Effects of Prolonged Pomace Contact on Color and Mouthfeel Characteristics in Merlot Wine During the Ageing Process Under Microwave Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Winemaking Process and Experiment Condition
2.3. Spectrophotometric Parameters
2.4. CIELab Parameters
2.5. Analysis of Total Polyphenol Content (TPC)
2.6. Analysis of Total Monomer Anthocyanins (TMA)
2.7. Analysis of Total Tannins (TT)
2.8. Analysis of Total Flavan-3-ols (TF-3-ols)
2.9. Analysis of Main Flavane-3-ol Compounds
2.10. Fluorescence Quenching Spectra Between Phenolic Compounds and BSA
2.11. Sensory Evaluation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Visible Spectrum of Wine Without Pomace and Prolonged Pomace Contact Under Microwave Irradiation and Storage Time
3.2. Changes in Color Characteristics During Storage
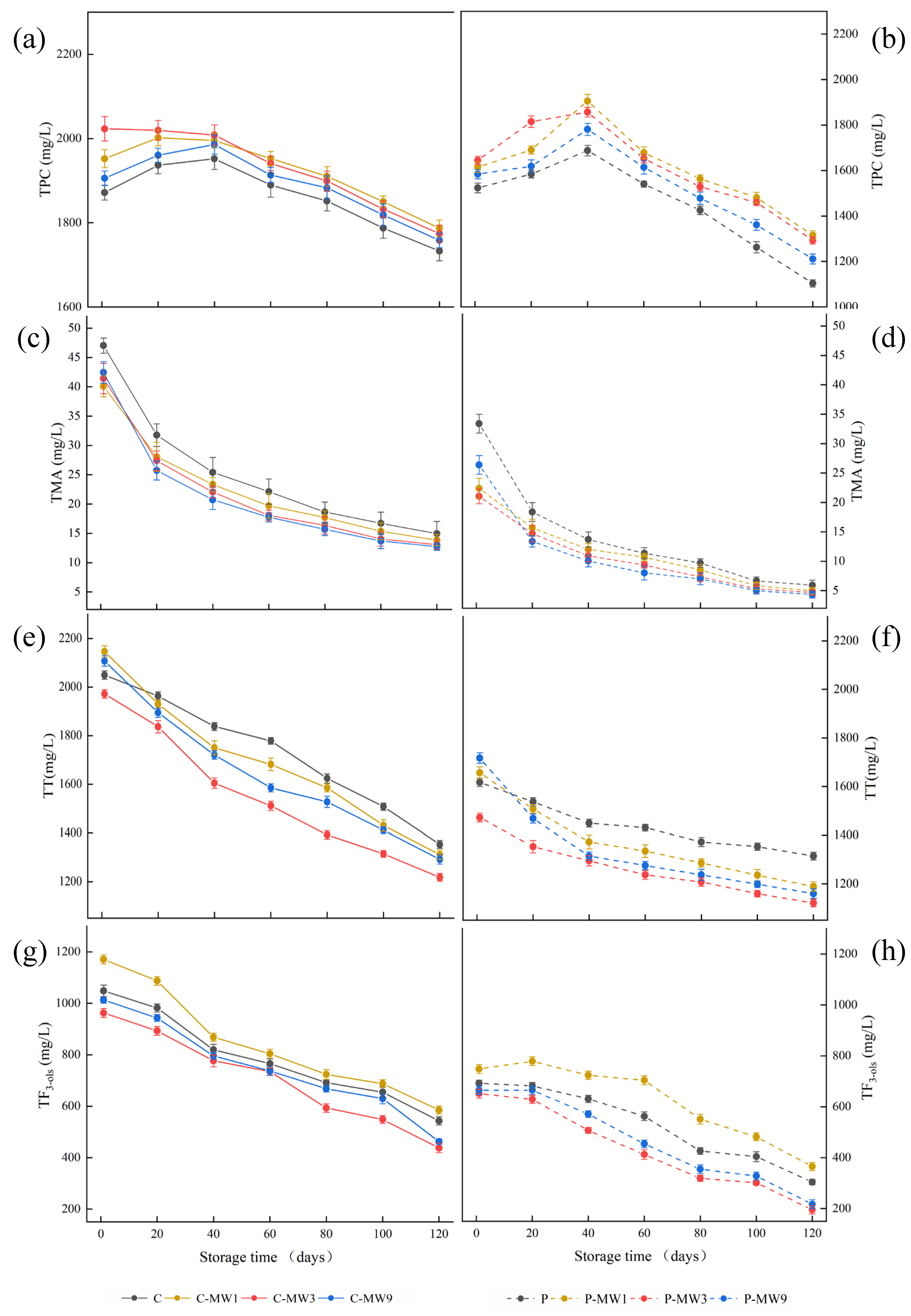
3.3. Changes in TPC, TMA, TT, and TF3-ols During Storage
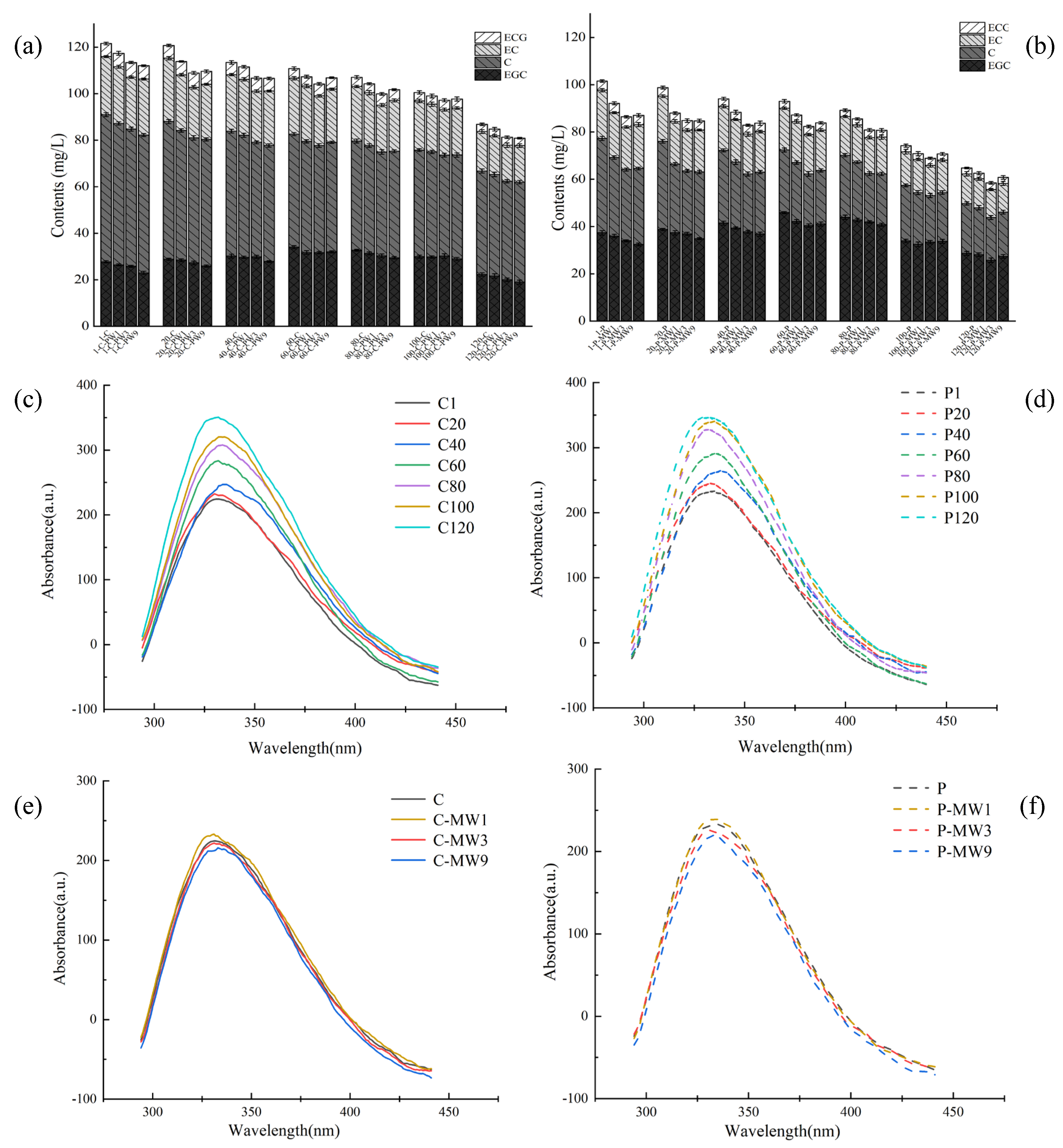
3.4. Identification, Quantification, and Analysis of Flavane-3-ol Monomers During Storage
3.5. Fluorescence Quenching of Wine Samples and BSA
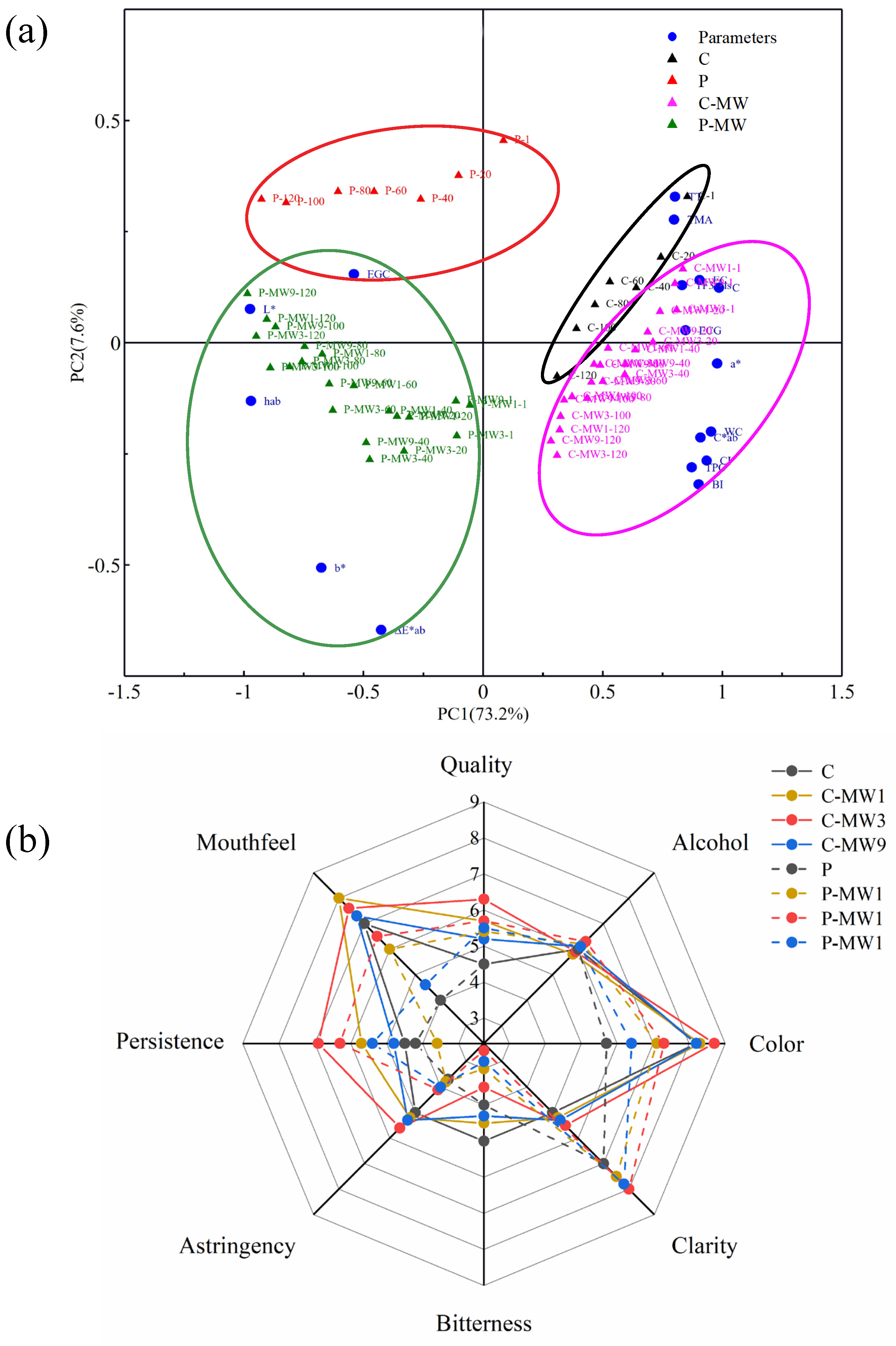
3.6. Principal Component Analysis and Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chira, K.; Lorrain, B.; Ky, I.; Teissedre, P.L. Tannin composition of Cabernet-Sauvignon and Merlot grapes from the bordeaux area for different vintages (2006 to 2009) and comparison to tannin profile of five 2009 vintage mediterranean grapes varieties. Molecules 2011, 16, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Beneficial and harmful effects of wine consumption on health: Phenolic compounds, biogenic amines and ochratoxin A. In Nutrition and Diet Reserch Progress: Appetite and Weight Loss; Nova Science Pub Inc.: New York, NY, USA, 2011; pp. 173–206. [Google Scholar]
- Dipalmo, T.; Crupi, P.; Pati, S.; Clodoveo, M.L.; Luccia, A.D. Studying the evolution of anthocyanin-derived pigments in a typical red wine of Southern Italy to assess its resistance to aging. LWT-Food Sci. Technol. 2016, 71, 1–9. [Google Scholar] [CrossRef]
- Li, L.X.; Sun, B.S. Grape and wine polymeric polyphenols: Their importance in enology. Crit. Rev. Food Sci. Nutr. 2017, 59, 563–579. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Sato, M.; Ueno, N.; Singleton, V.L. Colour and sensory characteristics of Merlot red wines caused by prolonged pomace contact. J. Wine Res. 2000, 11, 7–18. [Google Scholar] [CrossRef]
- Stockley, C.S.; Johnson, D.L. Adverse food reactions from consuming wine. Aust. J. Grape Wine Res. 2015, 21, 568–581. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Comparison of fining red wines with purified grape pomace versus commercial fining agents: Effect on wine chromatic characteristics and phenolic content. Int. J. Food Sci. Technol. 2018, 54, 1018–1026. [Google Scholar] [CrossRef]
- Tao, Y.; García, J.F.; Sun, D.W. Advances in wine aging technologies for enhancing wine quality and accelerating wine aging process. Crit. Rev. Food Sci. Nutr. 2013, 54, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Luchian, C.E.; Scutarașu, E.C.; Colibaba, L.C.; Motrescu, I.; Cotea, V.V. Non-thermal and thermal physical procedures—Optimistic solutions in the winemaking industry. Appl. Sci. 2024, 14, 7537. [Google Scholar] [CrossRef]
- Yuan, J.F.; Lai, Y.T.; Chen, Z.Y.; Song, H.X.; Zhang, J.; Wang, D.H.; Gong, M.G.; Sun, J.R. Microwave irradiation: Effects on the change of colour characteristics and main phenolic compounds of Cabernet Gernischt dry red wine during storage. Foods 2022, 11, 1778. [Google Scholar] [CrossRef] [PubMed]
- Tartian, A.C.; Cotea, V.V.; Niculaua, M.; Zamfir, C.I.; Colibaba, C.L.; Moroşanu, A.M. The influence of the different techniques of maceration on the aromatic and phenolic profile of the Busuioaca de Bohotin wine. BIO Web Conf. 2017, 9, 02032. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. Effect of continuous flow microwave and conventional heating on the bioactive compounds, colour, enzymes activity, microbial and sensory quality of strawberry Purée. Food Bioprocess Technol. 2015, 8, 1864–1876. [Google Scholar] [CrossRef]
- Yuan, J.F.; Hou, Z.C.; Wang, D.H.; Qiu, Z.J.; Gong, M.G.; Sun, J.R. Microwave irradiation: Effect on activities and properties of polyphenol oxidase in grape maceration stage. Food Biosci. 2021, 44, 101378. [Google Scholar] [CrossRef]
- Krishnaswamy, K.; Orsat, V.; Gariépy, Y.; Thangavel, K. Optimization of microwave-assisted extraction of phenolic antioxidants from grape seeds (Vitis vinifera). Food Bioprocess Technol. 2013, 6, 441–455. [Google Scholar] [CrossRef]
- Yuan, J.F.; Chen, Z.Y.; Wang, D.H.; Gong, M.G.; Qiu, Z.J. Microwave-induced free radicals production in red wine and model wine by electron paramagnetic resonance spin trapping. J. Food Process. Preserv. 2021, 45, 15407. [Google Scholar] [CrossRef]
- Carew, A.L.; Sparrow, A.M.; Curtin, C.D.; Close, D.C.; Dambergs, R.G. Microwave maceration of Pinot Noir grape must: Sanitation and extraction effects and wine phenolics outcomes. Food Bioprocess Technol. 2013, 7, 954–963. [Google Scholar] [CrossRef]
- Lai, Y.T.; Yuan, J.F.; Chen, Z.Y.; Wang, D.H.; Sun, J.R.; Ma, J.L. Microwave irradiation: Reduction of higher alcohols in wine and the effect mechanism by employing model wine. LWT-Food Sci. Technol. 2023, 181, 114765. [Google Scholar] [CrossRef]
- Yuan, J.F.; Wang, T.T.; Chen, Z.Y.; Wang, D.H.; Gong, M.G.; Li, P.Y. Microwave irradiation: Impacts on physicochemical properties of red wine. CyTA-J. Food 2020, 18, 281–290. [Google Scholar] [CrossRef]
- Yuan, J.F.; Wang, T.T.; Wang, D.H.; Zhou, G.H.; Zou, G.X.; Wang, Y.; Gong, M.G.; Zhang, B. Effect of microwave on changes of gallic acid and resveratrol in a model extraction solution. Food Bioprocess Technol. 2020, 13, 1246–1254. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Cancho-Grande, B.; Simal-Gándara, J. Garnacha Tintorera-based sweet wines: Chromatic properties and global phenolic composition by means of UV–Vis spectrophotometry. Food Chem. 2013, 140, 217–224. [Google Scholar] [CrossRef]
- Ivanova, V.; Stefova, M.; Vojnoski, B.; Dörnyei, Á.; Márk, L.; Dimovska, V.; Stafilov, T.; Kilár, F. Identification of polyphenolic compounds in red and white grape varieties grown in R. Macedonia and changes of their content during ripening. Food Res. Int. 2011, 44, 2851–2860. [Google Scholar] [CrossRef]
- Yuan, J.F.; Chen, Z.Y.; Lai, Y.T.; Qiu, Z.J.; Wang, D.H.; Zhao, J.F.; Sun, J.R.; Li, X. Microwave irradiation: The influence on the production of Xanthylium Cation pigments in model wine. Food Bioprocess Technol. 2022, 15, 2210–2225. [Google Scholar] [CrossRef]
- Celotti, E.; Stante, S.; Ferraretto, P.; Román, T.; Nicolini, G.; Natolino, A. High power ultrasound treatments of red young wines: Effect on anthocyanins and phenolic stability indices. Foods 2020, 9, 1344. [Google Scholar] [CrossRef]
- Guaita, M.; Motta, S.; Messina, S.; Casini, F.; Bosso, A. Polyphenolic profile and antioxidant activity of green extracts from grape pomace skins and seeds of Italian cultivars. Foods 2023, 12, 3880. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Wang, T.T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Coppola, M.; Moio, L. Aging of Aglianico and Sangiovese wine on mannoproteins: Effect on astringency and colour. LWT-Food Sci. Technol. 2019, 105, 233–241. [Google Scholar] [CrossRef]
- Gordillo, B.; Rivero, F.J.; Jara-Palacios, M.J.; González-Miret, M.L.; Heredia, F.J. Impact of a double post-fermentative maceration with ripe and overripe seeds on the phenolic composition and color stability of Syrah red wines from warm climate. Food Chem. 2021, 346, 128919. [Google Scholar] [CrossRef]
- Giuffrida de Esteban, M.L.; Ubeda, C.; Heredia, F.J.; Catania, A.A.; Assof, M.V.; Fanzone, M.L.; Jofre, V.P. Impact of closure type and storage temperature on chemical and sensory composition of Malbec wines (Mendoza, Argentina) during aging in bottle. Food Res. Int. 2019, 125, 108553. [Google Scholar] [CrossRef]
- Nicolle, P.; Marcotte, C.; Angers, P.; Pedneault, K. Pomace limits tannin retention in Frontenac wines. Food Chem. 2018, 277, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Marquez, A.; Serratosa, M.P.; Merida, J. Influence of bottle storage time on colour, phenolic composition and sensory properties of sweet red wines. Food Chem. 2013, 146, 507–514. [Google Scholar] [CrossRef]
- Lei, X.Q.; Zhu, Y.Y.; Wang, X.Y.; Zhao, P.T.; Liu, P.; Zhang, Q.T.; Chen, T.G.; Yuan, H.H.; Guo, Y.R. Wine polysaccharides modulating astringency through the interference on interaction of flavan-3-ols and BSA in model wine. Int. J. Biol. Macromol. 2019, 139, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Duan, C.Q. Astringency, bitterness and color changes in dry red wines before and during oak barrel aging: An updated phenolic perspective review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1840–1867. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Kanellopoulou, A.; Paraskevopoulos, I.; Kotseridis, Y.; Kallithraka, S. Characterization of grape and wine proanthocyanidins of Agiorgitiko (Vitis vinifera L. cv.) cultivar grown in different regions of Nemea. J. Food Compos. Anal. 2017, 63, 98–110. [Google Scholar] [CrossRef]
- Li, L.X.; Zhang, M.N.; Zhang, S.T.; Cui, Y.; Sun, B.S. Preparation and antioxidant activity of ethyl-Linked anthocyanin-flavanol pigments from model wine solutions. Molecules 2018, 23, 1066. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Navajas, M.P.; Ferreira, V.; Dizy, M.; Fernández-Zurbano, P. Characterization of taste-active fractions in red wine combining HPLC fractionation, sensory analysis and ultra performance liquid chromatography coupled with mass spectrometry detection. Anal. Chim. Acta 2010, 673, 151–159. [Google Scholar] [CrossRef]


| Parameters | Treatment Time | Storing Times (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 40 | 60 | 80 | 100 | 120 | ||
| BI (A420) | C | 0.362 ± 0.005 C,f | 0.376 ± 0.005 C,e | 0.381 ± 0.005 C,d | 0.402 ± 0.005 C,c | 0.410 ± 0.005 B,b | 0.413 ± 0.005 C,b | 0.416 ± 0.005 D,a |
| C-MW1 | 0.371 ± 0.002 B,g | 0.389 ± 0.005 B,f | 0.397 ± 0.007 B,e | 0.406 ± 0.007 B,d | 0.413 ± 0.005 B,c | 0.425 ± 0.005 B,b | 0.447 ± 0.005 B,a | |
| C-MW3 | 0.392 ± 0.005 A,e | 0.403 ± 0.005 A,d | 0.409 ± 0.002 A,d | 0.417 ± 0.005 A,c | 0.427 ± 0.002 A,b | 0.430 ± 0.002 A,b | 0.456 ± 0.002 A,a | |
| C-MW9 | 0.368 ± 0.002 B,f | 0.389 ± 0.002 B,e | 0.395 ± 0.002 B,e | 0.402 ± 0.005 C,d | 0.412 ± 0.005 B,c | 0.420 ± 0.002 B,b | 0.426 ± 0.007 C,a | |
| P | 0.297 ± 0.002 D,a | 0.264 ± 0.002 C,b | 0.266 ± 0.007 B,b | 0.249 ± 0.005 B,c | 0.231 ± 0.002 C,d | 0.186 ± 0.002 B,e | 0.150 ± 0.005 D,f | |
| P-MW1 | 0.364 ± 0.002 B,a | 0.274 ± 0.005 B,b | 0.269 ± 0.002 B,c | 0.256 ± 0.005 C,d | 0.241 ± 0.002 B,e | 0.232 ± 0.002 A,f | 0.187 ± 0.007 B,g | |
| P-MW3 | 0.371 ± 0.002 A,a | 0.301 ± 0.02 A,b | 0.292 ± 0.005 A,c | 0.269 ± 0.002 A,d | 0.249 ± 0.005 A,e | 0.230 ± 0.007 A,f | 0.196 ± 0.002 A,g | |
| P-MW9 | 0.334 ± 0.003 C,a | 0.273 ± 0.007 B,b | 0.268 ± 0.002 B,b | 0.252 ± 0.005 C,c | 0.244 ± 0.007 A,d | 0.232 ± 0.005 A,e | 0.165 ± 0.002 C,f | |
| WC (A520) | C | 0.381 ± 0.005 B,b | 0.382 ± 0.005 B,a | 0.385 ± 0.002 C,a | 0.383 ± 0.002 C,a | 0.378 ± 0.007 D,c | 0.383 ± 0.002 D,a | 0.385 ± 0.002 D,a |
| C-MW1 | 0.382 ± 0.005 B,e | 0.387 ± 0.005 B,d | 0.399 ± 0.002 B,c | 0.399 ± 0.007 B,c | 0.401 ± 0.005 B,b | 0.403 ± 0.002 B,b | 0.407 ± 0.005 B,a | |
| C-MW3 | 0.395 ± 0.005 A,d | 0.403 ± 0.005 A,c | 0.409 ± 0.005 A,b | 0.414 ± 0.005 A,a | 0.412 ± 0.002 A,b | 0.412 ± 0.005 A,b | 0.417 ± 0.002 A,a | |
| C-MW9 | 0.377 ± 0.002 C,e | 0.380 ± 0.007 B,d | 0.386 ± 0.005 C,c | 0.383 ± 0.002 C,c | 0.390 ± 0.005 C,b | 0.391 ± 0.005 C,b | 0.399 ± 0.005 C,a | |
| P | 0.222 ± 0.005 C,a | 0.186 ± 0.005 C,b | 0.173 ± 0.005 C,c | 0.168 ± 0.005 B,d | 0.148 ± 0.005 C,e | 0.114 ± 0.002 C,f | 0.086 ± 0.005 D,g | |
| P-MW1 | 0.271 ± 0.002 A,a | 0.195 ± 0.005 B,b | 0.184 ± 0.005 B,c | 0.173 ± 0.005 A,d | 0.156 ± 0.002 A,e | 0.144 ± 0.005 A,f | 0.116 ± 0.002 B,g | |
| P-MW3 | 0.278 ± 0.005 A,a | 0.216 ± 0.005 A,b | 0.198 ± 0.007 A,c | 0.177 ± 0.005 A,d | 0.158 ± 0.007 A,e | 0.146 ± 0.005 A,f | 0.121 ± 0.005 A,g | |
| P-MW9 | 0.244 ± 0.007 B,a | 0.193 ± 0.005 B,b | 0.182 ± 0.005 B,c | 0.169 ± 0.005 B,d | 0.153 ± 0.005 B,e | 0.136 ± 0.002 B,f | 0.103 ± 0.005 C,g | |
| CI (A420 + A520 + A620) | C | 0.828 ± 0.007 C,f | 0.833 ± 0.005 D,e | 0.849 ± 0.005 D,d | 0.853 ± 0.005 D,c | 0.856 ± 0.002 D,c | 0.866 ± 0.002 D,b | 0.877 ± 0.005 D,a |
| C-MW1 | 0.836 ± 0.005 B,g | 0.864 ± 0.005 B,f | 0.889 ± 0.002 B,e | 0.905 ± 0.002 B,d | 0.912 ± 0.002 B,c | 0.932 ± 0.002 B,b | 0.950 ± 0.005 B,a | |
| C-MW3 | 0.878 ± 0.002 A,f | 0.907 ± 0.002 A,e | 0.925 ± 0.005 A,d | 0.925 ± 0.005 A,d | 0.934 ± 0.002 A,c | 0.952 ± 0.005 A,b | 0.984 ± 0.005 A,a | |
| C-MW9 | 0.825 ± 0.005 C,f | 0.859 ± 0.005 C,e | 0.877 ± 0.005 C,d | 0.895 ± 0.005 C,c | 0.896 ± 0.005 C,c | 0.907 ± 0.005 C,b | 0.912 ± 0.002 C,a | |
| P | 0.572 ± 0.002 D,a | 0.508 ± 0.005 C,b | 0.486 ± 0.005 D,c | 0.446 ± 0.002 C,d | 0.415 ± 0.002 C,e | 0.307 ± 0.005 B,f | 0.232 ± 0.005 D,g | |
| P-MW1 | 0.717 ± 0.005 B,a | 0.545 ± 0.002 B,b | 0.512 ± 0.002 B,c | 0.476 ± 0.005 A,d | 0.435 ± 0.005 B,e | 0.411 ± 0.002 A,f | 0.324 ± 0.002 B,g | |
| P-MW3 | 0.738 ± 0.005 A,a | 0.571 ± 0.002 A,b | 0.535 ± 0.005 A,c | 0.480 ± 0.005 A,d | 0.442 ± 0.005 A,e | 0.412 ± 0.005 A,f | 0.341 ± 0.002 A,g | |
| P-MW9 | 0.640 ± 0.005 C,a | 0.511 ± 0.005 C,b | 0.493 ± 0.005 C,c | 0.456 ± 0.002 B,d | 0.437 ± 0.005 B,e | 0.410 ± 0.002 A,f | 0.269 ± 0.002 C,g | |
| L* (Lightness) | C | 45.79 ± 0.93 C,c | 45.32 ± 1.03 C,d | 44.64 ± 1.07 C,e | 44.37 ± 1.02 D,f | 44.17 ± 0.94 D,g | 46.07 ± 1.04 C,b | 46.55 ± 1.06 C,a |
| C-MW1 | 46.03 ± 0.99 B,b | 45.45 ± 0.97 B,c | 44.89 ± 1.01 A,d | 44.77 ± 1.04 B,e | 44.91 ± 0.82 B,d | 46.31 ± 0.94 B,b | 46.89 ± 0.94 B,a | |
| C-MW3 | 46.93 ± 0.92 A,b | 45.73 ± 1.05 A,d | 44.90 ± 1.02 A,f | 44.98 ± 1.04 A,f | 45.14 ± 0.95 A,e | 46.80 ± 0.85 A,c | 47.12 ± 0.83 A,a | |
| C-MW9 | 46.02 ± 0.84 B,c | 45.37 ± 0.92 C,d | 44.75 ± 1.01 B,f | 44.61 ± 0.98 C,f | 44.78 ± 0.92 C,e | 46.11 ± 0.96 C,b | 46.88 ± 0.84 B,a | |
| P | 55.04 ± 0.98 D,g | 55.72 ± 1.04 D,f | 59.88 ± 1.17 D,e | 62.67 ± 1.14 D,d | 65.51 ± 1.03 D,c | 71.26 ± 1.13 D,b | 73.55 ± 0.95 D,a | |
| P-MW1 | 57.76 ± 0.94 C,f | 59.09 ± 1.06 B,e | 62.08 ± 1.06 C,d | 64.35 ± 1.06 C,d | 66.51 ± 1.17 C,c | 72.77 ± 1.01 B,b | 74.61 ± 1.08 B,a | |
| P-MW3 | 59.32 ± 1.04 A,g | 60.36 ± 0.97 A,f | 65.17 ± 1.01 A,e | 66.80 ± 1.03 A,d | 68.55 ± 1.05 A,c | 74.61 ± 1.02 A,b | 75.31 ± 0.93 A,a | |
| P-MW9 | 59.10 ± 1.03 B,f | 58.19 ± 1.03 C,g | 64.63 ± 1.12 B,e | 66.05 ± 1.17 B,d | 67.77 ± 1.04 B,c | 72.62 ± 0.93 C,b | 74.36 ± 1.00 C,a | |
| a* (green/redcomponent) | C | 53.40 ± 0.21 A,a | 52.98 ± 0.20 A,b | 52.85 ± 0.36 A,b | 52.15 ± 0.21 A,c | 52.26 ± 0.34 A,c | 51.61 ± 0.25 A,d | 51.52 ± 0.23 A,e |
| C-MW1 | 52.82 ± 0.26 C,a | 52.67 ± 0.25 B,b | 52.42 ± 0.25 C,b | 52.08 ± 0.22 B,c | 51.96 ± 0.22 C,c | 50.61 ± 0.31 C,e | 51.18 ± 0.25 B,d | |
| C-MW3 | 52.90 ± 0.30 B,a | 52.68 ± 0.20 B,b | 52.57 ± 0.20 B,b | 52.16 ± 0.24 A,c | 52.05 ± 0.21 B,c | 50.75 ± 0.32 B,e | 51.21 ± 0.30 B,d | |
| C-MW9 | 52.85 ± 0.30 B,a | 52.67 ± 0.27 B,b | 52.53 ± 0.24 B,b | 52.15 ± 0.23 A,c | 52.04 ± 0.25 B,c | 50.74 ± 0.30 B,e | 51.16 ± 0.27 B,d | |
| P | 46.52 ± 0.40 A,a | 44.45 ± 0.40 A,b | 41.04 ± 0.44 A,c | 37.86 ± 0.40 A,d | 34.85 ± 0.44 A,e | 28.98 ± 0.30 A,f | 26.30 ± 0.46 A,g | |
| P-MW1 | 42.94 ± 0.35 B,a | 40.08 ± 0.25 B,b | 37.02 ± 0.35 B,c | 35.35 ± 0.23 B,d | 33.62 ± 0.35 B,e | 28.73 ± 0.25 B,f | 25.84 ± 0.45 B,g | |
| P-MW3 | 41.43 ± 0.20 D,a | 38.45 ± 0.30 C,b | 35.32 ± 0.40 D,c | 33.43 ± 0.40 D,d | 31.24 ± 0.40 D,e | 26.81 ± 0.30 D,f | 23.86 ± 0.40 D,g | |
| P-MW9 | 41.95 ± 0.35 C,a | 38.21 ± 0.25 D,b | 35.72 ± 0.35 C,c | 33.68 ± 0.35 C,d | 31.85 ± 0.15 C,e | 28.09 ± 0.25 C,f | 24.45 ± 0.45 C,g | |
| b* (yellow/bluecomponent) | C | 26.87 ± 0.37 B,g | 30.89 ± 0.35 D,f | 32.83 ± 0.34 D,e | 34.27 ± 0.35 D,d | 35.62 ± 0.25 B,c | 37.09 ± 0.35 D,b | 39.40 ± 0.31 D,a |
| C-MW1 | 28.67 ± 0.27 C,g | 32.00 ± 0.18 C,f | 34.33 ± 0.19 C,e | 35.74 ± 0.15 C,d | 36.96 ± 0.16 A,c | 38.55 ± 0.21 C,b | 40.51 ± 0.25 C,a | |
| C-MW3 | 28.85 ± 0.31 B,g | 32.19 ± 0.25 B,f | 34.55 ± 0.24 B,e | 35.99 ± 0.25 B,d | 36.98 ± 0.28 A,c | 38.66 ± 0.25 B,b | 40.63 ± 0.24 B,a | |
| C-MW9 | 28.96 ± 0.22 A,g | 32.58 ± 0.19 A,f | 34.62 ± 0.20 A,e | 36.05 ± 0.22 A,d | 37.07 ± 0.20 A,c | 38.81 ± 0.18 A,b | 40.81 ± 0.20 A,a | |
| P | 29.12 ± 0.21 C,g | 32.30 ± 0.20 D,f | 34.12 ± 0.20 D,e | 36.18 ± 0.22 C,d | 37.01 ± 0.20 D,c | 37.89 ± 0.23 D,b | 38.16 ± 0.30 D,a | |
| P-MW1 | 35.51 ± 0.16 A,f | 38.27 ± 0.14 A,e | 38.91 ± 0.16 A,d | 41.20 ± 0.24 A,c | 41.54 ± 0.26 A,b | 41.69 ± 0.18 A,b | 41.89 ± 0.26 A,a | |
| P-MW3 | 34.49 ± 0.23 B,e | 36.74 ± 0.21 C,d | 37.81 ± 0.23 C,c | 39.52 ± 0.23 B,b | 39.55 ± 0.21 C,b | 39.87 ± 0.33 C,a | 39.92 ± 0.23 C,a | |
| P-MW9 | 35.56 ± 0.15 A,g | 37.06 ± 0.15 B,f | 38.56 ± 0.22 B,e | 39.60 ± 0.25 B,d | 39.98 ± 0.25 B,c | 40.18 ± 0.23 B,b | 40.36 ± 0.25 B,a | |
| C*ab (chroma) | C | 59.78 ± 0.30 A,f | 61.33 ± 0.26 A,e | 62.22 ± 0.29 A,d | 62.40 ± 0.31 A,c | 62.96 ± 0.39 A,b | 63.56 ± 0.40 A,b | 64.86 ± 0.37 A,a |
| C-MW1 | 60.10 ± 0.23 A,f | 61.63 ± 0.25 A,e | 62.66 ± 0.30 A,d | 63.16 ± 0.26 A,c | 63.76 ± 0.36 A,b | 63.82 ± 0.36 A,b | 65.27 ± 0.38 A,a | |
| C-MW3 | 60.25 ± 0.29 A,f | 61.74 ± 0.27 A,e | 62.90 ± 0.32 A,d | 63.37 ± 0.20 A,c | 63.85 ± 0.34 A,b | 63.90 ± 0.38 A,b | 65.37 ± 0.33 A,a | |
| C-MW9 | 60.26 ± 0.26 A,f | 61.93 ± 0.31 A,e | 62.91 ± 0.31 A,d | 63.39 ± 0.28 A,c | 63.89 ± 0.38 A,b | 63.98 ± 0.43 A,b | 65.44 ± 0.35 A,a | |
| P | 54.88 ± 0.30 B,a | 54.95 ± 0.32 B,a | 53.37 ± 0.32 B,b | 52.37 ± 0.26 B,c | 50.84 ± 0.32 C,d | 46.12 ± 0.29 D,f | 48.28 ± 0.33 C,e | |
| P-MW1 | 55.72 ± 0.24 A,a | 55.42 ± 0.23 A,a | 53.71 ± 0.31 A,c | 54.29 ± 0.23 A,b | 53.44 ± 0.35 A,d | 48.62 ± 0.23 A,f | 50.80 ± 0.23 A,e | |
| P-MW3 | 53.91 ± 0.18 C,a | 53.18 ± 0.18 C,b | 51.74 ± 0.20 D,c | 51.76 ± 0.29 C,c | 50.40 ± 0.39 D,d | 46.46 ± 0.38 C,f | 48.09 ± 0.20 C,e | |
| P-MW9 | 54.99 ± 0.19 B,a | 53.23 ± 0.26 C,b | 52.56 ± 0.23 C,c | 51.99 ± 0.22 C,d | 51.12 ± 0.41 B,e | 47.03 ± 0.29 B,g | 49.17 ± 0.25 B,f | |
| hab (hue) | C | 26.71° ± 0.29 C,g | 30.24° ± 0.31 C,f | 31.85° ± 0.31 B,e | 33.31° ± 0.32 B,d | 34.45° ± 0.30 B,c | 35.70° ± 0.31 B,b | 37.41° ± 0.31 C,a |
| C-MW1 | 28.49° ± 0.26 B,g | 31.28° ± 0.27 B,f | 33.22° ± 0.26 A,e | 34.46° ± 0.26 A,d | 35.42° ± 0.25 A,c | 37.30° ± 0.27 A,b | 38.36° ± 0.25 A,a | |
| C-MW3 | 28.61° ± 0.42 A,g | 31.42° ± 0.40 B,f | 33.31° ± 0.41 A,e | 34.61° ± 0.47 A,d | 35.44° ± 0.34 A,c | 37.31° ± 0.30 A,b | 38.48° ± 0.40 A,a | |
| C-MW9 | 28.72° ± 0.30 A,g | 31.74° ± 0.30 A,f | 33.39° ± 0.41 A,e | 34.66° ± 0.30 A,d | 35.46° ± 0.31 A,c | 37.41° ± 0.30 A,b | 37.89° ± 0.30 B,a | |
| P | 32.05° ± 0.20 C,g | 36.00° ± 0.20 C,f | 39.74° ± 0.20 C,e | 43.70° ± 0.23 C,d | 46.72° ± 0.20 C,c | 52.59° ± 0.20 C,b | 53.11° ± 0.20 C,a | |
| P-MW1 | 39.59° ± 0.30 B,f | 43.68° ± 0.31 B,e | 46.43° ± 0.30 B,d | 49.15° ± 0.40 B,c | 51.02° ± 0.30 B,b | 55.43° ± 0.30 B,a | 55.56° ± 0.30 B,a | |
| P-MW3 | 39.78° ± 0.30 B,f | 43.70° ± 0.30 B,e | 46.95° ± 0.26 A,d | 49.77° ± 0.30 A,c | 51.70° ± 0.30 A,b | 56.08° ± 0.31 A,a | 56.11° ± 0.30 A,a | |
| P-MW9 | 40.29° ± 0.30 A,f | 44.12° ± 0.30 A,e | 47.07° ± 0.31 A,d | 49.82° ± 0.28 A,c | 51.76° ± 0.31 A,b | 56.09° ± 0.20 A,a | 56.16° ± 0.31 A,a | |
| ΔE*ab | C-MW1 | 1.90 ± 0.08 B,a | 1.16 ± 0.13 C,d | 1.58 ± 0.05 C,b | 1.53 ± 0.12 B,b | 1.52 ± 0.19 B,b | 1.78 ± 0.12 B,c | 1.21 ± 0.07 C,d |
| C-MW3 | 2.34 ± 0.22 A,a | 1.40 ± 0.15 B,d | 1.76 ± 0.08 B,c | 1.73 ± 0.10 A,c | 1.67 ± 0.02 A,c | 1.92 ± 0.06 A,b | 1.39 ± 0.07 B,d | |
| C-MW9 | 2.37 ± 0.08 A,a | 1.72 ± 0.14 A,d | 1.82 ± 0.05 A,c | 1.80 ± 0.16 A,c | 1.68 ± 0.03 A,d | 1.93 ± 0.04 A,b | 1.49 ± 0.03 A,e | |
| P-MW1 | 7.81 ± 0.09 C,b | 8.13 ± 0.12 C,a | 6.63 ± 0.11 C,c | 5.86 ± 0.15 C,d | 4.79 ± 0.13 B,e | 4.11 ± 0.09 B,f | 2.15 ± 0.17 B,g | |
| P-MW3 | 8.54 ± 0.12 A,b | 8.79 ± 0.08 A,a | 8.62 ± 0.11 A,a | 6.92 ± 0.11 A,c | 5.36 ± 0.12 A,d | 4.59 ± 0.07 A,e | 3.09 ± 0.12 A,f | |
| P-MW9 | 8.17 ± 0.18 B,b | 8.40 ± 0.11 B,a | 8.23 ± 0.17 B,a | 6.37 ± 0.14 B,c | 4.80 ± 0.14 B,d | 3.24 ± 0.06 C,e | 2.12 ± 0.24 B,f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.-F.; Qin, H.-M.; Wang, L.-J.; Yang, X.-W.; Li, Y.; Wan, N.-B.; Zhang, J. Effects of Prolonged Pomace Contact on Color and Mouthfeel Characteristics in Merlot Wine During the Ageing Process Under Microwave Irradiation. Foods 2025, 14, 507. https://doi.org/10.3390/foods14030507
Yuan J-F, Qin H-M, Wang L-J, Yang X-W, Li Y, Wan N-B, Zhang J. Effects of Prolonged Pomace Contact on Color and Mouthfeel Characteristics in Merlot Wine During the Ageing Process Under Microwave Irradiation. Foods. 2025; 14(3):507. https://doi.org/10.3390/foods14030507
Chicago/Turabian StyleYuan, Jiang-Feng, Hui-Min Qin, Li-Juan Wang, Xiao-Wen Yang, Yang Li, Ning-Bo Wan, and Jie Zhang. 2025. "Effects of Prolonged Pomace Contact on Color and Mouthfeel Characteristics in Merlot Wine During the Ageing Process Under Microwave Irradiation" Foods 14, no. 3: 507. https://doi.org/10.3390/foods14030507
APA StyleYuan, J.-F., Qin, H.-M., Wang, L.-J., Yang, X.-W., Li, Y., Wan, N.-B., & Zhang, J. (2025). Effects of Prolonged Pomace Contact on Color and Mouthfeel Characteristics in Merlot Wine During the Ageing Process Under Microwave Irradiation. Foods, 14(3), 507. https://doi.org/10.3390/foods14030507





