Construction and In Vitro Evaluation of Brain-Targeted Lutein Liposomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Materials
2.2. Preparation of Lactoferrin-Conjugated PEGylated Liposomes for Lutein Loading (Lf-LLips)
2.3. Determination of Lutein Content
2.4. Determination of Lactoferrin Content
2.5. Determination of Particle Size, Zeta Potential, and the Polydispersity Index
2.6. Determination of Lutein Encapsulation Efficiency
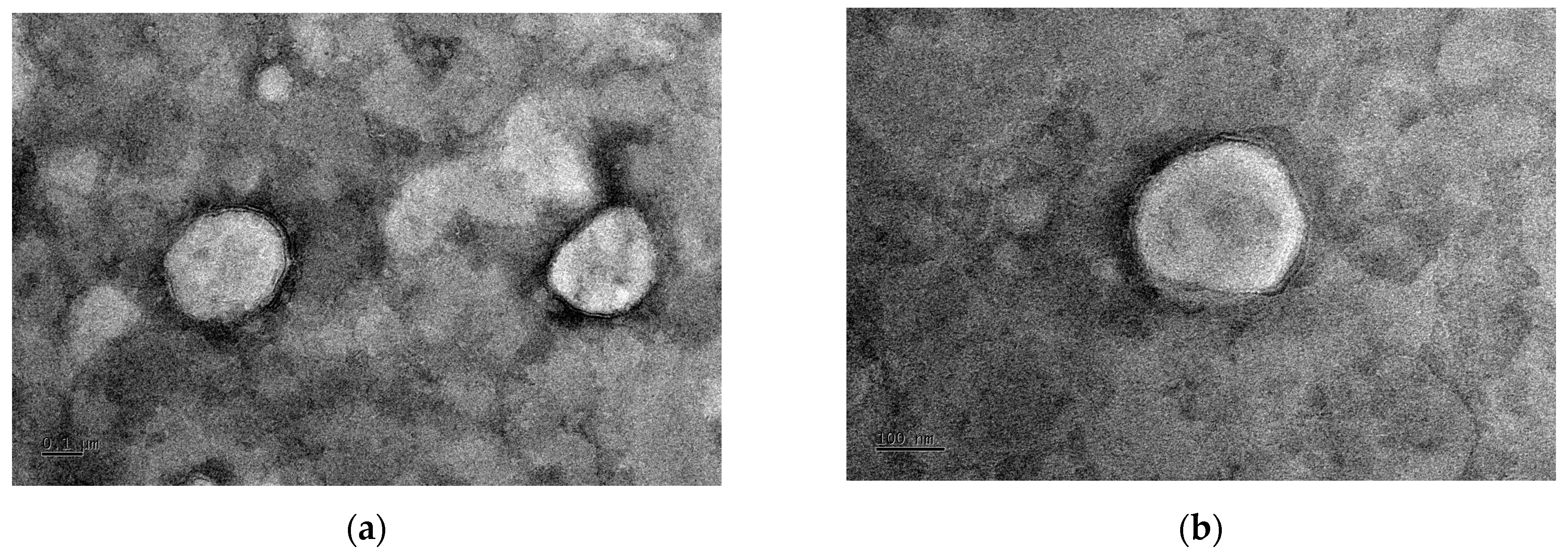
2.7. Transmission Electron Microscopy (TEM) Analysis
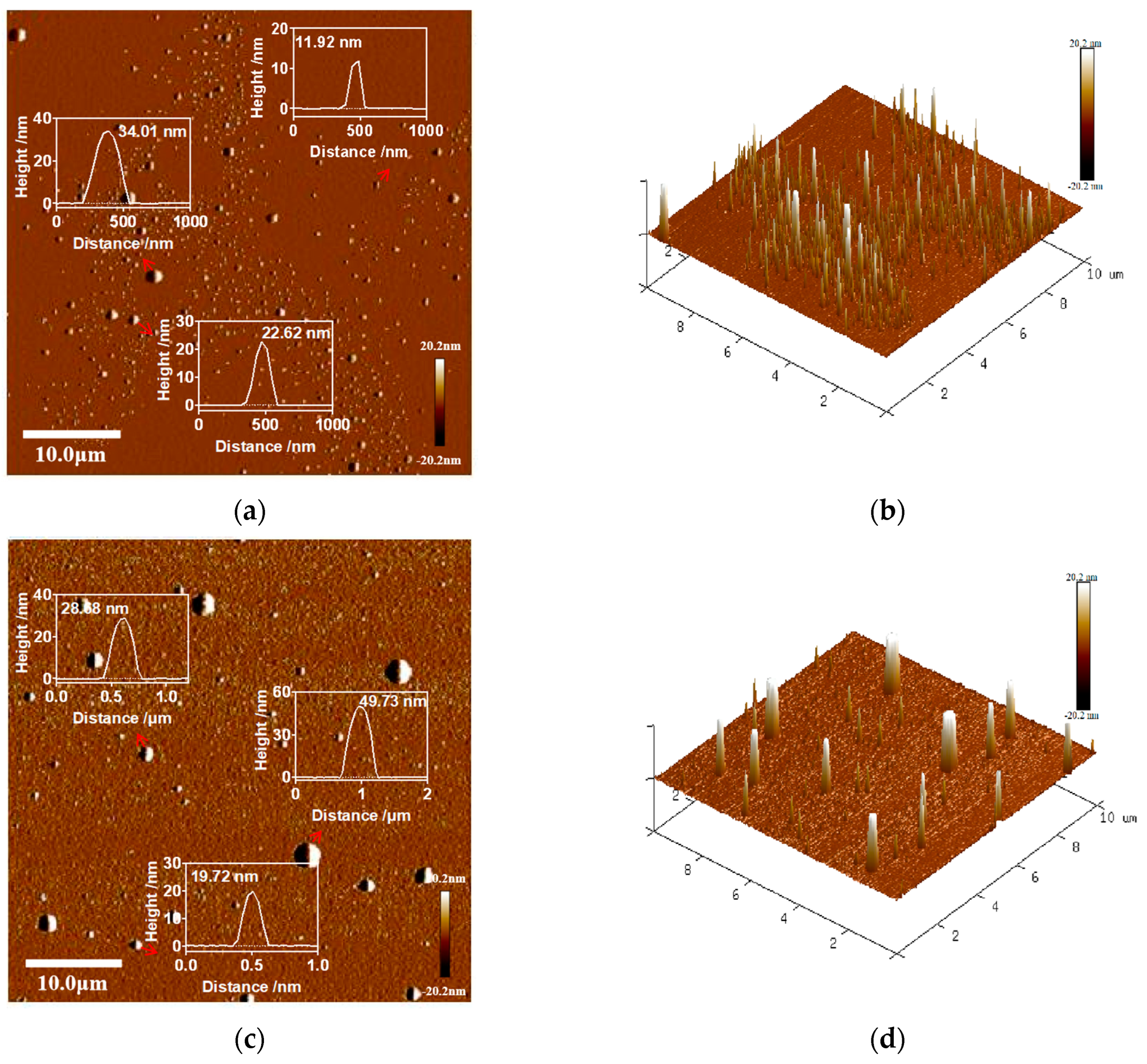
2.8. Atomic Force Microscopy (AFM) Analysis
2.9. Stability
2.10. Release Characteristics In Vitro
2.11. Brain Targeting In Vitro
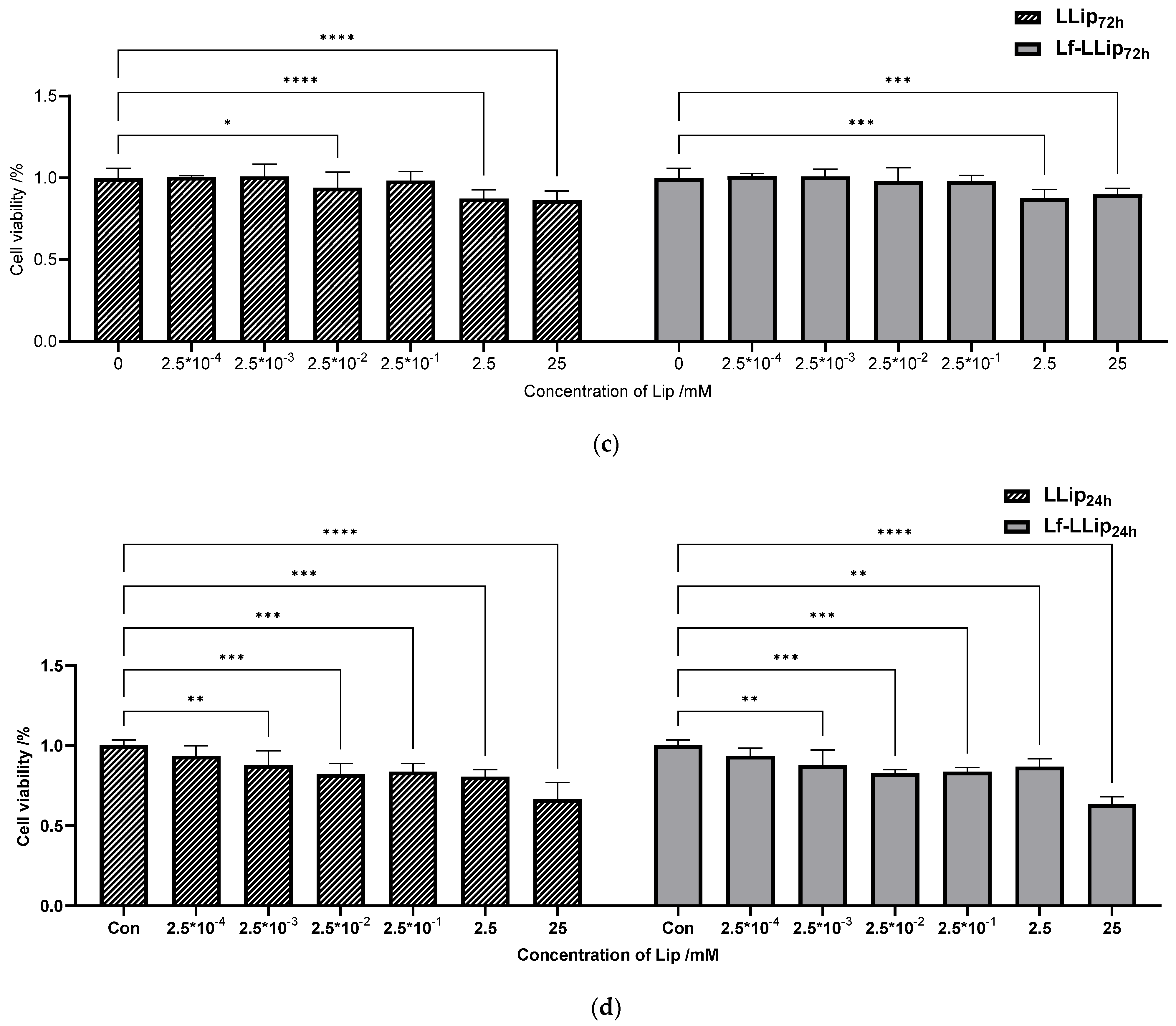
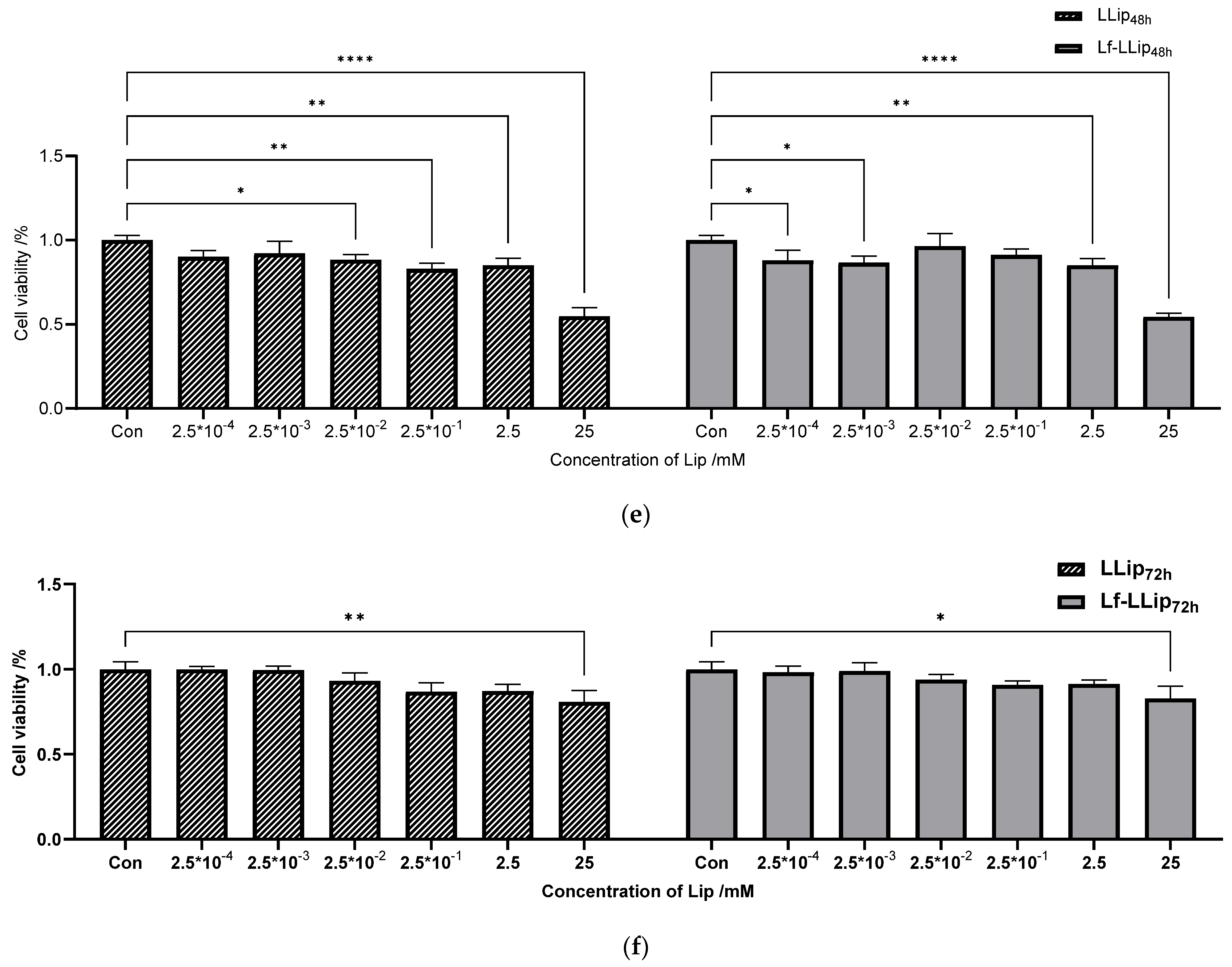
2.11.1. In Vitro Cytotoxicity
2.11.2. Establishment of the BBB Co-Culture Model
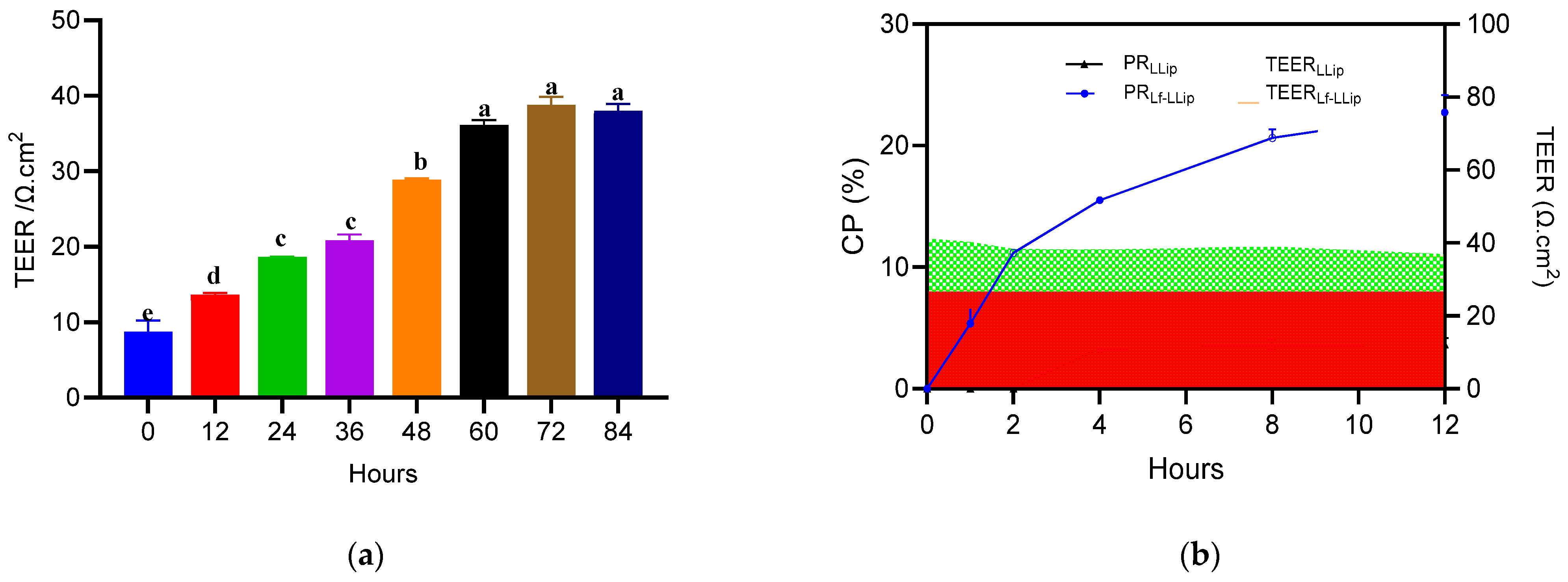
2.11.3. Permeability
2.12. Statistical Analysis
3. Results and Discussion
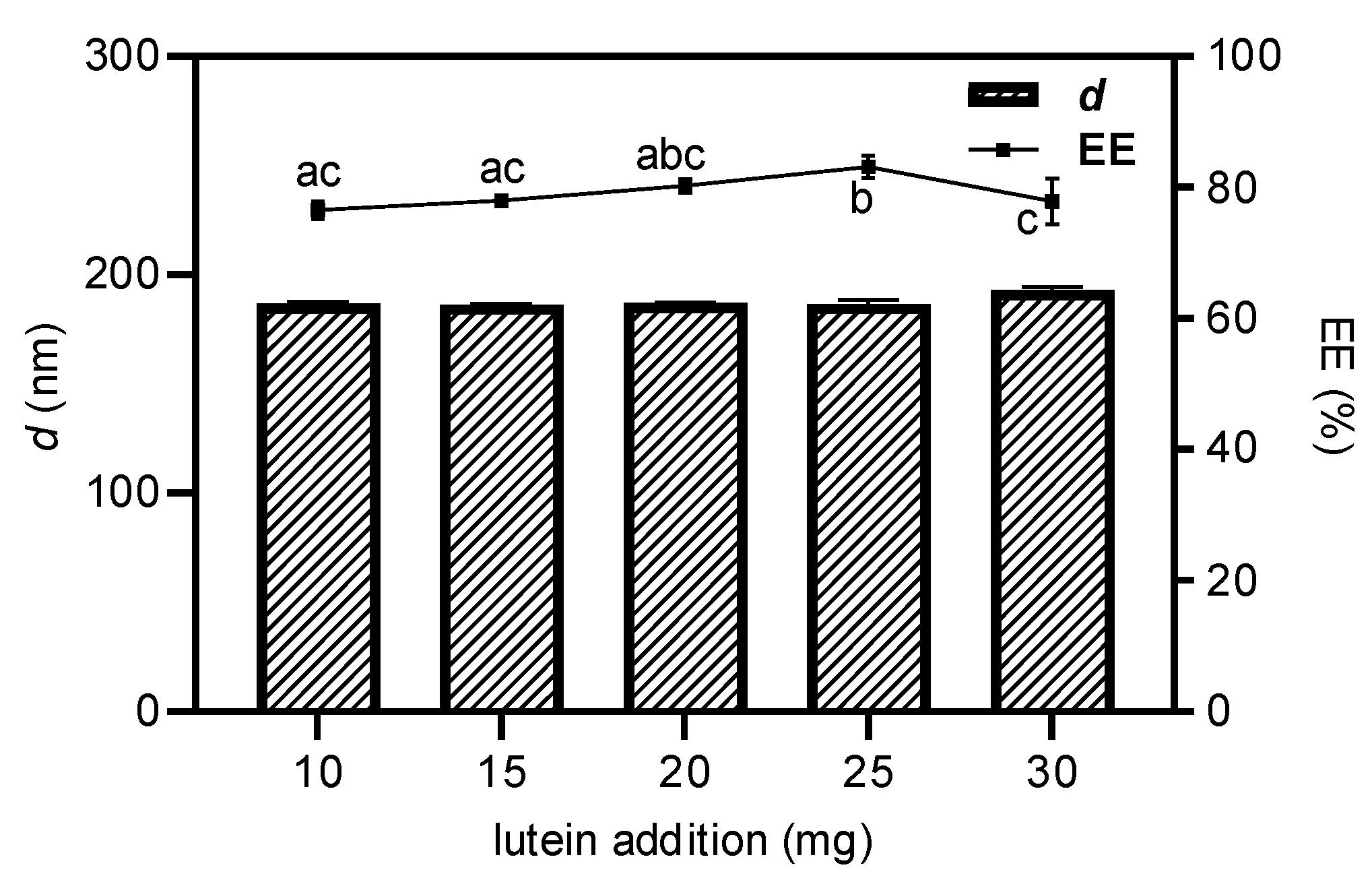
3.1. Optimization of Lactoferrin-Conjugated PEGylated Liposomes for Lutein Loading (Lf-LLips) Preparation Process and Characterization
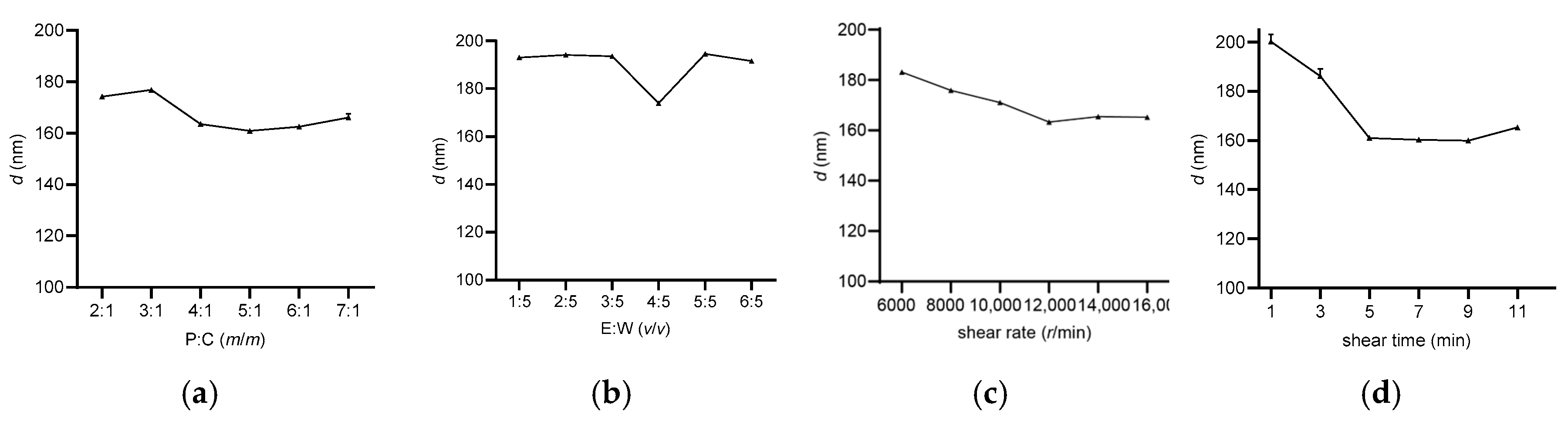
3.1.1. Optimization of Lf-BLips Preparation Conditions
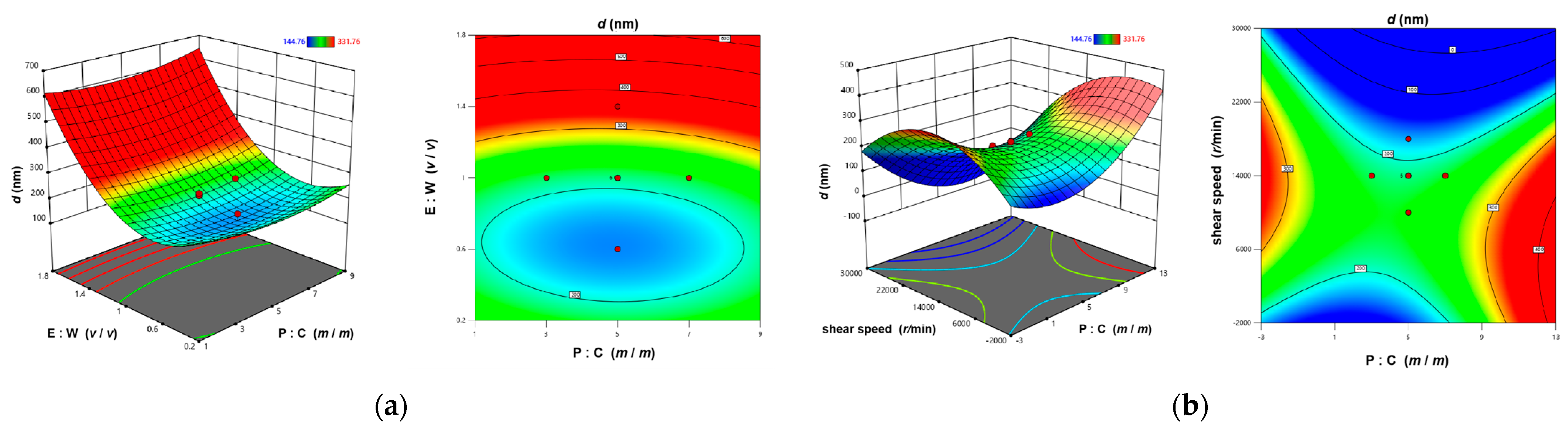
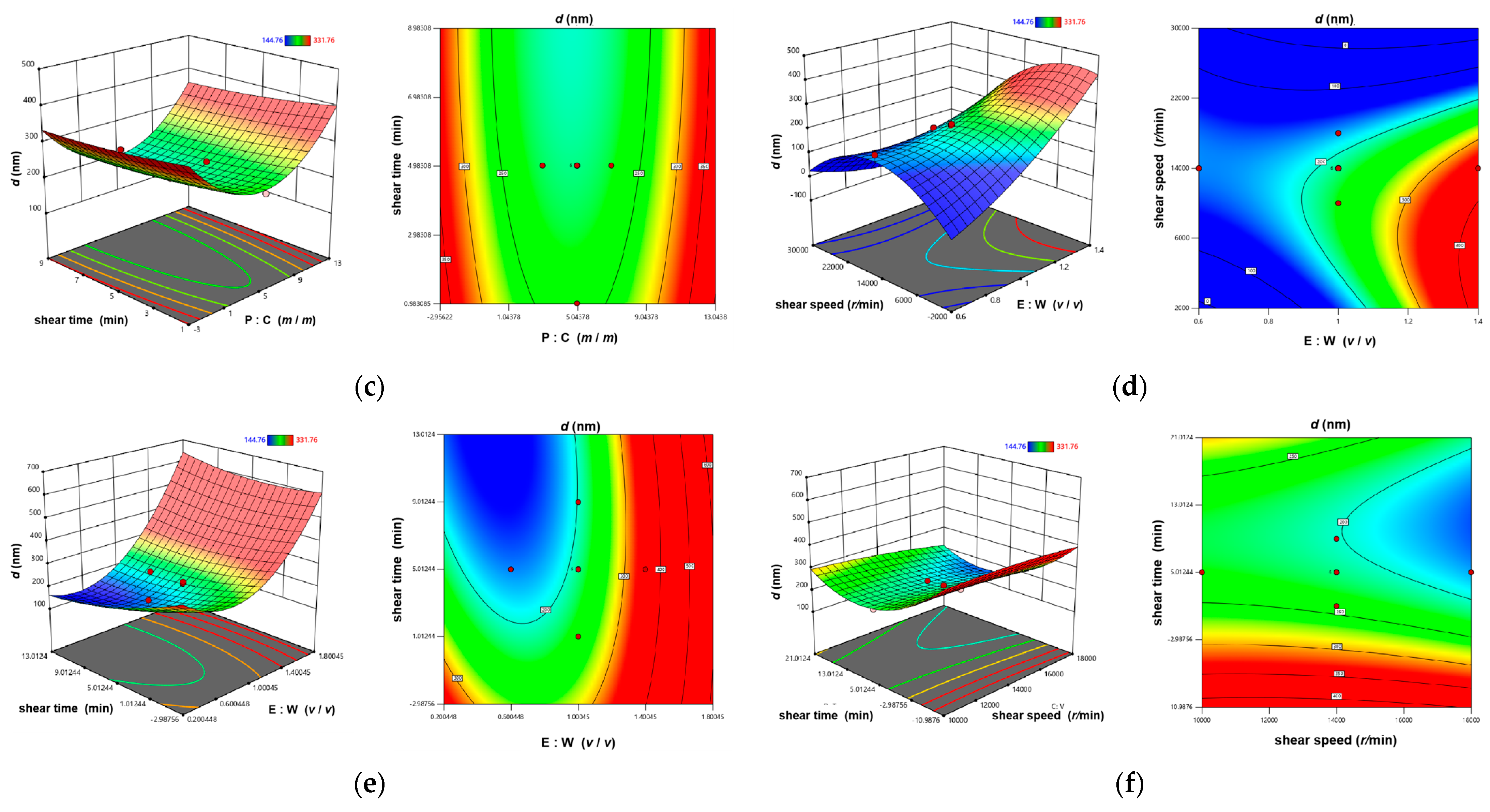
3.1.2. The CCD-RSM Analysis
3.2. Morphological Characterization of the Lutein Delivery System
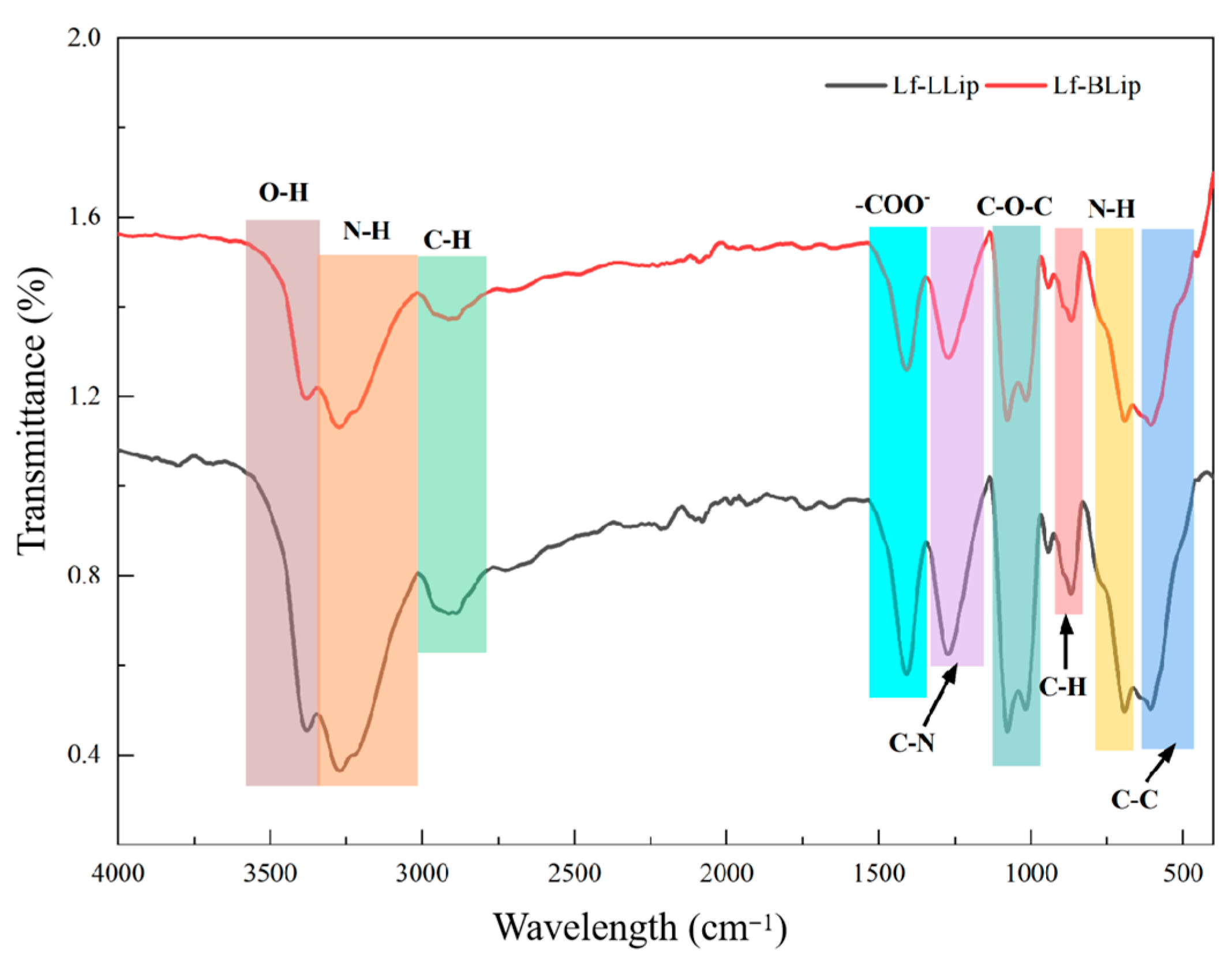
3.3. FTIR Analysis Results of the Lutein Delivery System
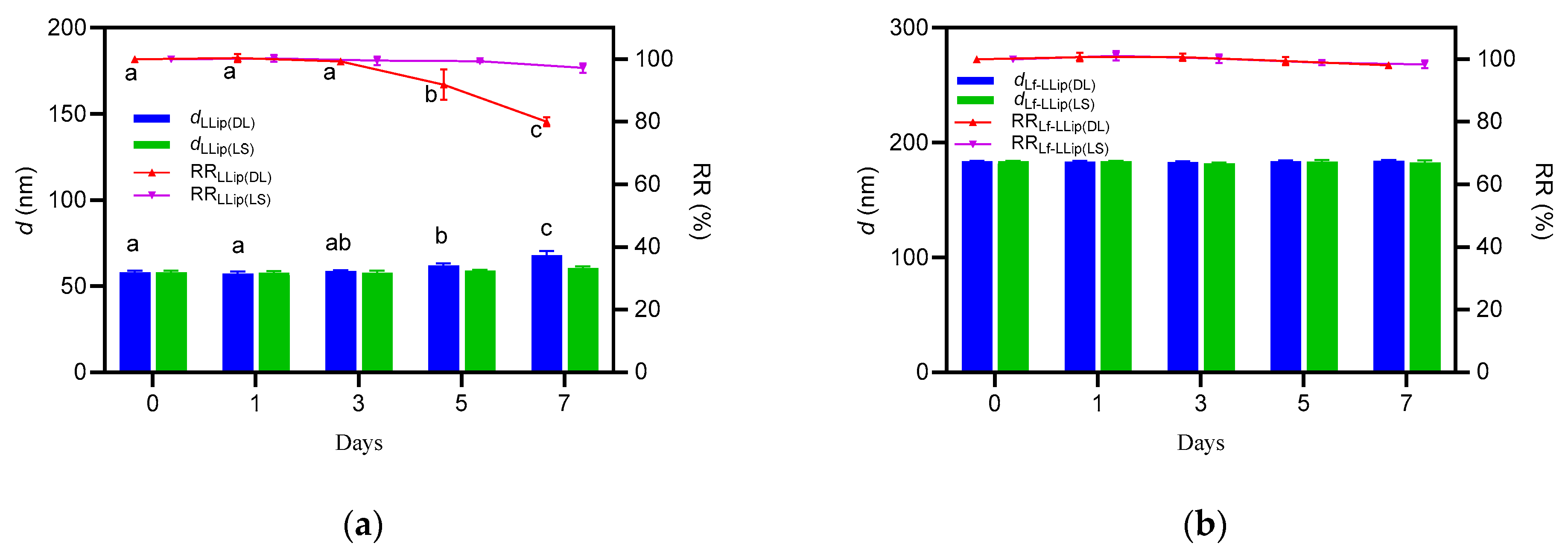
3.4. Stability of Lactoferrin-Modified Lutein Liposomes (Lf-LLips)
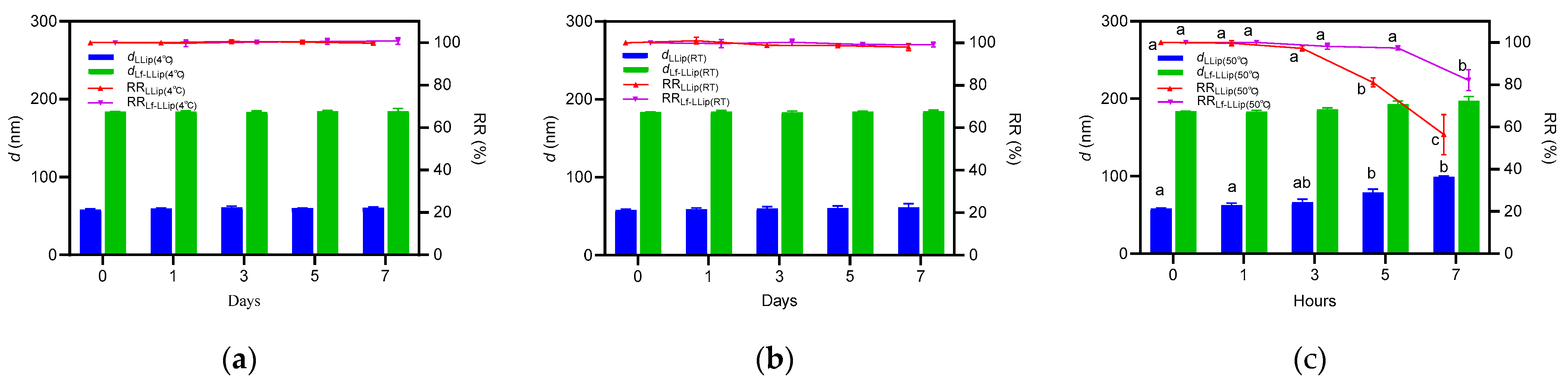
3.4.1. Effect of Light on the Stability of Lf-LLips
3.4.2. Effect of Temperature on the Stability of Lf-LLip
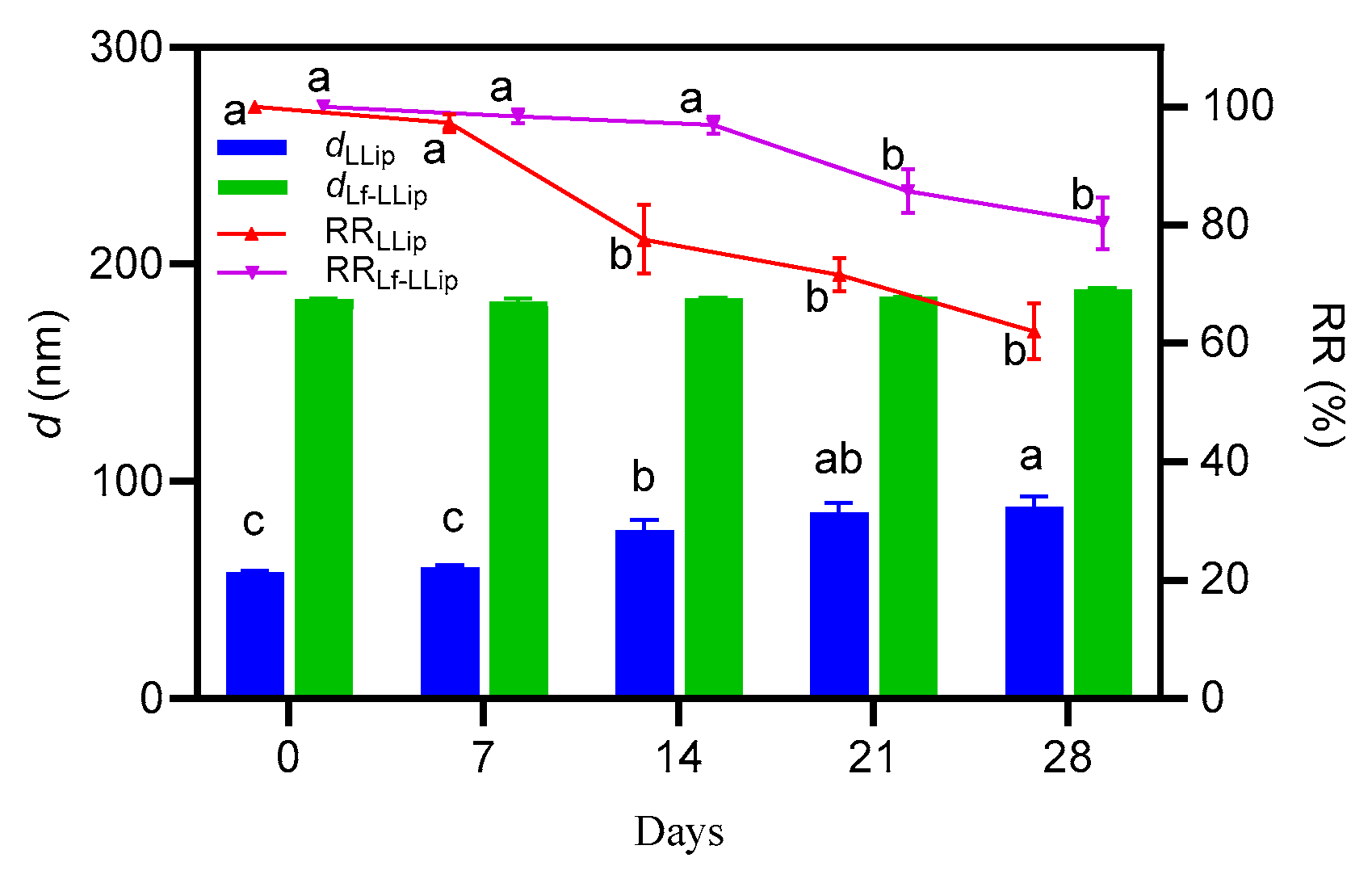
3.4.3. Effect of Storage Duration on the Stability of Lf-LLips
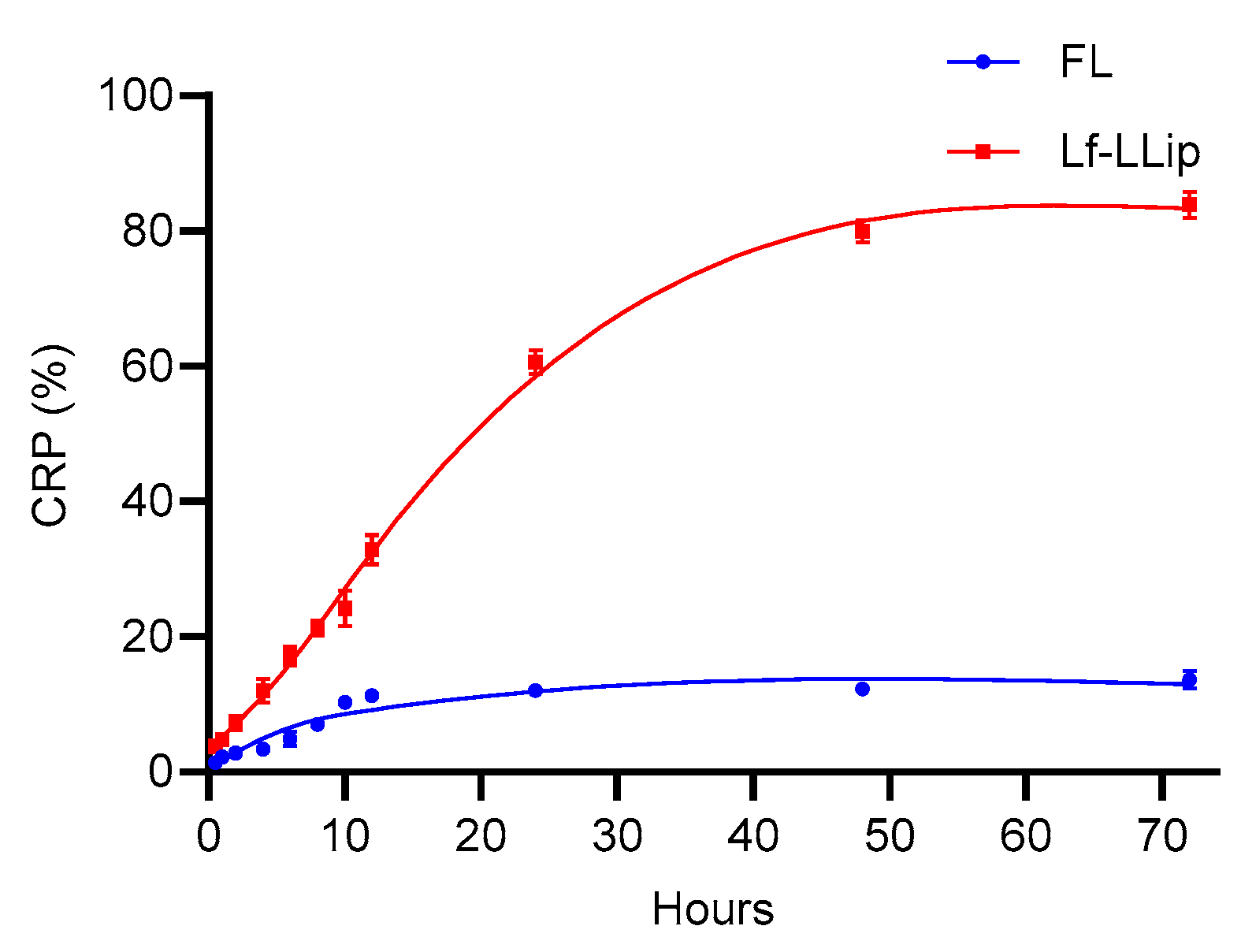
3.5. In Vitro Release Characteristics of Lf-LLips
3.6. Brain-Targeting Evaluation of Lf-LLips
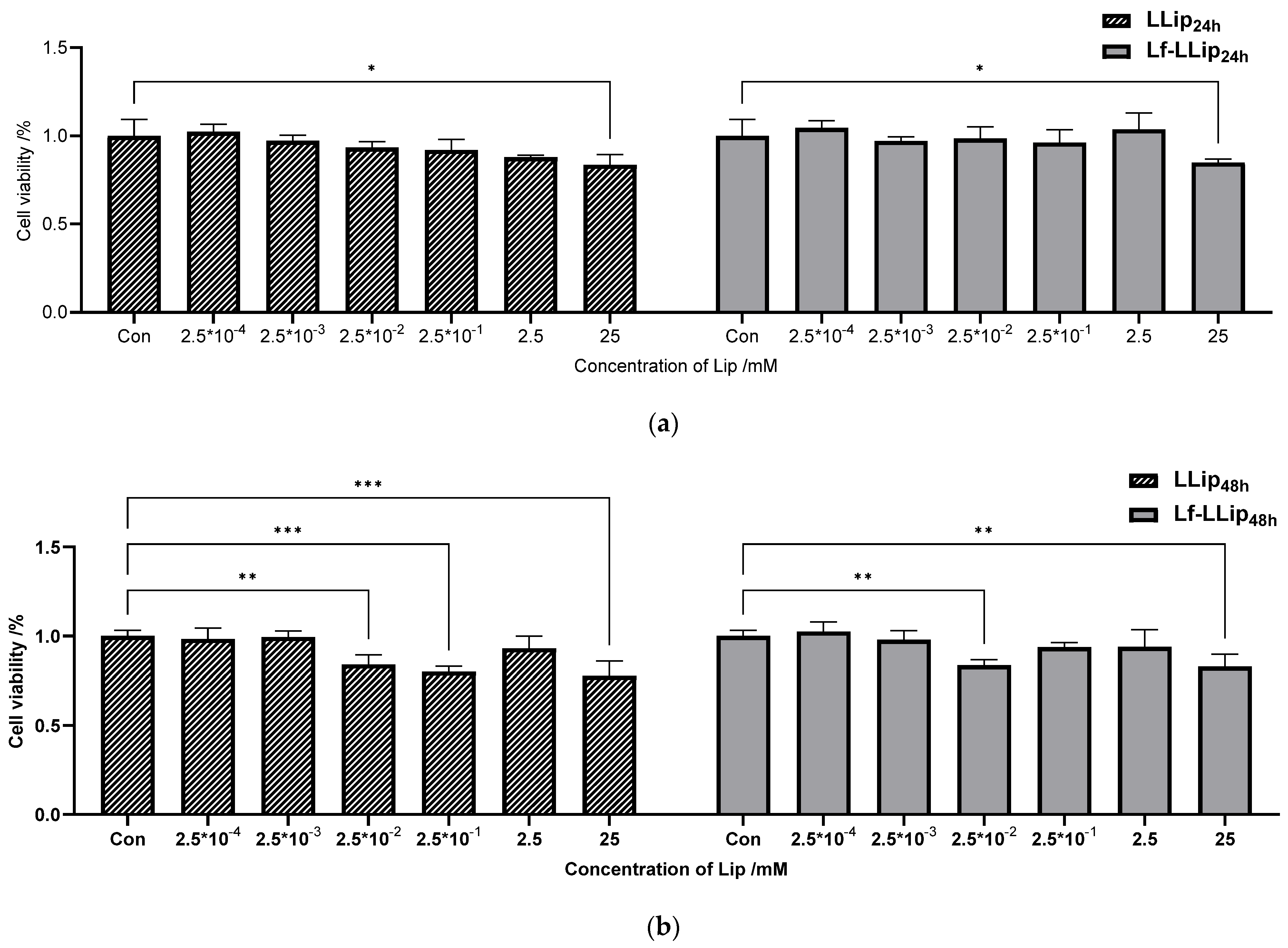
3.6.1. Cell Cytotoxicity
3.6.2. The Permeability Across the BBB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, S.; Lu, W.; Zhang, J.; Zhang, L.; Tao, F.; Zhang, Y.; Hu, X.; Liu, Q. The mechanisms, hallmarks, and therapies for brain aging and age-related dementia. Sci. Bull. 2024, 69, 3756–3776. [Google Scholar] [CrossRef] [PubMed]
- Sedlacik, J.; Boelmans, K.; Löbel, U.; Holst, B.; Siemonsen, S.; Fiehler, J. Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T—ScienceDirect. NeuroImage 2014, 84, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Chappus-McCendie, H.; Chevalier, L.; Roberge, C.; Plourde, M. Omega-3 PUFA metabolism and brain modifications during aging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109662. [Google Scholar] [CrossRef]
- Morley, J.E. Nutrition and the Brain. Clin. Geriatr. Med. 2010, 26, 89. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimer’s Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef]
- Nolan, J.M.; Power, R.; Howard, A.N.; Bergin, P.; Roche, W.; Prado-Cabrero, A.; Pope, G.; Cooke, J.; Power, T.; Mulcahy, R. Supplementation With Carotenoids, Omega-3 Fatty Acids, and Vitamin E Has a Positive Effect on the Symptoms and Progression of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 90, 233–249. [Google Scholar] [CrossRef]
- Ong, J.-S.; Lew, L.-C.; Hor, Y.-Y.; Liong, M.-T. Probiotics: The Next Dietary Strategy against Brain Aging. Prev. Nutr. Food Sci. 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Pan, Y.; Wallace, T.C.; Karosas, T.; Bennett, D.A.; Agarwal, P.; Chung, M. Association of Egg Intake With Alzheimer’s Dementia Risk in Older Adults: The Rush Memory and Aging Project. J. Nutr. 2024, 154, 2236–2243. [Google Scholar] [CrossRef]
- Dohgu, S.; Takata, F.; Yamauchi, A.; Nakagawa, S.; Egawa, T.; Naito, M.; Tsuruo, T.; Sawada, Y.; Niwa, M.; Kataoka, Y. Brain pericytes contribute to the induction and up-regulation of blood–brain barrier functions through transforming growth factor-β production. Brain Res. 2005, 1038, 208–215. [Google Scholar] [CrossRef]
- Cheng, S.; Gao, W.; Xu, X.; Fan, H.; Wu, Y. Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res. Bull. 2016, 127, 226–233. [Google Scholar] [CrossRef]
- Masotti, A.; Mossa, G.; Cametti, C.; Ortaggi, G.; Bianco, A.; Grosso, N.D.; Malizia, D.; Esposito, C. Comparison of different commercially available cationic liposome-DNA lipoplexes: Parameters influencing toxicity and transfection efficiency. Colloids Surf. B Biointerfaces 2009, 68, 136–144. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Johnson, E.J.; Vishwanathan, R.; Johnson, M.A.; Hausman, D.B.; Davey, A.; Scott, T.M.; Green, R.C.; Miller, L.S.; Gearing, M.; Woodard, J. Relationship between Serum and Brain Carotenoids, α-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. J. Aging Res. 2013, 2013, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Aziziha, H.; Hassanpour, S.; Zendehdel, M. Lutein Exerts Antioxidant and Neuroprotective Role on Schizophrenia-Like Behaviours in Mice. Int. J. Dev. Neurosci. 2025, 85, e10407. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Lee, C.M.; Lee, M.K. Lutein Modulates Th2 Immune Response in Ovalbumin-Induced Airway Inflammation. J. Life Sci. 2012, 22, 298–305. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Jagadeesh, K.D.; Hegde, M.L.; Rao, K.S. Carotenoids as Novel Therapeutic Molecules Against Neurodegenerative Disorders: Chemistry and Molecular Docking Analysis. Int. J. Mol. Sci. 2019, 20, 5553. [Google Scholar] [CrossRef]
- Matsui, M.; Murata, T.; Kurobe-Takashima, Y.; Ikeda, T.; Noguchi-Shinohara, M.; Ono, K.; Shidara, H.; Otsuka, K.; Kuriki, D.; Suzuki, M. Lutein from Chicken Eggs Prevents Amyloid β-Peptide Aggregation In Vitro and Amyloid β-Induced Inflammation in Human Macrophages (THP-1). ACS Omega 2024, 9, 12. [Google Scholar] [CrossRef]
- Liu, T.; Liu, W.H.; Zhao, J.S.; Meng, F.Z.; Wang, H. Lutein protects against β-amyloid peptide-induced oxidative stress in cerebrovascular endothelial cells through modulation of Nrf-2 and NF-κb. Cell Biol. Toxicol. 2017, 33, 57–67. [Google Scholar] [CrossRef]
- Fatani, A.J.; Parmar, M.Y.; Abuohashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S. Protective effect of lutein supplementation on oxidative stress and inflammatory progression in cerebral cortex of streptozotocin-induced diabetes in rats. Neurochem. J. 2016, 10, 69–76. [Google Scholar] [CrossRef]
- Lieblein-Boff, J.C.; Johnson, E.J.; Kennedy, A.D.; Chron-Si, L.; Kuchan, M.J.; Xuchu, W. Exploratory Metabolomic Analyses Reveal Compounds Correlated with Lutein Concentration in Frontal Cortex, Hippocampus, and Occipital Cortex of Human Infant Brain. PLoS ONE 2015, 10, e0136904. [Google Scholar] [CrossRef]
- Borel, P. Factors Affecting Intestinal Absorption of Highly Lipophilic Food Microconstituents (Fat-Soluble Vitamins, Carotenoids and Phytosterols). Clin. Chem. Lab. Med. 2003, 41, 979–994. [Google Scholar] [CrossRef]
- Shanmugam, S.; Park, J.H.; Kim, K.S.; Piao, Z.Z.; Yong, C.S.; Choi, H.G.; Woo, J.S. Enhanced bioavailability and retinal accumulation of lutein from self-emulsifying phospholipid suspension (SEPS). Int. J. Pharm. 2011, 412, 99–105. [Google Scholar] [CrossRef]
- Gandhi, S.; Roy, I. Lipid-Based Inhalable Micro- and Nanocarriers of Active Agents for Treating Non-Small-Cell Lung Cancer. Pharmaceutics 2023, 15, 1457. [Google Scholar] [CrossRef]
- Yeo, Y.; Tamam, H. Preparing Liposomes with High Drug Loading Capacity and the Use Thereof. Available online: https://patents.google.com/patent/WO2020150412A1/en (accessed on 23 July 2020).
- Yuan, D.; Wei, C.; Dan, S.; Gui-Ling, W.; Yu, H.; Shuai, M.; Jianhua, C.; Ying, X.; Zhiqiang, W. In vivo study of doxorubicin-loaded cell-penetrating peptide-modified pH-sensitive liposomes: Biocompatibility, bio-distribution, and pharmacodynamics in BALB/c nude mice bearing human breast tumors. Drug Des. Dev. Ther. 2017, 11, 3105–3117. [Google Scholar]
- Liang, X.; Gao, J.; Jiang, L.; Luo, J.; Jing, L.; Li, X.; Jin, Y.; Dai, Z. Nanohybrid Liposomal Cerasomes with Good Physiological Stability and Rapid Temperature Responsiveness for High Intensity Focused Ultrasound Triggered Local Chemotherapy of Cancer. ACS Nano 2015, 9, 1280–1293. [Google Scholar] [CrossRef]
- Li, S.; Xie, X.; Wang, W.; Jiang, S.; Mei, W.; Zhang, Y.; Liu, S.; Yu, X. Choline phosphate lipid as an intra-crosslinker in liposomes for drug and antibody delivery under guard. Nanoscale 2022, 14, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Solarska-Sciuk, K.; Pruchnik, H. A Critical View on the Biocompatibility of Silica Nanoparticles and Liposomes as Drug Delivery Systems. Mol. Pharm. 2025, 22, 2830–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Sun, X.L. Chemically-selective surface glyco-functionalization of liposomes through Staudinger ligation. Chem. Commun. 2009, 21, 3032–3034. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Dodwadkar, N.S.; Deshpande, P.P.; Parab, S.; Torchilin, V.P. Surface Functionalization of Doxorubicin-Loaded Liposomes with Octa-arginine for Enhanced Anticancer Activity. Eur. J. Pharm. Biopharm. 2013, 84, 517–525. [Google Scholar] [CrossRef]
- Wen, J.; Xu, J.; Hong, M.; Li, W.; Li, T. The application of glycosylated liposomes in targeted drug delivery. J. Drug Deliv. Sci. Technol. 2025, 105, 106638. [Google Scholar] [CrossRef]
- Umeda, I.; Ogata, M.; Kaneko, E.; Le, B.; Uehara, T.; Moribe, K.; Arano, Y.; Yamamoto, K.; Fujii, H. 99mTc-EC carrying liposome with rapid clearance from the reticuloendothelial system. Soc. Nucl. Med. Annu. Meet. Abstr. 2010, 51, 1107. [Google Scholar]
- Mihoub, A.B.; Elkhoury, K.; Nel, J.; Acherar, S.; Velot, E.; Malaplate, C.; Linder, M.; Latifi, S.; Kahn, C.; Huguet, M. Neuroprotective Effect of Curcumin-Loaded RGD Peptide-PEGylated Nanoliposomes. Pharmaceutics 2023, 15, 15. [Google Scholar]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002, 4, 95–99. [Google Scholar] [CrossRef]
- Rouf, M.A.; Vural, I.; Renoir, J.M.; Hincal, A.A. Development and characterization of liposomal formulations for rapamycin delivery and investigation of their antiproliferative effect on MCF7 cells. J. Liposome Res. 2009, 19, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Cui, B.; Yan, Q.; Qiu, C.; Zhu, Z.; Wen, L.; Chen, W. Liposomes-mediated enhanced antitumor effect of docetaxel with BRD4-PROTAC as synergist for breast cancer chemotherapy/ immunotherapy. Int. J. Pharm. 2025, 668, 124973. [Google Scholar] [CrossRef] [PubMed]
- Islam Alfreahat, M.; Nsairat, H.; Aldeeb, I.D.; Al-Samydai, A.; Alshaer, W. In Vitro Potentiation of Doxorubicin Cytotoxicity Utilizing Clarithromycin Loaded-PEGylated Liposomes. Technol. Cancer Res. Treat. 2025, 24, 15330338241312561. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Y.; Zhang, Q.; Jiang, W.; Tang, L.; Liu, J.; He, Q. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur. J. Pharm. Sci. 2011, 44, 164–173. [Google Scholar] [CrossRef]
- Di, Z.; Shuhe, Z.; Baoding, S.; Yihan, Z.; Guangming, J. Preparation of transferrin-modified IR820-loaded liposomes and its effect on photodynamic therapy of breast cancer. Discov. Oncol. 2024, 15, 611. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, S.; Zeng, X.; Meng, Y.; Chang, C.; Zheng, G. Angiopep-2 and cyclic RGD dual-targeting ligand modified micelles across the blood-brain barrier for improved anti-tumor activity. J. Appl. Polym. Sci. 2022, 139, 52358. [Google Scholar] [CrossRef]
- Bahar, A.; Mohammad, Y.; Maria, K.; Riaz, K. Plausible antioxidant biomechanics and anticonvulsant pharmacological activity of brain-targeted β-carotene nanoparticles. Int. J. Nanomed. 2012, 7, 4311–4322. [Google Scholar]
- Golshany, H.; Ni, Y.; Yu, Q.; Fan, L. Nano-Delivery Systems for Carotenoids: Enhancing Bioavailability and Therapeutic Potential in Neurodegenerative Diseases. Food Rev. Int. 2025, 41, 2007–2032. [Google Scholar] [CrossRef]
- Miyazawa, T.; Nakagawa, K.; Harigae, T.; Onuma, R.; Kimura, F.; Fujii, T.; Miyazawa, T. Distribution of β-carotene-encapsulated polysorbate 80-coated poly(D, L-lactide-co-glycolide) nanoparticles in rodent tissues following intravenous administration. Int. J. Nanomed. 2015, 10, 7223–7230. [Google Scholar]
- Khanna, K.; Sharma, N.; Rawat, S.; Khan, N.; Karwasra, R.; Hasan, N.; Kumar, A.; Jain, G.K.; Nishad, D.K.; Khanna, S.; et al. Intranasal solid lipid nanoparticles for management of pain: A full factorial design approach, characterization & Gamma Scintigraphy. Chem. Phys. Lipids 2021, 236, 105060. [Google Scholar] [CrossRef]
- Shirsath, K.; Agrawal, Y.O. A Potential Strategy for Treating Parkinson’s Disease Through Intranasal Nanoemulsions. Cns Neurol. Disord.-Drug Targets 2023, 22, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.A.; Lai, F.; Corrias, F.; Caboni, P.; Bimpisidis, Z.; Maccioni, E.; Fadda, A.M.; Di Chiara, G. Lactoferrin- and antitransferrin-modified liposomes for brain targeting of the NK3 receptor agonist senktide: Preparation and in vivo evaluation. Int. J. Pharm. 2015, 479, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, C.; Wang, T.; Sun, L.; Wu, F.; Yu, D. Combined multispectral analysis and molecular docking to research the interaction of soybean isolate protein with different kinds of phospholipid liposomes and its effect on liposome properties. Food Chem. 2025, 474, 143160. [Google Scholar] [CrossRef]
- Zhou, L.; Gong, J.; Wang, P.; Zou, M.; Xia, X. Optimization of Formulation Process of Paeonol Liver-Targeting Nano Liposomes and Evaluation of Anti-Liver Tumor Effect in Vivo. Chin. J. Pharm. 2025, 60, 1620–1631. [Google Scholar]
- Luo, L. Preparation of Curcumin Liposome Using Ultrasound and High-Speed Shear Coupling to Supercritical CO2 Method. Biol. Chem. Eng. 2022, 8, 83–86. [Google Scholar]
- Zhang, X.; Li, Q.; Liu, M.; Li, Y.; Xu, Z.; Zhu, J.; Zhang, L.; Yang, S.; Dou, J.; Li, Y. Optimazation of preparation of syringopicrosides PLGA nanopartides by central composite design-response surface methodology. Chin. Tradit. Herb. Drugs 2019, 50, 4108–4113. [Google Scholar]
- Aslan, N. Application of Response Surface Methodology and Central Composite Rotatable Design for Modeling and Optimization of a Multi-Gravity Separator for Chromite Concentration. Powder Technol. 2008, 185, 80–86. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, N.; Yu, Q.; Yuan, C.; Hu, K.; Li, W.; Chen, L.; Wang, N. Co-preparation of Proanthocyanidin Nanoliposomes by ReverseEvaporation-Ultrasonic Microwave Synergistic Preparationand Its Stability and Antioxidant Activity Analysis. Sci. Technol. Food Ind. 2025, 46, 235–248. [Google Scholar] [CrossRef]
- Sun, W.-X.; Zhang, C.; Yu, X.-N.; Guo, J.; Ma, H.; Liu, K.; Luo, P.; Ren, J. Preparation and pharmacokinetic study of diosmetin long-circulating liposomes modified with lactoferrin. J. Funct. Foods 2022, 91, 105027. [Google Scholar] [CrossRef]
- An, Z.; Zhang, Q.; Shi, C.; Lu, F.; Wang, J.; Li, W. Preparation and in Vitro Evaluation of Lactoferrin-modified Liposomes of Codonopsis PilosulaPolysaccharide. Chin. J. Mod. Appl. Pharm. 2023, 40, 1317–1329. [Google Scholar] [CrossRef]
- Xu, W. Study on Lactoferrin Modified Diosmetin BrainTargeting Long Circulating Liposomes. Master’s Thesis, Chengdu University, Chengdu, China, 2021. [Google Scholar]
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Khang, D.; Kim, S.Y.; Liu-Snyder, P.; Palmore, G.T.R.; Durbin, S.M.; Webster, T.J. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: Independent role of surface nano-roughness and associated surface energy. Biomaterials 2007, 28, 4756–4768. [Google Scholar] [CrossRef]
- Zhang, J. Preparation and Characterization ofAnthocyanin Nanoparticles Coated withLactoferrin-Carboxymethyl Cellulose Sodium. Master’s Thesis, Chengdu University, Chengdu, China, 2025. [Google Scholar]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal Nanocapsules in Food Science and Agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Qin, Y.; Zhang, X.; Sun, L.; Chang, J. Highly transparent, low haze and ultraviolet to blue light blocking cellulose films tailored by lutein as electronic screen protectors. Ind. Crops Prod. 2024, 217, 118897. [Google Scholar] [CrossRef]
- Wang, M.; Luo, W.; Wu, X.; Zhang, W.; Li, H.; Li, H.; Yu, J. Changes in the structure and functional properties of lactoferrin with different iron saturations before and after simulated high-temperature short-time heat treatments. Int. J. Dairy Technol. 2024, 77, 575–585. [Google Scholar] [CrossRef]
- Vargha-Butler, E.I.; Foldvari, M.; Mezei, M. Study of the sedimentation behaviour of liposomal drug delivery system. Colloids Surf. 1989, 42, 375–389. [Google Scholar] [CrossRef]
- Li, Y.; Sun, K.; Chen, S.; Zhao, J.; Lei, Y.; Geng, L. Nano-Resveratrol Liposome: Physicochemical Stability, In Vitro Release, and Cytotoxicity. Appl. Biochem. Biotechnol. 2023, 195, 5950–5965. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Dengra, B.; Gonzalez-Alvarez, I.; Bermejo, M.; Gonzalez-Alvarez, M. Access to the CNS: Strategies to overcome the BBB. Int. J. Pharm. 2023, 636, 122759. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Barbosa, D.J.; Remiao, F.; Silva, R. Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies. Biomedicines 2024, 12, 626. [Google Scholar] [CrossRef]
- Park, J.S.; Choe, K.; Khan, A.; Jo, M.H.; Park, H.Y.; Kang, M.H.; Park, T.J.; Kim, M.O. Establishing Co-Culture Blood-Brain Barrier Models for Different Neurodegeneration Conditions to Understand Its Effect on BBB Integrity. Int. J. Mol. Sci. 2023, 24, 5283. [Google Scholar] [CrossRef]
- Ensaf, P.K.; Goodarzi, M.T.; Tabrizi, M.H.; Neamati, A.; Hosseinyzadeh, S.S. Novel formulation of parthenolide-loaded liposome coated with chitosan and evaluation of its potential anticancer effects in vitro. Mol. Biol. Rep. 2024, 51, 369. [Google Scholar] [CrossRef]
- Jasim, A.J.; Albukhaty, S.; Sulaiman, G.M.; Al-Karagoly, H.; Jabir, M.S.; Abomughayedh, A.M.; Mohammed, H.A.; Abomughaid, M.M. Liposome Nanocarriers Based on γ Oryzanol: Preparation, Characterization, and In Vivo Assessment of Toxicity and Antioxidant Activity. ACS Omega 2024, 9, 3554–3564. [Google Scholar] [CrossRef]
- Setyawati, D.R.; Azzahra, K.; Mardliyati, E.; Tarwadi; Maharani, B.Y.; Nurmeilis. Box-Behnken design assisted approach in optimizing lipid composition for cationic liposome formulation as gene carrier. Biochim. Biophys. Acta-Gen. Subj. 2024, 1868, 130705. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Guo, P.; Song, X.; Zhang, Y.; Li, N.; Yu, C.; Xing, B.; Liu, R.; Jia, X.; Zhang, Q.; et al. Nuclear Targeted Peptide Combined With Gambogic Acid for Synergistic Treatment of Breast Cancer. Front. Chem. 2022, 9, 821426. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Xu, J.; Xu, S.; Liu, H. pH/Redox-Controlled Interaction between Lipid Membranes and Peptide Derivatives with a "Helmet". J. Phys. Chem. B 2019, 123, 6784–6791. [Google Scholar] [CrossRef]
- Shahbazi, S.; Tafvizi, F.; Naseh, V. Enhancing the efficacy of letrozole-loaded PEGylated nanoliposomes against breast cancer cells: In vitro study. Heliyon 2024, 10, e30503. [Google Scholar] [CrossRef] [PubMed]
- Rakshitha, D.M.; Ganesh, N.S.; Nesalin, J.A.J.; Chandy, V. Transfersomes: A versatile tool for drug delivery and targeting. World J. Biol. Pharm. Health Sci. 2025, 21, 358–371. [Google Scholar] [CrossRef]
- Berna, M.; Dalzoppo, D.; Pasut, G.; Manunta, M.; Izzo, L.; Jones, A.T.; Duncan, R.; Veronese, F.M. Novel monodisperse PEG-dendrons as new tools for targeted drug delivery: Synthesis, characterisation and cellular uptake. Biomacromolecules 2006, 7, 146. [Google Scholar] [CrossRef]
- Du, Q.; Liu, Y.; Fan, M.; Wei, S.; Ismail, M.; Zheng, M. PEG length effect of peptide-functional liposome for blood brain barrier (BBB) penetration and brain targeting. J. Control. Release 2024, 372, 85–94. [Google Scholar] [CrossRef]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef]
- Wu, L.; Huang, W.; Peng, K.; Wang, Y.; Chen, Q.; Lu, B. Enhancing the stability, BBB permeability and neuroprotective activity of verbascoside in vitro using lipid nanocapsules in combination with menthol. Food Chem. 2023, 414, 135682. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Elnaggar, Y.S.R.; Abdallah, O.Y. Pectin-stabilized nanoceria double coated with lactoferrin/chitosan for management of experimental autoimmune encephalomyelitis. Colloids Surf. B-Biointerfaces 2025, 245, 114271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, S.C.; Kim, H.J.; Lee, D.Y. Inhibition of DAMP actions in the tumoral microenvironment using lactoferrin-glycyrrhizin conjugate for glioblastoma therapy. Biomater. Res. 2023, 27, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, X.; Yang, H.; Tan, J.; Gong, R.; Mei, C.; Cai, X.; Su, Z.; Kong, F. Lactoferrin/CD133 antibody conjugated nanostructured lipid carriers for dual targeting of blood-brain-barrier and glioblastoma stem cells. Biomed. Mater. 2024, 19, 055041. [Google Scholar] [CrossRef]

| NO. | P:C | E:W | Shear Speed | Shear Time |
|---|---|---|---|---|
| 1 | 0 | 0 | −2 | 0 |
| 2 | −1 | 1 | 1 | −1 |
| 3 | 1 | −1 | 1 | −1 |
| 4 | −1 | 1 | −1 | −1 |
| 5 | 1 | −1 | −1 | −1 |
| 6 | 0 | 0 | 0 | 2 |
| 7 | −1 | 1 | 1 | 1 |
| 8 | 1 | 1 | 1 | 1 |
| 9 | −1 | −1 | 1 | 1 |
| 10 | −1 | −1 | 1 | −1 |
| 11 | 0 | 0 | 0 | 0 |
| 12 | −1 | −1 | −1 | 1 |
| 13 | 0 | −2 | 0 | 0 |
| 14 | −1 | −1 | −1 | −1 |
| 15 | 0 | 0 | 0 | 0 |
| 16 | 1 | −1 | 1 | 1 |
| 17 | 0 | 0 | 0 | 0 |
| 18 | −1 | 1 | −1 | 1 |
| 19 | 0 | 0 | 0 | 0 |
| 20 | 1 | 1 | −1 | −1 |
| 21 | 0 | 0 | 0 | −2 |
| 22 | 1 | −1 | −1 | 1 |
| 23 | −2 | 0 | 0 | 0 |
| 24 | 0 | 0 | 2 | 0 |
| 25 | 1 | 1 | 1 | −1 |
| 26 | 0 | 0 | 0 | 0 |
| 27 | 2 | 0 | 0 | 0 |
| 28 | 0 | 2 | 0 | 0 |
| 29 | 1 | 1 | −1 | 1 |
| 30 | 0 | 0 | 0 | 0 |
| Factors | Levels | |||
|---|---|---|---|---|
| Low (−1) | High (1) | −alpha | +alpha | |
| A—P:C (m/m) | 4 | 6 | 3 | 7 |
| B—E:W (v/v) | 0.8 | 1.2 | 0.6 | 1.4 |
| C—shear speed (r/min) | 12,000 | 16,000 | 10,000 | 18,000 |
| D—shear time (min) | 3 | 7 | 1 | 9 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 63,820.32 | 14 | 4558.59 | 22.52 | <0.0001 | Y |
| A-P:C | 68.41 | 1 | 68.41 | 0.3379 | 0.5697 | N |
| B-E:W | 51,307.9 | 1 | 51,307.9 | 253.44 | <0.0001 | Y |
| C-V | 4241.11 | 1 | 4241.11 | 20.95 | 0.0004 | Y |
| D-T | 2265.93 | 1 | 2265.93 | 11.19 | 0.0044 | Y |
| AB | 5.24 | 1 | 5.24 | 0.0259 | 0.8743 | N |
| AC | 46.38 | 1 | 46.38 | 0.2291 | 0.6391 | N |
| AD | 0.0625 | 1 | 0.0625 | 0.0003 | 0.9862 | N |
| BC | 1009.33 | 1 | 1009.33 | 4.99 | 0.0412 | Y |
| BD | 186.32 | 1 | 186.32 | 0.9204 | 0.3526 | N |
| CD | 13.62 | 1 | 13.62 | 0.0673 | 0.7989 | N |
| A2 | 142.17 | 1 | 142.17 | 0.7023 | 0.4152 | N |
| B2 | 4105.37 | 1 | 4105.37 | 20.28 | 0.0004 | Y |
| C2 | 182.69 | 1 | 182.69 | 0.9024 | 0.3572 | N |
| D2 | 110.72 | 1 | 110.72 | 0.5469 | 0.471 | N |
| Residual | 3036.68 | 15 | 202.45 | |||
| Lack of Fit | 2688.01 | 10 | 268.8 | 3.85 | 0.0747 | N |
| Pure Error | 348.67 | 5 | 69.73 | |||
| Cor Total | 66,857 | 29 |
| Experiment Number | A—P:C | B—E:W | C—Shear Speed | D—Shear Time | d (nm) |
|---|---|---|---|---|---|
| NO.1 | 5:1 (m/m) | 4:5 (v/v) | 16,000 r/min | 7 min | 163.17 |
| NO.2 | 158.22 | ||||
| NO.3 | 159.31 | ||||
| Average of d value | 160.23 ± 2.60 |
| Model Name | Model Equation | Fitting Equation of FL | Fitting Equation of Lf-LLips |
|---|---|---|---|
| Zero-Order Kinetics Model | y = a + bx | y = 5.1976 + 1.3788x R2 = 0.9061 | y = 4.1664 + 0.1849x R2 = 0.5805 |
| First-Order Kinetics Model | y = a × (1 − e−bx) | y = 93.1526 × (1 − e−0.0377x) R2 = 0.9880 | y = 12.7943 × (1 − e−0.1226x) R2 = 0.9469 |
| Higuchi Equation Model | y = a × (x^1/2) + b | y = 11.3368x1/2 − 5.9282 R2 = 0.9674 | y = 1.7765x1/2 − 1.3442 R2 = 0.7865 |
| Ritger–Peppas Equation Model | y = a × (x^b) | y = 6.0674x0.6439 (CRP ≤ 60%) R2 = 0.8012 | y = 3.2277x0.3660 (CRP ≤ 60%) R2 = 0.9909 |
| Weibull Distribution Model | y = a − (a − b) × e−(kx)^d | y = 12.3933 − 10.5388e(−0.1146x)^2.5986 R2 = 0.9867 | y = 83.8645 − 80.3023e(−0.0474x)^1.3967 R2 = 0.9989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, T.; Na, Z.; Zhao, R.; Ma, Y. Construction and In Vitro Evaluation of Brain-Targeted Lutein Liposomes. Foods 2025, 14, 3611. https://doi.org/10.3390/foods14213611
You T, Na Z, Zhao R, Ma Y. Construction and In Vitro Evaluation of Brain-Targeted Lutein Liposomes. Foods. 2025; 14(21):3611. https://doi.org/10.3390/foods14213611
Chicago/Turabian StyleYou, Tingting, Zhiguo Na, Ruobing Zhao, and Yongqiang Ma. 2025. "Construction and In Vitro Evaluation of Brain-Targeted Lutein Liposomes" Foods 14, no. 21: 3611. https://doi.org/10.3390/foods14213611
APA StyleYou, T., Na, Z., Zhao, R., & Ma, Y. (2025). Construction and In Vitro Evaluation of Brain-Targeted Lutein Liposomes. Foods, 14(21), 3611. https://doi.org/10.3390/foods14213611




