Mangosteen Seed Fat: A Typical 1,3-Distearoyl-Sn-2-Linoleoyl-Glycerol-Rich Fat and Its Effects on Delaying Chocolate Fat Bloom
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Fat Acid Composition
2.3. Analysis of Triacylglycerol Species
2.4. Determination of Solid Fat Content
2.5. Determination of Crystal Morphology
2.6. Preparation of Chocolates
2.7. Chocolate Bloom Test During Storage
2.8. Analysis of Chocolate Hardness
2.9. Determination of Differential Scanning Calorimetry (DSC)
2.10. Statistics
3. Results and Discussion
3.1. Lipid Compositions of Mangosteen Seed Fat
3.2. Solid Fat Contents of the Binary Fats
3.3. Crystal Morphologies of the Binary Fat Crystals
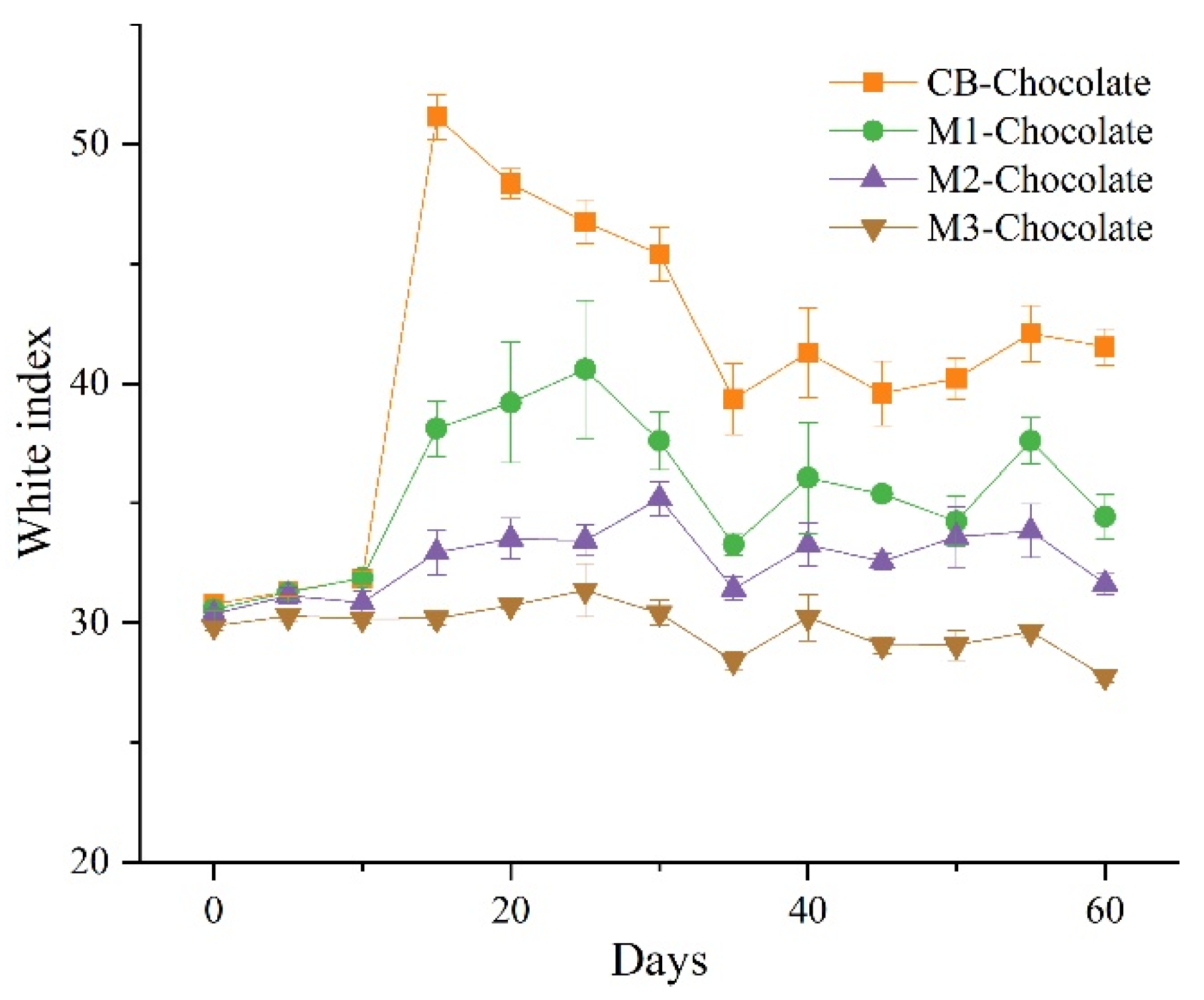
3.4. Bloom Behaviors of Mangosteen Seed Fat-Based Chocolates
3.5. Texture Characteristics of Mangosteen Seed Fat-Chocolates
3.6. Thermal Behaviors of Mangosteen Seed Fat-Chocolates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Tm (°C) | ΔHm (kJ/mol) | LS (nm) | SS (nm) | ||
|---|---|---|---|---|---|
| StOSt | α | 23.5 | 47.7 | 4.8 | 0.421 |
| γ | 35.4 | 98.5 | 7.1 | 0.472 0.450 0.388 | |
| β’ | 36.5 | 104.8 | 7.0 | 0.424 0.390 | |
| β2 | 41.0 | 143.0 | 6.5 | 0.458 0.367 | |
| β1 | 43.0 | 151.0 | 6.5 | 0.458 0.365 | |
| StLSt | sub-α₂ | −5.3 | 3.7 | — | 0.419 0.374 |
| sub-α₁ | 8.8 | 4.2 | — | 0.421 0.385 | |
| α | 20.8 | 40.9 | 5.4 | 0.412 | |
| γ | 34.5 | 137.4 | 7.3 | 0.474 0.450 0.381 |
References
- Marty-Terrade, S.; Marangoni, A.G. Impact of cocoa butter origin on crystal behavior. In Cocoa Butter and Related Compounds; AOCS Press: Champaign, IL, USA, 2012; pp. 245–274. [Google Scholar]
- Ghazani, S.M.; Marangoni, A.G. The triclinic polymorphism of cocoa butter is dictated by its major molecular species, 1-palmitoyl, 2-oleoyl, 3-stearoyl glycerol (POS). Cryst. Growth Des. 2018, 19, 90–97. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Chocolate Science and Technology, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2016. [Google Scholar]
- Co, E.D.; Ghazani, S.M.; Pink, D.A.; Marangoni, A.G. Heterogeneous nucleation of 1,3-distearoyl-2-oleoylglycerol on tristearin surfaces. ACS Omega 2019, 4, 6273–6282. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Takagi, M.; Hondoh, H.; Michikawa, S.; Hirai, Y.; Ueno, S. Control of phase separation for CBS-based compound chocolates focusing on growth kinetics. Cryst. Growth Des. 2022, 22, 6879–6885. [Google Scholar] [CrossRef]
- Koizumi, H.; Kimura, K.; Takagi, M.; Michikawa, S.; Hirai, Y.; Sato, K.; Ueno, S. Effect of accumulated strain on fat bloom in CBS-based compound chocolates. CrystEngComm 2023, 25, 4562–4567. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Akoh, C.C.; Wang, X. StOSt-rich fats in the manufacture of heat-stable chocolates and their potential impacts on fat bloom behaviors. Trends Food Sci. Technol. 2021, 118, 418–430. [Google Scholar] [CrossRef]
- Jin, J.; Jin, Q.; Wang, X.; Akoh, C.C. Improving heat and fat bloom stabilities of “dark chocolates” by addition of mango kernel fat-based chocolate fats. J. Food Eng. 2019, 246, 33–41. [Google Scholar] [CrossRef]
- Fukami, Y.; Iwaoka, E.; Kuriyama, M.; Sato, K. Physical properties of bloom-resistant chocolate using palm-based no-trans cocoa butter replacers. J. Am. Oil Chem. Soc. 2024. [Google Scholar] [CrossRef]
- Ketsa, S.; Paull, R.E. Mangosteen (Garcinia mangostana L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Woodhead Publishing: Cambridge, UK, 2011; pp. 1–30, 31e–32e. [Google Scholar]
- Cheok, C.Y.; Mohd Adzahan, N.; Abdul Rahman, R.; Zainal Abedin, N.H.; Hussain, N.; Sulaiman, R.; Chong, G.H. Current trends of tropical fruit waste utilization. Crit. Rev. Food Sci. Nutr. 2018, 58, 335–361. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, Y.; Hou, X.; Chen, Y.; Chi, J.; Jin, J.; Jin, Q.; Wang, X. Characteristics of Garcinia seed fats and their potential usages as functional ingredients: A review. Int. J. Food Sci. Technol. 2024, 59, 673–684. [Google Scholar] [CrossRef]
- Ajayi, I.A.; Oderinde, R.A.; Ogunkoya, B.O.; Egunyomi, A.; Taiwo, V.O. Chemical analysis and preliminary toxicological evaluation of Garcinia mangostana seeds and seed oil. Food Chem. 2007, 101, 999–1004. [Google Scholar] [CrossRef]
- Hiranrangsee, L.; Kumaree, K.K.; Sadiq, M.B.; Anal, A.K. Extraction of anthocyanins from pericarp and lipids from seeds of mangosteen (Garcinia mangostana L.) by Ultrasound-assisted extraction (UAE) and evaluation of pericarp extract enriched functional ice-cream. J. Food Sci. Technol. 2016, 53, 3806–3813. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Zheng, X.; Jin, J.; Jin, Q.; Akoh, C.C.; Wang, X. Novel disaturated triacylglycerol-rich fat from Garcinia mangostana: Lipid compositions, fatty acid distributions, triacylglycerol species and thermal characteristics. Ind. Crop. Prod. 2023, 197, 116506. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ueno, A.; Yano, J.; Floter, E.; Sato, K. Polymorphic transformation of 1, 3-distearoyl-sn-2-linoleoyl-glycerol. J. Am. Oil Chem. Soc. 2000, 77, 1243–1250. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ueno, S.; Flöter, E.; Sato, K. Binary phase behavior of 1, 3-distearoyl-2-oleoyl-sn-glycerol (SOS) and 1, 3-distearoyl-2-linoleoyl-sn-glycerol (SLS). J. Am. Oil Chem. Soc. 2002, 79, 627–632. [Google Scholar] [CrossRef]
- Hou, X.; Gao, Z.; Elbarbary, A.; Jin, J. Chemical compositions and crystallization characteristics of SOS-rich fats. J. Am. Oil Chem. Soc. 2024, 101, 1267–1276. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zheng, L.; Jin, Q.; Wang, X. Synthesis of 1, 3-distearoyl-2-oleoylglycerol by enzymatic acidolysis in a solvent-free system. Food Chem. 2017, 228, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Yamoneka, J.; Malumba, P.; Lognay, G.; Béra, F.; Blecker, C.; Danthine, S. Enzymatic inter-esterification of binary blends containing Irvingia gabonensis seed fat to produce cocoa butter substitute. Eur. J. Lipid Sci. Technol. 2018, 120, 1700423. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Zhang, W.; Tan, C.P.; Lan, D.; Wang, Y. Characteristics and feasibility of olive oil-based diacylglycerol plastic fat for use in compound chocolate. Food Chem. 2022, 391, 133254. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, G.; Chen, X.; Chen, J.; Liu, W. Interaction of monopalmitate and carnauba wax on the properties and crystallization behavior of soybean oleogel. Grain Oil Sci. Technol. Engl. 2020, 3, 8. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Zeng, W.; Gu, X.; Jin, Q.; Wang, X. Classification and feasibility of mango kernel fat and its stepwise fractionated stearins for use in heat-stable chocolate fats. Int. J. Food Sci. Technol. 2023, 58, 557–566. [Google Scholar] [CrossRef]
- Kadanthottu, S.J.; Bolla, S.; Joshi, K.; Bhat, M.; Naik, K.; Patil, S.; Bendre, S.; Gangappa, B.; Haibatti, V.; Payamalle, S.; et al. Determination of chemical composition and nutritive value with fatty acid compositions of African mangosteen (Garcinia livingstonei). Erwerbs-Obstbau 2017, 59, 195–202. [Google Scholar]
- Kadivar, S.; De Clercq, N.; Danthine, S.; Dewettinck, K. Crystallization and polymorphic behavior of enzymatically produced sunflower oil based cocoa butter equivalents. Eur. J. Lipid Sci. Technol. 2016, 118, 1521–1538. [Google Scholar] [CrossRef]
- Alishevich, K.; Berčíková, M.; Kyselka, J.; Sasínová, K.; Honzíková, T.; Šimicová, P.; Šmidrkal, J.; Rohlíček, J.; Filip, V. Binary Phase Behavior of 2-oleoyl-1-palmitoyl-3-stearoyl-rac-glycerol (POS) and 2-linoleoyl-1-palmitoyl-3-stearoyl-rac-glycerol (PLS). Food Biophys. 2023, 18, 161–173. [Google Scholar] [CrossRef]
- Marangoni, A.G.; McGauley, S.E. Relationship between crystallization behavior and structure in cocoa butter. Cryst. Growth Des. 2003, 3, 95–108. [Google Scholar] [CrossRef]
- Ramel, P.R.; Campos, R.; Marangoni, A.G. Effects of shear and cooling rate on the crystallization behavior and structure of cocoa butter: Shear applied during the early stages of nucleation. Cryst. Growth Des. 2018, 18, 1002–1011. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Zou, L.; Rakitsky, W.G.; Marangoni, A.G. Algal butter, a novel cocoa butter equivalent: Chemical composition, physical properties, and functionality in chocolate. J. Am. Oil Chem. Soc. 2018, 95, 1239–1251. [Google Scholar] [CrossRef]
- Shen, L.; Jin, J.; Ye, X.; Li, Y.; Zhang, C.; Jiang, L.; Zhao, L. Effects of sucrose particle size on the microstructure and bloom behavior of chocolate model systems. Food Struct. 2023, 36, 100323. [Google Scholar] [CrossRef]
- Kinta, Y.; Hatta, T. Morphology of chocolate fat bloom. In Cocoa Butter and Related Compounds; AOCS Press: Urbana, IL, USA, 2012; pp. 195–212. [Google Scholar]
- Sato, S.; Hondoh, H.; Ueno, S. Fat bloom caused by partial DE-oiling on chocolate surfaces after high-temperature exposure. J. Am. Oil Chem. Soc. 2021, 98, 269–280. [Google Scholar] [CrossRef]
- De Clercq, N.; Kadivar, S.; Van de Walle, D.; De Pelsmaeker, S.; Ghellynck, X.; Dewettinck, K. Functionality of cocoa butter equivalents in chocolate products. Eur. Food Res. Technol. 2017, 243, 309–321. [Google Scholar] [CrossRef]
- Hřivna, L.; Machálková, L.; Burešová, I.; Nedomová, Š.; Gregor, T. Texture, color, and sensory changes occurring in chocolate bars with filling during storage. Food Sci. Nutr. 2021, 9, 4863–4873. [Google Scholar] [CrossRef]
- Watanabe, S.; Yoshikawa, S.; Sato, K. Formation and properties of dark chocolate prepared using fat mixtures of cocoa butter and symmetric/asymmetric stearic-oleic mixed-acid triacylglycerols: Impact of molecular compound crystals. Food Chem. 2021, 339, 127808. [Google Scholar] [CrossRef]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Mansoor, A.H.; Norliza, J.; Patricia, M.; Ramlah George, M.R.; Noorakmar, A.W.; Lee, J.S.; Fan, H.Y. Trends in blending vegetable fats and oils for cocoa butter alternative application: A review. Trends Food Sci. Technol. 2021, 116, 102–114. [Google Scholar] [CrossRef]
- Chen, J.; Ghazani, S.M.; Stobbs, J.A.; Marangoni, A.G. Tempering of cocoa butter and chocolate using minor lipidic components. Nat. Commun. 2021, 12, 5018. [Google Scholar] [CrossRef] [PubMed]

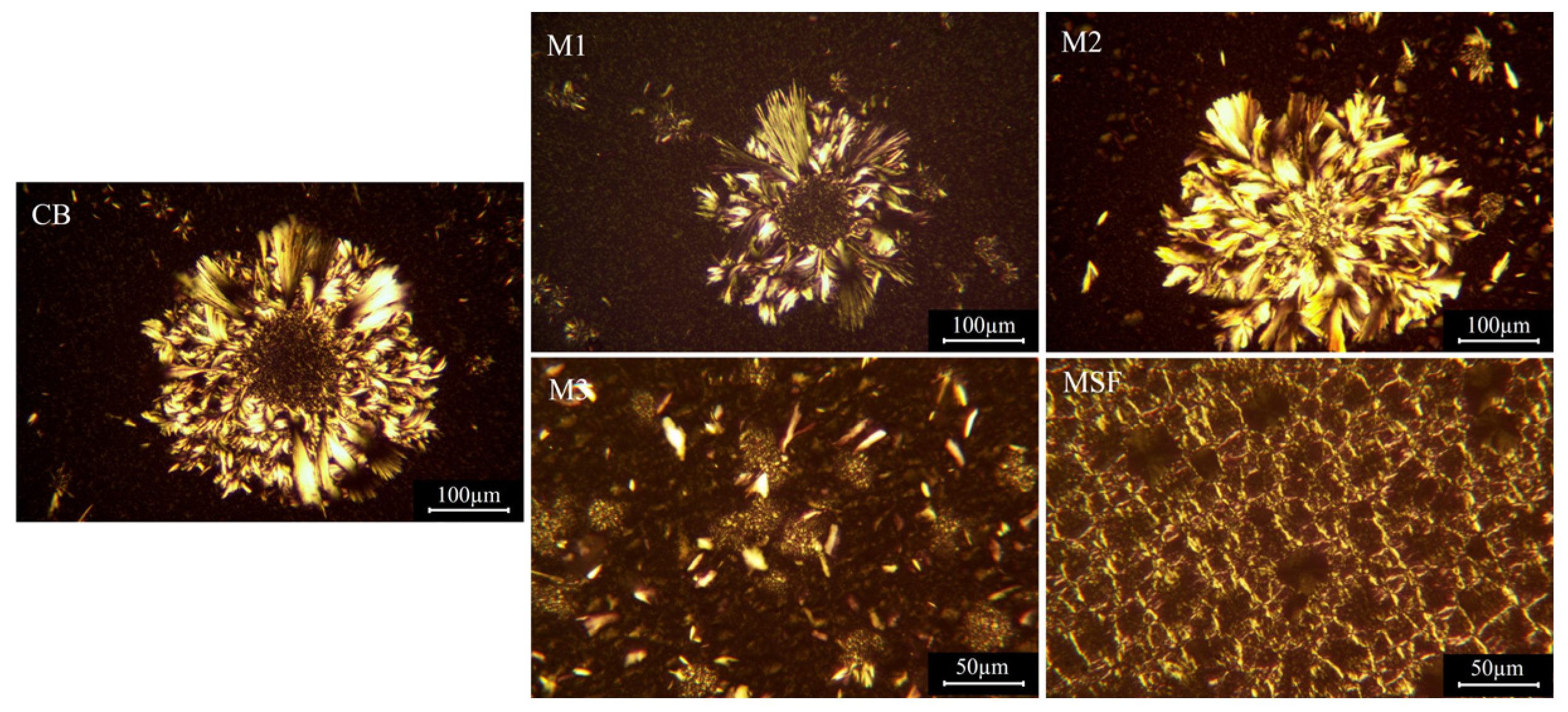

| CB | M1 | M2 | M3 | MSF | |
|---|---|---|---|---|---|
| P | 25.85 ± 0.01 | 24.90 ± 0.05 | 20.94 ± 0.04 | 13.77 ± 0.05 | 5.08 ± 0.01 |
| St | 36.63 ± 0.06 | 37.52 ± 0.01 | 41.44 ± 0.01 | 48.47 ± 0.07 | 56.98 ± 0.08 |
| O | 32.61 ± 0.04 | 32.19 ± 0.01 | 30.31 ± 0.04 | 26.91 ± 0.01 | 22.85 ± 0.04 |
| L | 2.35 ± 0.01 | 2.89 ± 0.00 | 5.06 ± 0.01 | 9.04 ± 0.01 | 13.83 ± 0.07 |
| Ln | 1.29 ± 0.01 | 1.26 ± 0.01 | 1.16 ± 0.00 | 0.98 ± 0.01 | 0.75 ± 0.00 |
| A | 0.22 ± 0.00 | 0.22 ± 0.00 | 0.19 ± 0.01 | 0.12 ± 0.01 | 0.04 ± 0.01 |
| Others | 1.06 ± 0.03 | 1.03 ± 0.01 | 0.92 ± 0.01 | 0.73 ± 0.01 | 0.48 ± 0.01 |
| Triacylglycerol (%) | CB | MSF |
|---|---|---|
| PLP | 0.29 ± 0.05 | 1.33 ± 0.06 |
| POO | 1.05 ± 0.01 | 0.37 ± 0.07 |
| PLSt | 1.24 ± 0.06 | 3.59 ± 0.19 |
| POP | 18.36 ± 0.16 | 5.04 ± 0.19 |
| StOO | 1.34 ± 0.00 | 4.00 ± 0.09 |
| POSt | 48.25 ± 0.49 | 6.51 ± 0.67 |
| StLSt | — | 30.43 ± 0.41 |
| PPSt | 0.66 ± 0.08 | — |
| StOSt | 27.64 ± 0.58 | 48.04 ± 0.19 |
| StOA | 0.54 ± 0.08 | — |
| Others | 0.66 ± 0.04 | 0.68 ± 0.06 |
| Hardness (g) | CB-Chocolate | M1-Chocolate | M2-Chocolate | M3-Chocolate |
|---|---|---|---|---|
| 15 °C | 5041.63 ± 765.63 | 4898.82 ± 575.11 | 5180.04 ± 504.82 | 4227.98 ± 1224.89 |
| 25 °C | 2337.87 ± 126.92 | 1996.69 ± 105.30 * | 1888.76 ± 176.70 * | 1758.25 ± 87.73 * |
| 32 °C | 136.28 ± 15.73 | 163.12 ± 29.76 | 151.83 ± 26.81 | 124.88 ± 17.99 |
| Onset Temp. (°C) | Peak Temp. (°C) | Enthalpy (J/g) | |
|---|---|---|---|
| CB-chocolate | 33.05 ± 0.35 | 35.36 ± 0.11 | 25.01 ± 3.26 |
| 25.28 ± 0.45 | 29.91 ± 0.10 | 15.57 ± 4.47 | |
| M1-chocolate | 27.83 ± 0.25 | 34.07 ± 0.88 | 37.47 ± 1.07 |
| M2-chocolate | 29.73 ± 0.49 | 33.53 ± 0.11 | 38.83 ± 0.13 |
| M3-chocolate | 29.08 ± 0.42 | 33.95 ± 0.01 | 35.00 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Chen, Y.; Wei, L.; Jin, J. Mangosteen Seed Fat: A Typical 1,3-Distearoyl-Sn-2-Linoleoyl-Glycerol-Rich Fat and Its Effects on Delaying Chocolate Fat Bloom. Foods 2025, 14, 557. https://doi.org/10.3390/foods14040557
Hou X, Chen Y, Wei L, Jin J. Mangosteen Seed Fat: A Typical 1,3-Distearoyl-Sn-2-Linoleoyl-Glycerol-Rich Fat and Its Effects on Delaying Chocolate Fat Bloom. Foods. 2025; 14(4):557. https://doi.org/10.3390/foods14040557
Chicago/Turabian StyleHou, Xueying, Yuhang Chen, Lai Wei, and Jun Jin. 2025. "Mangosteen Seed Fat: A Typical 1,3-Distearoyl-Sn-2-Linoleoyl-Glycerol-Rich Fat and Its Effects on Delaying Chocolate Fat Bloom" Foods 14, no. 4: 557. https://doi.org/10.3390/foods14040557
APA StyleHou, X., Chen, Y., Wei, L., & Jin, J. (2025). Mangosteen Seed Fat: A Typical 1,3-Distearoyl-Sn-2-Linoleoyl-Glycerol-Rich Fat and Its Effects on Delaying Chocolate Fat Bloom. Foods, 14(4), 557. https://doi.org/10.3390/foods14040557








