Arginine-Enhanced Termitomyces Mycelia: Improvement in Growth and Lignocellulose Degradation Capabilities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culture
2.2. Measurement of Physical and Chemical Indicators
2.3. Measurement of Enzyme Activity
2.4. Structural and Chemical Analysis
2.5. RNA Isolation and Sequencing
2.6. Gene Expression Analysis
2.7. Validation of the RNA-Seq Results by RT-qPCR
2.8. Statistics
3. Results and Discussion
3.1. Screening of Vital Amino Acids for Mycelial Growth of Termitomyces
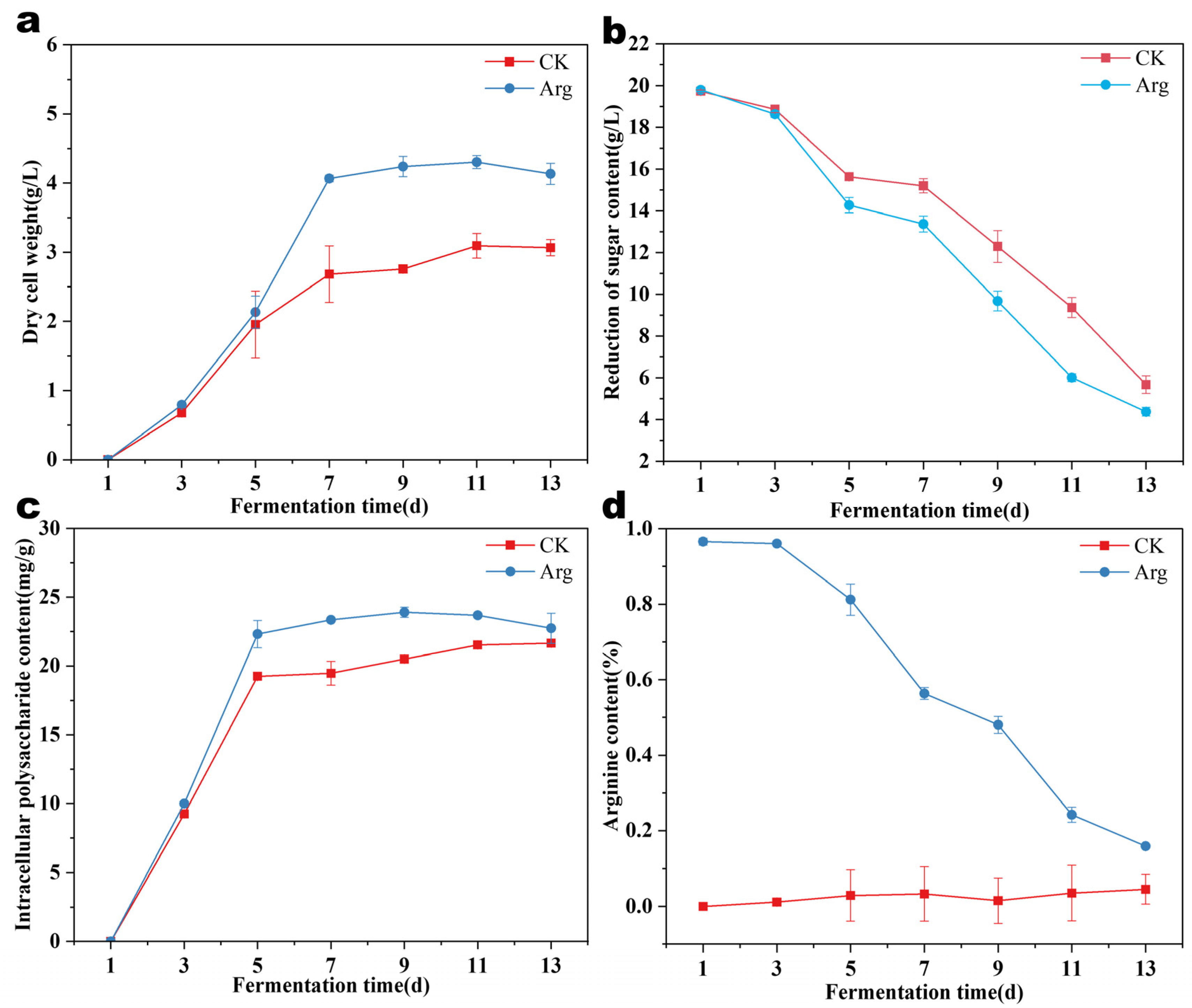
3.2. Effect of Arginine on Physicochemical Indices of Mycelial Growth
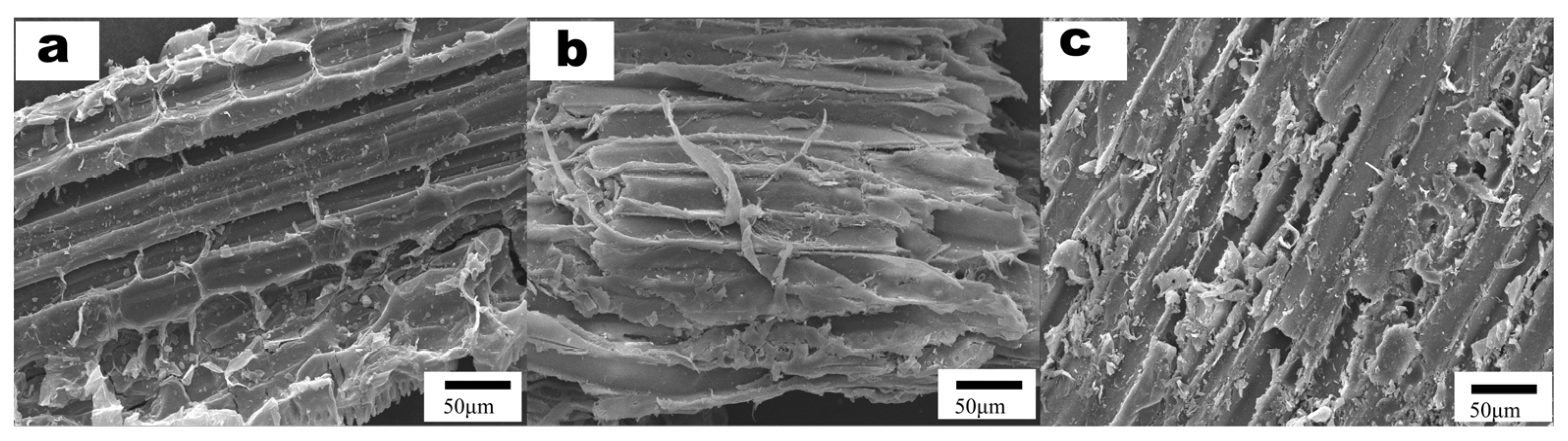
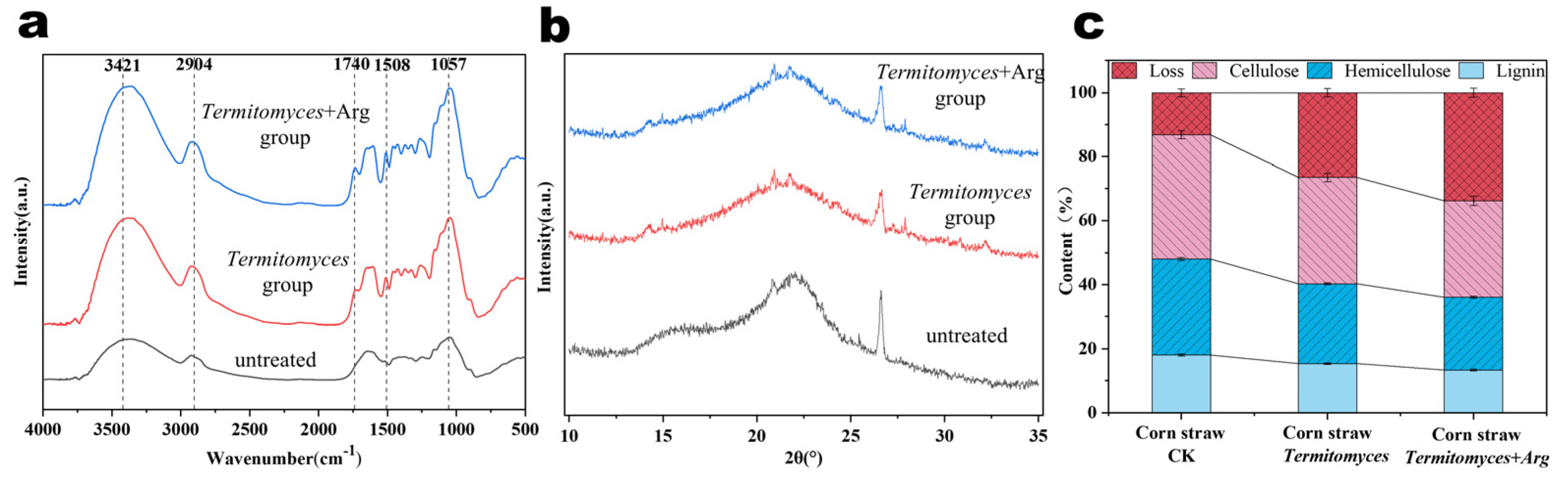
3.3. Characterization and Chemical Compositional Analysis of Corn Straw Structure
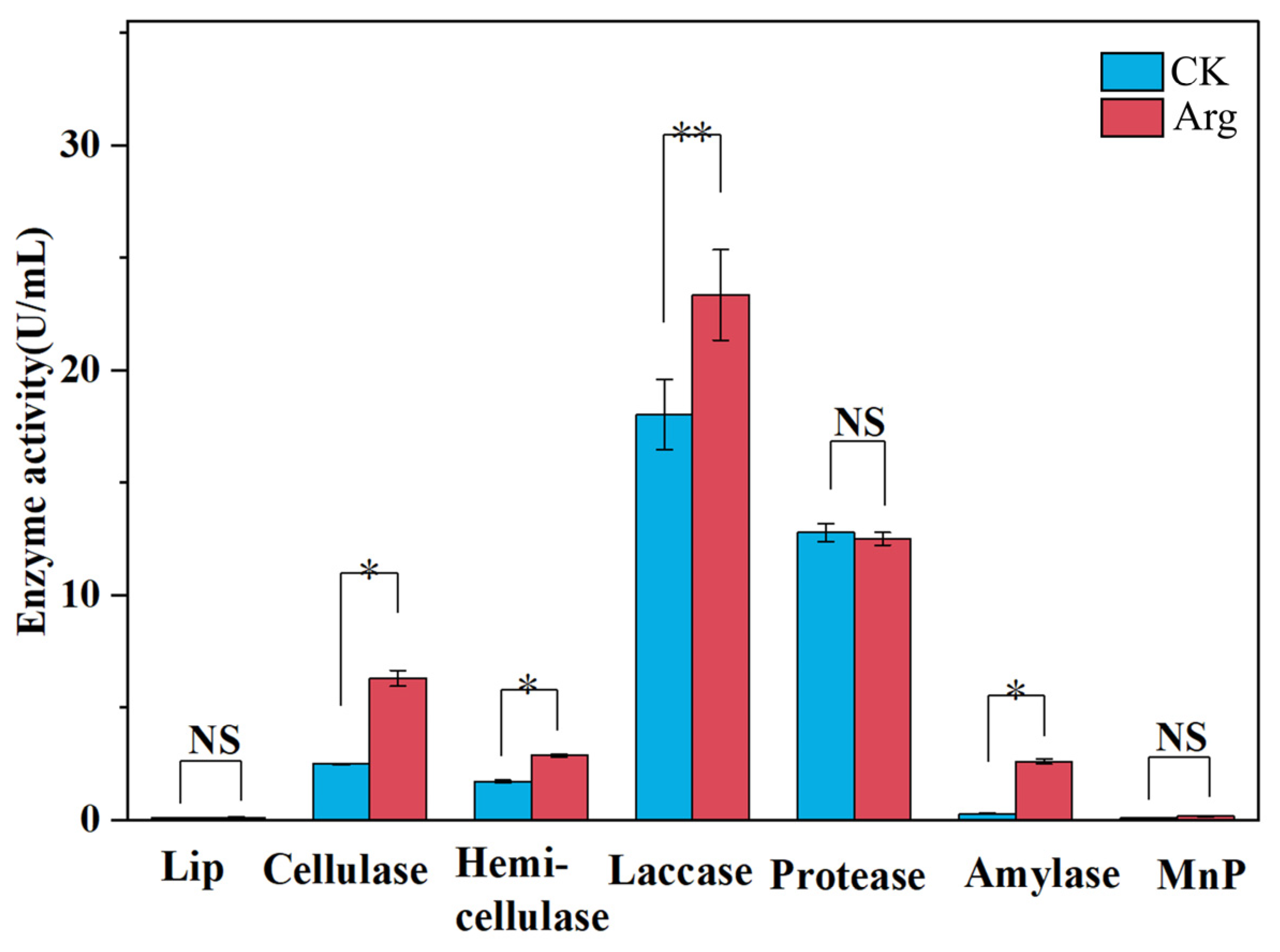
3.4. Enzyme Activity Assay
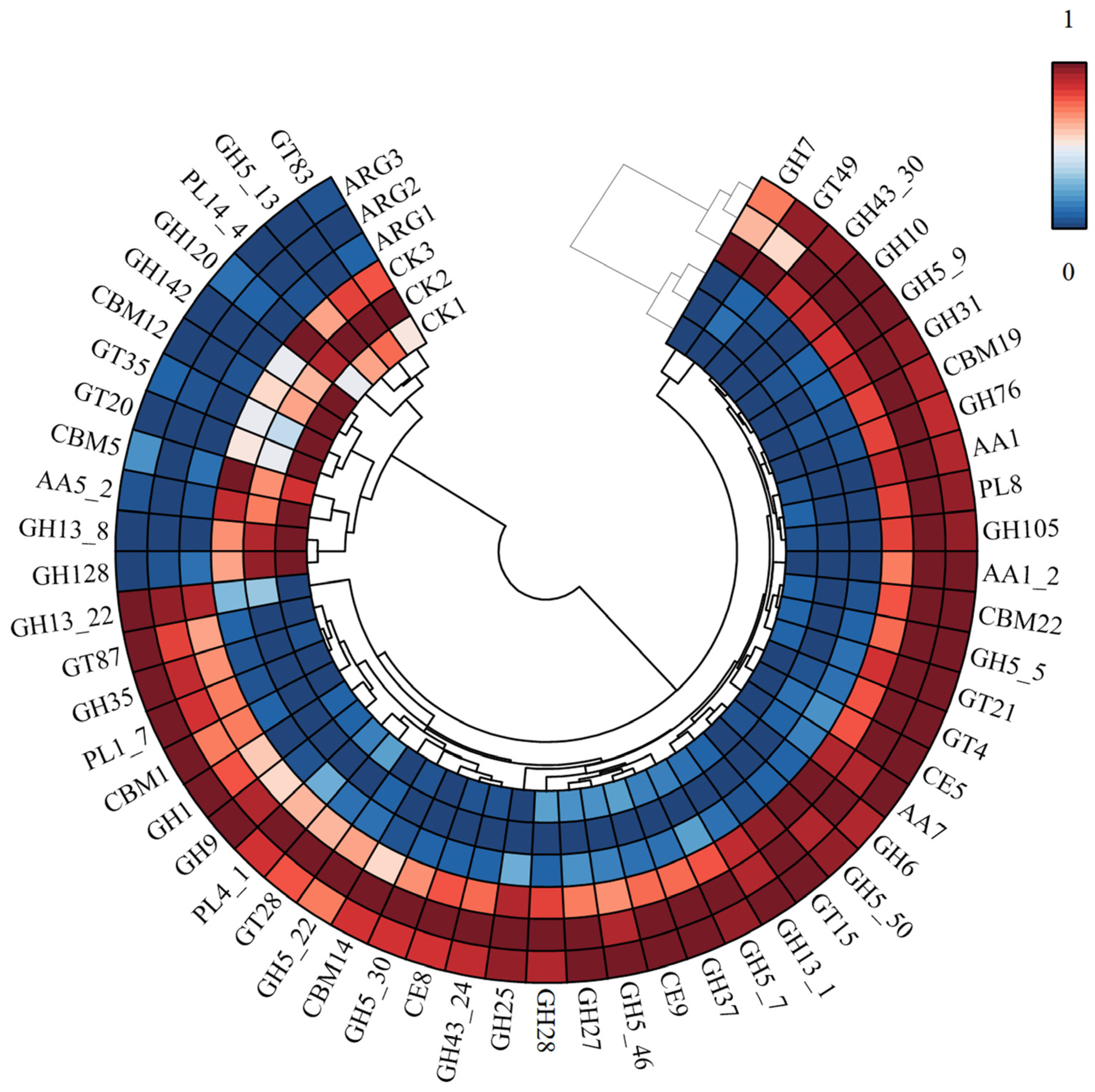
3.5. Analysis of CAZyme DEGs Related to the Decomposition of Corn Straw in Arginine
3.6. GO Enrichment Analysis
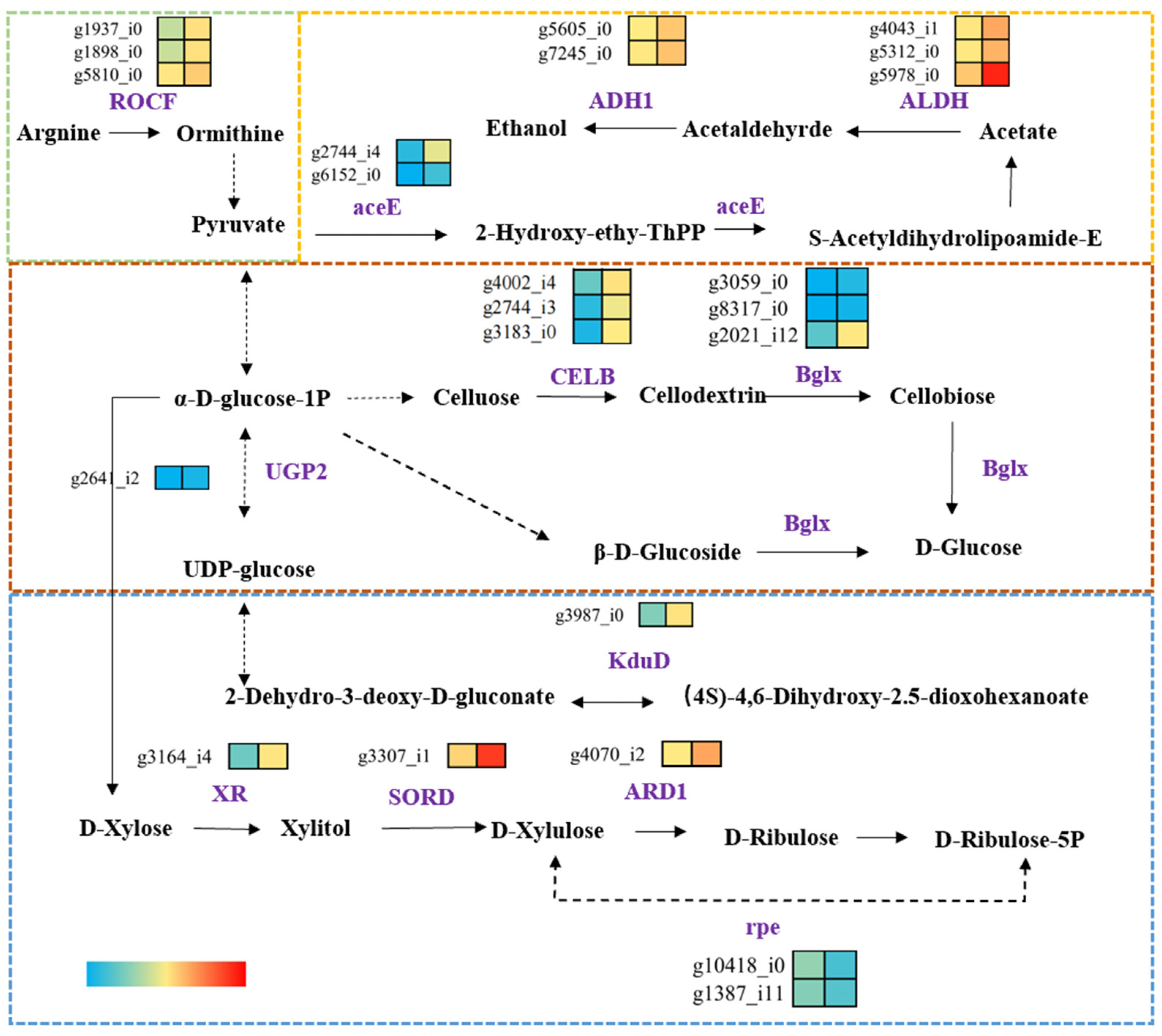
3.7. Analysis of Carbohydrate Metabolic Enrichment Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, G.Y.; Guo, M.X.; Yu, B.T.; Hu, Y.; Mo, J.C. A Review of the Studies of Termite-Symbiotic Termitomyces. Mycosystema 2019, 38, 1747–1759. [Google Scholar] [CrossRef]
- Hojo, M. Distribution Pattern of Termitomyces Types Symbiotic with the Fungus-growing Termite Odontotermes formosanus on Okinawa Island. Entomol. Sci. 2019, 22, 398–403. [Google Scholar] [CrossRef]
- Kreuzenbeck, N.B.; Dhiman, S.; Roman, D.; Burkhardt, I.; Conlon, B.H.; Fricke, J.; Guo, H.; Blume, J.; Görls, H.; Poulsen, M.; et al. Isolation, (Bio)Synthetic Studies and Evaluation of Antimicrobial Properties of Drimenol-Type Sesquiterpenes of Termitomyces Fungi. Commun. Chem. 2023, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Wu, J.; Georgiev, M.I.; Xu, B.; Wong, K.-H.; Bai, W.; Tian, L. Isolation, Structural Properties, and Bioactivities of Polysaccharides from Mushrooms Termitomyces: A Review. J. Agric. Food Chem. 2022, 70, 21–33. [Google Scholar] [CrossRef]
- Tharavecharak, S.; D’Alessandro-Gabazza, C.N.; Toda, M.; Yasuma, T.; Tsuyama, T.; Kamei, I.; Gabazza, E.C. Culture Conditions for Mycelial Growth and Anti-Cancer Properties of Termitomyces. Mycobiology 2023, 51, 94–108. [Google Scholar] [CrossRef]
- Ahmad, F.; Yang, G.; Zhu, Y.; Poulsen, M.; Li, W.; Yu, T.; Mo, J. Tripartite Symbiotic Digestion of Lignocellulose in the Digestive System of a Fungus-Growing Termite. Microbiol. Spectr. 2022, 10, e01234-22. [Google Scholar] [CrossRef]
- da Costa, R.R.; Hu, H.; Pilgaard, B.; Vreeburg, S.M.E.; Schückel, J.; Pedersen, K.S.K.; Kračun, S.K.; Busk, P.K.; Harholt, J.; Sapountzis, P.; et al. Enzyme Activities at Different Stages of Plant Biomass Decomposition in Three Species of Fungus-Growing Termites. Appl. Environ. Microbiol. 2017, 84, e01815–e01817. [Google Scholar] [CrossRef]
- Nobre, T.; Aanen, D.K. Fungiculture or Termite Husbandry? The Ruminant Hypothesis. Insects 2012, 3, 307–323. [Google Scholar] [CrossRef]
- Schalk, F.; Gostinčar, C.; Kreuzenbeck, N.B.; Conlon, B.H.; Sommerwerk, E.; Rabe, P.; Burkhardt, I.; Krüger, T.; Kniemeyer, O.; Brakhage, A.A.; et al. The Termite Fungal Cultivar Termitomyces Combines Diverse Enzymes and Oxidative Reactions for Plant Biomass Conversion. mBio 2021, 12, e03551-20. [Google Scholar] [CrossRef]
- Johjima, T.; Taprab, Y.; Noparatnaraporn, N.; Kudo, T.; Ohkuma, M. Large-Scale Identification of Transcripts Expressed in a Symbiotic Fungus (Termitomyces) During Plant Biomass Degradation. Appl. Microbiol. Biotechnol. 2006, 73, 195–203. [Google Scholar] [CrossRef]
- Ono, K.; Hata, T.; Yoshimura, T.; Kinjo, K. Wood Decaying Properties of the Termite Mushroom Termitomyces eurrhizus. J. Wood Sci. 2017, 63, 83–94. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Wang, X.; Wang, Y.; Song, R.; Yuan, S.; Fan, Z.; Meng, D. Methyl Jasmonate Differentially and Tissue-Specifically Regulated the Expression of Arginine Catabolism–Related Genes and Proteins in Agaricus bisporus Mushrooms During Storage. Fungal Genet. Biol. 2024, 170, 103864. [Google Scholar] [CrossRef]
- Nguyen Van Long, N.; Vasseur, V.; Coroller, L.; Dantigny, P.; Le Panse, S.; Weill, A.; Mounier, J.; Rigalma, K. Temperature, Water Activity and pH during Conidia Production Affect the Physiological State and Germination Time of Penicillium Species. Int. J. Food Microbiol. 2017, 241, 151–160. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, M.; Wang, Q.; Wang, F.; Zheng, H.; Gu, Z.; Li, Y.; Shi, G.; Ding, Z. Development of an Efficient Strategy to Improve Extracellular Polysaccharide Production of Ganoderma Lucidum Using L-Phenylalanine as an Enhancer. Front. Microbiol. 2019, 10, 2306. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Zhang, Y.; Xu, X.; Zhao, Y.; Jiang, X.; Zhang, R.; Gui, Z. Characterization of Cellulose-Degrading Bacteria Isolated from Silkworm Excrement and Optimization of Its Cellulase Production. Polymers 2023, 15, 4142. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Y.; Cheng, X.; He, X.; Bei, Q.; Wang, Y.; Zhou, Y.; Zhu, B.; Zhang, K.; Tian, X.; et al. Straw Mulch Improves Soil Carbon and Nitrogen Cycle by Mediating Microbial Community Structure and Function in the Maize Field. Front. Microbiol. 2023, 14, 1217966. [Google Scholar] [CrossRef]
- Pinto, P.A.; Dias, A.A.; Fraga, I.; Marques, G.; Rodrigues, M.A.M.; Colaço, J.; Sampaio, A.; Bezerra, R.M.F. Influence of Ligninolytic Enzymes on Straw Saccharification during Fungal Pretreatment. Bioresour. Technol. 2012, 111, 261–267. [Google Scholar] [CrossRef]
- Li, N.-J.; Zeng, G.-M.; Huang, D.-L.; Hu, S.; Feng, C.-L.; Zhao, M.-H.; Lai, C.; Huang, C.; Wei, Z.; Xie, G.-X. Oxalate Production at Different Initial Pb2+ Concentrations and the Influence of Oxalate During Solid-State Fermentation of Straw with Phanerochaete chrysosporium. Bioresour. Technol. 2011, 102, 8137–8142. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Biologically Pretreated Sugarcane Top as a Potential Raw Material for the Enhancement of Gaseous Energy Recovery by Two Stage Biohythane Process. Bioresour. Technol. 2016, 218, 1090–1097. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, H.; Meng, F. Pleurotus Ostreatus Decreases Cornstalk Lignin Content, Potentially Improving Its Suitability for Animal Feed. J. Sci. Food Agric. 2016, 97, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, W.; Tong, S.; Zhang, C.; Liu, P. Evaluation of the Effects of Isolated Lignin on Cellulose Enzymatic Hydrolysis of Corn Stover Pretreatment by NaOH Combined with Ozone. Molecules 2018, 23, 1495. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Wojcieszak, D.; Waśkiewicz, A.; Szentner, K.; Przybył, J.; Borysiak, S.; Goliński, P. Chemical and Structural Characterization of Maize Stover Fractions in Aspect of Its Possible Applications. Materials 2021, 14, 1527. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce Acceleration-Supported Software for Integrated Quality Control and Preprocessing of High-Throughput Sequencing Data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B.; et al. TIGR Gene Indices Clustering Tools (TGICL): A Software system for Fast Clustering of Large EST Datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A Universal Tool for Annotation, Visualization and Analysis in Functional Genomics Research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Park, B.H.; Karpinets, T.V.; Syed, M.H.; Leuze, M.R.; Uberbacher, E.C. CAZymes Analysis Toolkit (CAT): Web Service for Searching and Analyzing Carbohydrate-Active Enzymes in a Newly Sequenced Organism Using CAZy Database. Glycobiology 2010, 20, 1574–1584. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Li, J.; Zhang, X.-N.; Li, H.-H.; Xu, X.-F. Comparative Transcriptome Profiling of Termitomyces sp. between Monocultures In Vitro and Link-Stipe of Fungus-Combs In Situ. Lett. Appl. Microbiol. 2022, 74, 429–443. [Google Scholar] [CrossRef]
- Yuan, B.-H.; Li, H.; Liu, L.; Du, X.-H. Successful Induction and Recognition of Conidiation, Conidial Germination and Chlamydospore Formation in Pure Culture of Morchella. Fungal Biol. 2021, 125, 285–293. [Google Scholar] [CrossRef]
- Aanen, D.K. As You Reap, so Shall You Sow: Coupling of Harvesting and Inoculating Stabilizes the Mutualism between Termites and Fungi. Biol. Lett. 2006, 2, 209–212. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Correa, R.C.G.; Peralta, R.A.; Moreira, R.d.F.P.M.; Polizeli, M.d.L.T.d.M.; de Souza, C.G.M.; Peralta, R.M. Biological Pretreatment of Eucalyptus grandis Sawdust with White-Rot Fungi: Study of Degradation Patterns and Saccharification Kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Lu, X.; Li, F.; Zhou, X.; Hu, J.; Liu, P. Biomass, Lignocellulolytic Enzyme Production and Lignocellulose Degradation Patterns by Auricularia Auricula during Solid State Fermentation of Corn Stalk Residues under Different Pretreatments. Food Chem. 2022, 384, 132622. [Google Scholar] [CrossRef]
- Huang, W. Modification of Corn Stover for Improving Biodegradability and Anaerobic Digestion Performance by Ceriporiopsis Subvermispora. Bioresour. Technol. 2019, 283, 76–85. [Google Scholar] [CrossRef]
- Takada, M.; Chandra, R.P.; Saddler, J.N. The Influence of Lignin Migration and Relocation during Steam Pretreatment on the Enzymatic Hydrolysis of Softwood and Corn Stover Biomass Substrates. Biotechnol. Bioeng. 2019, 116, 2864–2873. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, G.; Yang, M.; Yang, X.; Li, T.; Chen, G. De Novo Transcriptome Analysis of Pleurotus djamor to Identify Genes Encoding CAZymes Related to the Decomposition of Corn Stalk Lignocellulose. J. Biosci. Bioeng. 2019, 128, 529–536. [Google Scholar] [CrossRef]
- Gado, J.E.; Harrison, B.E.; Sandgren, M.; Ståhlberg, J.; Beckham, G.T.; Payne, C.M. Machine Learning Reveals Sequence-Function Relationships in Family 7 Glycoside Hydrolases. J. Biol. Chem. 2021, 297, 100931. [Google Scholar] [CrossRef]
- Dahiya, S.; Rapoport, A.; Singh, B. Biotechnological Potential of Lignocellulosic Biomass as Substrates for Fungal Xylanases and Its Bioconversion into Useful Products: A Review. Fermentation 2024, 10, 82. [Google Scholar] [CrossRef]
- Karnaouri, A.; Matsakas, L.; Topakas, E.; Rova, U.; Christakopoulos, P. Development of Thermophilic Tailor-Made Enzyme Mixtures for the Bioconversion of Agricultural and Forest Residues. Front. Microbiol. 2016, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Schink, S.J.; Christodoulou, D.; Mukherjee, A.; Athaide, E.; Brunner, V.; Fuhrer, T.; Bradshaw, G.A.; Sauer, U.; Basan, M. Glycolysis/Gluconeogenesis Specialization in Microbes Is Driven by Biochemical Constraints of Flux Sensing. Mol. Syst. Biol. 2022, 18, e10704. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, W.; Zhou, J.; Xiao, Q.; Zhong, W.; Xu, X. Arginine-Enhanced Termitomyces Mycelia: Improvement in Growth and Lignocellulose Degradation Capabilities. Foods 2025, 14, 361. https://doi.org/10.3390/foods14030361
Yi W, Zhou J, Xiao Q, Zhong W, Xu X. Arginine-Enhanced Termitomyces Mycelia: Improvement in Growth and Lignocellulose Degradation Capabilities. Foods. 2025; 14(3):361. https://doi.org/10.3390/foods14030361
Chicago/Turabian StyleYi, Wenhui, Jingfei Zhou, Qiwei Xiao, Wujie Zhong, and Xuefeng Xu. 2025. "Arginine-Enhanced Termitomyces Mycelia: Improvement in Growth and Lignocellulose Degradation Capabilities" Foods 14, no. 3: 361. https://doi.org/10.3390/foods14030361
APA StyleYi, W., Zhou, J., Xiao, Q., Zhong, W., & Xu, X. (2025). Arginine-Enhanced Termitomyces Mycelia: Improvement in Growth and Lignocellulose Degradation Capabilities. Foods, 14(3), 361. https://doi.org/10.3390/foods14030361






