Frankfurters Manufactured with Valorized Grape Pomace as a Substitute of Nitrifying Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Raw Materials for the Production of Grape Pomace
2.1.2. Manufacturing Process of Grape Pomace
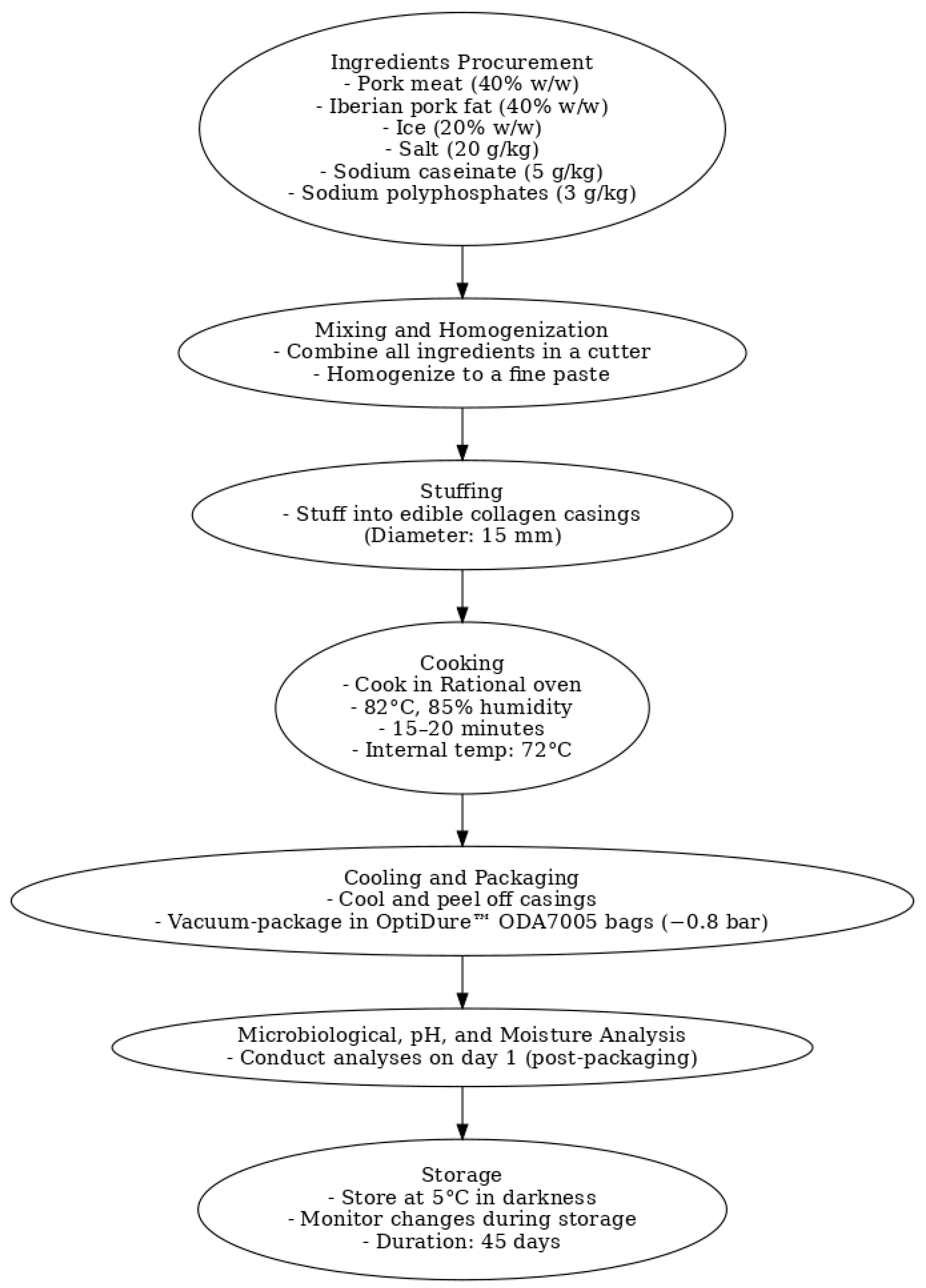
2.1.3. Manufacturing, Packaging, and Storage of Frankfurters
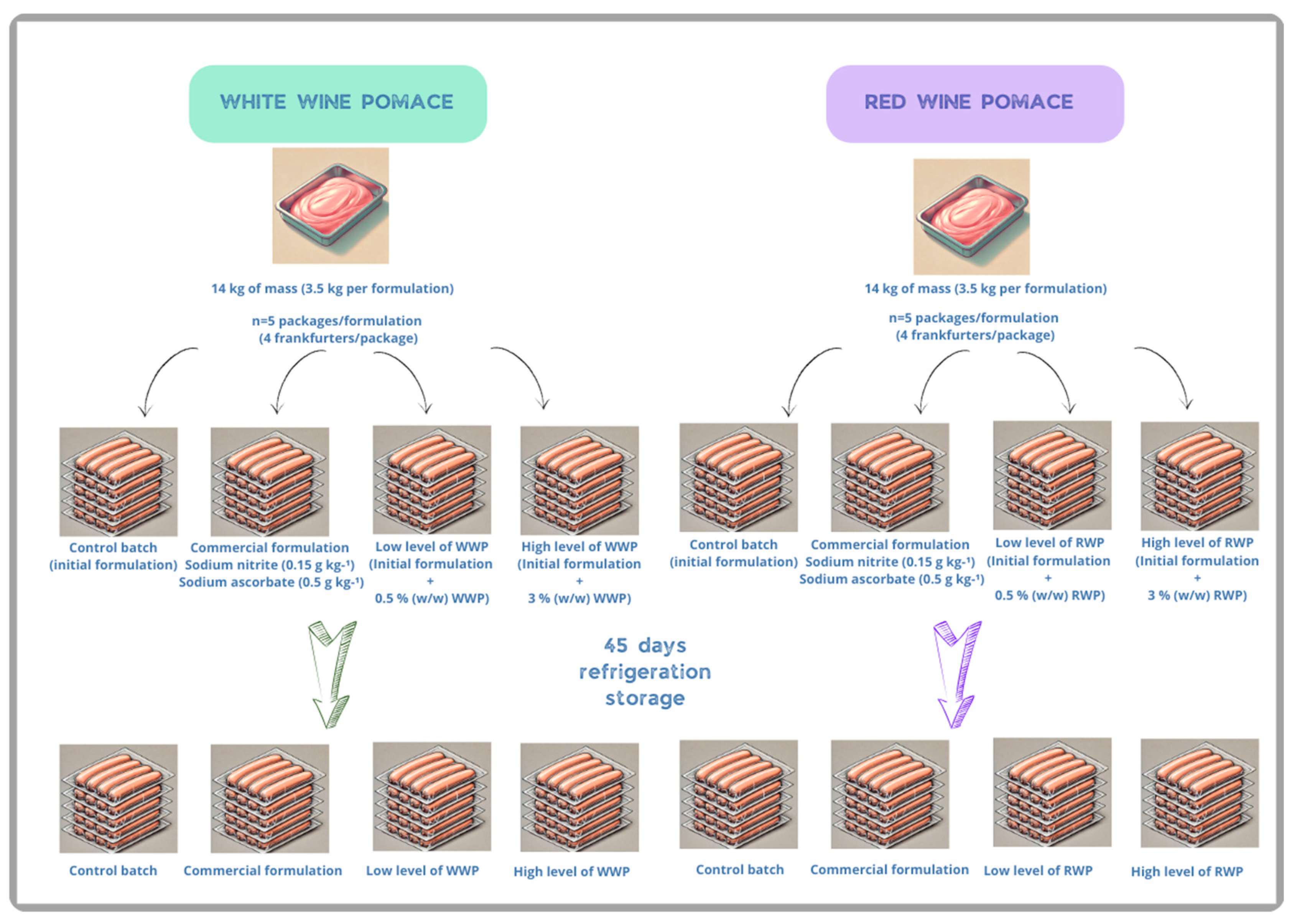
2.2. Experimental Design
2.3. Methods
2.3.1. Physico-Chemical Characterization of Grape Pomace and Frankfurters
2.3.2. Microbiological Analysis of Frankfurters
2.4. Instrumental Color
2.5. Oxidative Stability
2.6. Texture Profile Analysis
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Tejeda, J.F.; Hernández-Matamoros, A.; García-Cascos, J.M.; González, E. Variability in Subcutaneous Fat Composition of Iberian Pigs Reared in Free-Range Conditions in the Southwest of the Iberian Peninsula. Can. J. Anim. Sci. 2020, 100, 665–673. [Google Scholar] [CrossRef]
- Delgado Adámez, J.; Gamero Samino, E.; Valdés Sánchez, E.; González-Gómez, D. In Vitro Estimation of the Antibacterial Activity and Antioxidant Capacity of Aqueous Extracts from Grape-Seeds (Vitis vinifera L.). Food Control 2012, 24, 136–141. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A. Los Polifenoles de Los Alimentos y La Salud; Arán Ediciones: Madrid, Spain, 2003; Volume 10, No. 2; pp. 42–53. [Google Scholar]
- Sachi, K.L.; Bisson, L.F.; Adams, D.O. Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Perez-Manuera, I.; Anton, A.; Lluch, M.A. Extraction and Characterization of the Color in Vinification Residues from Valencia (Spain). Alimentaria 1998, 295, 97–101. [Google Scholar]
- Silva, M.L.; Macedo, A.C.; Malcata, F.X. Review: Steam Distilled Spirits from Fermented Grape Pomace. Food Sci. Technol. Int. 2000, 6, 285–300. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Ladas, D.; Mavromatis, A. Potential Uses and Applications of Treated Wine Waste: A Review. Int. J. Food Sci. Technol. 2006, 41, 475–487. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Martín-Mateos, M.J.; Delgado-Adámez, J.; Moreno-Cardona, D.; Valdés-Sánchez, M.E.; Ramírez-Bernabé, M.R. Application of White-Wine-Pomace-Derived Ingredients in Extending Storage Stability of Fresh Pork Burgers. Foods 2023, 12, 4468. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, R.; Delgado, J.; Rocha-Pimienta, J.; Valdés, M.E.; Martín-Mateos, M.J.; Ayuso-Yuste, M.C. Preservation of White Wine Pomace by High Hydrostatic Pressure. Heliyon 2023, 9, e21199. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Delgado-Adámez, J.; Rocha-Pimienta, J.; Valdés-Sánchez, M.E.; Ramírez-Bernabé, M.R. Integral Use of Red Wine Pomace after Hydrostatic High Pressure: Application of Two Consecutive Cycles of Treatment. Foods 2024, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K. Food Processing by High Hydrostatic Pressure. Biosci. Biotechnol. Biochem. 2017, 81, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Cascaes Teles, A.S.; Hidalgo Chávez, D.W.; Zarur Coelho, M.A.; Rosenthal, A.; Fortes Gottschalk, L.M.; Tonon, R.V. Combination of Enzyme-Assisted Extraction and High Hydrostatic Pressure for Phenolic Compounds Recovery from Grape Pomace. J. Food Eng. 2020, 288, 110128. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Color and Pigment. In Encyclopedia of Meat Sciences; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 244–251. ISBN 9780123847317. [Google Scholar]
- Donald, M.; Gray, J.I.; Gibbins, N. Role of Nitrite in Cured Meat Flavor: Antioxidant Role of Nitrite. J. Food Sci. 1980, 45, 893–897. [Google Scholar] [CrossRef]
- Stoica, M.; Antohi, V.M.; Alexe, P.; Ivan, A.S.; Stanciu, S.; Stoica, D.; Zlati, M.L.; Stuparu-Cretu, M. New Strategies for the Total/Partial Replacement of Conventional Sodium Nitrite in Meat Products: A Review. Food Bioprocess. Technol. 2022, 15, 514–538. [Google Scholar] [CrossRef]
- Ferysiuk, K.; Wójciak, K.M. Reduction of Nitrite in Meat Products through the Application of Various Plant-Based Ingredients. Antioxidants 2020, 9, 711. [Google Scholar] [CrossRef] [PubMed]
- Armenteros, M.; Morcuende, D.; Ventanas, S.; Estévez, M. Application of Natural Antioxidants from Strawberry Tree (Arbutus unedo L.) and Dog Rose (Rosa canina L.) to Frankfurters Subjected to Refrigerated Storage. J. Integr. Agric. 2013, 12, 1972–1981. [Google Scholar] [CrossRef]
- Riazi, F.; Student, M.S.; Zeynali, F.; Hoseini, E.; Behmadi, H. Effect of Dry Red Grape Pomace as a Nitrite Substitute on the Microbiological and Physicochemical Properties and Residual Nitrite of Dry-Cured Sausage. Nutr. Food Sci. Res. 2016, 3, 37–44. [Google Scholar] [CrossRef]
- Özvural, E.B.; Vural, H. Grape Seed Flour Is a Viable Ingredient to Improve the Nutritional Profile and Reduce Lipid Oxidation of Frankfurters. Meat Sci. 2011, 88, 179–183. [Google Scholar] [CrossRef]
- Özvural, E.B.; Vural, H. The Effects of Grape Seed Extract on Quality Characteristics of Frankfurters. J. Food Process. Preserv. 2012, 36, 291–297. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides Fromo Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, M.J.; Barragán, R. Determinación Cuantitativa de La Fracción Hidrocarbonada En Alimentos. Anal. Bromatol. XXXVII 1985, 37, 61–77. [Google Scholar]
- Sorensen, G.; Jorgensen, S.S. A Critical Examination of Some Experimental Variables in the 2.Thiobarbituric Acid (TBA) Test for Lipid Oxidation in Meat Products; Springer: Berlin/Heidelberg, Germany, 1996; Volume 202. [Google Scholar]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-Related Changes in Oxidized Proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [CrossRef]
- Pereira, A.; Lee, H.C.; Lammert, R.; Wolberg, C.; Ma, D.; Immoos, C.; Casassa, F.; Kang, I. Effects of Red-Wine Grape Pomace on the Quality and Sensory Attributes of Beef Hamburger Patty. Int. J. Food Sci. Technol. 2022, 57, 1814–1823. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Goñi, I. Effect of Grape Antioxidant Dietary Fiber on the Lipid Oxidation of Raw and Cooked Chicken Hamburgers. LWT 2009, 42, 971–976. [Google Scholar] [CrossRef]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Overview of Plant Extracts as Natural Preservatives in Meat. J. Food Process. Preserv. 2022, 46, e16796. [Google Scholar] [CrossRef]
- Abdelhakam, O.S.; Elsebaie, E.M.; Ghazi, A.K.; Gouda, M.S. Quality Characteristics of Beef Hamburger Enriched with Red Grape Pomace Powder during Freezing Storage. Slov. Vet. Res. 2019, 56, 333–340. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; Cabeza De Vaca, M.; Vázquez, F.M.; Cava, R. Effect of Production System on Physical-Chemical, Antioxidant and Fatty Acids Composition of Longissimus Dorsi and Serratus Ventralis Muscles from Iberian Pig. Food Chem. 2012, 133, 293–299. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Cava, R. Extensively Reared Iberian Pigs versus Intensively Reared White Pigs for the Manufacture of Frankfurters. Meat Sci. 2006, 72, 356–364. [Google Scholar] [CrossRef]
- Hammad, S.; Pu, S.; Jones, P.J. Current Evidence Supporting the Link Between Dietary Fatty Acids and Cardiovascular Disease. Lipids 2016, 51, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gong, H.; Li, H.; Zhang, Y.; Lan, T.; Chen, Z. Characterization of Chinese Grape Seed Oil by Physicochemical Properties, Fatty Acid Composition, Triacylglycrol Profiles, and Sterols and Squalene Composition. Int. J. Food Eng. 2019, 15, 20190031. [Google Scholar] [CrossRef]
- Skibsted, L.H. Nitric Oxide and Quality and Safety of Muscle Based Foods. Nitric Oxide 2011, 24, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Hesari, J.; Nemati, Z.; Munekata, P.E.S.; Barba, F.J.; Lorenzo, J.M. Combined Effect of Natural Antioxidants and Antimicrobial Compounds during Refrigerated Storage of Nitrite-Free Frankfurter-Type Sausage. Food Res. Int. 2019, 120, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.K.; Kim, Y.J.; Park, J.H.; Hur, I.C.; Nam, S.H.; Shin, D. Effects of Purple-Fleshed Sweet Potato (Ipomoera Batatas Cultivar Ayamurasaki) Powder Addition on Color and Texture Properties and Sensory Characteristics of Cooked Pork Sausages during Storage. Asian-Australas. J. Anim. Sci. 2012, 25, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Park, J.; Lee, Y.; Do, B.; Lee, J.; Kwon, H. Effect of Cooking Method on the Concentrations of Volatile N-Nitrosamines in Various Food Products. J. Food Process. Preserv. 2022, 46, e16590. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of Plant Extracts on Microbial Growth, Color Change, and Lipid Oxidation in Cooked Beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Thimothe, J.; Bonsi, I.A.; Padilla-Zakour, O.I.; Koo, H. Chemical Characterization of Red Wine Grape (Vitis vinifera and Vitis Interspecific Hybrids) and Pomace Phenolic Extracts and Their Biological Activity against Streptococcus mutans. J. Agric. Food Chem. 2007, 55, 10200–10207. [Google Scholar] [CrossRef] [PubMed]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and Antimicrobial Properties of Grape Seed Extract, Nisin, and EDTA Incorporated Soy Protein Edible Films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Chung, K.-T.; Lu, Z.; Chou, M.W. Mechanism of Inhibition of Tannic Acid and Related Compounds on the Growth of Intestinal Bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Shibambo, S.L. The Anti-Fungal and Anti-Oxidant Properties of Polyphenols Extracted from the Resurrection Plants. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2008. [Google Scholar]
- Scalbert, A. Antimicrobial Properties of Tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Higuero, N.; Moreno, I.; Lavado, G.; Vidal-Aragón, M.C.; Cava, R. Reduction of Nitrate and Nitrite in Iberian Dry Cured Loins and Its Effects during Drying Process. Meat Sci. 2020, 163, 108062. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.S.; Shim, K.S.; Shin, D. Effect of Grape Pomace Powder Addition on TBARS and Color of Cooked Pork Sausages during Storage. Korean J. Food Sci. Anim. Resour. 2014, 34, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Carrapiso, A.I.; Martín-Mateos, M.J.; D’Arrigo, M.; Delgado-Adámez, J.; Saraiva, J.A.; Ramírez-Bernabé, M.R. High-Hydrostatic-Pressure-Stabilized White Grape Pomace to Improve the Oxidative Stability of Dry-Cured Sausages (“Salchichón”). Foods 2024, 13, 687. [Google Scholar] [CrossRef]
- D’Arrigo, M.; Petrón, M.J.; Delgado-Adámez, J.; García-Parra, J.J.; Martín-Mateos, M.J.; Ramírez-Bernabé, M.R. Dry-Cured Sausages “Salchichón” Manufactured with a Valorized Ingredient from Red Grape Pomace (Var. Tempranillo). Foods 2024, 13, 3133. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol Screening of Pomace from Red and White Grape Varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Jara-Palacios, M.J.; Hernández-Hierro, J.M.; Heredia, F.J. Evaluation of the Influence of White Grape Seed Extracts as Copigment Sources on the Anthocyanin Extraction from Grape Skins Previously Classified by near Infrared Hyperspectral Tools. Food Chem. 2017, 221, 1685–1690. [Google Scholar] [CrossRef]
- Stevanato, R.; Fabris, S.; Momo, F. New Enzymatic Method for the Determination of Total Phenolic Content in Tea and Wine. J. Agric. Food Chem. 2004, 52, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.; O’Grady, M.N.; O’Callaghan, Y.C.; O’Brien, N.M.; Kerry, J.P. Evaluation of the Antioxidant Potential of Grape Seed and Bearberry Extracts in Raw and Cooked Pork. Meat Sci. 2007, 76, 604–610. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Health Aspects of Functional Grape Seed Constituents. Trends Food Sci. Technol. 2004, 15, 422–433. [Google Scholar] [CrossRef]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Enzymatic Synthesis of Hydrophilic and Hydrophobic Derivatives of Natural Phenolic Acids in Organic Media. J. Mol. Catal. B Enzym. 2001, 11, 323–328. [Google Scholar] [CrossRef]
- Jongberg, S.; Skov, S.H.; Tørngren, M.A.; Skibsted, L.H.; Lund, M.N. Effect of White Grape Extract and Modified Atmosphere Packaging on Lipid and Protein Oxidation in Chill Stored Beef Patties. Food Chem. 2011, 128, 276–283. [Google Scholar] [CrossRef]
- Estevez, M.; Ventanas, S.; Cava, R. Protein Oxidation in Frankfurters with Increasing Levels of Added Rosemary Essential Oil: Effect on Color and Texture Deterioration. J. Food Sci. 2005, 70, c427–c432. [Google Scholar] [CrossRef]
- Feng, X.; Li, C.; Jia, X.; Guo, Y.; Lei, N.; Hackman, R.M.; Chen, L.; Zhou, G. Influence of Sodium Nitrite on Protein Oxidation and Nitrosation of Sausages Subjected to Processing and Storage. Meat Sci. 2016, 116, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Vossen, E.; De Smet, S. Protein Oxidation and Protein Nitration Influenced by Sodium Nitrite in Two Different Meat Model Systems. J. Agric. Food Chem. 2015, 63, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Berardo, A.; Claeys, E.; Vossen, E.; Leroy, F.; De Smet, S. Protein Oxidation Affects Proteolysis in a Meat Model System. Meat Sci. 2015, 106, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.; Parra, V.; Estévez, M. Oxidative and Nitrosative Stress Induced in Myofibrillar Proteins by a Hydroxyl-Radical-Generating System: Impact of Nitrite and Ascorbate. J. Agric. Food Chem. 2014, 62, 2158–2164. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P.; Abad, A.; Pegg, R.B. Food and Bioactive Encapsulation. In Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2020; pp. 529–596. [Google Scholar]
- Stadtman, E.R.; Levine, R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein Oxidation in Aging, Disease, and Oxidative Stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.-L.; Kang, T.U.; Guo, L.-Y.; Yang, J.-L.; Wang, H.; Chen, Y.-Y. The effect of sodium nitrite on the textural properties of cooked sausage during cold storage. J. Texture Stud. 2007, 38, 537–554. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein Oxidation in Muscle Foods: A Review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef] [PubMed]
| Red Wine Pomace | White Wine Pomace | p-Value | |
|---|---|---|---|
| pH | 3.94 ± 0.03 | 3.96 ± 0.03 | ns |
| Aw | 0.980 ± 0.003 | 0.971 ± 0.013 | ns |
| Proximate composition (g 100 g−1) | |||
| Moisture | 57.4 ± 0.6 | 69.2 ± 1.5 | ns |
| Protein | 4.3 ± 0.6 | 2.3 ± 0.2 | ns |
| Fiber | 25.4 ± 1.7 | 18.0 ± 0.9 | * |
| Fat | 3.9 ± 0.2 | 1.7 ± 0.1 | ** |
| Phenolic compounds (mg 100 g−1) | 486.0 ± 24.5 | 766.7 ± 15.6 | ** |
| Antioxidant activity (mM Trolox mL−1) | 45.3 ± 3.5 | 65.9 ± 1.9 | ** |
| Control | Commercial | Low Level | High Level | p-Formulation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine pomace (WWP) | ||||||||||||||
| Moisture | 46.7 | ± | 1.2 | 46.8 | ± | 2.0 | 46.1 | ± | 1.8 | 47.9 | ± | 1.8 | ns | |
| Protein | 15.1 | ± | 1.4 | 13.7 | ± | 0.9 | 13.7 | ± | 1.5 | 14.5 | ± | 0.4 | ns | |
| Fat | 20.1 | ± | 3.7 | 21.2 | ± | 1.7 | 19.7 | ± | 1.8 | 22.4 | ± | 1.4 | ns | |
| pH | 5.94b | ± | 0.02 | 5.98a | ± | 0.01 | 5.94b | ± | 0.01 | 5.86c | ± | 0.01 | *** | |
| Fatty acids profile (%) | ||||||||||||||
| Lauric ac. (C12:0) | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | ns | |
| Myristic ac. (C14:0) | 1.2 | ± | 0.0 | 1.2 | ± | 0.0 | 1.2 | ± | 0.1 | 1.2 | ± | 0.2 | ns | |
| Palmitic ac. (C16:0) | 22.2 | ± | 0.1 | 22.1 | ± | 0.2 | 22.8 | ± | 0.9 | 22.8 | ± | 1.4 | ns | |
| Palmitoleic ac. (C16:1) | 1.9 | ± | 0.0 | 1.9 | ± | 0.0 | 2.0 | ± | 0.1 | 1.8 | ± | 0.1 | ns | |
| Margaric ac. (C17:0) | 0.3 | ± | 0.0 | 0.2 | ± | 0.0 | 0.3 | ± | 0.0 | 0.3 | ± | 0.1 | ns | |
| Margaroleic ac. (C17:1) | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | ns | |
| Stearic ac. (C18:0) | 11.6 | ± | 0.0 | 11.3 | ± | 0.1 | 11.7 | ± | 0.3 | 11.6 | ± | 0.5 | ns | |
| Oleic ac. (C18:1) | 50.8ab | ± | 0.1 | 51.0a | ± | 0.2 | 50.0b | ± | 0.5 | 50.1ab | ± | 0.9 | * | |
| Linoleic ac. (C18:2) | 9.9 | ± | 0.1 | 10.0 | ± | 0.1 | 10.0 | ± | 0.2 | 10.0 | ± | 0.2 | ns | |
| Linolenic ac. (C18:3) | 0.4b | ± | 0.0 | 0.5ab | ± | 0.0 | 0.5a | ± | 0.0 | 0.5b | ± | 0.0 | ** | |
| Arachidic ac. (C20:0) | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | ns | |
| Gadoleic ac. (C20:1) | 1.4 | ± | 0.0 | 1.4 | ± | 0.0 | 1.2 | ± | 0.2 | 1.3 | ± | 0.3 | ns | |
| White wine pomace (WWP) | ||||||||||||||
| Moisture | 44.8 | ± | 1.1 | 43.0 | ± | 0.6 | 43.1 | ± | 1.4 | 43.9 | ± | 0.8 | ns | |
| Protein | 13.6 | ± | 0.7 | 13.4 | ± | 0.9 | 13.3 | ± | 0.8 | 13.6 | ± | 0.7 | ns | |
| Fat | 22.2a | ± | 1.7 | 19.1b | ± | 2.1 | 20.1ab | ± | 1.1 | 17.1b | ± | 1.9 | ** | |
| pH | 5.90b | ± | 0.02 | 5.93a | ± | 0.01 | 5.94a | ± | 0.00 | 5.86c | ± | 0.00 | *** | |
| Fatty acids profile (%) | ||||||||||||||
| Lauric ac. (C12:0) | 0.1a | ± | 0.0 | 0.1b | ± | 0.0 | 0.1b | ± | 0.0 | 0.1b | ± | 0.0 | *** | |
| Myristic ac. (C14:0) | 1.3a | ± | 0.0 | 1.2c | ± | 0.0 | 1.2bc | ± | 0.0 | 1.2b | ± | 0.0 | *** | |
| Palmitic ac. (C16:0) | 27.9a | ± | 3.3 | 22.2b | ± | 0.4 | 22.5b | ± | 0.5 | 22.8b | ± | 0.2 | *** | |
| Palmitoleic ac. (C16:1) | 1.9b | ± | 0.2 | 1.9ab | ± | 0.0 | 2.0b | ± | 0.1 | 2.1a | ± | 0.0 | * | |
| Margaric ac. (C17:0) | 0.3a | ± | 0.0 | 0.3b | ± | 0.0 | 0.3b | ± | 0.0 | 0.3b | ± | 0.0 | *** | |
| Margaroleic ac. (C17:1) | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | 0.2 | ± | 0.0 | ns | |
| Stearic ac. (C18:0) | 14.1a | ± | 1.8 | 11.7b | ± | 0.2 | 11.5b | ± | 0.1 | 11.1b | ± | 0.1 | *** | |
| Oleic ac. (C18:1) | 43.3b | ± | 3.9 | 50.7a | ± | 0.5 | 50.9a | ± | 0.4 | 50.3a | ± | 0.3 | *** | |
| Linoleic ac. (C18:2) | 9.5 | ± | 0.9 | 9.8 | ± | 0.1 | 9.5 | ± | 0.0 | 10.2 | ± | 0.0 | ns | |
| Linolenic ac. (C18:3) | 0.5ab | ± | 0.1 | 0.5ab | ± | 0.0 | 0.4b | ± | 0.0 | 0.5a | ± | 0.0 | * | |
| Arachidic ac. (C20:0) | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | ns | |
| Gadoleic ac. (C20:1) | 0.9c | ± | 0.1 | 1.3a | ± | 0.0 | 1.3a | ± | 0.1 | 1.2b | ± | 0.0 | *** | |
| Control | Commercial | Low Level | High Level | p-Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine pomace (RWP) | |||||||||||||
| Mesophiles | 1.7 | ± | 0.9 | 2.0 | ± | 0.9 | 2.4 | ± | 0.6 | 1.8 | ± | 0.6 | ns |
| Psychrophiles | 0.9 | ± | 0.5 | 1.0 | ± | 0.2 | 1.0 | ± | 0.2 | >1 | ns | ||
| Molds and yeasts | 1.1 | ± | 0.2 | 1.1 | ± | 0.3 | 1.2 | ± | 0.4 | 0.9 | ± | 0.0 | ns |
| S. aureus | 2.0 | ± | 0.2 | <2 | 2.1 | ± | 0.3 | 2.1 | ± | 0.2 | ns | ||
| Cl. perfringens | <2 | <2 | <2 | <2 | ns | ||||||||
| Total coliforms | 1.1 | ± | 0.4 | <1 | 0.9 | ± | 0.0 | <1 | ns | ||||
| E. coli | <1 | <1 | 0.9 | ± | 0.0 | <1 | ns | ||||||
| White wine pomace (WWP) | |||||||||||||
| Mesophiles | 2.5 | ± | 0.5 | 2.2 | ± | 0.2 | 2.9 | ± | 0.5 | 2.9 | ± | 0.7 | ns |
| Psychrophiles | <1 | <1 | 1.1 | ± | 0.2 | 1.0 | ± | 0.3 | ns | ||||
| Molds and yeasts | 1.2 | ± | 0.6 | <1 | 1.0 | ± | 0.0 | 1.1 | ± | 0.4 | ns | ||
| S. aureus | 2.1 | ± | 0.2 | 2.0 | ± | 0.2 | 2.1 | ± | 0.2 | <2 | ns | ||
| Cl. perfringens | <2 | <2 | <2 | <2 | ns | ||||||||
| Total coliforms | <1 | <1 | 0.9 | ± | 0.0 | 1.2 | ± | 0.6 | ns | ||||
| E. coli | <1 | <1 | <1 | <1 | ns | ||||||||
| Storage (Days) | Control | Commercial | Low Level | High Level | p-Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine pomace (RWP) | ||||||||||||||
| L* | T0 | 69.9a | ± | 1.5 | 69.6a | ± | 0.9 | 65.7b | ± | 1.9 | 59.9c | ± | 0.6 | *** |
| T1 | 68.4a | ± | 1.3 | 70.4a | ± | 1.0 | 68.5a | ± | 2.0 | 60.3b | ± | 0.5 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| a* | T0 | 2.0b | ± | 0.1 | 8.3a | ± | 0.2 | 1.4c | ± | 0.1 | 1.9b | ± | 0.1 | *** |
| T1 | 2.4b | ± | 0.1 | 8.3a | ± | 0.2 | 1.7d | ± | 0.3 | 2.1c | ± | 0.1 | *** | |
| p-storage | *** | ns | ns | * | ||||||||||
| b* | T0 | 14.3a | ± | 0.1 | 10.8c | ± | 0.2 | 12.3b | ± | 0.6 | 8.7d | ± | 0.2 | *** |
| T1 | 14.0a | ± | 0.3 | 10.5c | ± | 0.2 | 11.6b | ± | 0.3 | 8.5d | ± | 0.2 | *** | |
| p-storage | * | ns | ns | ns | ||||||||||
| Chroma | T0 | 14.4a | ± | 0.1 | 13.6b | ± | 0.3 | 12.3c | ± | 0.6 | 8.9d | ± | 0.2 | *** |
| T1 | 14.2a | ± | 0.3 | 13.4b | ± | 0.2 | 11.8c | ± | 0.3 | 8.8d | ± | 0.2 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Hue | T0 | 82.1b | ± | 0.4 | 52.5d | ± | 0.3 | 83.4a | ± | 0.3 | 77.5c | ± | 0.7 | *** |
| T1 | 80.1b | ± | 0.2 | 51.9d | ± | 0.4 | 81.6a | ± | 1.3 | 76.3c | ± | 0.7 | *** | |
| p-storage | *** | * | * | * | ||||||||||
| White wine pomace (WWP) | ||||||||||||||
| L* | T0 | 71.5a | ± | 1.1 | 66.7c | ± | 0.9 | 68.6b | ± | 1.7 | 66.3c | ± | 0.7 | *** |
| T1 | 72.5a | ± | 0.9 | 67.4c | ± | 1.2 | 69.5b | ± | 0.7 | 66.5c | ± | 0.8 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| a* | T0 | 1.5c | ± | 0.1 | 8.4a | ± | 0.4 | 1.7c | ± | 0.0 | 2.7b | ± | 0.1 | *** |
| T1 | 1.7d | ± | 0.2 | 8.2a | ± | 0.5 | 2.2c | ± | 0.2 | 2.8b | ± | 0.1 | *** | |
| p-storage | ** | ns | *** | ns | ||||||||||
| b* | T0 | 14.3a | ± | 0.1 | 9.9d | ± | 0.2 | 12.9c | ± | 0.3 | 13.7b | ± | 0.1 | *** |
| T1 | 14.0a | ± | 0.3 | 10.1d | ± | 0.2 | 12.7c | ± | 0.1 | 13.5b | ± | 0.3 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Chroma | T0 | 14.4a | ± | 0.1 | 13.0c | ± | 0.4 | 13.0c | ± | 0.3 | 13.9b | ± | 0.1 | *** |
| T1 | 14.1a | ± | 0.3 | 13.1b | ± | 0.3 | 12.9b | ± | 0.1 | 13.8a | ± | 0.3 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Hue | T0 | 84.2a | ± | 0.3 | 49.7d | ± | 0.9 | 82.6b | ± | 0.1 | 78.9c | ± | 0.2 | *** |
| T1 | 83.0a | ± | 0.7 | 51.0d | ± | 2.0 | 80.3b | ± | 0.9 | 78.1c | ± | 0.8 | *** | |
| p-storage | ** | ns | *** | ns | ||||||||||
| Storage (Days) | Control | Commercial | Low Level | High Level | p-Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine pomace (RWP) | ||||||||||||||
| Lipid oxidation | T0 | 1.0a | ± | 0.1 | 0.1c | ± | 0.0 | 0.2b | ± | 0.0 | 0.1ab | ± | 0.0 | *** |
| T1 | 1.0a | ± | 0.1 | 0.0d | ± | 0.0 | 0.4b | ± | 0.0 | 0.1c | ± | 0.0 | *** | |
| p-storage | ns | ns | *** | ns | ||||||||||
| Protein oxidation | T0 | 2.7a | ± | 0.6 | 2.5a | ± | 0.5 | 1.7b | ± | 0.2 | 1.6b | ± | 0.3 | *** |
| T1 | 2.7a | ± | 0.6 | 2.7a | ± | 0.4 | 1.8b | ± | 0.4 | 1.7b | ± | 0.1 | *** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Phenolic compounds | T0 | 10.4c | ± | 1.3 | 60.3a | ± | 3.6 | 15.1b | ± | 1.2 | 18.1b | ± | 0.3 | *** |
| T1 | 11.6d | ± | 1.3 | 69.3a | ± | 3.2 | 15.0c | ± | 1.4 | 22.5b | ± | 1.1 | *** | |
| p-storage | ns | ** | ns | *** | ||||||||||
| White wine pomace(WWP) | ||||||||||||||
| Lipid oxidation | T0 | 0.6a | ± | 0.1 | 0.0b | ± | 0.0 | 0.2b | ± | 0.0 | 0.1b | ± | 0.0 | *** |
| T1 | 0.6a | ± | 0.0 | 0.1d | ± | 0.1 | 0.4b | ± | 0.0 | 0.2c | ± | 0.0 | *** | |
| p-storage | ns | ns | *** | *** | ||||||||||
| Protein oxidation | T0 | 2.5a | ± | 0.5 | 2.3a | ± | 0.2 | 1.7b | ± | 0.4 | 1.5b | ± | 0.2 | *** |
| T1 | 1.8b | ± | 0.3 | 5.6a | ± | 0.6 | 2.5b | ± | 1.0 | 2.2b | ± | 0.5 | *** | |
| * | *** | ns | * | |||||||||||
| Phenolic compounds | T0 | 17.6b | ± | 3.6 | 67.6a | ± | 5.0 | 14.4b | ± | 1.6 | 19.4b | ± | 3.1 | *** |
| T1 | 14.b | ± | 3.2 | 42.6a | ± | 3.9 | 13.7b | ± | 2.1 | 13.2b | ± | 2.3 | *** | |
| p-storage | *** | *** | ns | ** | ||||||||||
| Storage (Days) | Control | Commercial | Low Level | High Level | p-Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red wine pomace (RWP) | ||||||||||||||
| Hardness (N cm2) | T0 | 15.8ab | ± | 1.5 | 17.1ab | ± | 0.2 | 14.6b | ± | 1.9 | 17.8a | ± | 0.5 | * |
| T1 | 19.6b | ± | 2.6 | 25.5a | ± | 2.8 | 16.7b | ± | 0.8 | 19.0 | ± | 2.2 | ** | |
| p-storage | ns | ** | ns | ns | ||||||||||
| Springiness (cm) | T0 | 0.8ab | ± | 0.0 | 0.9a | ± | 0.0 | 0.8ab | ± | 0.0 | 0.7b | ± | 0.0 | * |
| T1 | 0.9 | ± | 0.0 | 0.9 | ± | 0.0 | 0.9 | ± | 0.0 | 0.9 | ± | 0.0 | ns | |
| p-storage | ns | ns | ** | * | ||||||||||
| Cohesiveness | T0 | 0.4b | ± | 0.0 | 0.5a | ± | 0.0 | 0.4ab | ± | 0.0 | 0.4b | ± | 0.0 | * |
| T1 | 0.4b | ± | 0.0 | 0.5a | ± | 0.0 | 0.4bc | ± | 0.0 | 0.3c | ± | 0.0 | ** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Gumminess (N cm s2) | T0 | 6.0 | ± | 1.0 | 7.8 | ± | 0.6 | 5.8 | ± | 1.1 | 6.4 | ± | 0.1 | ns |
| T1 | 8.0b | ± | 0.8 | 12.3a | ± | 1.8 | 6.4b | ± | 0.4 | 6.5b | ± | 0.8 | ** | |
| p-storage | ns | * | ns | ns | ||||||||||
| Chewiness (N cm s2) | T0 | 4.8b | ± | 0.7 | 6.6a | ± | 0.6 | 4.6b | ± | 0.8 | 4.7b | ± | 0.3 | * |
| T1 | 6.8b | ± | 0.5 | 10.9a | ± | 1.9 | 5.6b | ± | 0.5 | 5.6b | ± | 0.8 | ** | |
| p-storage | * | * | ns | ns | ||||||||||
| Resilience | T0 | 0.1b | ± | 0.0 | 0.2a | ± | 0.0 | 0.1b | ± | 0.0 | 0.1b | ± | 0.0 | ** |
| T1 | 0.2b | ± | 0.0 | 0.2a | ± | 0.0 | 0.2b | ± | 0.0 | 0.1b | ± | 0.0 | ** | |
| p-storage | ns | ns | ns | ns | ||||||||||
| White wine pomace (WWP) | ||||||||||||||
| Hardness (N cm2) | T0 | 17.8b | ± | 0.5 | 20.4a | ± | 1.0 | 17.7b | ± | 1.0 | 17.6b | ± | 0.1 | ns |
| T1 | 23.5b | ± | 0.5 | 28.7a | ± | 0.7 | 23.7b | ± | 0.5 | 23.2b | ± | 0.1 | *** | |
| p-storage | *** | *** | ** | *** | ||||||||||
| Springiness (cm) | T0 | 0.8 | ± | 0.0 | 0.9 | ± | 0.0 | 0.8 | ± | 0.0 | 0.9 | ± | 0.0 | ns |
| T1 | 0.9 | ± | 0.0 | 0.9 | ± | 0.1 | 0.9 | ± | 0.0 | 0.9 | ± | 0.0 | ns | |
| p-storage | ns | ns | ns | ns | ||||||||||
| Cohesiveness | T0 | 0.5b | ± | 0.0 | 0.6a | ± | 0.0 | 0.4b | ± | 0.0 | 0.4b | ± | 0.0 | ** |
| T1 | 0.5bc | ± | 0.0 | 0.6a | ± | 0.0 | 0.5b | ± | 0.0 | 0.4c | ± | 0.0 | *** | |
| p-storage | ns | ns | * | ns | ||||||||||
| Gumminess (N cm s2) | T0 | 8.6b | ± | 1.0 | 11.7a | ± | 0.7 | 7.0b | ± | 0.3 | 7.5b | ± | 0.5 | *** |
| T1 | 11.1b | ± | 0.6 | 16.8a | ± | 0.4 | 12.1b | ± | 0.4 | 10.1c | ± | 0.3 | *** | |
| p-storage | * | *** | *** | ** | ||||||||||
| Chewiness (N cm s2) | T0 | 7.2b | ± | 0.7 | 10.7a | ± | 1.37b | 5.9b | ± | 0.2 | 6.2b | ± | 0.3 | *** |
| T1 | 9.5b | ± | 0.9 | 15.2a | ± | 0.6 | 10.3b | ± | 0.7 | 8.9b | ± | 0.5 | *** | |
| p-storage | * | ** | ** | ** | ||||||||||
| Resilience | T0 | 0.2b | ± | 0.0 | 0.3a | ± | 0.0 | 0.2b | ± | 0.0 | 0.2b | ± | 0.0 | *** |
| T1 | 0.2c | ± | 0.0 | 0.3a | ± | 0.0 | 0.2b | ± | 0.0 | 0.2c | ± | 0.0 | *** | |
| p-storage | ns | ns | ** | ns | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Mateos, M.J.; Delgado-Adámez, J.; Díaz-Ponce, M.; Tejerina, D.; Ramírez-Bernabé, M.R. Frankfurters Manufactured with Valorized Grape Pomace as a Substitute of Nitrifying Salts. Foods 2025, 14, 391. https://doi.org/10.3390/foods14030391
Martín-Mateos MJ, Delgado-Adámez J, Díaz-Ponce M, Tejerina D, Ramírez-Bernabé MR. Frankfurters Manufactured with Valorized Grape Pomace as a Substitute of Nitrifying Salts. Foods. 2025; 14(3):391. https://doi.org/10.3390/foods14030391
Chicago/Turabian StyleMartín-Mateos, María Jesús, Jonathan Delgado-Adámez, María Díaz-Ponce, David Tejerina, and María Rosario Ramírez-Bernabé. 2025. "Frankfurters Manufactured with Valorized Grape Pomace as a Substitute of Nitrifying Salts" Foods 14, no. 3: 391. https://doi.org/10.3390/foods14030391
APA StyleMartín-Mateos, M. J., Delgado-Adámez, J., Díaz-Ponce, M., Tejerina, D., & Ramírez-Bernabé, M. R. (2025). Frankfurters Manufactured with Valorized Grape Pomace as a Substitute of Nitrifying Salts. Foods, 14(3), 391. https://doi.org/10.3390/foods14030391







