Characterization of Plant-Based Raw Materials Used in Meat Analog Manufacture
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Material
2.3. Physicochemical Quality Parameters
- m0—mass of the sample weight for drying, g;
- m1—mass of the sample and the aluminum moisture box before drying, g;
- m2—mass of the sample and the aluminum moisture box after drying, g.
- DSM—mass of the dry starch, g;
- RMM—mass of the raw material, g.
2.4. Amino Acid Profile
2.5. Mineral Profile
2.6. Granulosity
2.7. Water-Holding Capacity
- G0—mass of dry bottle, g;
- G2—mass of bottle after decanting, g;
- Gm—total analyzed material mass, g.
2.8. Color Parameters
2.9. Antioxidant Activity
2.10. Antioxidant Activity In Vitro Digestion Model
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Quality Indicators
3.2. Results of Antioxidant Activity In Vitro Digestion Model
4. Conclusions
5. Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.; Evans, N.M.; Liu, H.; Shao, S. A review of research on plant-based meat alternatives: Driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.J.; Lee, E.Y.; Hwang, Y.H.; Jo, S.T. Traditional plant-based meat alternatives, current and a future perspective: A review. J. Agric. Life Sci. 2021, 55, 1–11. [Google Scholar] [CrossRef]
- Anum, I.; Shafeeqa, I.; Arooba, S.; Nauman, K. Plant-based meat analogs: A review with reference to formulation andgastrointestinal fate. CRFS 2022, 5, 973–983. [Google Scholar]
- Poshadri, A.; Pawar, V.S. The Potential of Production of Meat Analogue by using Indian Legumes—A review. Biol. Forum—Inter. J. 2021, 13, 557–564. [Google Scholar]
- Singh, A.; Sit, N. Meat Analogues: Types, Methods of Production and Their Effect on Attributes of Developed Meat Analogues. Food Bioprocess Technol. 2022, 15, 2664–2682. [Google Scholar] [CrossRef]
- Shubhangi, A.; Priyanka, K.; Mans, N.; Ishika, T.; Vaishnavi, S.; Faraz, A.; Shafiul, H.; Moya, S.; Esra, C.; Rahul, V.; et al. Comprehensive Review on the Role of Plant Protein As a Possible Meat Analogue: Framing the Future of Meat. ACS Omega 2023, 8, 23305–23319. [Google Scholar]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Bronzato, S.; Durante, A.A. Contemporary review of the relationship between red meat consumption and cardiovascular risk. Int. J. Prev. Med. 2017, 8, 40. [Google Scholar] [PubMed]
- Niva, M.; Vainio, A.; Jallinoja, P. 10 Barriers to Increasing Plant Protein Consumption in Western Populations. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Academic Press: Cambridge, MA, USA; Elsever: Amsterdam, The Netherlands, 2017; pp. 157–171. [Google Scholar]
- Yang, Y.; Zheng, Y.; Ma, W.; Zhang, Y.; Sun, C.; Fang, Y. Meat and plant-based meat analogues: Nutritional profile and in vitro digestion comparison. Food Hydrocoll. 2023, 143, 108886. [Google Scholar] [CrossRef]
- Yuliarti, O.; Kovis, T.; Yi, N. Structuring the meat analogue by using plant-based derived composites. J. Food Eng. 2020, 288, 110138. [Google Scholar] [CrossRef]
- Palanisamy, M.; Toepfl, S.; Aganovic, K.; Berger, R. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT-Food Sci. Technol. 2017, 87C, 546–552. [Google Scholar] [CrossRef]
- Jinchuang, Z.; Qiongling, C.; David, L.K.; Qiang, W. High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: Science, technology, and prospect. Trends Food Sci. 2022, 128, 202–216. [Google Scholar]
- Du, M.; Zhang, Y.; Zhao, Y.; Fang, Y. Agarose/konjac glucomannan double network hydrogels to mimic the texture of beef tripe. Food Hydrocoll. 2023, 135, 108173. [Google Scholar] [CrossRef]
- Lei, S.; Youling, L.X. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. 2020, 102, 51–61. [Google Scholar]
- Schmid, E.-M.; Farahnaky, A.; Adhikari, B.; Torley, P.J. High moisture extrusion cooking of meat analogues: A review of mechanismsof protein texturization. Compr Rev. Food Sci Food Saf. 2022, 21, 4573–4609. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczak, K.; Onopiuk, A.; Szpicer, A.; Poltorak, A. Meat Analogues in the Perspective of Recent Scientific Research: A Review. Foods 2022, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Doss, A.; Esther, A.; Rajalakshmi, R. Influence of UV-B Treatment on the Accumulation of Free Phenols and Tannins in the Legumes of Abrus Precatorius L. and Vigna Mungo (L.) Hepper. Phytomed. Plus 2022, 2, 100189. [Google Scholar] [CrossRef]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef]
- Bursa, K.; Toker, O.S.; Palabiyik, I.; Yaman, M.; Kian-Pour, N.; Konar, N.; Kilicli, M. Valorization of hazelnut cake in compound chocolate: The effect of formulation on rheological and physical properties. LWT 2021, 139, 110609. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Du, W.; Cai, Y.; Yang, Z.; Yin, Y.; Wakisaka, M.; Wang, J.; Zhou, Z.; Liu, D.; et al. Leveraging microalgae as a sustainable ingredient for meat analogues. Food Chem. 2024, 450, 139360. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Ge, J.; He, J.; Gan, R.; Fang, Y. Processing, Quality, Safety, and Acceptance of Meat Analogue Products. Engineering 2021, 7, 674–678. [Google Scholar] [CrossRef]
- Schreuders, F.K.G.; Sagis, L.M.C.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Mapping the Texture of Plant Protein Blends for Meat Analogues. Food Hydrocoll. 2021, 118, 106753. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Shen, Y.; Wang, J.; Zhou, D. Legume Consumption and All-Cause and Cardiovascular Disease Mortality. Biomed. Res. Int. 2017, 2017, 8450618. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. Association of Official Analytical Chemists International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- Tasie, M.; Gebreyes, B. Characterization of Nutritional, Antinutritional, and Mineral Contents of Thirty-Five Sorghum Varieties Grown in Ethiopia. Int. J. Food Sci. 2020, 11, 8243617. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Carvalho, C.W.P.; Velasco, J.I.; Fakhouri, F.M. Extraction and characterization of starches from pigmented rice. Int. J. Biol. Macromol. 2020, 156, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.B.; Silva Freire Neto, R.; Collantes, N.F.; Chávez, D.W.H.; Queiroz, V.A.V.; de Carvalho, C.W.P. Exploring starches from varied sorghum genotypes compared to commercial maize starch. J. Food Process Eng. 2022, 46, e14251. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Bulgaru, V.; Netreba, N.; Ghendov-Mosanu, A. Pre-Treatment of Vegetable Raw Materials (Sorghum Oryzoidum) for Use in Meat Analog Manufacture. Appl. Sci. 2025, 15, 349. [Google Scholar] [CrossRef]
- Heywood, A.A.; Myers, D.J.; Bailey, T.B.; Johnson, L.A. Functional properties of low-fat soy flour produced by an extrusion-expelling system. J. Am. Oil Chem. Soc. 2002, 79, 1249–1253. [Google Scholar] [CrossRef]
- Lin, C.S.; Zayas, J.F. Functionality of defatted corn germ proteins in a model system: Fat binding capacity and water retention. J. Food Sci. 1987, 52, 1308–1311. [Google Scholar] [CrossRef]
- Traynham, T.; Myers, D.; Carriquiry, A.; Johnson, L. Evaluation of Water-Holding Capacity for Wheat–Soy Flour Blends. J. Am. Oil Chem. Soc. 2007, 84, 151–155. [Google Scholar] [CrossRef]
- Netreba, N.; Sergheeva, E.; Gurev, A.; Dragancea, V.; Codină, G.G.; Sturza, R.; Ghendov-Mosanu, A. The Influence of Pomace Powder of Musky Squash on the Characteristics of Foamy Confectionery Products during Storage. Appl. Sci. 2024, 4, 6671. [Google Scholar] [CrossRef]
- Paulpriya, K.; Packia Lincy, M.; Tresina Soris, P.; Veerabahu Ramasamy, M. In vitro antioxidant activity, total phenolic and total flavonoid contents of aerial part extracts of Daphniphyllum neilgherrense (wt.) Rosenth. Ethnopharm. J. Biol. Innov. 2015, 4, 257–268. [Google Scholar]
- Arnao, M.B.; Cano, A.; Alcolea, J.F.; Acosta, M. Estimation of free radical-quenching activity of leaf pigment extracts. Phytochem. Anal. 2001, 12, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Popescu, L.; Cojocari, D.; Ghendov-Mosanu, A.; Lung, I.; Soran, M.-L.; Opriş, O.; Kacso, I.; Ciorîta, A.; Balan, G.; Pintea, A.; et al. The Effect of Aromatic Plant Extracts Encapsulated in Alginate on the Bioactivity, Textural Characteristics and Shelf Life of Yogurt. Antioxidants 2023, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. JMLR 2011, 12, 2825–2830. [Google Scholar]
- Joshi, V.K.; Kumar, S. Meat Analogues: Plant based alternatives to meat productsA review. Intl. J. Food. Ferment. Technol. 2015, 5, 107–119. [Google Scholar] [CrossRef]
- Nolden, A.A.; Forde, C.G. The Nutritional Quality of Plant-Based Foods. Sustainability 2023, 15, 3324. [Google Scholar] [CrossRef]
- Acan, B.G.; Toker, O.S.; Palabiyik, I.; Pirouzian, H.R.; Bursa, K.; Kilicli, M.; Yaman, M.; Er, T.; Konar, N. Physicochemical properties of chocolate spread with hazelnut cake: Comparative study and optimization. LWT 2021, 147, 111548. [Google Scholar] [CrossRef]
- Xiao, S.; Li, Z.; Zhou, K.; Fu, Y. Chemical composition of Kabuli and desi chickpea (Cicerarietinum L.) cultivars grown in Xinjiang, China. Food Sci. Nutr. 2023, 11, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Pycia, K.; Juszczak, L. Rheological Characteristics of Wheat Dough Containing Powdered Hazelnuts or Walnuts Oil Cakes. Foods 2024, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Goksu, A.G.; Cakir, B.; Gulseren, I. Industrial Utilization of Bioactive Hazelnut Peptide Fractions in the Manufacture of Functional Hazelnut Paste: ACE-Inhibition and Allergy Suppression. Waste Biomass Valori. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Van der Sman, R.G.M.; Van der Goot, A.J. Hypotheses concerning structuring of extruded meat analogues. CRFS 2023, 6, 100510. [Google Scholar] [PubMed]
- Ozdemir, M.B.; Kılıçarslan, E.; Demir, H.; Koca, E.; Salum, P.; Berktaş, S.; Çam, M.; Erbay, Z.; Aydemir, L.Y. Upgrading the Bioactive Potential of Hazelnut Oil Cake by Aspergillus oryzae under Solid-State Fermentation. Molecules 2024, 29, 4237. [Google Scholar] [CrossRef] [PubMed]
- Badia-Olmos, C.; Laguna, L.; Haros, C.M.; Tárrega, A. Techno-Functional and Rheological Properties of Alternative Plant-Based Flours. Foods 2023, 12, 1411. [Google Scholar] [CrossRef]
- Farshi, P.; Mirmohammadali, S.N.; Rajpurohit, B.; Smith, J.S.; Li, Y. Pea protein and starch: Functional properties and applications in edible films. J. Agric. Res. 2024, 15, 100927. [Google Scholar] [CrossRef]
- Wang, F.; Gu, X.; Lü, M.; Huang, Y.; Zhu, Y.; Sun, Y.; Zhu, X. Structural Analysis and Study of Gel Properties of Thermally-Induced Soybean Isolate–Potato Protein Gel System. Foods 2022, 11, 3562. [Google Scholar] [CrossRef] [PubMed]
- Alviola, J.N.A.; Monterde, V.G. Physicochemical and functional properties of wheat (Triticum aestivum) and selected local flours in the Philippines. Philipp. J. Sci. 2018, 147, 419–430. [Google Scholar]
- Katyal, M.; Singh, N.; Virdi, A.S.; Kaur, A.; Chopra, N.; Ahlawat, A.K.; Singh, A.M. Extraordinarily soft, medium-hard and hard Indian wheat varieties: Composition, protein profile, dough and baking properties. Int. Food Res. 2017, 100, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Jake, A.P.; Yanyun, Z.; McGorrin, R.J. Chemical composition, phytochemical content, and antioxidant activity of hazelnut (Corylus avellana L.) skins from Oregon. LWT 2024, 201, 116204. [Google Scholar]
- Ceylan, F.D.; Adrar, N.; Bolling, B.W.; Capanoglu, E. Valorisation of Hazelnut By-Products: Current Applications and Future Potential. Biotechnol. Genet. Eng. Rev. 2022, 39, 586–621. [Google Scholar] [CrossRef]
- Robbani, R.B.; Hossen, M.M.; Mitra, K.; Haque, M.Z.; Abu Zubair, M.; Khan, S.; Uddin, N.M. Nutritional, Phytochemical, and In Vitro Antioxidant Activity Analysis of Different States of Soy Products. Int. J. Food Sci. 2022, 2022, 9817999. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Kwon, S.-J.; Qu, S.; Kim, D.-G.; Eom, S.H. Antioxidant Contributors in Seed, Seed Coat, and Cotyledon of γ-ray-Induced Soybean Mutant Lines with Different Seed Coat Colors. Antioxidants 2021, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Summo, C.; Centrone, M.; Rybicka, I.; D’Agostino, M.; Annicchiarico, P.; Caponio, F.; StePavan, S.; Tamma, G.; Antonella Pasqualone, A. Macro- and Micro-Nutrient Composition and Antioxidant Activity of Chickpea and Pea Accessions. Pol. J. Food Nutr. Sci. 2021, 71, 177–185. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszycska, A. Antioxidant Activity of Extract of Pea And its Fractions of Low Molecular Phenolics and Tannins. Pol. J. Food Nutr. Sci. 2003, 53, 10–15. [Google Scholar]
- Zia-Ul-Haq, M.; Amarowicz, R.; Ahmad, S.; Riaz, M. Antioxidant Potential of Some Pea (Pisum sativum L.) Cultivars Commonly Consumed in Pakistan. Oxid. Commun. 2013, 36, 1046–1057. [Google Scholar]
- Nilsson, J.; Stegmark, R.; Åkesson, B. Total antioxidant capacity in different pea (Pisum sativum) varieties after blanching and freezing. Food Chem. 2004, 86, 501–507. [Google Scholar] [CrossRef]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT-Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, L.; Zhang, X.; Wang, M.; Liu, H.; Zhu, Y. Nutritional components, volatile constituents and antioxidant activities of 6 chickpea species. Food Biosci. 2021, 41, 100964. [Google Scholar] [CrossRef]
- Newman, C.W.; Roth, N.R.; Lockerman, R.H. Protein quality of chickpea (Cicer arietinum L.). Nutr. Rep. Int. 1987, 36, 1–5. [Google Scholar]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional composition, health benefits and bio-active compounds of chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.W.; Zeb, A.; Mahood, F.; Tariq, S.; Khattak, A.; Shah, H. Comparative sprout quality characteristics of desi and kabuli type chickpea cultivars (Cicer arietinum L.). LWT-Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Lloris, F.; Giménez, R.; Olalla, M.; López, M.C. Mineral content in legumes and nuts: Contribution to the Spanish dietary intake. Sci. Total Environ. 2003, 308, 1–14. [Google Scholar] [CrossRef]
- Ziarati, P.; Amini-Noori, F. Chemical Composition of Native Hazelnut (Corylus avellana L.) Varieties in Iran, Association with Ecological Conditions. Biosci. Biotechnol. Res. Asia 2015, 12, 2053–2060. [Google Scholar]
- Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Differential Effects of In Vitro Simulated Digestion on Antioxidant Activity and Bioaccessibility of Phenolic Compounds in Purple Rice Bran Extracts. Molecules 2024, 29, 2994. [Google Scholar] [CrossRef]
- Muzolf, M.; Szymusiak, H.; Swiglo, A.G.; Rietjens, I.M.; Tyrakowska, B. pH-Dependent radical scavenging capacity of green tea catechins. J. Agric. Food Chem. 2008, 56, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Cel, K.; Sroka, Z. The mechanistic insights into the role of pH and solvent on antiradical and prooxidant properties of polyphenols—Nine compounds case study. Food Chem. 2023, 407, 134677. [Google Scholar] [CrossRef] [PubMed]
- Kut, K.; Bartosz, G.; Sadowska-Bartosz, I. Denaturation and Digestion Increase the Antioxidant Capacity of Proteins. Processes 2023, 11, 1362. [Google Scholar] [CrossRef]

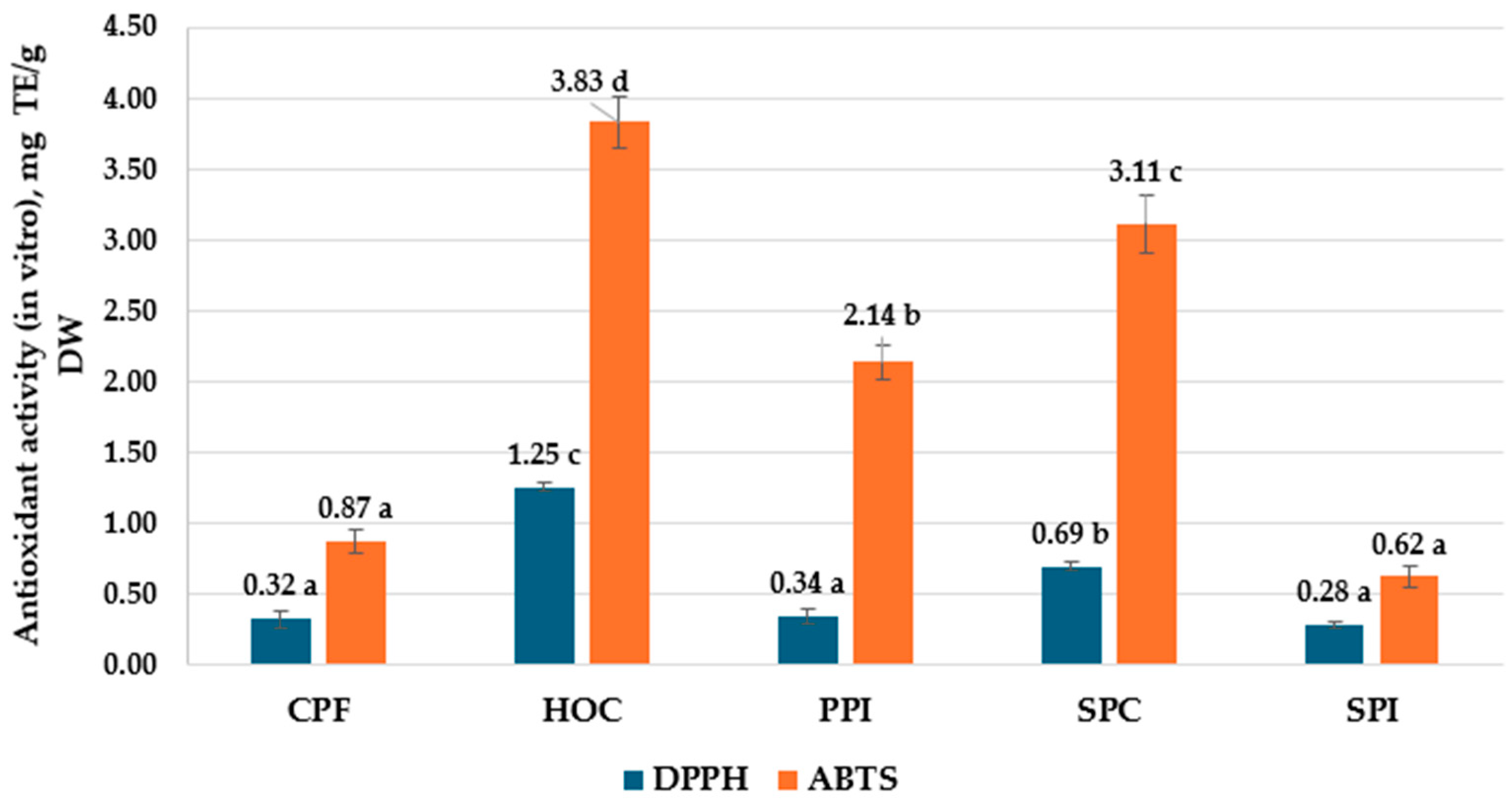
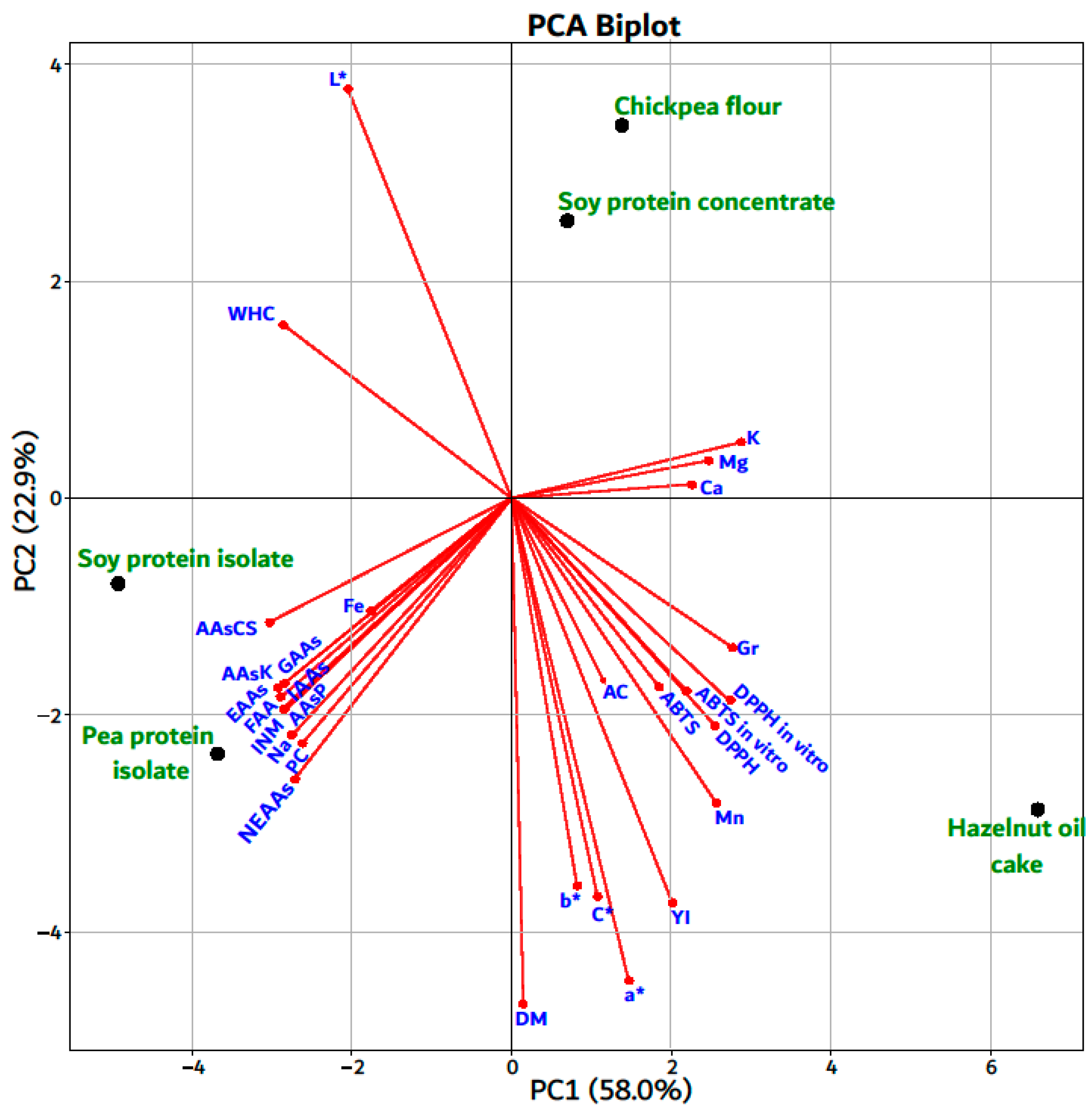
| Indices | Analyzed Plant-Based Materials | ||||
|---|---|---|---|---|---|
| CPF | HOC | PPI | SPC | SPI | |
| DM, % | 91.60 ± 0.11 b | 93.20 ± 0.09 d | 93.00 ± 0.07 d | 90.80 ± 0.15 a | 92.40 ± 0.13 c |
| AC, % | 2.99 ± 0.03 a | 6.57 ± 0.09 d | 4.10 ± 0.03 b | 6.44 ± 0.05 d | 5.33 ± 0.07 c |
| FC, % | 5.25 ± 0.03 d | 3.12 ± 0.06 c | 8.13 ± 0.05 e | 2.21 ± 0.04 b | 0.92 ± 0.02 a |
| PC, % | 22.05 ± 0.31 a | 34.98 ± 0.26 b | 76.00 ± 0.42 d | 52.80 ± 0.34 c | 80.50 ± 0.39 d |
| CFC, % | 1.96 ± 0.0 b | 1.98 ± 0.0 b | 0.19 ± 0.01 a | 0.20 ± 0.01 a | 4.03 ± 0.04 c |
| SY, % | 35.15 ± 0.16 | - | - | - | - |
| WHC, g water/g material | 3.88 ± 0.07 b | 2.17 ± 0.05 a | 4.62 ± 0.03 c | 4.97 ± 0.06 d | 5.54 ± 0.04 d |
| Gr, µm | 170 ± 4 b | 205 ± 4 c | 140 ± 1 a | 134 ± 4 a | 135 ± 3 a |
| L* | 90.59 ± 1.15 c | 51.62 ± 0.76 a | 81.12 ± 0.56 b | 88.87 ± 1.04 c | 83.44 ± 0.53 b |
| a* | −1.00 ± 0.03 a | 6.97 ± 0.09 d | 2.65 ± 0.06 c | −1.06 ± 0.04 a | 1.20 ± 0.05 b |
| b* | 19.64 ± 0.07 b | 22.45 ± 0.10 c | 21.55 ± 0.09 c | 14.98 ± 0.16 a | 18.51 ± 0.13 b |
| C* | 19.67 ± 0.04 b | 23.51 ± 0.09 d | 21.71 ± 0.06 c | 15.02 ± 0.10 a | 18.55 ± 0.07 b |
| YI | 30.97 ± 0.11 b | 62.13 ± 0.57 d | 37.95 ± 0.31 c | 24.08 ± 0.24 a | 31.69 ± 0.51 b |
| AA (DPPH), mg TE/g DW | 0.61 ± 0.04 a | 2.09 ± 0.03 d | 0.99 ± 0.01 b | 1.36 ± 0.01 c | 0.45 ± 0.05 a |
| AA (ABTS), mg TE/g DW | 0.92 ± 0.03 a | 4.07 ± 0.10 d | 2.7 ± 0.08 b | 3.76 ± 0.05 c | 0.90 ± 0.04 a |
| Amino Acids, g/kg | Analyzed Plant-Based Material | ||||
|---|---|---|---|---|---|
| CPF | HOC | PPI | SPC | SPI | |
| Aspartame | 20.13 ± 1.03 a | 15.74 ± 0.97 a | 62.43 ± 1.32 c | 40.69 ± 1.28 b | 63.16 ± 2.13 c |
| Threonine | 7.91 ± 0.17 a | 7.58 ± 0.32 a | 18.17 ± 0.25 c | 13.70 ± 0.41 b | 21.26 ± 0.18 d |
| Serin | 7.69 ± 0.21 a | 12.33 ± 0.15 b | 26.42 ± 0.29 d | 20.30 ± 0.11 c | 30.19 ± 0.45 e |
| Glutamic | 25.72 ± 0.34 a | 37.62 ± 1.11 b | 80.24 ± 1.78 d | 46.70 ± 0.56 c | 78.58 ± 1.47 d |
| Proline | 10.62 ± 0.21 a | 15.86 ± 0.75 b | 28.92 ± 0.67 c,d | 25.02 ± 0.32 c | 39.55 ± 0.89 e |
| Glycine | 7.56 ± 0.42 a | 10.72 ± 0.64 b | 23.80 ± 1.02 e | 14.77 ± 0.43 c | 20.75 ± 0.47 d |
| Alanine | 8.79 ± 0.15 b | 4.71 ± 0.42 a | 25.44 ± 0.85 d | 16.17 ± 0.31 c | 26.21 ± 0.56 d |
| Valin | 8.35 ± 0.22 a | 13.30 ± 0.41 b | 25.51 ± 1.03 c | 14.95 ± 0.67 b | 27.32 ± 0.81 d |
| Cysteine | 1.84 ± 0.06 a | 1.49 ± 0.03 a | 4.14 ± 0.26 c | 2.48 ± 0.05 b | 6.61 ± 0.09 d |
| Methionine | 3.04 ± 0.08 b | 1.97 ± 0.05 a | 6.21 ± 0.07 e | 4.20 ± 0.09 c | 5.70 ± 0.14 d |
| Isoleucine | 14.83 ± 0.19 b | 9.26 ± 0.11 a | 24.63 ± 0.24 d | 16.75 ± 0.37 b,c | 25.99 ± 0.17 e |
| Leucine | 15.08 ± 0.26 a | 14.57 ± 0.32 a | 66.71 ± 1.08 d | 23.49 ± 0.56 b | 43.42 ± 0.98 c |
| Tyrosine | 4.77 ± 0.31 a | 8.36 ± 0.20 a | 24.52 ± 0.96 d | 11.02 ± 0.54 b | 21.39 ± 0.56 c,d |
| Phenylalanine | 11.29 ± 0.13 a | 9.94 ± 0.06 a | 27.67 ± 0.43 c | 17.85 ± 0.62 b | 35.72 ± 0.60 d |
| γ-aminobutyric | 0.29 ± 0.02 a | 0.55 ± 0.02 b | nd | nd | nd |
| Lysine | 17.02 ± 0.17 b | 5.03 ± 0.41 a | 45.81 ± 0.57 e | 25.81 ± 0.28 c | 35.91 ± 0.20 d |
| Histidine | 8.05 ± 0.08 b | 4.99 ± 0.12 a | 16.26 ± 0.19 c | 9.79 ± 0.06 b | 16.57 ± 0.34 c |
| Arginine | 16.54 ± 0.29 a | 18.43 ± 0.16 a | 58.94 ± 0.51 d | 25.84 ± 0.18 b | 39.75 ± 0.41 c |
| Ammonia | 1.89 ± 0.05 a | 4.87 ± 0.08 c | 4.94 ± 0.13 c | 3.21 ± 0.07 b | 5.97 ± 0.08 d |
| FAAs | 189.6 ± 1.3 a | 192.5 ± 0.9 a | 565.9 ± 1.4 c | 329.6 ± 1.6 b | 538.1 ± 1.1 c |
| INM | 191.5 ± 0.9 a | 197.3 ± 1.8 a | 570.8 ± 1.9 c | 332.8 ± 1.2 b | 544.1 ± 2.4 c |
| NEAAs | 87.14 ± 0.78 a | 106.8 ± 1.3 a | 275.9 ± 1.8 b | 117.2 ± 0.9 a | 286.5 ± 1.7 b |
| EAAs | 102.2 ± 0.8 a | 85.08 ± 0.76 a | 289.9 ± 1.5 d | 152.4 ± 1.1 b | 251.6 ± 1.3 c |
| IAAs | 80.74 ± 0.73 a | 93.33 ± 1.04 a | 242.4 ± 0.9 c | 155.0 ± 0.7 b | 253.4 ± 0.8 c |
| GAAs | 60.45 ± 0.21 a | 64.38 ± 0.19 a | 181.8 ± 1.1 c | 120.6 ± 0.8 b | 188.9 ± 0.6 c |
| AAsK | 62.99 ± 0.35 b | 47.17 ± 0.56 a | 189.3 ± 1.0 e | 94.94 ± 0.27 c | 162.4 ± 0.51 d |
| AAsP | 189.3 ± 1.3 a | 191.9 ± 1.1 a | 565.9 ± 2.4 c | 329.6 ± 1.9 b | 538.1 ± 1.5 c |
| AAsCS | 4.93 ± 0.07 b | 3.46 ± 0.11 a | 10.35 ± 0.25 d | 6.68 ± 0.18 c | 12.32 ± 0.11 e |
| Minerals, mg/kg | Analyzed Plant-Based Materials | ||||
|---|---|---|---|---|---|
| CPF | HOC | PPI | SPC | SPI | |
| Sodium (Na) | 32.8 ± 1.6 a | 11.0 ± 0.9 a | 10,537 ± 23 b | 192.5 ± 11.6 a | 15,375 ± 29 c |
| Potasium (K) | 12,085 ± 15 b | 29,219 ± 41 d | 860.0 ± 12.1 a | 23,925 ± 37 c | 2262 ± 16 a |
| Magnesium (Mg) | 1405 ± 9 b | 3792 ± 15 c | 696.9 ± 8.9 a | 3798 ± 11 c | 1042 ± 19 a |
| Calcium (Ca) | 1254 ± 31 a | 3656 ± 17 b | 1230 ± 12 a | 4012 ± 10 b | 951.3 ± 6.2 a |
| Manganese (Mn) | 25.0 ± 1.2 a | 1320 ± 23 b | 30.6 ± 1.4 a | 45.3 ± 1.0 a | 17.3 ± 0.4 a |
| Iron (Fe) | 55.0 ± 0.8 a | 137.5 ± 3.5 b | 202.5 ± 5.1 c | 263.3 ± 3.7 d | 270.0 ± 2.9 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulgaru, V.; Mazur, M.; Netreba, N.; Paiu, S.; Dragancea, V.; Gurev, A.; Sturza, R.; Şensoy, İ.; Ghendov-Mosanu, A. Characterization of Plant-Based Raw Materials Used in Meat Analog Manufacture. Foods 2025, 14, 483. https://doi.org/10.3390/foods14030483
Bulgaru V, Mazur M, Netreba N, Paiu S, Dragancea V, Gurev A, Sturza R, Şensoy İ, Ghendov-Mosanu A. Characterization of Plant-Based Raw Materials Used in Meat Analog Manufacture. Foods. 2025; 14(3):483. https://doi.org/10.3390/foods14030483
Chicago/Turabian StyleBulgaru, Viorica, Mihail Mazur, Natalia Netreba, Sergiu Paiu, Veronica Dragancea, Angela Gurev, Rodica Sturza, İlkay Şensoy, and Aliona Ghendov-Mosanu. 2025. "Characterization of Plant-Based Raw Materials Used in Meat Analog Manufacture" Foods 14, no. 3: 483. https://doi.org/10.3390/foods14030483
APA StyleBulgaru, V., Mazur, M., Netreba, N., Paiu, S., Dragancea, V., Gurev, A., Sturza, R., Şensoy, İ., & Ghendov-Mosanu, A. (2025). Characterization of Plant-Based Raw Materials Used in Meat Analog Manufacture. Foods, 14(3), 483. https://doi.org/10.3390/foods14030483






