Soybean Soluble Polysaccharides: Composition, Structure, and Protein Stabilization Mechanism in Acidic Milk Drinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fractionation of SSPS
2.3. Monosaccharide Composition
2.4. Measurement of Molecular Weight and Distribution
2.5. Zeta Potential and Particle Size Measurement
2.6. Rheological Analysis
2.7. Amino Acid Analysis
2.8. Atomic Force Microscopy
2.9. Preparation of AMDs
2.10. Measurement of AMDs Stability by LUMi-Sizer
2.11. Statistical Analysis
3. Results and Discussion
3.1. Molecular Weight and Particle Size of SSPS
3.2. Monosaccharide Compositions and Protein Content
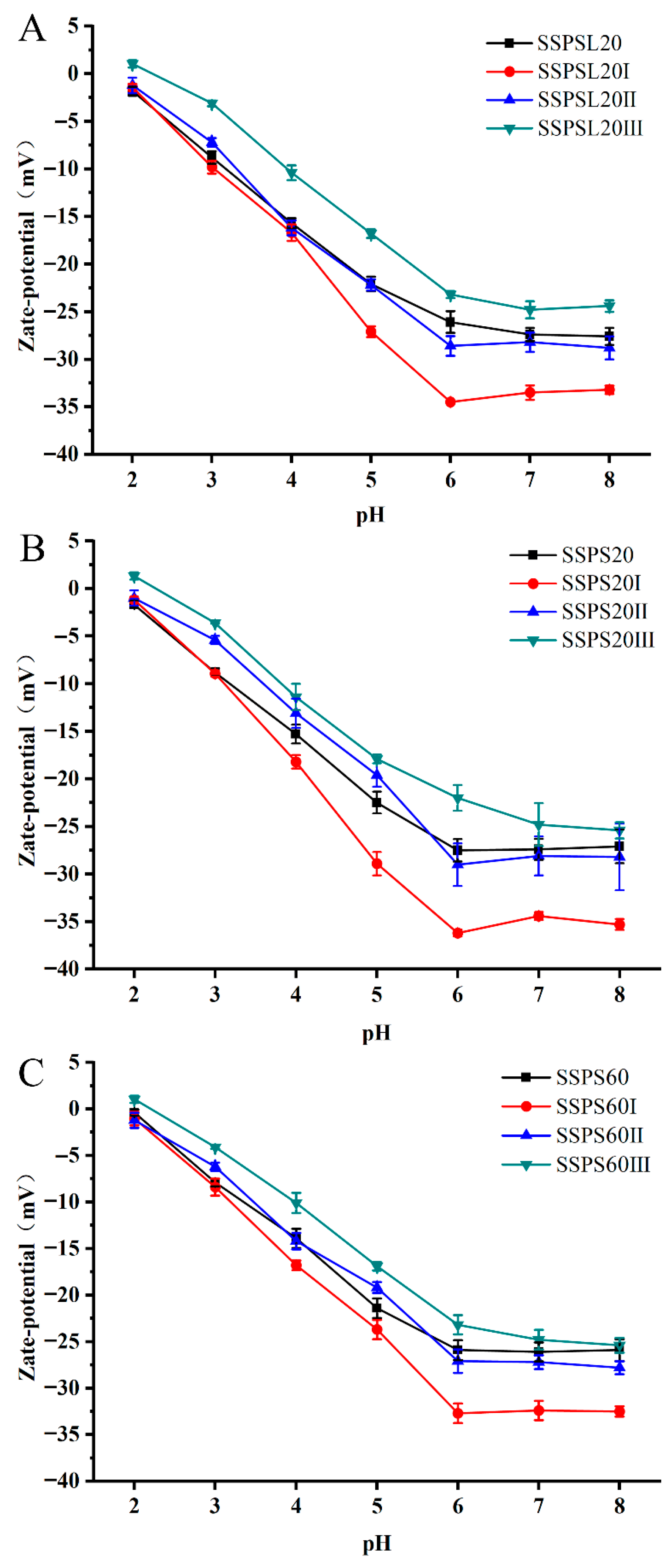
3.3. Zeta Potential
3.4. Amino Acid Composition Analysis
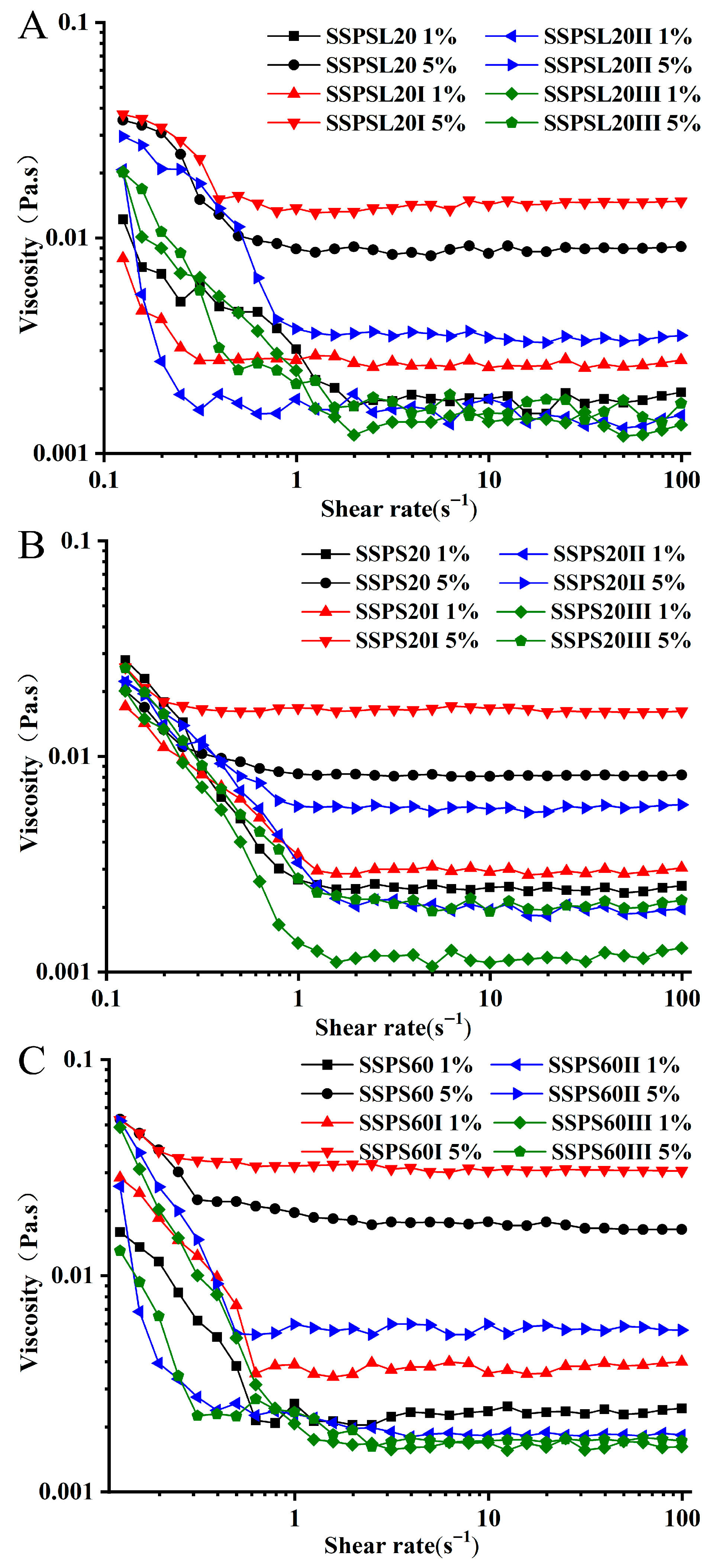
3.5. Apparent Viscosity Analysis
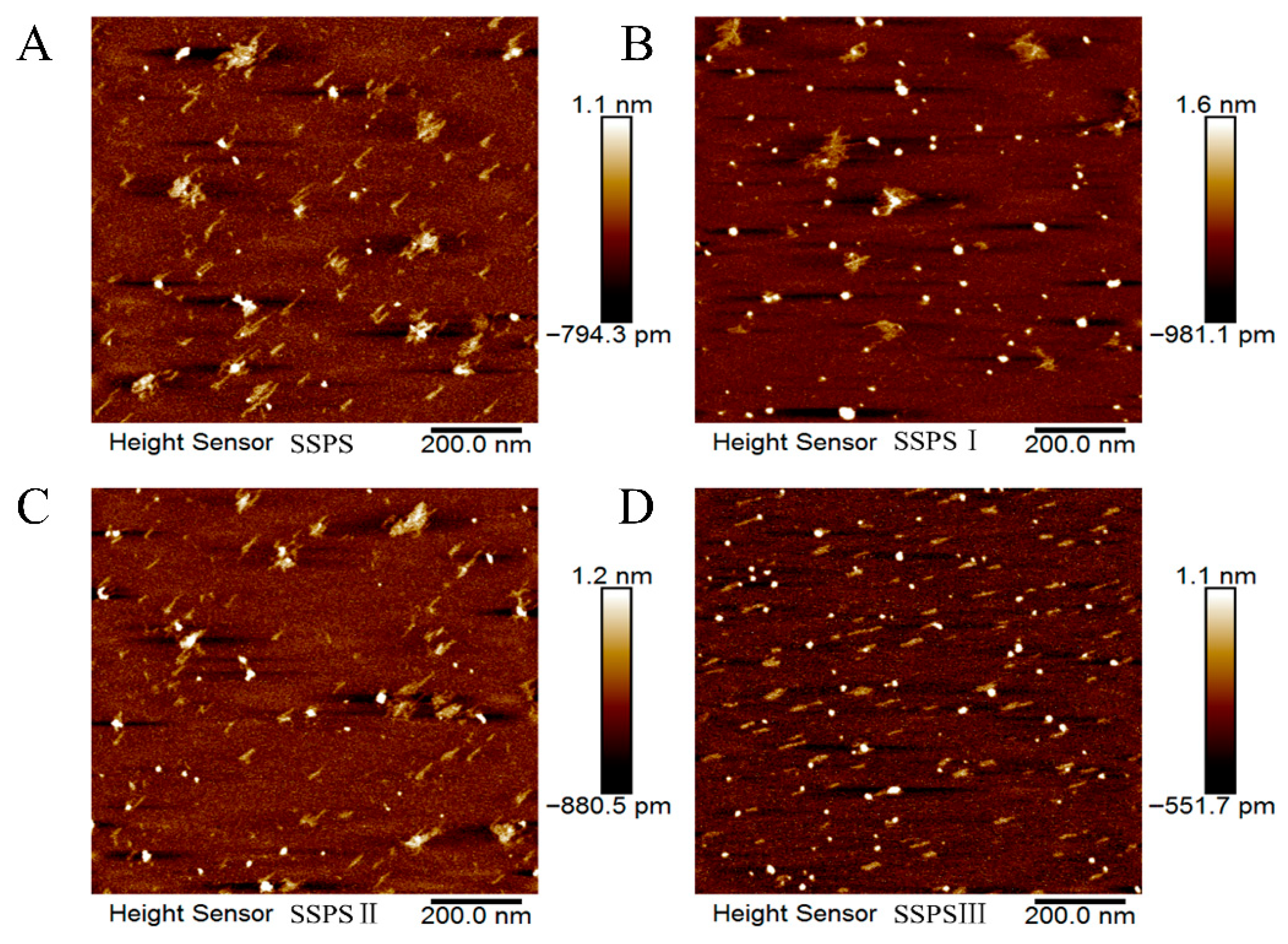
3.6. AFM Analysis of SSPS
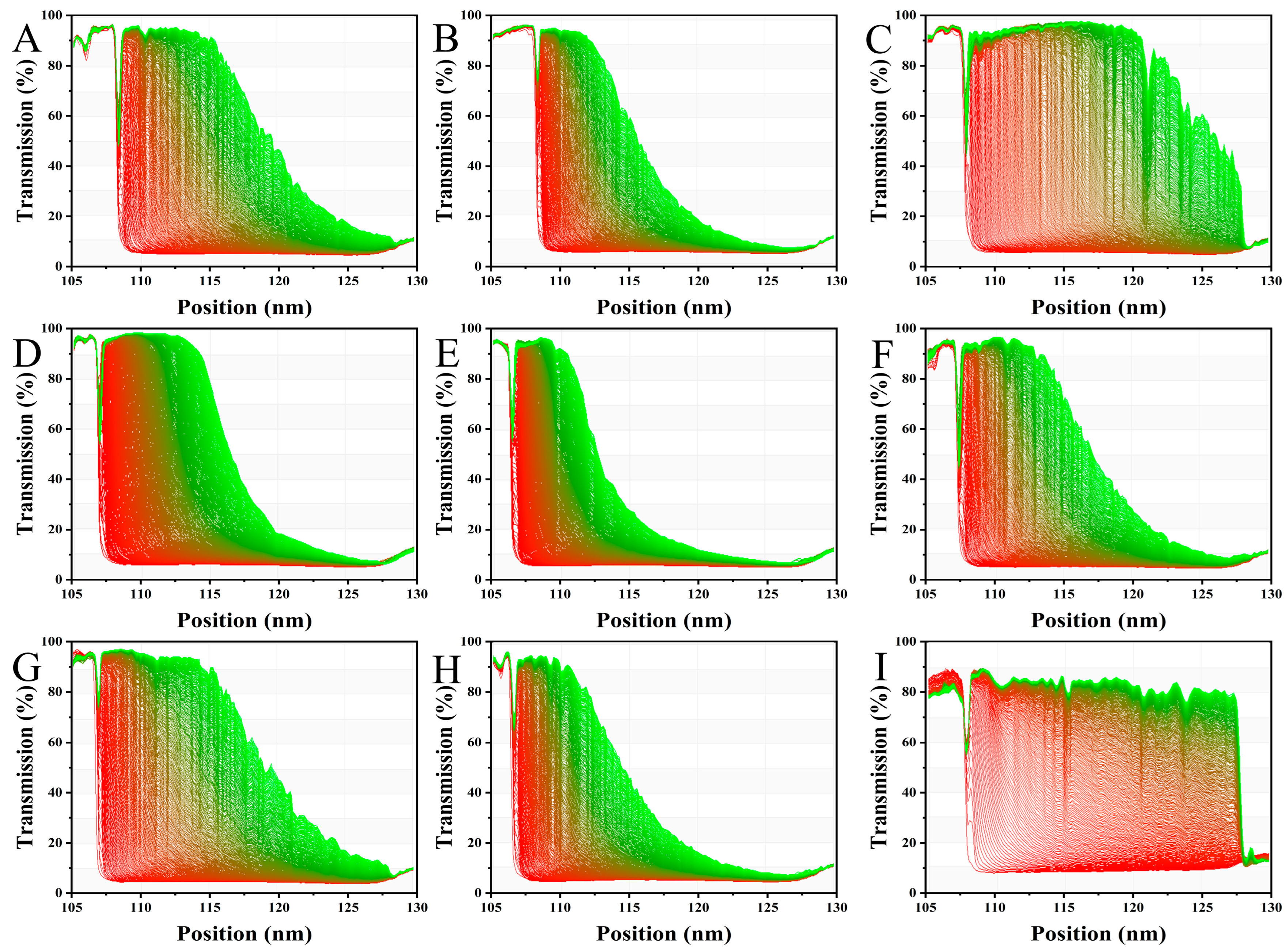
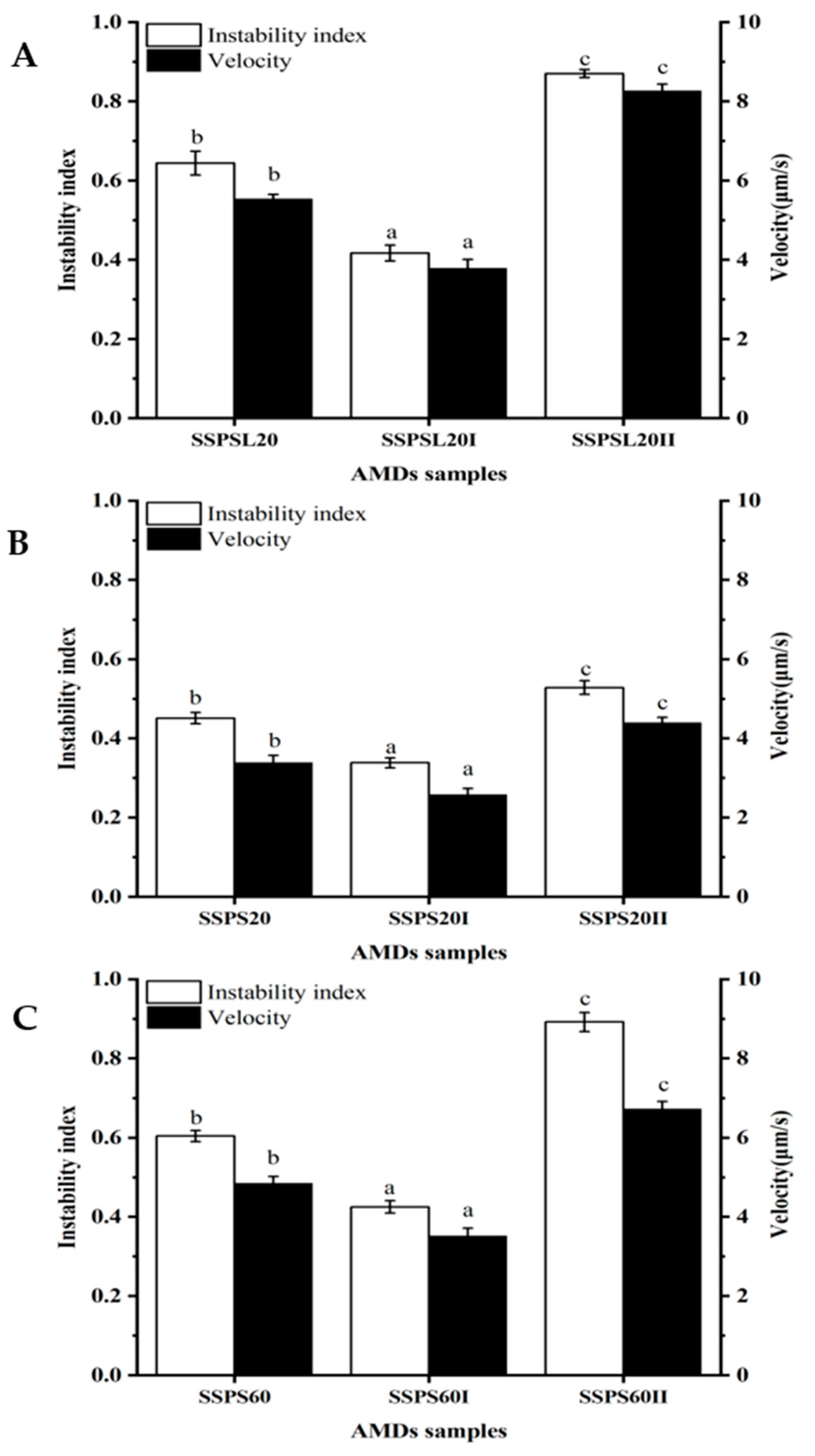
3.7. Physical Stability Analysis of AMDs by LUMi-Sizer
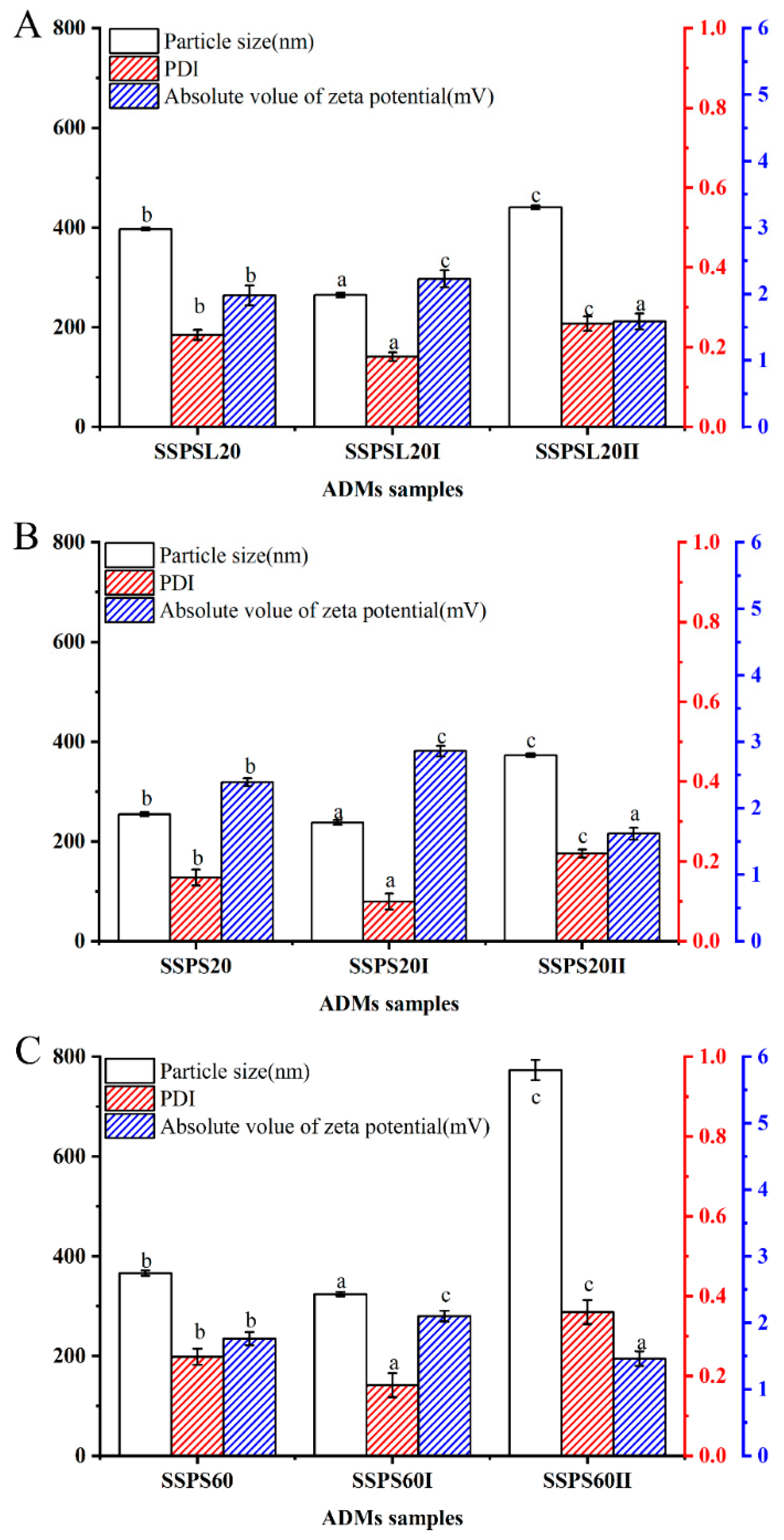
3.8. Particle Size and Zeta Potential of Casein Particles Coated by Different Fractions of SSPS
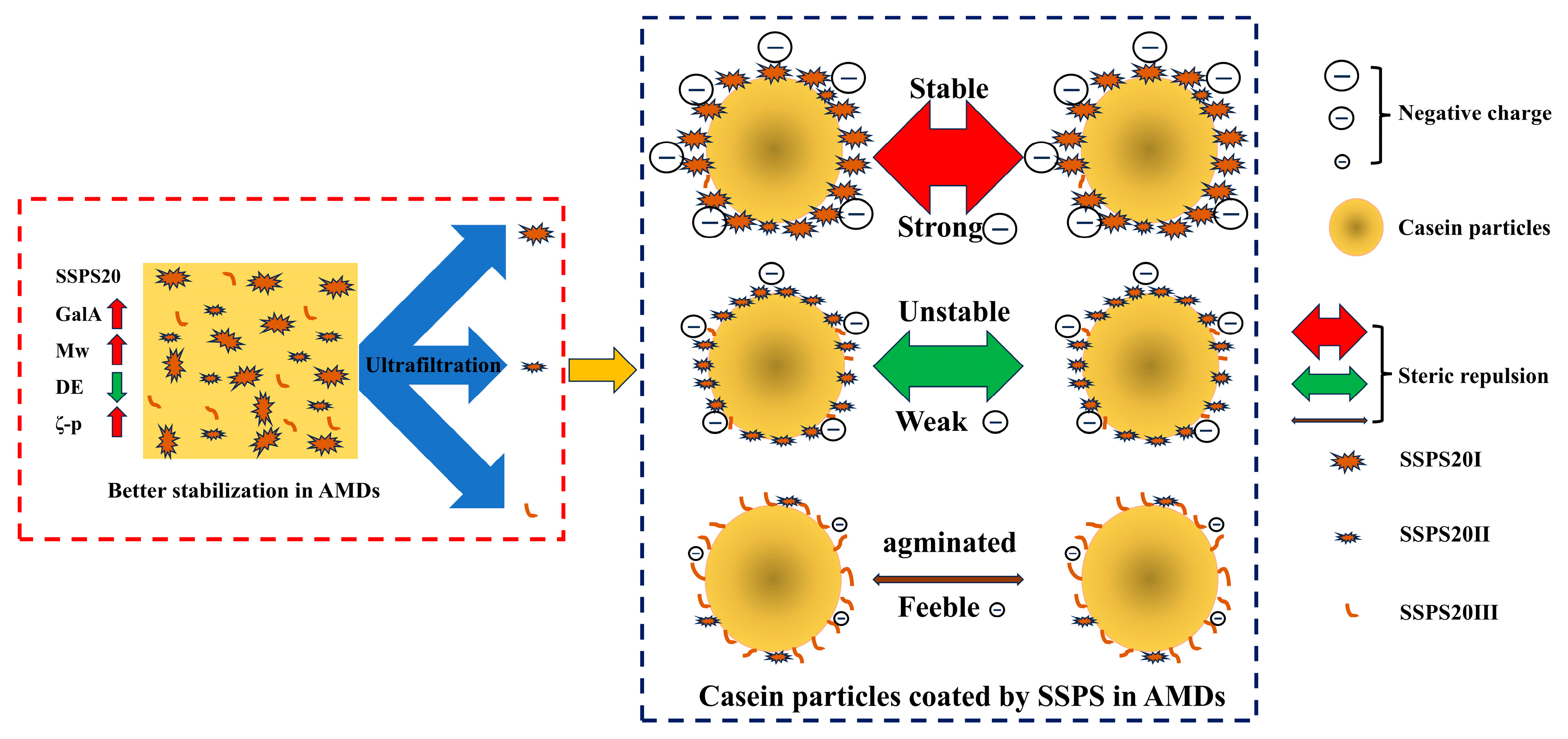
3.9. Protein Dispersing and Stabilizing Mechanism of AMDs by Using SSPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSPS | Soybean Soluble Polysaccharide |
| AMDs | acidified milk beverages |
| DE | degree of esterification |
| MW | molecular weight |
| GalA | galacturonic acid |
Appendix A

References
- Maeda, H.; Nakamura, A. Soluble Soybean Polysaccharide. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009; pp. 693–709. [Google Scholar]
- Nakamura, A.; Furuta, H.; Maeda, H.; Takao, T.; Nagamatsu, Y. Structural Studies by Stepwise Enzymatic Degradation of the Main Backbone of Soybean Soluble Polysaccharides Consisting of Galacturonan and Rhamnogalacturonan. Biosci. Biotechnol. Biochem. 2002, 66, 1301–1313. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Maeda, H.; Nagamatsu, Y.; Yoshimoto, A. Analysis of Structural Components and Molecular Construction of Soybean Soluble Polysaccharides by Stepwise Enzymatic Degradation. Biosci. Biotechnol. Biochem. 2001, 65, 2249–2258. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K.; Sahi, A.A.; Al-Sharifi, A.S.N. Effect of Feeding Soy Milk Fermented by Probiotic Bacteria on Some Blood Criteria and Weight of Experimental Animals. Probiotics Antimicrob. Proteins 2017, 9, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Fujii, N.; Tobe, J.; Adachi, N.; Hirotsuka, M. Characterization and Functional Properties of Soybean High-Molecular-Mass Polysaccharide Complex. Food Hydrocoll. 2012, 29, 75–84. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, L.; Chen, Y.; Ruan, Q.; Zhang, C.; Hua, Y. Effects of Alkali Treatment and Subsequent Acidic Extraction on the Properties of Soybean Soluble Polysaccharides. Food Bioprod. Process. 2015, 94, 239–247. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, Y.; Guo, Y.; Ma, A.; Zhang, H. Influence of the Degree of Esterification of Soluble Soybean Polysaccharide on the Stability of Acidified Milk Drinks. Food Hydrocoll. 2020, 108, 106052. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, J.; Du, B.; Zhang, H. Impact of Distribution of Carboxymethyl Substituents in the Stabilizer of Carboxymethyl Cellulose on the Stability of Acidified Milk Drinks. Food Hydrocoll. 2018, 76, 150–157. [Google Scholar] [CrossRef]
- Cai, Y.; Cai, B.; Ikeda, S. Stabilization of Milk Proteins in Acidic Conditions by Pectic Polysaccharides Extracted from Soy Flour. J. Dairy Sci. 2017, 100, 7793–7801. [Google Scholar] [CrossRef]
- Liu, J.R.; Nakamura, A.; Corredig, M. Addition of Pectin and Soy Soluble Polysaccharide Affects the Particle Size Distribution of Casein Suspensions Prepared from Acidified Skim Milk. J. Agric. Food Chem. 2006, 54, 6241–6246. [Google Scholar] [CrossRef]
- Nakamura, A.; Furuta, H.; Kato, M.; Maeda, H.; Nagamatsu, Y. Effect of Soybean Soluble Polysaccharides on the Stability of Milk Protein under Acidic Conditions. Food Hydrocoll. 2003, 17, 333–343. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Cabezas, D.M.; Palazolo, G.G. Effects of PH, Protein: Polysaccharide Ratio, and NaCl-Added Concentration on Whey Protein Isolate and Soluble Soybean Polysaccharides Electrostatic-Complexes Formation. Food Chem. Adv. 2022, 1, 100123. [Google Scholar] [CrossRef]
- Tang, W.; Liu, C.; Liu, J.; Hu, L.; Huang, Y.; Yuan, L.; Liu, F.; Pan, S.; Chen, S.; Bian, S.; et al. Purification of Polysaccharide from Lentinus Edodes Water Extract by Membrane Separation and Its Chemical Composition and Structure Characterization. Food Hydrocoll. 2020, 105, 105851. [Google Scholar] [CrossRef]
- Atgié, M.; Garrigues, J.C.; Chennevière, A.; Masbernat, O.; Roger, K. Gum Arabic in Solution: Composition and Multi-Scale Structures. Food Hydrocoll. 2019, 91, 319–330. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological Properties and Chain Conformation of Soy Hull Water-Soluble Polysaccharide Fractions Obtained by Gradient Alcohol Precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Nakamura, A.; Yoshida, R.; Maeda, H.; Furuta, H.; Corredig, M. Study of the Role of the Carbohydrate and Protein Moieties of Soy Soluble Polysaccharides in Their Emulsifying Properties. J. Agric. Food Chem. 2004, 52, 5506–5512. [Google Scholar] [CrossRef]
- Ikeda, S.; Funami, T.; Zhang, G. Visualizing Surface Active Hydrocolloids by Atomic Force Microscopy. Carbohydr. Polym. 2005, 62, 192–196. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Comparative Studies on the Stabilization of Pea Protein Dispersions by Using Various Polysaccharides. Food Hydrocoll. 2020, 98, 105233. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, Q.; He, Z.; Wang, Z.; Qin, F.; Zeng, M.; Chen, J. Effects of Molecular Weight and Degree of Esterification of Soluble Soybean Polysaccharide on the Stability of Casein under Acidic Conditions. Foods 2021, 10, 686. [Google Scholar] [CrossRef]
- Liang, W.-L.; Liao, J.S.; Qi, J.R.; Jiang, W.-X.; Yang, X.-Q. Physicochemical Characteristics and Functional Properties of High Methoxyl Pectin with Different Degree of Esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Food Emulsions: Principles, Practices, and Techniques, Julian McClements, 2nd edition, CRC Press, Boca Raton, FL (2005), pp. 609, $149.95. Food Hydrocoll. 2006, 20, 137. [Google Scholar] [CrossRef]
- Li, J.; Matsumoto, S.; Nakamura, A.; Maeda, H.; Matsumura, Y. Characterization and Functional Properties of Sub-Fractions of Soluble Soybean Polysaccharides. Biosci. Biotechnol. Biochem. 2009, 73, 2568–2575. [Google Scholar] [CrossRef][Green Version]
- Wagoner, T.B.; Foegeding, E.A. Whey Protein–Pectin Soluble Complexes for Beverage Applications. Food Hydrocoll. 2017, 63, 130–138. [Google Scholar] [CrossRef]
- Zhao, R.X.; Qi, J.R.; Liu, Q.R.; Zeng, W.Q.; Yang, X.Q. Fractionation and Characterization of Soluble Soybean Polysaccharide Esterified of Octenyl Succinic Anhydride and Its Effect as a Stabilizer in Acidified Milk Drinks. Food Hydrocoll. 2018, 85, 215–221. [Google Scholar] [CrossRef]
- Nobuhara, T.; Matsumiya, K.; Nambu, Y.; Nakamura, A.; Fujii, N.; Matsumura, Y. Stabilization of Milk Protein Dispersion by Soybean Soluble Polysaccharide under Acidic PH Conditions. Food Hydrocoll. 2014, 34, 39–45. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, C.; Li, Q. Interaction Between the Polysaccharides and Proteins in Semisolid Food Systems. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 439–445. ISBN 978-0-12-814045-1. [Google Scholar]
- Nakamura, A.; Naeki, R.; Kikuchi, M.; Corredig, M.; Shima, Y.; Fujii, N. Molecular Structures of High- and Low-Methoxy Water-Soluble Polysaccharides Derived from Peas and Their Functions for Stabilizing Milk Proteins under Acidic Conditions. Food Res. Int. 2023, 165, 112390. [Google Scholar] [CrossRef] [PubMed]
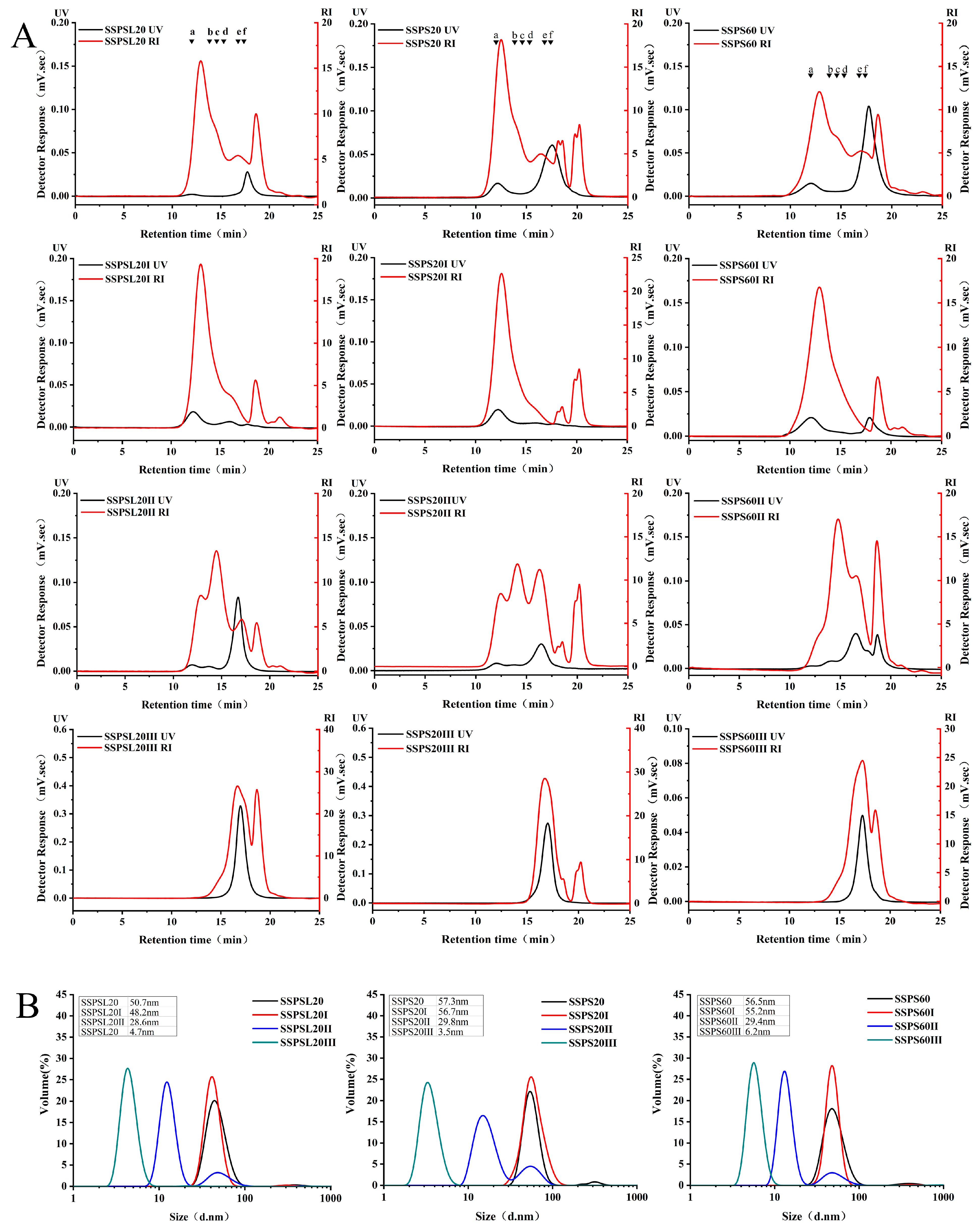
| Sample | MW (kDa) | Molecular Weight Distribution Range (%) | Particle Size (nm) | |||
|---|---|---|---|---|---|---|
| >500 kDa | 500~100 kDa | 100~10 kDa | <10 kDa | |||
| SSPS20 * | 475 | 54.12 | 15.67 | 14.39 | 15.82 | 57.3 ± 0.7 |
| SSPS20I | 847 | 72.19 | 15.04 | 1.98 | 10.79 | 56.7 ± 0.9 |
| SSPS20II | 380 | 18.65 | 31.01 | 30.47 | 19.87 | 29.8 ± 0.7 |
| SSPS20III | 44 | 0.00 | 2.15 | 81.88 | 15.97 | 3.5 ± 0.1 |
| SSPS60 * | 483 | 46.90 | 18.45 | 16.63 | 18.02 | 56.5 ± 0.6 |
| SSPS60I | 859 | 73.49 | 14.61 | 2.17 | 9.74 | 55.2 ± 0.6 |
| SSPS60II | 369 | 7.27 | 52.76 | 20.08 | 19.90 | 29.4 ± 0.5 |
| SSPS60III | 45 | 0.00 | 4.53 | 73.80 | 21.67 | 6.2 ± 0.1 |
| SSPSL20 * | 395 | 42.15 | 19.87 | 19.01 | 18.97 | 50.7 ± 0.5 |
| SSPSL20I | 716 | 67.92 | 17.42 | 2.36 | 14.20 | 48.2± 0.6 |
| SSPSL20II | 305 | 16.46 | 42.19 | 22.48 | 18.87 | 28.6 ± 0.3 |
| SSPSL20III | 41 | 0.00 | 3.58 | 69.26 | 27.16 | 4.7 ± 0.2 |
| Sample | Neutral Sugar (%) | GalA | GlcA | Ara/Rha | Protein (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fuc | Ara | Rha | Gal | Glc | Xyl | Man | |||||
| SSPS20 | 2.47 | 15.30 | 2.86 | 64.66 | 3.78 | 4.46 | 0.29 | 5.97 | 0.21 | 5.35 | 3.11 ± 0.30 |
| SSPS20I | 3.52 | 16.28 | 3.21 | 63.01 | - | 5.59 | 1.00 | 7.25 | 0.14 | 5.07 | 0.83 ± 0.11 |
| SSPS20II | 2.50 | 14.66 | 2.62 | 68.21 | 1.66 | 4.01 | 0.41 | 5.72 | 0.21 | 5.60 | 1.13 ± 0.08 |
| SSPS20III | 0.89 | 4.04 | 0.42 | 68.25 | 18.6 | 2.85 | 1.14 | 3.64 | 0.15 | 9.62 | 3.07 ± 0.12 |
| SSPS60 | 3.90 | 17.13 | 4.28 | 58.84 | 1.06 | 1.72 | 7.65 | 5.04 | 0.73 | 4.00 | 3.80 ± 0.10 |
| SSPS60I | 5.67 | 18.85 | 4.06 | 56.61 | - | - | 8.1 | 6.08 | 0.63 | 4.64 | 2.15 ± 0.13 |
| SSPS60II | 2.58 | 19.17 | 4.12 | 64.85 | 0.49 | 0.50 | 4.33 | 3.50 | 0.45 | 4.65 | 0.69 ± 0.15 |
| SSPS60III | 2.25 | 9.93 | 1.78 | 77.63 | 1.02 | 3.64 | 0.39 | 2.81 | 0.54 | 5.58 | 4.23 ± 0.17 |
| SSPSL20 | 3.29 | 16.37 | 4.15 | 63.79 | 2.07 | 4.51 | 0.20 | 5.33 | 0.29 | 3.94 | 2.70 ± 0.10 |
| SSPSL20I | 4.36 | 16.20 | 4.03 | 61.83 | 0.14 | - | 6.70 | 6.48 | 0.25 | 4.02 | 0.57 ± 0.10 |
| SSPSL20II | 2.14 | 15.96 | 3.34 | 70.67 | 0.18 | 3.47 | 0.25 | 3.83 | 0.16 | 4.78 | 0.80 ± 0.12 |
| SSPSL20III | 1.32 | 4.98 | 0.56 | 70.35 | 15.1 | 4.36 | 0.76 | 2.31 | 0.19 | 8.89 | 3.58 ± 0.15 |
| Amino Acid (g/100 g) | SSPSL20 | SSPSL20I | SSPSL20II | SSPSL20III | SSPS20 | SSPS20I | SSPS20II | SSPS20III | SSPS60 | SSPS60I | SSPS60II | SSPS60III |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp | 0.412 | 0.099 | 0.140 | 0.508 | 0.499 | 0.169 | 0.192 | 0.614 | 0.543 | 0.211 | 0.110 | 0.699 |
| Glu | 0.655 | 0.143 | 0.285 | 1.44 | 0.762 | 0.139 | 0.278 | 1.820 | 0.817 | 0.288 | 0.208 | 1.72 |
| Ser | 0.113 | 0.03 | 0.029 | 0.116 | 0.141 | 0.028 | 0.037 | 0.155 | 0.157 | 0.092 | 0.031 | 0.153 |
| His | 0.100 | 0.031 | 0.037 | 0.172 | 0.097 | 0.022 | 0.042 | 0.180 | 0.133 | 0.079 | 0.035 | 0.153 |
| Gly | 0.112 | 0.029 | 0.029 | 0.121 | 0.136 | 0.023 | 0.037 | 0.149 | 0.138 | 0.081 | 0.033 | 0.136 |
| Thr | 0.077 | 0.021 | 0.017 | 0.062 | 0.119 | 0.022 | 0.033 | 0.103 | 0.115 | 0.078 | 0.021 | 0.093 |
| Arg | 0.089 | 0.024 | 0.050 | 0.281 | 0.144 | 0.048 | 0.068 | 0.360 | 0.192 | 0.100 | 0.059 | 0.266 |
| Ala | 0.087 | 0.026 | 0.017 | 0.052 | 0.129 | 0.039 | 0.039 | 0.076 | 0.127 | 0.100 | 0.022 | 0.074 |
| Tyr | 0.048 | 0.012 | 0.011 | 0.04 | 0.068 | 0.008 | 0.009 | 0.042 | 0.041 | 0.028 | 0.011 | 0.055 |
| Cys | 0.002 | 0.000 | 0.003 | 0.003 | 0.001 | 0.000 | 0.001 | 0.001 | 0.005 | 0.002 | 0.002 | 0.002 |
| Val | 0.075 | 0.024 | 0.019 | 0.054 | 0.105 | 0.032 | 0.027 | 0.066 | 0.111 | 0.096 | 0.021 | 0.078 |
| Met | 0.013 | 0.003 | 0.002 | 0.009 | 0.018 | 0.003 | 0.001 | 0.022 | 0.007 | 0.004 | 0.004 | 0.014 |
| Phe | 0.057 | 0.018 | 0.016 | 0.058 | 0.090 | 0.024 | 0.023 | 0.077 | 0.083 | 0.067 | 0.017 | 0.065 |
| Ile | 0.057 | 0.018 | 0.015 | 0.056 | 0.080 | 0.021 | 0.019 | 0.069 | 0.086 | 0.077 | 0.016 | 0.077 |
| Leu | 0.086 | 0.029 | 0.016 | 0.053 | 0.130 | 0.004 | 0.028 | 0.060 | 0.136 | 0.129 | 0.021 | 0.078 |
| Lys | 0.131 | 0.039 | 0.046 | 0.220 | 0.184 | 0.005 | 0.057 | 0.280 | 0.221 | 0.134 | 0.044 | 0.226 |
| Pro | 0.107 | 0.032 | 0.072 | 0.333 | 0.281 | 0.089 | 0.106 | 0.509 | 0.275 | 0.082 | 0.037 | 0.334 |
| Protein content | 2.221 | 0.578 | 0.804 | 3.578 | 2.984 | 0.676 | 0.997 | 4.583 | 3.188 | 1.648 | 0.692 | 4.223 |
| Sample | Concentration | K (Pa Sn) | n | R2 |
|---|---|---|---|---|
| SSPSL20 | 1% | 0.0012 ± 0.0001 | 1.010 ± 0.0001 | 0.997 |
| 5% | 0.0082 ± 0.0001 | 1.022 ± 0.0001 | 0.999 | |
| SSPSL20I | 1% | 0.0020 ± 0.0001 | 1.062 ± 0.0001 | 0.999 |
| 5% | 0.0139 ± 0.0001 | 1.012 ± 0.0001 | 0.999 | |
| SSPSL20II | 1% | 0.0011 ± 0.0001 | 1.013 ± 0.0001 | 0.997 |
| 5% | 0.0054 ± 0.0001 | 1.012 ± 0.0001 | 0.999 | |
| SSPSL20III | 1% | 0.008 ± 0.0001 | 0.979 ± 0.0001 | 0.995 |
| 5% | 0.0018 ± 0.0001 | 0.987 ± 0.0001 | 0.996 | |
| SSPS20 | 1% | 0.0020 ± 0.0001 | 0.996 ± 0.006 | 0.998 |
| 5% | 0.0083 ± 0.0002 | 0.985 ± 0.005 | 0.999 | |
| SSPS20I | 1% | 0.0022 ± 0.0001 | 0.993 ± 0.002 | 0.999 |
| 5% | 0.017 ± 0.0005 | 0.983 ± 0.007 | 0.999 | |
| SSPS20II | 1% | 0.0017 ± 0.0001 | 0.996 ± 0.008 | 0.997 |
| 5% | 0.0051 ± 0.0001 | 0.994 ± 0.006 | 0.999 | |
| SSPS20III | 1% | 0.0005 ± 0.0001 | 0.998 ± 0.003 | 0.997 |
| 5% | 0.0010 ± 0.0001 | 1.021 ± 0.0001 | 0.997 | |
| SSPS60 | 1% | 0.0019 ± 0.0001 | 1.051 ±0.003 | 0.999 |
| 5% | 0.0196 ± 0.0002 | 0.959 ± 0.004 | 0.999 | |
| SSPS60I | 1% | 0.0019 ± 0.0001 | 1.061 ± 0.0001 | 0.999 |
| 5% | 0.0314 ± 0.0001 | 0.994 ± 0.0001 | 0.999 | |
| SSPS60II | 1% | 0.0025 ± 0.0001 | 1.010 ± 0.0002 | 0.999 |
| 5% | 0.0098 ± 0.0001 | 0.9912 ± 0.0004 | 0.999 | |
| SSPS60III | 1% | 0.0006 ± 0.0002 | 0.9944 ± 0.0001 | 0.999 |
| 5% | 0.0010 ± 0.0001 | 1.021 ± 0.0001 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liang, G.; Wang, Z.; Zeng, M.; He, Z.; Chen, Q.; Qin, F.; Chen, J. Soybean Soluble Polysaccharides: Composition, Structure, and Protein Stabilization Mechanism in Acidic Milk Drinks. Foods 2025, 14, 3629. https://doi.org/10.3390/foods14213629
Li Y, Liang G, Wang Z, Zeng M, He Z, Chen Q, Qin F, Chen J. Soybean Soluble Polysaccharides: Composition, Structure, and Protein Stabilization Mechanism in Acidic Milk Drinks. Foods. 2025; 14(21):3629. https://doi.org/10.3390/foods14213629
Chicago/Turabian StyleLi, Yujian, Guijiang Liang, Zhaojun Wang, Maomao Zeng, Zhiyong He, Qiuming Chen, Fang Qin, and Jie Chen. 2025. "Soybean Soluble Polysaccharides: Composition, Structure, and Protein Stabilization Mechanism in Acidic Milk Drinks" Foods 14, no. 21: 3629. https://doi.org/10.3390/foods14213629
APA StyleLi, Y., Liang, G., Wang, Z., Zeng, M., He, Z., Chen, Q., Qin, F., & Chen, J. (2025). Soybean Soluble Polysaccharides: Composition, Structure, and Protein Stabilization Mechanism in Acidic Milk Drinks. Foods, 14(21), 3629. https://doi.org/10.3390/foods14213629








