Determined by High-Performance Liquid Chromatography. Agriculture 2022, 12, 1495. [Google Scholar] [CrossRef]
- Nyalala, I.; Okinda, C.; Chao, Q.; Mecha, P.; Korohou, T.; Yi, Z.; Nyalala, S.; Jiayu, Z.; Chao, L.; Kunjie, C. Weight and Volume Estimation of Single and Occluded Tomatoes Using Machine Vision. Int. J. Food Prop. 2021, 24, 818–832. [Google Scholar] [CrossRef]
- Pedro, A.M.K.; Ferreira, M.M.C. Nondestructive Determination of Solids and Carotenoids in Tomato Products by Near-Infrared Spectroscopy and Multivariate Calibration. Anal. Chem. 2005, 77, 2505–2511. [Google Scholar] [CrossRef]
- Shao, Y.; He, Y.; Gómez, A.H.; Pereir, A.G.; Qiu, Z.; Zhang, Y. Visible/near Infrared Spectrometric Technique for Nondestructive Assessment of Tomato ‘Heatwave’ (Lycopersicum esculentum) Quality Characteristics. J. Food Eng. 2007, 81, 672–678. [Google Scholar] [CrossRef]
- Sirisomboon, P.; Tanaka, M.; Kojima, T.; Williams, P. Nondestructive Estimation of Maturity and Textural Properties on Tomato ‘Momotaro’ by near Infrared Spectroscopy. J. Food Eng. 2012, 112, 218–226. [Google Scholar] [CrossRef]
- Tiwari, G.; Slaughter, D.C.; Cantwell, M. Nondestructive Maturity Determination in Green Tomatoes Using a Handheld Visible and near Infrared Instrument. Postharvest Biol. Technol. 2013, 86, 221–229. [Google Scholar] [CrossRef]
- Torres, I.; Pérez-Marín, D.; Haba, M.-J.D.L.; Sánchez, M.-T. Fast and Accurate Quality Assessment of Raf Tomatoes Using NIRS Technology. Postharvest Biol. Technol. 2015, 107, 9–15. [Google Scholar] [CrossRef]
- Lu, H.; Wang, F.; Liu, X.; Wu, Y. Rapid Assessment of Tomato Ripeness Using Visible/Near-Infrared Spectroscopy and Machine Vision. Food Anal. Methods 2017, 10, 1721–1726. [Google Scholar] [CrossRef]
- Jun, S.; Yating, L.; Xiaohong, W.; Chunxia, D.; Yong, C. SSC Prediction of Cherry Tomatoes Based on IRIV-CS-SVR Model and near Infrared Reflectance Spectroscopy. J. Food Process Eng. 2018, 41, e12884. [Google Scholar] [CrossRef]
- Ibáñez, G.; Cebolla-Cornejo, J.; Martí, R.; Roselló, S.; Valcárcel, M. Non-Destructive Determination of Taste-Related Compounds in Tomato Using NIR Spectra. J. Food Eng. 2019, 263, 237–242. [Google Scholar] [CrossRef]
- Sheng, R.; Cheng, W.; Li, H.; Ali, S.; Akomeah Agyekum, A.; Chen, Q. Model Development for Soluble Solids and Lycopene Contents of Cherry Tomato at Different Temperatures Using Near-Infrared Spectroscopy. Postharvest Biol. Technol. 2019, 156, 110952. [Google Scholar] [CrossRef]
- Brito, A.A.D.; Campos, F.; Nascimento, A.D.R.; Corrêa, G.D.C.; Silva, F.A.D.; Teixeira, G.H.D.A.; Cunha Júnior, L.C. Determination of Soluble Solid Content in Market Tomatoes Using Near-Infrared Spectroscopy. Food Control 2021, 126, 108068. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Y.; Chen, G.; Tian, X.; Wang, Z.; Fan, S.; Xin, Z. Nondestructive Evaluation of Soluble Solids Content in Tomato with Different Stage by Using Vis/NIR Technology and Multivariate Algorithms. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2021, 248, 119139. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Xu, Y.; Chen, K. Prediction of Tomato Firmness Using Spatially-Resolved Spectroscopy. Postharvest Biol. Technol. 2018, 140, 18–26. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Assessment of Tomato Soluble Solids Content and pH by Spatially-Resolved and Conventional Vis/NIR Spectroscopy. J. Food Eng. 2018, 236, 19–28. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Chen, W.; Yang, J.; Zheng, L. Feasibility in Multispectral Imaging for Predicting the Content of Bioactive Compounds in Intact Tomato Fruit. Food Chem. 2015, 173, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, S.; Bian, B.; Li, Y.; Sun, Y.; Wang, X. Discrimination of Tomato Maturity Using Hyperspectral Imaging Combined with Graph-Based Semi-Supervised Method Considering Class Probability Information. Food Anal. Methods 2021, 14, 968–983. [Google Scholar] [CrossRef]
- Tan, F.; Mo, X.; Ruan, S.; Yan, T.; Xing, P.; Gao, P.; Xu, W.; Ye, W.; Li, Y.; Gao, X.; et al. Combining Vis-NIR and NIR Spectral Imaging Techniques with Data Fusion for Rapid and Nondestructive Multi-Quality Detection of Cherry Tomatoes. Foods 2023, 12, 3621. [Google Scholar] [CrossRef]
- Dai, C.; Sun, J.; Huang, X.; Zhang, X.; Tian, X.; Wang, W.; Sun, J.; Luan, Y. Application of Hyperspectral Imaging as a Nondestructive Technology for Identifying Tomato Maturity and Quantitatively Predicting Lycopene Content. Foods 2023, 12, 2957. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Tian, Y.; Ma, H.; Tian, X.; Zhu, Y.; Huang, Y.; Cao, Y.; Wu, L. Determination of Soluble Solids Content in Tomatoes with Different Nitrogen Levels Based on Hyperspectral Imaging Technique. J. Food Sci. 2024, 89, 5724–5733. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S. Nondestructive Evaluation of Internal Maturity of Tomatoes Using Spatially Offset Raman Spectroscopy. Postharvest Biol. Technol. 2012, 71, 21–31. [Google Scholar] [CrossRef]
- Fu, X.; He, X.; Xu, H.; Ying, Y. Nondestructive and Rapid Assessment of Intact Tomato Freshness and Lycopene Content Based on a Miniaturized Raman Spectroscopic System and Colorimetry. Food Anal. Methods 2016, 9, 2501–2508. [Google Scholar] [CrossRef]
- Wang, S.-X.; Hu, R.-F.; Gao, K.; Wali, F.; Zan, G.-B.; Wang, D.-J.; Pan, Z.-Y.; Wei, S.-Q. Non-Destructive Study of Fruits Using Grating-Based X-Ray Imaging. Nucl. Sci. Tech. 2017, 28, 24. [Google Scholar] [CrossRef]
- Borba, K.R.; Oldoni, F.C.A.; Monaretto, T.; Colnago, L.A.; Ferreira, M.D. Selection of Industrial Tomatoes Using TD-NMR Data and Computational Classification Methods. Microchem. J. 2021, 164, 106048. [Google Scholar] [CrossRef]
- Gómez, A.H.; Hu, G.; Wang, J.; Pereira, A.G. Evaluation of Tomato Maturity by Electronic Nose. Comput. Electron. Agric. 2006, 54, 44–52. [Google Scholar] [CrossRef]
- Khorramifar, A.; Sharabiani, V.R.; Karami, H.; Kisalaei, A.; Lozano, J.; Rusinek, R.; Gancarz, M. Investigating Changes in pH and Soluble Solids Content of Potato during the Storage by Electronic Nose and Vis/NIR Spectroscopy. Foods 2022, 11, 4077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; You, Z.; Chen, H.; Wang, X.; Ying, Y.; Wang, Y. Integrated Fruit Ripeness Assessment System Based on an Artificial Olfactory Sensor and Deep Learning. Foods 2024, 13, 793. [Google Scholar] [CrossRef]
- Hong, X.; Wang, J. Use of Electronic Nose and Tongue to Track Freshness of Cherry Tomatoes Squeezed for Juice Consumption: Comparison of Different Sensor Fusion Approaches. Food Bioprocess. Technol. 2015, 8, 158–170. [Google Scholar] [CrossRef]
- Shiu, J.W.; Slaughter, D.C.; Boyden, L.E.; Barrett, D.M. Effect of the Shear-to-Compressive Force Ratio in Puncture Tests Quantifying Watermelon Mechanical Properties. J. Food Eng. 2015, 150, 125–131. [Google Scholar] [CrossRef]
- García-Ramos, F.J.; Valero, C.; Homer, I.; Ortiz-Cañavate, J.; Ruiz-Altisent, M. Non-Destructive Fruit Firmness Sensors: A Review. Span. J. Agric. Res. 2005, 3, 61. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Huang, J.; Zhang, X.; Zhu, Y.; Zhang, S.; Liu, H.; Gao, L.; Chen, M. Comparison of Apple Firmness Prediction Models Based on Non-destructive Acoustic Signal. Int. J. Food Sci. Technol. 2021, 56, 6443–6450. [Google Scholar] [CrossRef]
- De Ketelaere, B.; Howarth, M.S.; Crezee, L.; Lammertyn, J.; Viaene, K.; Bulens, I.; De Baerdemaeker, J. Postharvest Firmness Changes as Measured by Acoustic and Low-Mass Impact Devices: A Comparison of Techniques. Postharvest Biol. Technol. 2006, 41, 275–284. [Google Scholar] [CrossRef]
- Mizrach, A. Ultrasonic Technology for Quality Evaluation of Fresh Fruit and Vegetables in Pre- and Postharvest Processes. Postharvest Biol. Technol. 2008, 48, 315–330. [Google Scholar] [CrossRef]
- Morrison, D.S.; Abeyratne, U.R. Ultrasonic Technique for Non-Destructive Quality Evaluation of Oranges. J. Food Eng. 2014, 141, 107–112. [Google Scholar] [CrossRef]
- Yao, K.; Sun, J.; Zhou, X.; Nirere, A.; Tian, Y.; Wu, X. Nondestructive Detection for Egg Freshness Grade Based on Hyperspectral Imaging Technology. J. Food Process Eng. 2020, 43, e13422. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Sun, J.; Zhou, X.; Yao, K.; Nirere, A. Nondestructive Determination of the Total Mold Colony Count in Green Tea by Hyperspectral Imaging Technology. J. Food Process Eng. 2020, 43, e13570. [Google Scholar] [CrossRef]
- Huang, X.; Yu, S.; Xu, H.; Aheto, J.H.; Bonah, E.; Ma, M.; Wu, M.; Zhang, X. Rapid and Nondestructive Detection of Freshness Quality of Postharvest Spinaches Based on Machine Vision and Electronic Nose. J. Food Saf. 2019, 39, e12708. [Google Scholar] [CrossRef]
- Xu, Z.; Han, Y.; Zhao, D.; Li, K.; Li, J.; Dong, J.; Shi, W.; Zhao, H.; Bai, Y. Research Progress on Quality Detection of Livestock and Poultry Meat Based on Machine Vision, Hyperspectral and Multi-Source Information Fusion Technologies. Foods 2024, 13, 469. [Google Scholar] [CrossRef]
- Huo, L.; Liu, Y.; Yang, Y.; Zhuang, Z.; Sun, M. Review: Research on Product Surface Quality Inspection Technology Based on 3D Point Cloud. Adv. Mech. Eng. 2023, 15, 16878132231159523. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, C.; Yuan, W.; Zhou, Y.; Jiang, X.; Zhou, H. The Utility of Fourier Transform Near-Infrared Spectroscopy to Identify Geographical Origins of Chinese Pears. J. Food Meas. Charact. 2024, 18, 2674–2684. [Google Scholar] [CrossRef]
- Paz, P.; Sánchez, M.-T.; Pérez-Marín, D.; Guerrero, J.-E.; Garrido-Varo, A. Nondestructive Determination of Total Soluble Solid Content and Firmness in Plums Using Near-Infrared Reflectance Spectroscopy. J. Agric. Food Chem. 2008, 56, 2565–2570. [Google Scholar] [CrossRef]
- Maniwara, P.; Nakano, K.; Ohashi, S.; Boonyakiat, D.; Seehanam, P.; Theanjumpol, P.; Poonlarp, P. Evaluation of NIRS as Non-Destructive Test to Evaluate Quality Traits of Purple Passion Fruit. Sci. Hortic. 2019, 257, 108712. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Prediction of Firmness Parameters of Tomatoes by Portable Visible and Near-Infrared Spectroscopy. J. Food Eng. 2018, 222, 185–198. [Google Scholar] [CrossRef]
- Tang, T.; Luo, Q.; Yang, L.; Gao, C.; Ling, C.; Wu, W. Research Review on Quality Detection of Fresh Tea Leaves Based on Spectral Technology. Foods 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhou, H.; Zhou, Y.; Zhang, C.; Jiang, X.; Jiang, H. In-Field and Non-Destructive Determination of Comprehensive Maturity Index and Maturity Stages of Camellia Oleifera Fruits Using a Portable Hyperspectral Imager. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2024, 315, 124266. [Google Scholar] [CrossRef]
- Yang, C.; Lee, W.S.; Gader, P. Hyperspectral Band Selection for Detecting Different Blueberry Fruit Maturity Stages. Comput. Electron. Agric. 2014, 109, 23–31. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Yao, K.; Wu, X.; Shen, J.; Cao, Y.; Zhou, X. Nondestructive Detection of Total Soluble Solids in Grapes Using VMD-RC and Hyperspectral Imaging. J. Food Sci. 2022, 87, 326–338. [Google Scholar] [CrossRef]
- Zhao, M.; Cang, H.; Chen, H.; Zhang, C.; Yan, T.; Zhang, Y.; Gao, P.; Xu, W. Determination of Quality and Maturity of Processing Tomatoes Using Near-Infrared Hyperspectral Imaging with Interpretable Machine Learning Methods. LWT 2023, 183, 114861. [Google Scholar] [CrossRef]
- Li, T.; Wei, W.; Xing, S.; Min, W.; Zhang, C.; Jiang, S. Deep Learning-Based Near-Infrared Hyperspectral Imaging for Food Nutrition Estimation. Foods 2023, 12, 3145. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the Optical Properties of Fruits and Vegetables Using Spatially Resolved Hyperspectral Diffuse Reflectance Imaging Technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Hu, D.; Jia, T.; Sun, X.; Zhou, T.; Huang, Y.; Sun, Z.; Zhang, C.; Sun, T.; Zhou, G. Applications of Optical Property Measurement for Quality Evaluation of Agri-Food Products: A Review. Crit. Rev. Food Sci. Nutr. 2023, 64, 12599–12619. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Pan, W.; Kutsanedzie, F.; Chen, Q. Feasibility Study on Nondestructively Sensing Meat’s Freshness Using Light Scattering Imaging Technique. Meat Sci. 2016, 119, 102–109. [Google Scholar] [CrossRef]
- Lu, R.; Van Beers, R.; Saeys, W.; Li, C.; Cen, H. Measurement of Optical Properties of Fruits and Vegetables: A Review. Postharvest Biol. Technol. 2020, 159, 111003. [Google Scholar] [CrossRef]
- Ma, T.; Inagaki, T.; Tsuchikawa, S. Development of a Sensitivity-Enhanced Fluorescence Lifetime Spectroscopic Method for Nondestructive Quality Monitoring of Mature Tomatoes during Storage. Sci. Hortic. 2024, 330, 113059. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, D.; Zhou, T.; Sun, X.; Xie, L.; Ying, Y. Development of a Multispectral Spatial-Frequency Domain Imaging System for Property and Quality Assessment of Fruits and Vegetables. Comput. Electron. Agric. 2023, 214, 108251. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, D.; Wang, Z.; Xie, L.; Ying, Y. Spatial-Frequency Domain Imaging: An Emerging Depth-Varying and Wide-Field Technique for Optical Property Measurement of Biological Tissues. Photonics 2021, 8, 162. [Google Scholar] [CrossRef]
- Cubeddu, R.; D’Andrea, C.; Pifferi, A.; Taroni, P.; Torricelli, A.; Valentini, G.; Dover, C.; Johnson, D.; Ruiz-Altisent, M.; Valero, C. Nondestructive Quantification of Chemical and Physical Properties of Fruits by Time-Resolved Reflectance Spectroscopy in the Wavelength Range 650–1000 Nm. Appl. Opt. 2001, 40, 538. [Google Scholar] [CrossRef]
- Haskell, R.C.; Svaasand, L.O.; Tsay, T.-T.; Feng, T.-C.; Tromberg, B.J.; McAdams, M.S. Boundary Conditions for the Diffusion Equation in Radiative Transfer. J. Opt. Soc. Am. A 1994, 11, 2727. [Google Scholar] [CrossRef]
- Ma, T.; Inagaki, T.; Tsuchikawa, S. Validation Study on Light Scattering Changes in Kiwifruit during Postharvest Storage Using Time-Resolved Transmittance Spectroscopy. Sci. Rep. 2023, 13, 16556. [Google Scholar] [CrossRef]
- Huang, Y.; Si, W.; Chen, K.; Sun, Y. Assessment of Tomato Maturity in Different Layers by Spatially Resolved Spectroscopy. Sensors 2020, 20, 7229. [Google Scholar] [CrossRef]
- Zhu, Q.; He, C.; Lu, R.; Mendoza, F.; Cen, H. Ripeness Evaluation of ‘Sun Bright’ Tomato Using Optical Absorption and Scattering Properties. Postharvest Biol. Technol. 2015, 103, 27–34. [Google Scholar] [CrossRef]
- Lu, Y.; Saeys, W.; Kim, M.; Peng, Y.; Lu, R. Hyperspectral Imaging Technology for Quality and Safety Evaluation of Horticultural Products: A Review and Celebration of the Past 20-Year Progress. Postharvest Biol. Technol. 2020, 170, 111318. [Google Scholar] [CrossRef]
- Si, W.; Xiong, J.; Huang, Y.; Jiang, X.; Hu, D. Quality Assessment of Fruits and Vegetables Based on Spatially Resolved Spectroscopy: A Review. Foods 2022, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Lohner, S.A.; Biegert, K.; Nothelfer, S.; Hohmann, A.; McCormick, R.; Kienle, A. Determining the Optical Properties of Apple Tissue and Their Dependence on Physiological and Morphological Characteristics during Maturation. Part 1: Spatial Frequency Domain Imaging. Postharvest Biol. Technol. 2021, 181, 111647. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Yang, Z.; Wang, X.; Wang, W.; Hui, T. Rapid Non-Destructive Detection Technology in the Field of Meat Tenderness: A Review. Foods 2024, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Jiang, H. Monitoring of Chlorpyrifos Residues in Corn Oil Based on Raman Spectral Deep-Learning Model. Foods 2023, 12, 2402. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Zou, X.; Meng, G.; Wu, N. Raman Spectroscopy for Food Quality Assurance and Safety Monitoring: A Review. Curr. Opin. Food Sci. 2022, 47, 100910. [Google Scholar] [CrossRef]
- Xu, S.; Huang, X.; Lu, H. Advancements and Applications of Raman Spectroscopy in Rapid Quality and Safety Detection of Fruits and Vegetables. Horticulturae 2023, 9, 843. [Google Scholar] [CrossRef]
- Tao, M.; Fang, H.; Feng, X.; He, Y.; Liu, X.; Shi, Y.; Wei, Y.; Hong, Z. Rapid Trace Detection of Pesticide Residues on Tomato by Surface-Enhanced Raman Spectroscopy and Flexible Tapes. J. Food Qual. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Ying, X.; Barlow, N.J.; Feuston, M.H. Micro-CT and Volumetric Imaging in Developmental Toxicology. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 983–1000. ISBN 978-0-12-382032-7. [Google Scholar]
- Kim, T.; Lee, J.; Sun, G.-M.; Park, B.-G.; Park, H.-J.; Choi, D.-S.; Ye, S.-J. Comparison of X-Ray Computed Tomography and Magnetic Resonance Imaging to Detect Pest-Infested Fruits: A Pilot Study. Nucl. Eng. Technol. 2022, 54, 514–522. [Google Scholar] [CrossRef]
- Du, Z.; Zeng, X.; Li, X.; Ding, X.; Cao, J.; Jiang, W. Recent Advances in Imaging Techniques for Bruise Detection in Fruits and Vegetables. Trends Food Sci. Technol. 2020, 99, 133–141. [Google Scholar] [CrossRef]
- Du, Z.; Hu, Y.; Ali Buttar, N.; Mahmood, A. X-ray Computed Tomography for Quality Inspection of Agricultural Products: A Review. Food Sci. Nutr. 2019, 7, 3146–3160. [Google Scholar] [CrossRef]
- Tian, S.; Xu, H. Nondestructive Methods for the Quality Assessment of Fruits and Vegetables Considering Their Physical and Biological Variability. Food Eng. Rev. 2022, 14, 380–407. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Talluri, S.; Beebi, S.K.; Rajesh Kumar, B. Magnetic Resonance Imaging for Quality Evaluation of Fruits: A Review. Food Anal. Methods 2018, 11, 2943–2960. [Google Scholar] [CrossRef]
- Zhang, L.; Barrett, D.M.; McCarthy, M.J. Characterization of the Red Layer and Pericarp of Processing Tomato Using Magnetic Resonance Imaging. J. Food Sci. 2013, 78, E50–E55. [Google Scholar] [CrossRef] [PubMed]
- Sequi, P.; Dell’Abate, M.T.; Valentini, M. Identification of Cherry Tomatoes Growth Origin by Means of Magnetic Resonance Imaging. J. Sci. Food Agric. 2007, 87, 127–132. [Google Scholar] [CrossRef]
- Patel, K.K.; Khan, M.A.; Kar, A. Recent Developments in Applications of MRI Techniques for Foods and Agricultural Produce—An Overview. J. Food Sci. Technol. 2015, 52, 1–26. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Fusion of Artificial Senses as a Robust Approach to Food Quality Assessment. J. Food Eng. 2016, 171, 230–239. [Google Scholar] [CrossRef]
- Turasan, H.; Kokini, J. Novel Nondestructive Biosensors for the Food Industry. Annu. Rev. Food Sci. Technol. 2021, 12, 539–566. [Google Scholar] [CrossRef]
- Zhou, W.; Lian, J.; Zhang, J.; Mei, Z.; Gao, Y.; Hui, G. Tomato Storage Quality Predicting Method Based on Portable Electronic Nose System Combined with WOA-SVM Model. J. Food Meas. Charact. 2023, 17, 3654–3664. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Baldwin, E.A.; Plotto, A.; Rosskopf, E.; Hong, J.C.; Bai, J. Electronic Tongue Discrimination of Four Tomato Cultivars Harvested at Six Maturities and Exposed to Blanching and Refrigeration Treatments. Postharvest Biol. Technol. 2018, 136, 42–49. [Google Scholar] [CrossRef]
- Hongwiangjan, J.; Terdwongworakul, A.; Krisanapook, K. Evaluation of Pomelo Maturity Based on Acoustic Response and Peel Properties. Int. J. Food Sci. Technol. 2015, 50, 782–789. [Google Scholar] [CrossRef]
- Ding, C.; Feng, Z.; Wang, D.; Cui, D.; Li, W. Acoustic Vibration Technology: Toward a Promising Fruit Quality Detection Method. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1655–1680. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, F.; Özdemir, A.T.; Uluışık, S. Evaluation Performance of Ultrasonic Testing on Fruit Quality Determination. J. Food Qual. 2019, 2019, 6810865. [Google Scholar] [CrossRef]
- Park, B.; Lu, R. (Eds.) Hyperspectral Imaging Technology in Food and Agriculture; Food Engineering Series; Springer: New York, NY, USA, 2015; ISBN 978-1-4939-2835-4. [Google Scholar]
- Sun, Y.; Huang, Y.; Pan, L.; Wang, X. Evaluation of the Changes in Optical Properties of Peaches with Different Maturity Levels during Bruising. Foods 2021, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Sugimori, H.; Koseki, S.; Koyama, K. Automated Detection of Internal Fruit Rot in Hass Avocado via Deep Learning-Based Semantic Segmentation of X-Ray Images. Postharvest Biol. Technol. 2023, 203, 112390. [Google Scholar] [CrossRef]
- Arendse, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-Destructive Characterization and Volume Estimation of Pomegranate Fruit External and Internal Morphological Fractions Using X-Ray Computed Tomography. J. Food Eng. 2016, 186, 42–49. [Google Scholar] [CrossRef]
- Aouadi, B.; Zaukuu, J.-L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical Evolution and Food Control Achievements of Near Infrared Spectroscopy, Electronic Nose, and Electronic Tongue—Critical Overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Wang, Q.; Sun, D.-W. Applications of Machine Learning Techniques for Enhancing Nondestructive Food Quality and Safety Detection. Crit. Rev. Food Sci. Nutr. 2023, 63, 1649–1669. [Google Scholar] [CrossRef]
- Ye, M.; Yan, X.; Jiang, D.; Xiang, L.; Chen, N. MIFDELN: A Multi-Sensor Information Fusion Deep Ensemble Learning Network for Diagnosing Bearing Faults in Noisy Scenarios. Knowl.-Based Syst. 2024, 284, 111294. [Google Scholar] [CrossRef]
- Sun, J.; He, X.; Ge, X.; Wu, X.; Shen, J.; Song, Y. Detection of Key Organs in Tomato Based on Deep Migration Learning in a Complex Background. Agriculture 2018, 8, 196. [Google Scholar] [CrossRef]
- Adir, O.; Poley, M.; Chen, G.; Froim, S.; Krinsky, N.; Shklover, J.; Shainsky-Roitman, J.; Lammers, T.; Schroeder, A. Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine. Adv. Mater. 2020, 32, 1901989. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, J.; Lin, T.; Ying, Y. Food and Agro-Product Quality Evaluation Based on Spectroscopy and Deep Learning: A Review. Trends Food Sci. Technol. 2021, 112, 431–441. [Google Scholar] [CrossRef]
- Ye, M.; Yan, X.; Hua, X.; Jiang, D.; Xiang, L.; Chen, N. MRCFN: A Multi-Sensor Residual Convolutional Fusion Network for Intelligent Fault Diagnosis of Bearings in Noisy and Small Sample Scenarios. Expert. Syst. Appl. 2025, 259, 125214. [Google Scholar] [CrossRef]
- Liang, X.; Li, C.; Tian, L. Generative Adversarial Network for Semi-Supervised Image Captioning. Comput. Vis. Image Underst. 2024, 249, 104199. [Google Scholar] [CrossRef]
- Akkem, Y.; Biswas, S.K.; Varanasi, A. A Comprehensive Review of Synthetic Data Generation in Smart Farming by Using Variational Autoencoder and Generative Adversarial Network. Eng. Appl. Artif. Intell. 2024, 131, 107881. [Google Scholar] [CrossRef]
- Wu, G.; Li, C.; Yin, L.; Wang, J.; Zheng, X. Compared between Support Vector Machine (SVM) and Deep Belief Network (DBN) for Multi-Classification of Raman Spectroscopy for Cervical Diseases. Photodiagnosis Photodyn. Ther. 2023, 42, 103340. [Google Scholar] [CrossRef]
- You, J.; Li, D.; Wang, Z.; Chen, Q.; Ouyang, Q. Prediction and Visualization of Moisture Content in Tencha Drying Processes by Computer Vision and Deep Learning. J. Sci. Food Agric. 2024, 104, 5486–5494. [Google Scholar] [CrossRef]
- Nithya, R.; Santhi, B.; Manikandan, R.; Rahimi, M.; Gandomi, A.H. Computer Vision System for Mango Fruit Defect Detection Using Deep Convolutional Neural Network. Foods 2022, 11, 3483. [Google Scholar] [CrossRef]
- Zhao, S.; Tu, K.; Ye, S.; Tang, H.; Hu, Y.; Xie, C. Land Use and Land Cover Classification Meets Deep Learning: A Review. Sensors 2023, 23, 8966. [Google Scholar] [CrossRef]
- Ye, S.; Zhao, S.; Hu, Y.; Xie, C. Single-Image Super-Resolution Challenges: A Brief Review. Electronics 2023, 12, 2975. [Google Scholar] [CrossRef]
- Da Costa, A.Z.; Figueroa, H.E.H.; Fracarolli, J.A. Computer Vision Based Detection of External Defects on Tomatoes Using Deep Learning. Biosyst. Eng. 2020, 190, 131–144. [Google Scholar] [CrossRef]
- Kim, T.; Lee, D.-H.; Kim, K.-C.; Choi, T.; Yu, J.M. Tomato Maturity Estimation Using Deep Neural Network. Appl. Sci. 2022, 13, 412. [Google Scholar] [CrossRef]
- Khan, A.; Hassan, T.; Shafay, M.; Fahmy, I.; Werghi, N.; Mudigansalage, S.; Hussain, I. Tomato Maturity Recognition with Convolutional Transformers. Sci. Rep. 2023, 13, 22885. [Google Scholar] [CrossRef]
- Liu, J.; Pi, J.; Xia, L. A Novel and High Precision Tomato Maturity Recognition Algorithm Based on Multi-Level Deep Residual Network. Multimed. Tools Appl. 2020, 79, 9403–9417. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, J.; Zhou, X.; Yao, K.; Tang, N. Detection of Soluble Solid Content in Apples Based on Hyperspectral Technology Combined with Deep Learning Algorithm. J. Food Process. Preserv. 2022, 46, e16414. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Zhou, X.; Tang, N.; Shen, J.; Wu, X. Research on Nondestructive Identification of Grape Varieties Based on EEMD-DWT and Hyperspectral Image. J. Food Sci. 2021, 86, 2011–2023. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Tian, Y.; Lu, B.; Hang, Y.; Chen, Q. Hyperspectral Technique Combined with Deep Learning Algorithm for Detection of Compound Heavy Metals in Lettuce. Food Chem. 2020, 321, 126503. [Google Scholar] [CrossRef]
- Yuanyuan, C.; Zhibin, W. Quantitative Analysis Modeling of Infrared Spectroscopy Based on Ensemble Convolutional Neural Networks. Chemom. Intell. Lab. Syst. 2018, 181, 1–10. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, Q.; Su, Z.; Zhang, L.; Chen, Z.; Zhou, G.; Yao, Z.; Xuan, Q.; Cheng, Y. Deep Learning and Hyperspectral Images Based Tomato Soluble Solids Content and Firmness Estimation. Front. Plant Sci. 2022, 13, 860656. [Google Scholar] [CrossRef]
- Yin, W.; He, C.; Wu, Q. A Tomato Quality Identification Method Based on Raman Spectroscopy and Convolutional Neural Network. J. Phys. Conf. Ser. 2020, 1438, 012029. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Hu, T.; He, Q.; Chen, Z.S.; Wang, J.; Liu, L.; Fang, C.; Luo, J.; Fu, L.; et al. Nondestructive 3D Phenotyping Method of Passion Fruit Based on X-Ray Micro-Computed Tomography and Deep Learning. Front. Plant Sci. 2023, 13, 1087904. [Google Scholar] [CrossRef]
- Pessoa, H.P.; Copati, M.G.F.; Azevedo, A.M.; Dariva, F.D.; Almeida, G.Q.D.; Gomes, C.N. Combining Deep Learning and X-Ray Imaging Technology to Assess Tomato Seed Quality. Sci. Agric. 2023, 80, e20220121. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Q.; Xia, J.; Zhao, L.; Du, X.; Li, S.; Chen, Y.; Wang, X. Non-Destructive Fruit Firmness Evaluation Using a Soft Gripper and Vision-Based Tactile Sensing. Comput. Electron. Agric. 2023, 214, 108256. [Google Scholar] [CrossRef]
- Wang, A.; Qian, W.; Li, A.; Xu, Y.; Hu, J.; Xie, Y.; Zhang, L. NVW-YOLOv8s: An Improved YOLOv8s Network for Real-Time Detection and Segmentation of Tomato Fruits at Different Ripeness Stages. Comput. Electron. Agric. 2024, 219, 108833. [Google Scholar] [CrossRef]
- Quach, L.-D.; Quoc, K.N.; Quynh, A.N.; Ngoc, H.T.; Thai-Nghe, N. Tomato Health Monitoring System: Tomato Classification, Detection, and Counting System Based on YOLOv8 Model With Explainable MobileNet Models Using Grad-CAM++. IEEE Access 2024, 12, 9719–9737. [Google Scholar] [CrossRef]
- Chen, W.; Liu, M.; Zhao, C.; Li, X.; Wang, Y. MTD-YOLO: Multi-Task Deep Convolutional Neural Network for Cherry Tomato Fruit Bunch Maturity Detection. Comput. Electron. Agric. 2024, 216, 108533. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Q.; Zhao, Y.; Wan, H.; Wang, R.; Zhang, X.; Cheng, Y. Quantitative Extraction and Evaluation of Tomato Fruit Phenotypes Based on Image Recognition. Front. Plant Sci. 2022, 13, 859290. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Tseng, C.-Y.; Nguyen, D.-M.; Lin, Y.-D. YOLOv4-Driven Appearance Grading Filing Mechanism: Toward a High-Accuracy Tomato Grading Model through a Deep-Learning Framework. Mathematics 2022, 10, 3398. [Google Scholar] [CrossRef]
- Fawzia Rahim, U.; Mineno, H. Highly Accurate Tomato Maturity Recognition: Combining Deep Instance Segmentation, Data Synthesis and Color Analysis. In Proceedings of the 2021 4th Artificial Intelligence and Cloud Computing Conference, Kyoto Japan, 17–19 December 2021; pp. 16–23. [Google Scholar]
- Liu, Y.; Wei, C.; Yoon, S.-C.; Ni, X.; Wang, W.; Liu, Y.; Wang, D.; Wang, X.; Guo, X. Development of Multimodal Fusion Technology for Tomato Maturity Assessment. Sensors 2024, 24, 2467. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, H.; Sun, M.; Zhou, Y.; Zhang, C.; Zhou, H. Geographical Origin Identification of Chinese Tomatoes Using Long-Wave Fourier-Transform Near-Infrared Spectroscopy Combined with Deep Learning Methods. Food Anal. Methods 2023, 16, 664–676. [Google Scholar] [CrossRef]
- Qi, H.; Li, H.; Chen, L.; Chen, F.; Luo, J.; Zhang, C. Hyperspectral Imaging Using a Convolutional Neural Network with Transformer for the Soluble Solid Content and pH Prediction of Cherry Tomatoes. Foods 2024, 13, 251. [Google Scholar] [CrossRef]
- Nasution, A.M.T.; Wicaksono, P.E.; Suyanto, H. Determination of Coffee Bean’s Moisture Content Using Laser-Induced Breakdown Spectroscopy (LIBS). In Proceedings of the Third International Seminar on Photonics, Optics, and Its Applications (ISPhOA 2018), Surabaya, Indonesia, 1–2 August 2018; SPIE: Bellingham, WA, USA, 2019; Volume 11044, pp. 84–87. [Google Scholar]
- Shukla, N.; Bharti, A.S.; Srivastava, S.; Uttam, K.N. Determination of Elements in Carrot Root by Laser Induced Breakdown Spectroscopy. Natl. Acad. Sci. Lett. 2017, 40, 47–51. [Google Scholar] [CrossRef]
- Ranulfi, A.C.; Romano, R.A.; Magalhaes, A.B.; Ferreira, E.J.; Villas-Boas, P.R.; Bastos Pereira Milori, D.M. Evaluation of the Nutritional Changes Caused by Huanglongbing (HLB) to Citrus Plants Using Laser-Induced Breakdown Spectroscopy. Appl. Spectrosc. 2017, 71, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Meng, L.; Yang, L.; Wang, J.; Fu, X.; Du, X.; Li, S.; He, Y.; Huang, L. Feasibility of Laser-Induced Breakdown Spectroscopy and Hyperspectral Imaging for Rapid Detection of Thiophanate-Methyl Residue on Mulberry Fruit. Int. J. Mol. Sci. 2019, 20, 2017. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Liu, M.; Huang, L.; Zhao, J. Rapid Detection of Heavy Metal Contents in Fruits by Laser Induced Breakdown Spectroscopy. In Proceedings of the International Symposium on Computer Science and Computational Technology (ISCSCT 2009), Shanghai, China, 20–22 December 2008; Yu, F., Yue, G., Shu, J., Liu, Y., Eds.; Academy Publisher: Oulu, Finland, 2009; pp. 98–101. [Google Scholar]
- Jiang, W.; Wang, J.; Lin, R.; Chen, R.; Chen, W.; Xie, X.; Hsiung, K.-L.; Chen, H.-Y. Machine Learning-Based Non-Destructive Terahertz Detection of Seed Quality in Peanut. Food Chem. X 2024, 23, 101675. [Google Scholar] [CrossRef]
- Nakajima, S.; Shiraga, K.; Suzuki, T.; Kondo, N.; Ogawa, Y. Quantification of Starch Content in Germinating Mung Bean Seedlings by Terahertz Spectroscopy. Food Chem. 2019, 294, 203–208. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Wang, X.; Wang, H. Research of Pesticide Residues on Fruit by Terahertz Spectroscopy Technology. In Proceedings of the 2011 International Conference on Optical Instruments and Technology: Optoelectronic Measurement Technology and Systems, Beijing, China, 6–9 November 2011; SPIE: Bellingham, WA, USA, 2011; Volume 8201, pp. 535–539. [Google Scholar]
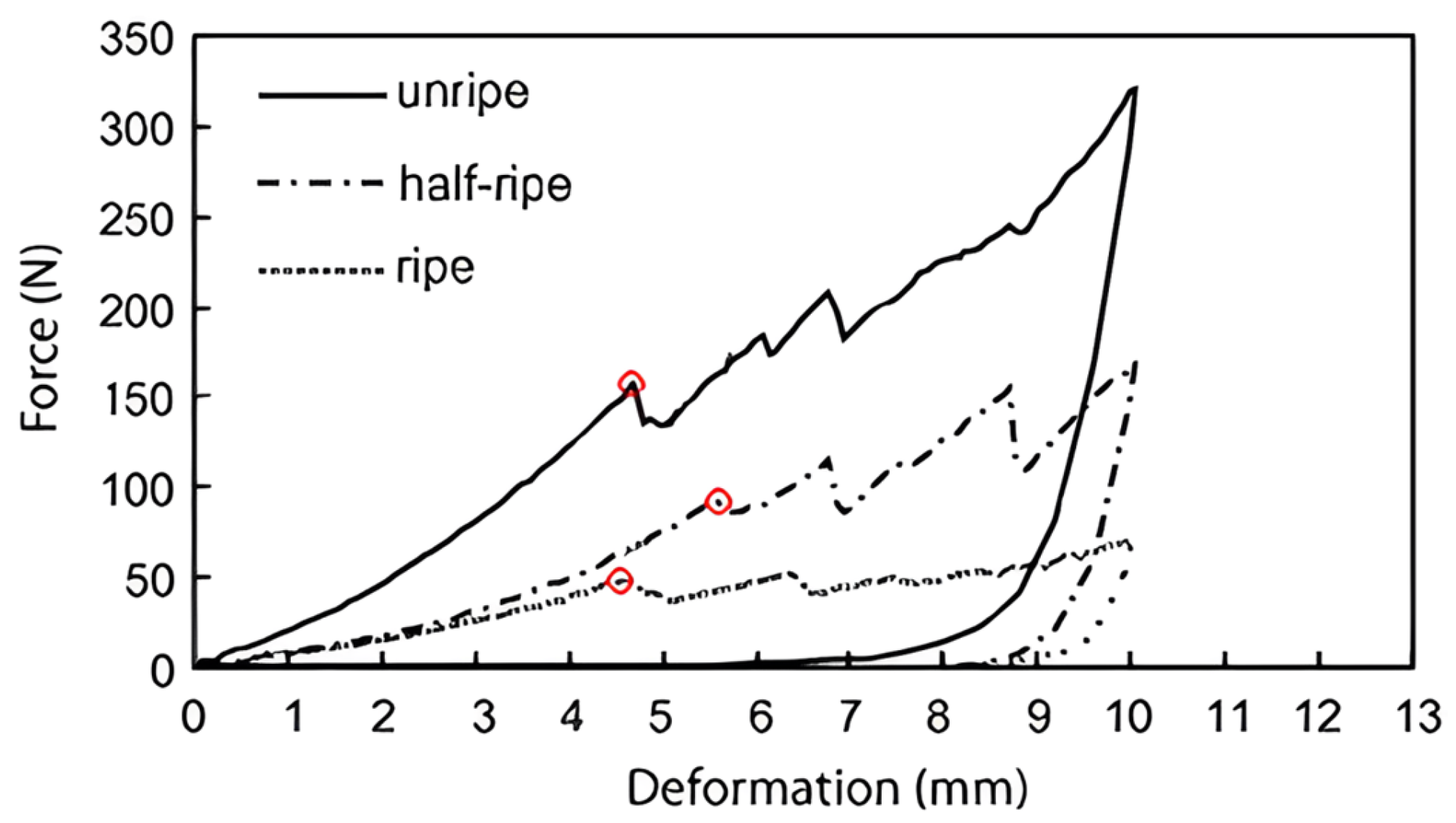
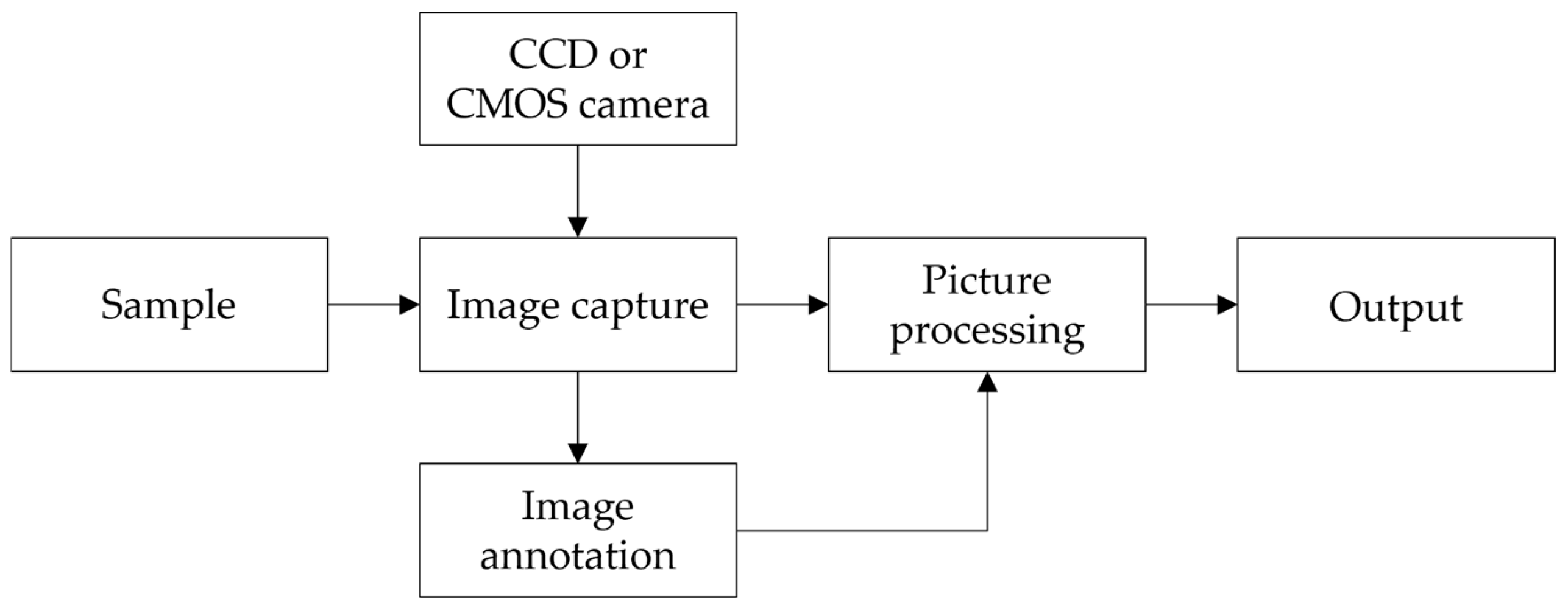
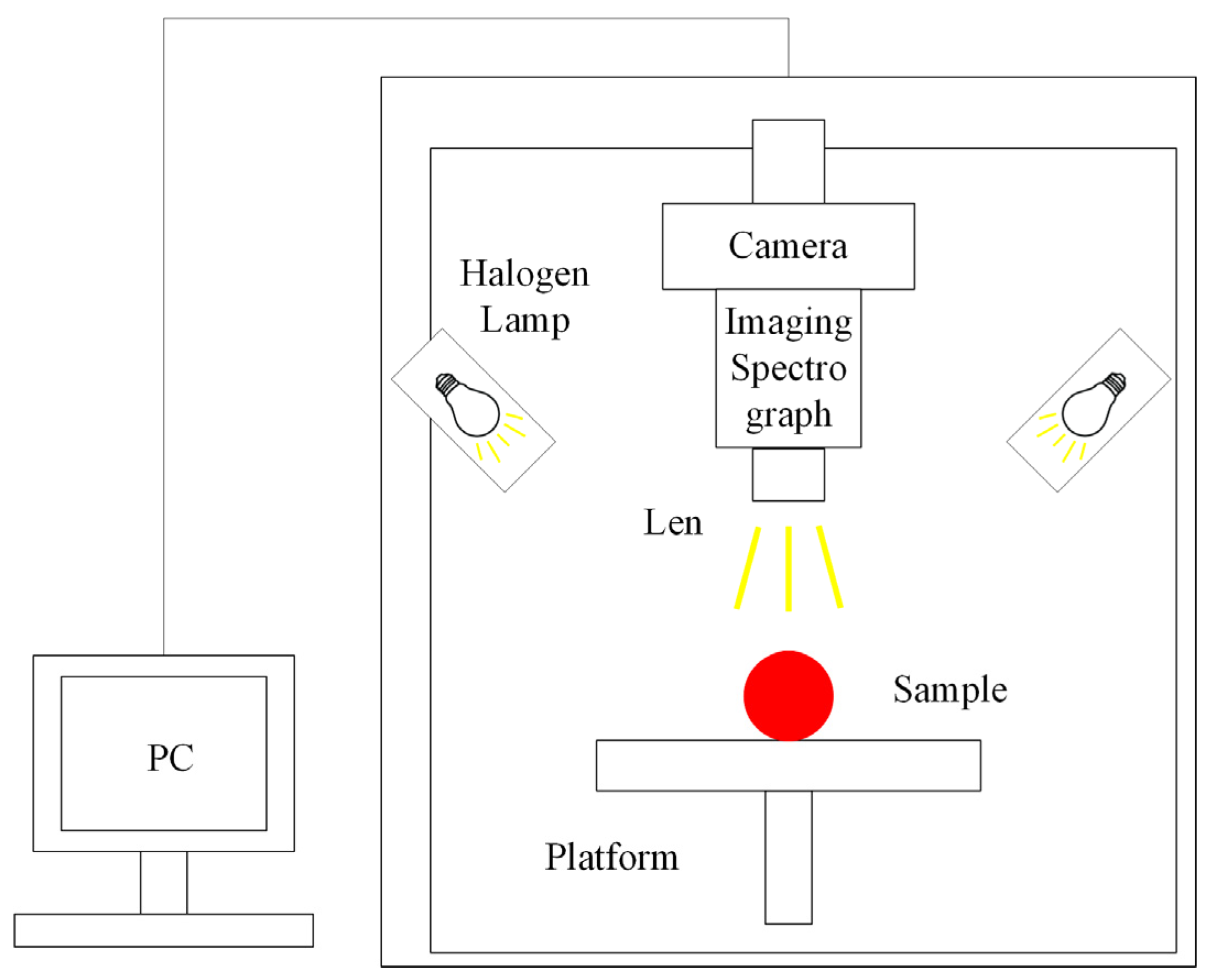
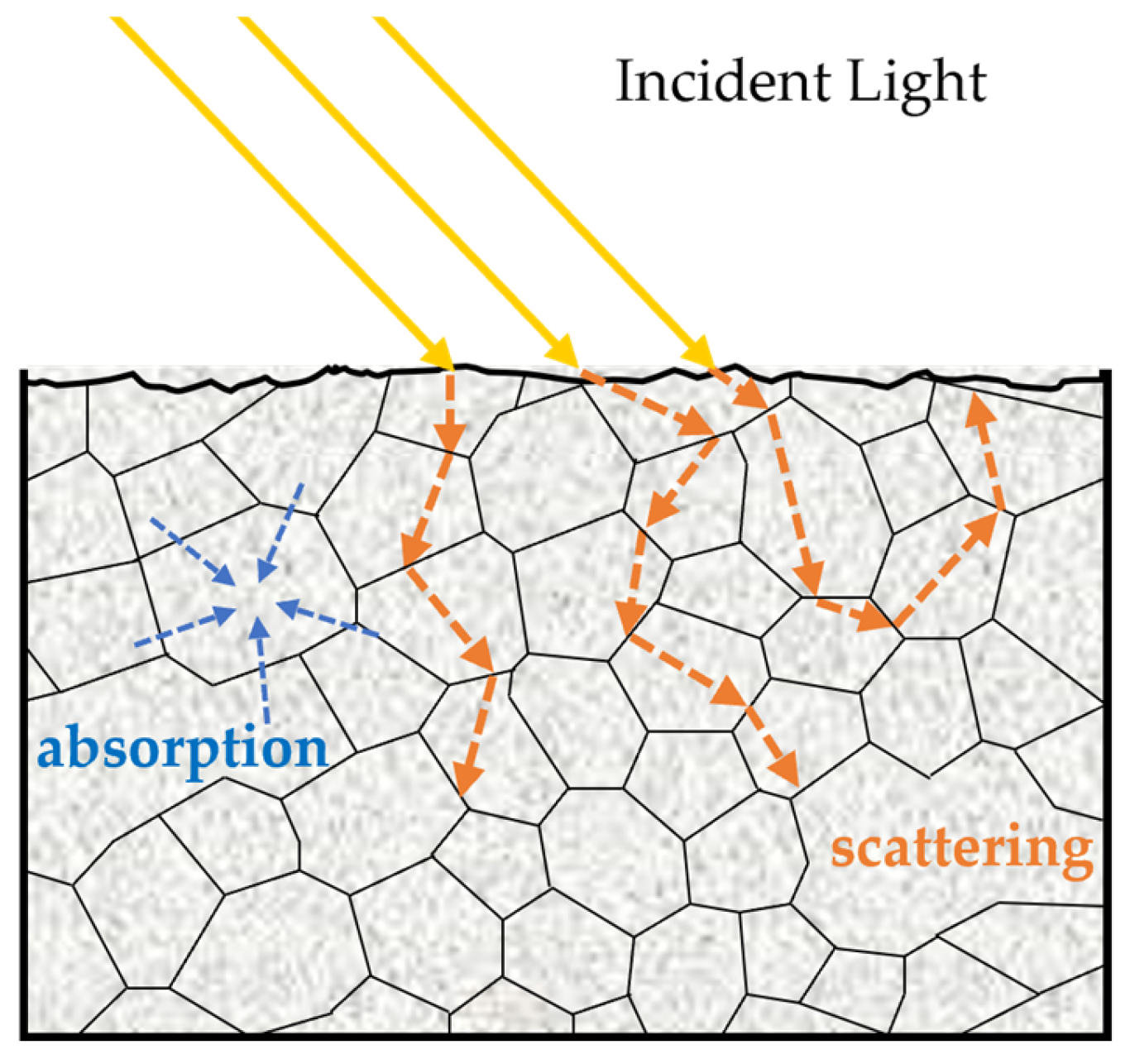
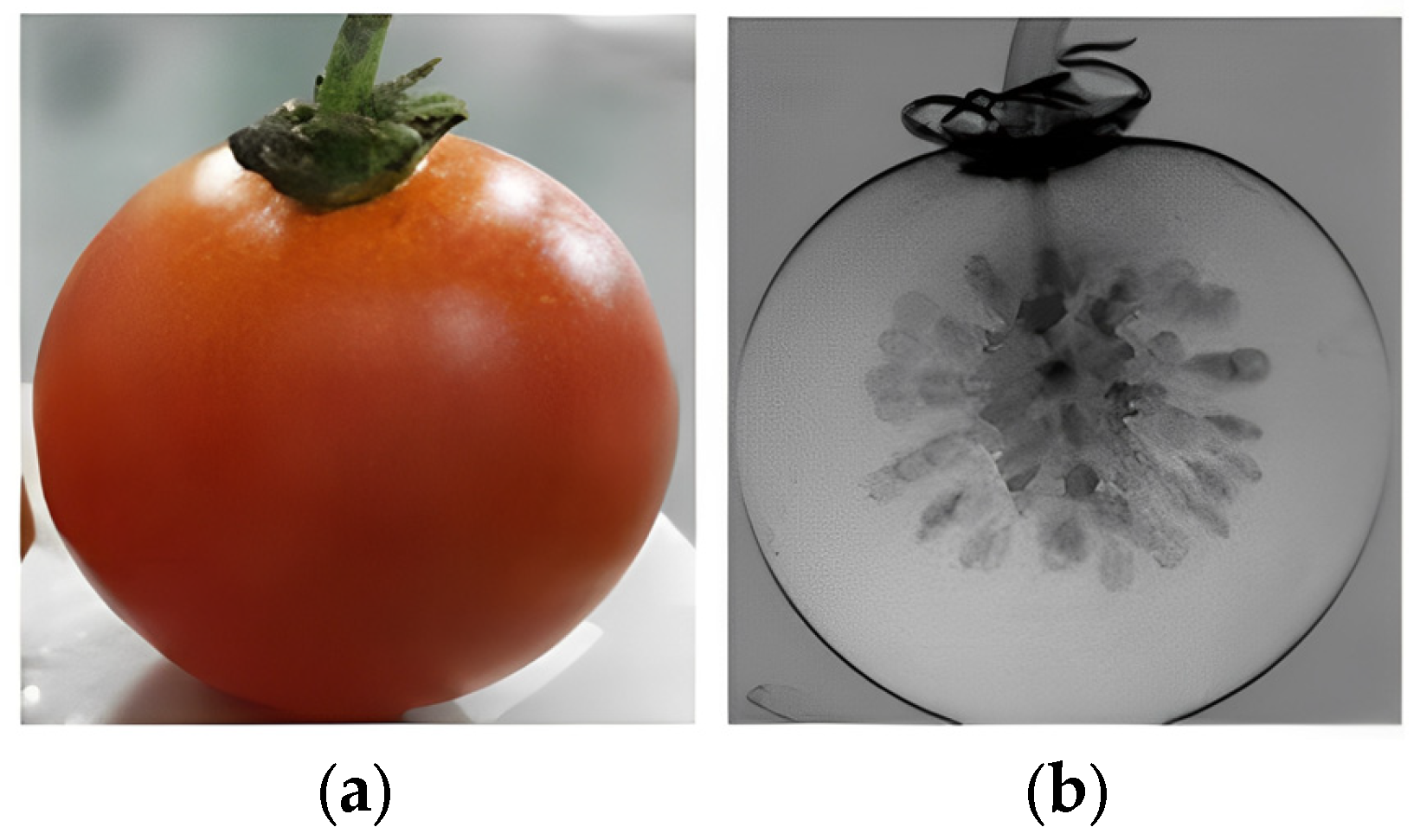
| Technique | Index | Model | RMSEP | Result | Year | References |
|---|---|---|---|---|---|---|
| Mechanical sensor | Firmness | PLS | r2 = 0.912 | 1996 | [48] | |
| Nondestructive impact | maturity | CA | 0.6448 | Acc = 0.823 | 2009 | [49] |
| Acoustic impact | Ripening | Bayesian classifier | Acc = 0.89 | 2008 | [50] | |
| Ultrasonic | Firmness | R2 = 0.916 | 2007 | [51] | ||
| Machine vision | Grading | SVM | Acc = 0.9774 | 2020 | [52] | |
| Weight | Bayesian regularization network | 1.468 | R2 = 0.971 | 2021 | [53] | |
| Volume | 1.2683 | R2 = 0.982 | ||||
| Vis/NIR spectroscopy | TSC | (1000~2500 nm) | 0.4157 | r = 0.9998 | 2005 | [54] |
| SSC | 0.6333 | r = 0.9996 | ||||
| Lycopene | 21.5779 | r = 0.9996 | ||||
| β-carotene | 0.7455 | r = 0.9981 | ||||
| Vis/NIR spectroscopy | Firmness SSC pH | PLS (350~2500 nm) | 1.48 | r = 0.81 | 2007 | [55] |
| 0.16 | r = 0.91 | |||||
| 0.09 | r = 0.85 | |||||
| PCR | 1.43 | r = 0.82 | ||||
| 0.19 | r = 0.86 | |||||
| 0.09 | r = 0.83 | |||||
| Maturity | PLS (1100~1800 nm) | Acc = 0.9685 | 2012 | [56] | ||
| Firmness | r2 = 0.90 | |||||
| Maturity | Bayesian classifier | Acc = 0.85 | 2013 | [57] | ||
| SSC | PLS (400~2500 nm) | 0.65 | r2 = 0.75 | 2015 | [58] | |
| Total acidity | 0.06 | r2 = 0.69 | ||||
| Ripeness | PLS (550~750 nm) | 0.18 | Acc = 0.9067 | 2017 | [59] | |
| SSC | IRIV-CS-SVR (950~1650 nm) | 0.1707 | r2 = 0.9718 | 2018 | [60] | |
| SSC | PLS (902~2094 nm) | 0.14 | r2 = 0.92 | 2019 | [61] | |
| Acids | 0.31 | r2 = 0.88 | ||||
| SSC | EPO (780~2500 nm) | 0.292 | r = 0.8988 | 2019 | [62] | |
| lycopene | 7.45 | r = 0.8023 | ||||
| SSC | PLS (840~1050 nm) | 0.3227 | R2 = 0.6665 | 2021 | [63] | |
| SSC | PLS (500~1400 nm) | 0.316 | R = 0.830 | 2021 | [64] | |
| Spatially resolved spectroscopy (550~1650 nm) | Firmness | PLS | 0.52 | r = 0.948 | 2018 | [65] |
| SSC | PLS | 0.38 | r = 0.809 | 2018 | [66] | |
| pH | 0.11 | r = 0.819 | ||||
| Multispectral imaging (405~970 nm) | Lycopene Total phenolics | PLS | 6.502 | R2 = 0.501 | 2015 | [67] |
| 2.329 | R2 = 0.343 | |||||
| LS-SVM | 2.602 | R2 = 0.910 | ||||
| 0.865 | R2 = 0.921 | |||||
| BPNN | 2.292 | R2 = 0.938 | ||||
| 0.308 | R2 = 0.965 | |||||
| Hyperspectral imaging | Moisture pH SSC | PLS (1000~1550 nm) | 0.63 | r = 0.81 | 2017 | [44] |
| 0.06 | r = 0.69 | |||||
| 0.33 | r = 0.74 | |||||
| Maturity | CSR (400~2500 nm) | Acc = 0.9678 | 2021 | [68] | ||
| Firmness SSC TA | PCR (950~1650 nm) | 0.847 | R2 = 0.736 | 2023 | [69] | |
| 0.279 | R2 = 0.813 | |||||
| 0.079 | R2 = 0.84 | |||||
| PLSR | 0.783 | R2 = 0.785 | ||||
| 0.258 | R2 = 0.864 | |||||
| 0.081 | R2 = 0.817 | |||||
| SVR | 0.647 | R2 = 0.862 | ||||
| 0.234 | R2 = 0.917 | |||||
| 0.077 | R2 = 0.874 | |||||
| BP | 0.709 | R2 = 0.816 | ||||
| 0.193 | R2 = 0.938 | |||||
| 0.069 | R2 = 0.919 | |||||
| Hyperspectral imaging | Maturity | SVC (480~1002 nm) | Acc = 0.9583 | 2023 | [70] | |
| Lycopene | SVR | 0.0166 | R2 = 0.9652 | |||
| PLSR | 7.9826 | R2 = 0.9589 | ||||
| SSC | CNN (400~1000 nm) | 0.4029 | r = 0.7932 | 2024 | [71] | |
| RCNN | 0.4199 | r = 0.7025 | ||||
| GCNN | 0.4125 | r = 0.7651 | ||||
| PCNN | 0.4129 | r = 0.7977 | ||||
| Optical properties (550~1300 nm) | Firmness | PLS | 0.62 | r = 0.923 | 2018 | [45] |
| SSC | 0.50 | r = 0.623 | ||||
| pH | 0.12 | r = 0.769 | ||||
| Raman spectroscopic (3703 nm) | Lycopene | PLS | R2 = 0.91 | 2006 | [72] | |
| Maturity | Acc = 0.938 | 2012 | [73] | |||
| Freshness | PLSR | Acc = 0.856 | 2016 | [74] | ||
| Lycopene | 14.2 | r = 0.57 | ||||
| X-ray | Internal structure | 2016 | [75] | |||
| Time-domain NMR | Maturity SSC | SIMCA | Acc = 0.88 | 2021 | [76] | |
| Acc = 0.87 | ||||||
| PLS-DA | Acc = 0.85 | |||||
| Acc = 0.90 | ||||||
| SVM | Acc = 0.97 | |||||
| Acc = 1.00 | ||||||
| KNN | Acc = 0.94 | |||||
| Acc = 1.00 | ||||||
| Electronic nose | Maturity | PCA | Acc = 0.9579 | 2006 | [77] | |
| LDA | Acc = 1.00 | |||||
| SSC pH | PCR | 0.136 | R2 = 0.877 | 2022 | [78] | |
| 0.184 | R2 = 0.748 | |||||
| PLS | 0.085 | R2 = 0.865 | ||||
| 0.185 | R2 = 0.747 | |||||
| SVR | 0.345 | R2 = 0.958 | ||||
| 0.134 | R2 = 0.877 | |||||
| Ripeness | DCNN | Acc = 0.8220 | 2024 | [79] | ||
| Electronic nose and tongue | Freshness | LVQ | E-nose Acc = 0.86 Acc = 0.968 | 2015 | [80] | |
| Lib-SVM | E-tongue Acc = 0.9688 Acc = 0.9816 |
| Classification | Technique | Advantages | Drawbacks |
|---|---|---|---|
| Mechanical characteristic technology | Mechanical loading | Directly measure the mechanical properties of the fruit | Risk of damaging fruit |
| Fast detection speed | Fruit shape easily affects results | ||
| Little influence by external factors | |||
| Impact | Simplicity of operator | Reflects the local firmness | |
| Relatively cheap | |||
| Acoustic | Reflects the overall firmness Suitable for samples with irregular shape or uneven size | Reduces detection accuracy by ambient noise or vibration during signal acquisition | |
| Ultrasonic | High sensitivity to the change in tissue density, lacuna, and water content in fruit | Needs calibration for varying acoustic characteristics of different fruit | |
| Electromagnetic technology | Vis/NIR spectroscopy | Good detection effect on chemical components and physical properties | Point detection Lack of comprehensive spatial information |
| No sample pretreatment | |||
| Optical properties | Reflects the scattering and absorption characteristics in fruit | Generates errors due to small values of the optical absorption and scattering coefficients | |
| Electromagnetic technology | Spatially resolved spectroscopy | Detects the internal and external quality characteristics simultaneously | Different kinds of samples require different mathematical models |
| Hyperspectral imaging | Provides continuous spectral information to detect a variety of components; | Expensive equipment | |
| Captures spectral and spatial information to analyze the internal and surface quality simultaneously; | Large amount of data and complicated data analysis | ||
| Without sample pretreatment | Slow acquisition speed for data | ||
| Raman spectroscopic | High sensitivity to chemical composition and can detect the molecular characteristics of trace substances; | Slow detection speed High cost of the equipment Requires high power laser and high sensitivity detector because of weak Raman scattering signals | |
| Enables distinguishing similar chemical compositions | |||
| X-ray | Provides high-resolution images for detailed quality analysis | Requires expensive instrument | |
| Visualizes internal defects and structural anomalies | Necessitates strict safety measures to protect operators from radiation exposure | ||
| less suitable for assessing chemical compositions | |||
| Time-domain NMR | Provides accurate data on the content and distribution of components such as water and protein | Slow detection speed | |
| Qualitative analysis of various inorganic substances and organic compounds | The cost of the equipment is expensive | ||
| Electrochemical sensor technology | Electronic nose and tongue | High sensitivity | Low accuracy by the external environment influence |
| Relatively cheap | Only suitable for liquid samples using the electronic tongue | ||
| Fast detection speed | High sensor requirements |
| Technique | Index | DL Model | RMSEP | Results | Reference |
|---|---|---|---|---|---|
| Vision-based tactile sensing | Firmness | CNN–LSTM | Acc = 0.846 | [168] | |
| Ripeness | 1.839 | R2 = 0.795 | |||
| Machine vision | Ripeness | NVW-YOLOv8s | Acc = 0.914 | [169] | |
| Quality grades | MobileNetV3 | Acc = 0.9669 | [170] | ||
| Ripeness | MTD-YOLOv7 | Acc = 0.866 | [171] | ||
| Phenotype | R-CNN | Acc = 0.95 | [172] | ||
| Maturity | DNN | Acc = 0.93 | [157] | ||
| Appearance grade | YOLOv4 | Acc = 0.999 | [173] | ||
| Maturity | R-CNN | Acc = 0.921 | [174] | ||
| Maturity | DenseNet | Acc = 0.9126 | [159] | ||
| Quality grades | DSSAEs | Acc = 0.955 | [39] | ||
| Vis/NIR spectroscopy | Ripeness | FCNN (350–1100 nm) | Acc = 0.993 | [175] | |
| Geographical Origin | SAE-SSA-SVM (1000–2500 nm) | Acc = 0.956 | [176] | ||
| Hyperspectral imaging | SSC | CNN-Transformer (900–1700 nm) | 0.56 | R2 = 0.84 | [177] |
| pH | 0.12 | R2 = 0.6 | |||
| Firmness | 0.94 | R2 = 0.92 | [100] | ||
| SSC | RNN | 0.19 | R2 = 0.88 | ||
| Lycopene | (980–1660 nm) | 0.73 | R2 = 0.94 | ||
| TA | 0.03 | R2 = 0.87 | |||
| SSC | Con1dResNet | 0.018 | R2 = 0.901 | [164] | |
| Firmness | (400–1000 nm) | R2 = 0.532 | |||
| Raman spectroscopy | Quality | CNN | R2 = 0.946 | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Z.; Bian, Z.; Jin, H.; Zheng, G.; Hu, D.; Sun, Y.; Fan, C.; Xie, W.; Fang, H. Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes. Foods 2025, 14, 286. https://doi.org/10.3390/foods14020286
Huang Y, Li Z, Bian Z, Jin H, Zheng G, Hu D, Sun Y, Fan C, Xie W, Fang H. Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes. Foods. 2025; 14(2):286. https://doi.org/10.3390/foods14020286
Chicago/Turabian StyleHuang, Yuping, Ziang Li, Zhouchen Bian, Haojun Jin, Guoqing Zheng, Dong Hu, Ye Sun, Chenlong Fan, Weijun Xie, and Huimin Fang. 2025. "Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes" Foods 14, no. 2: 286. https://doi.org/10.3390/foods14020286
APA StyleHuang, Y., Li, Z., Bian, Z., Jin, H., Zheng, G., Hu, D., Sun, Y., Fan, C., Xie, W., & Fang, H. (2025). Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes. Foods, 14(2), 286. https://doi.org/10.3390/foods14020286







