Immunomodulation of Glycyrrhiza Polysaccharides In Vivo Based on Microbiome and Metabolomics Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Glycyrrhiza Polysaccharides
2.3. Measurement of Molecular Weight
2.4. Monosaccharide Composition Analysis
2.5. Fourier Transform Infrared Spectroscopy Analysis
2.6. Scanning Electron Microscopy Analysis
2.7. Animal Experiment
2.8. Histological and Immunofluorescence Analysis
2.9. Measurement of Inflammatory Cytokines
2.10. Faecal Sample Preparation and 16S rRNA Sequencing
2.11. Preparation of Serum Samples for Metabolomics Analysis
2.12. Untargeted Serum Metabolomics
2.13. Targeted Serum and Faecal Metabolomics
2.14. Statistical Analysis
3. Results and Discussion
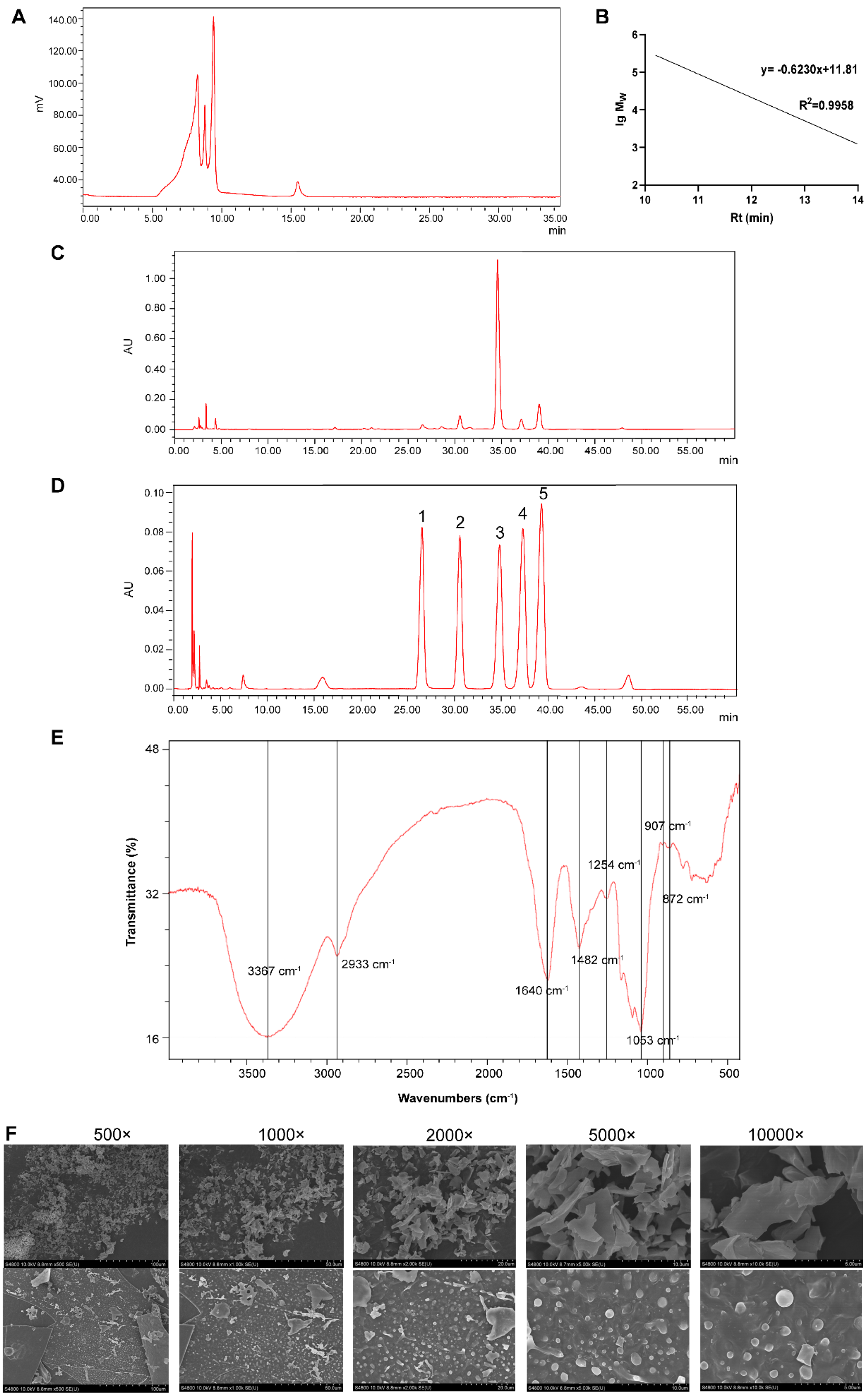
3.1. Characterisation of Glycyrrhiza Polysaccharide
3.1.1. Chemical Characterisation of GPs
3.1.2. FT-IR Analysis of GPs
3.1.3. Surface Morphological Observation
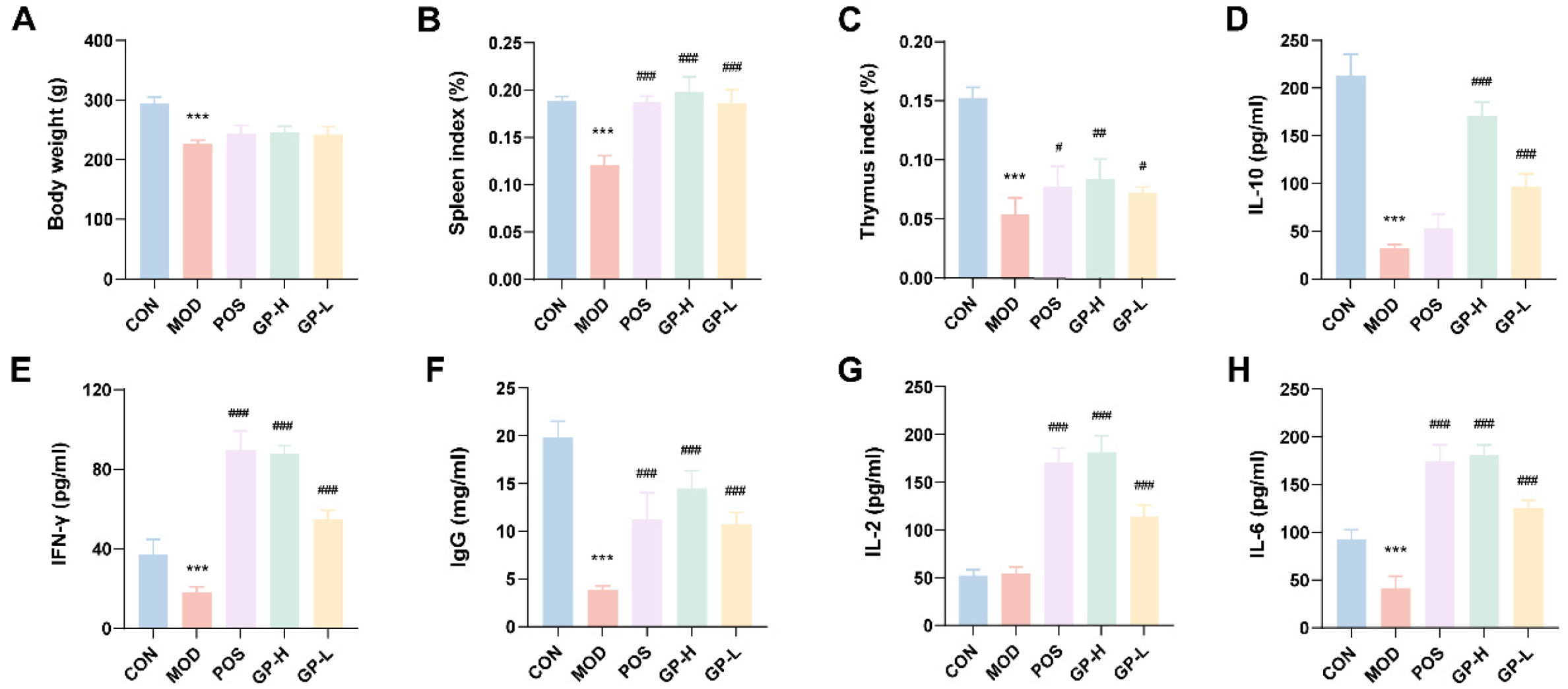
3.2. GPs Improve the Organ Index in Immunocompromised Rats
3.3. GPs Affect Cytokine Secretion in Immunocompromised Rats
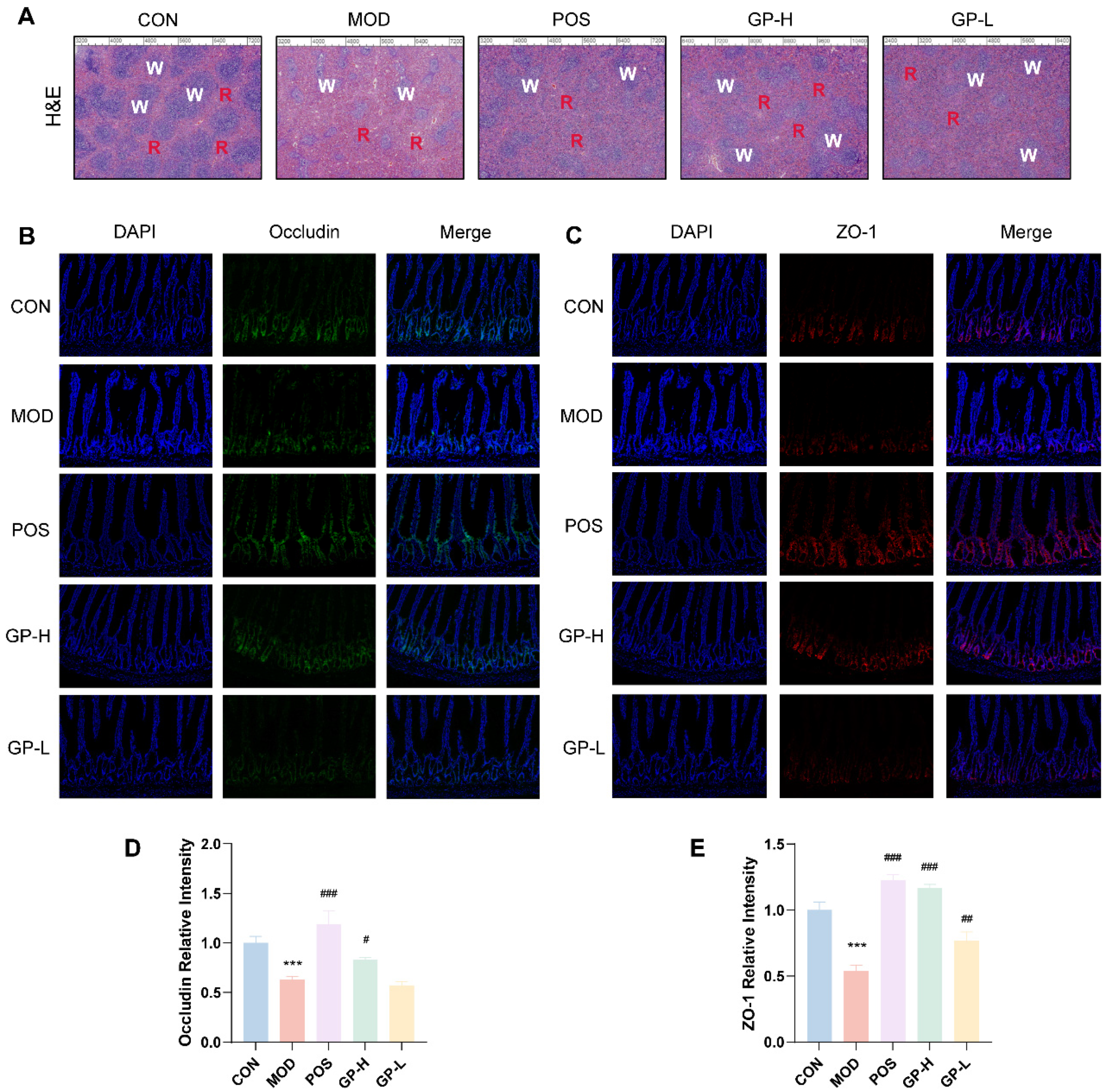
3.4. GPs Alleviate the Spleen and Intestinal Damage
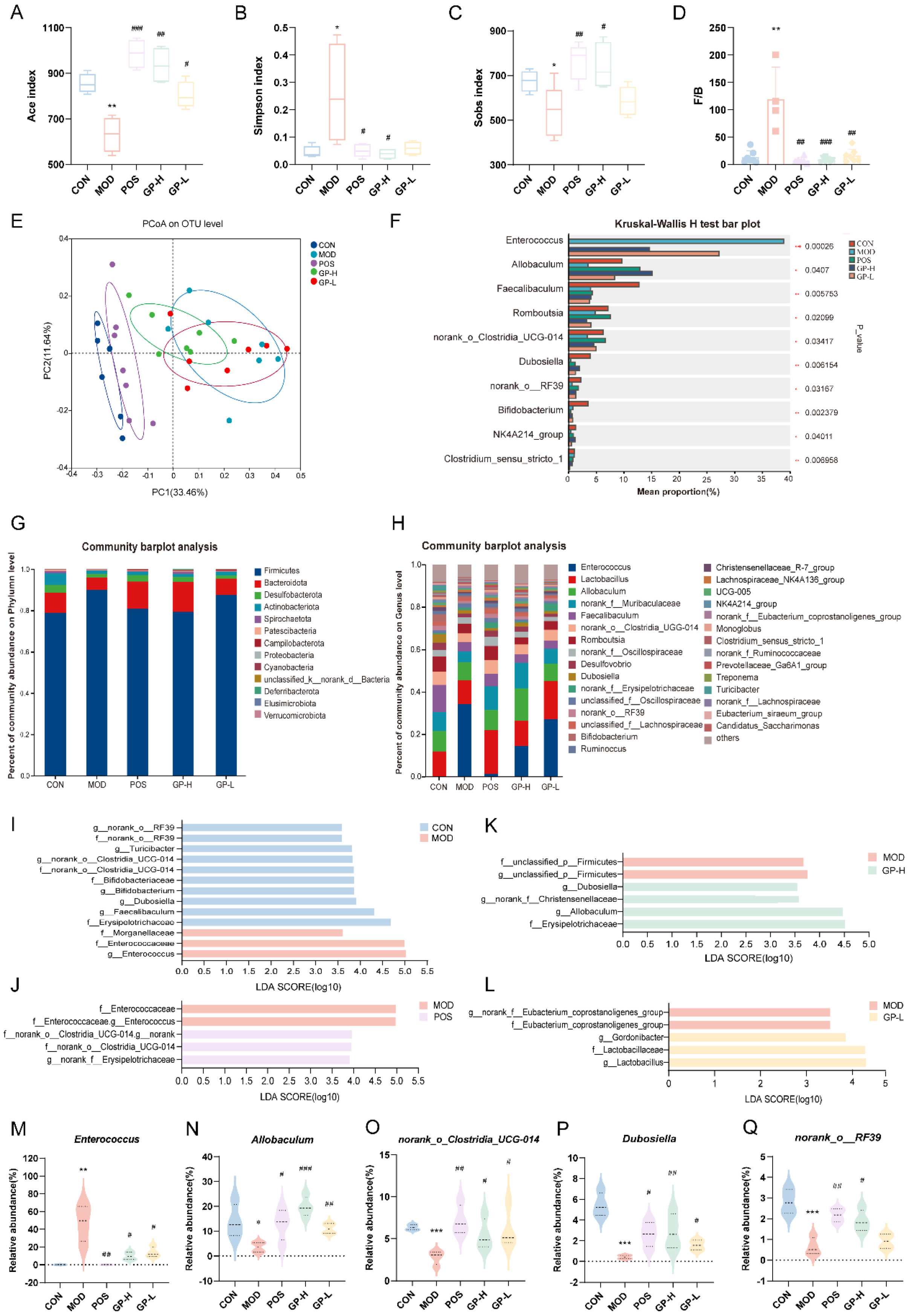
3.5. GPs Regulate the Composition of Intestinal Flora in Immunocompromised Rats
3.6. Serum Metabolic Profiling and Metabolic Pathway Analysis
3.6.1. Serum Metabolic Profiling
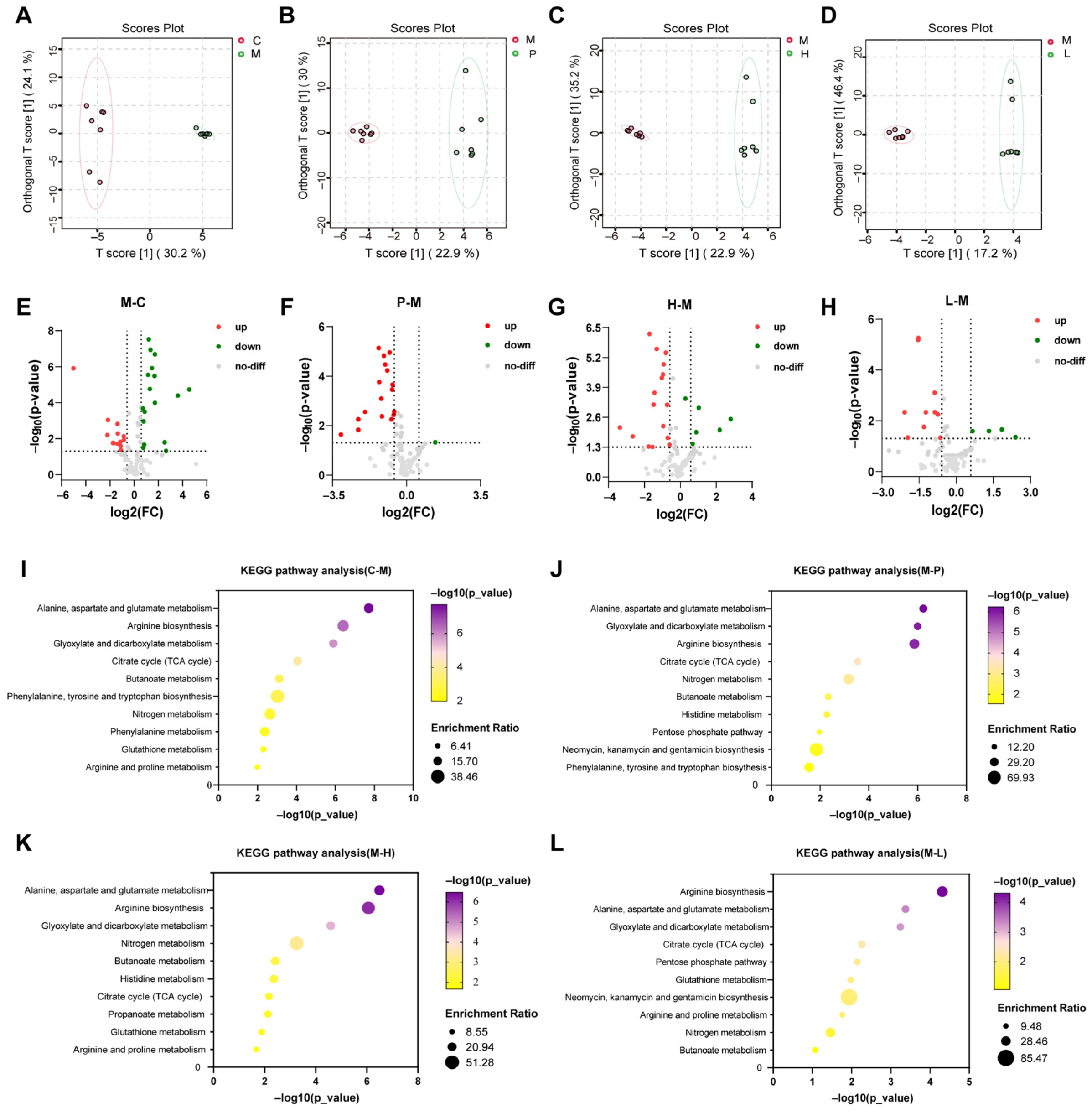
3.6.2. Metabolic Pathway Analysis
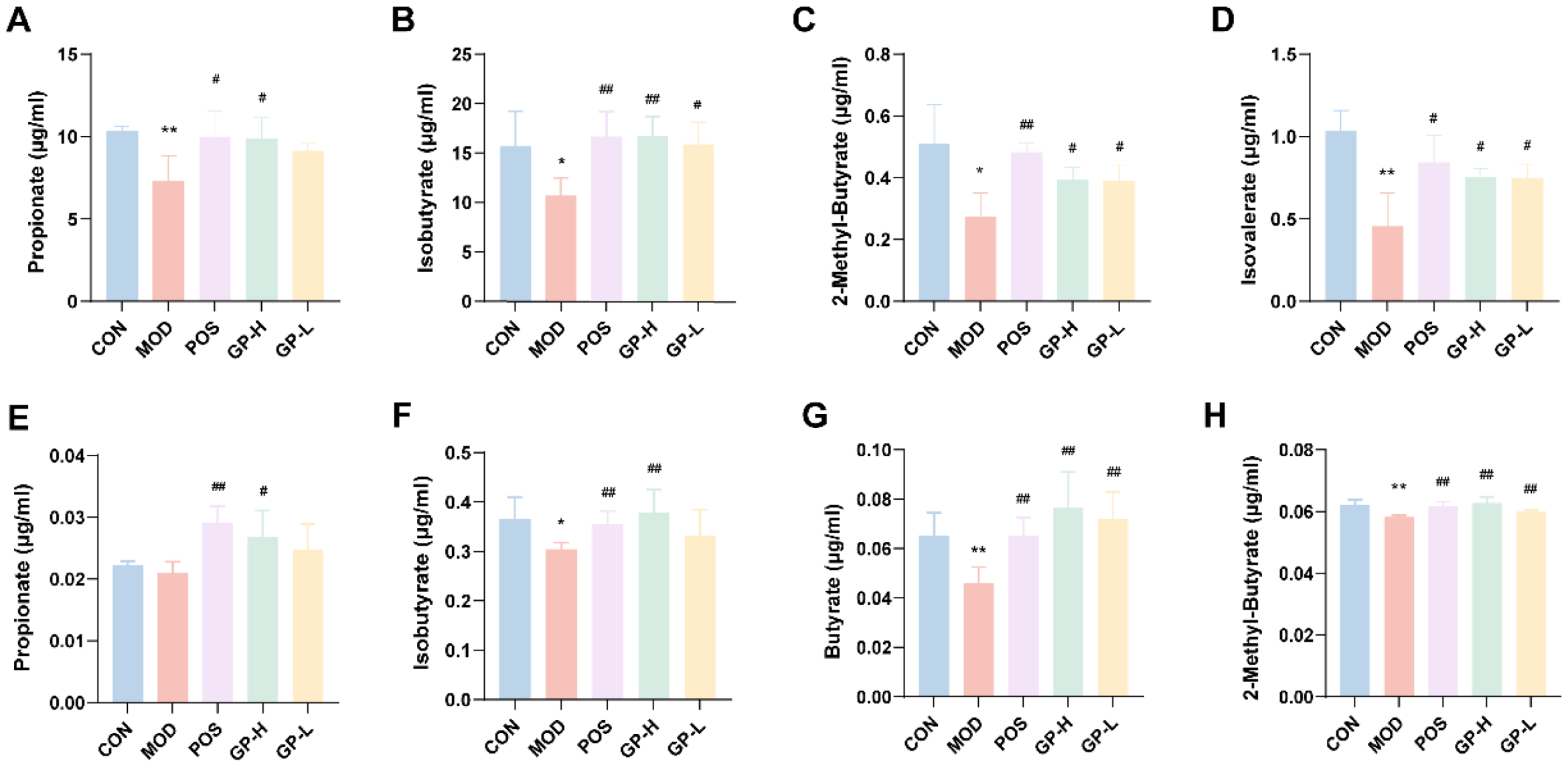
3.7. Analysis of SCFAs Content in Serum and Faeces
3.8. Correlation Analysis of Metabolites with Bacterial Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Dinteren, S.; Meijerink, J.; Witkamp, R.; van Ieperen, B.; Vincken, J.P.; Araya-Cloutier, C. Valorisation of liquorice (Glycyrrhiza) roots: Antimicrobial activity and cytotoxicity of prenylated (iso)flavonoids and chalcones from liquorice spent (G. glabra, G. inflata, and G. uralensis). Food Funct. 2022, 13, 12105–12120. [Google Scholar] [CrossRef] [PubMed]
- Bakr, A.F.; Shao, P.; Farag, M.A. Recent advances in glycyrrhizin metabolism, health benefits, clinical effects and drug delivery systems for efficacy improvement; a comprehensive review. Phytomedicine 2022, 99, 153999. [Google Scholar] [CrossRef] [PubMed]
- Simayi, Z.; Rozi, P.; Yang, X.J.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.W.; Ma, S.J.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Aipire, A.; Yuan, P.F.; Aimaier, A.; Cai, S.S.; Mahabati, M.; Lu, J.; Ying, T.L.; Zhang, B.H.; Li, J.Y. Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules 2020, 10, 159. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wang, X.J.; Zhang, K.; Zhang, X.; Li, S.W.; Li, Y.L.; Fan, W.G.; Leng, F.F.; Yang, M.J.; Chen, J.X. Extraction kinetics, thermodynamics, rheological properties and anti-BVDV activity of the hot water assisted extraction of Glycyrrhiza polysaccharide. Food Funct. 2020, 11, 4067–4080. [Google Scholar] [CrossRef]
- Cheng, A.; Wan, F.; Jin, Z.; Wang, J.; Xu, X. Nitrite oxide and inducible nitric oxide synthase were regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J. Ethnopharmacol. 2008, 118, 59–64. [Google Scholar] [CrossRef]
- Aipire, A.; Mahabati, M.; Cai, S.S.; Wei, X.X.; Yu, P.F.; Aimaier, A.; Wang, X.H.; Li, J.Y. The immunostimulatory activity of polysaccharides from Glycyrrhiza uralensis. PeerJ 2020, 8, e8294. [Google Scholar] [CrossRef]
- Li, J.; Jia, J.; Teng, Y.; Wang, X.; Xia, X.; Song, S.; Zhu, B.; Xia, X. Polysaccharides from Sea Cucumber (Stichopus japonicus) Synergize with Anti-PD1 Immunotherapy to Reduce MC-38 Tumor Burden in Mice Through Shaping the Gut Microbiome. Foods 2025, 14, 387–401. [Google Scholar] [CrossRef]
- Geng, X.; Tian, W.; Zhuang, M.; Shang, H.; Gong, Z.; Li, J. Green Radish Polysaccharides Ameliorate Hyperlipidemia in High-Fat-Diet-Induced Mice via Short-Chain Fatty Acids Production and Gut Microbiota Regulation. Foods 2024, 13, 4113–4132. [Google Scholar] [CrossRef]
- Ayeka, P.A.; Bian, Y.H.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef]
- Cheng, Z.R.; Zheng, Q.; Duan, Y.Q.; Cai, M.H.; Zhang, H.H. Effect of subcritical water temperature on the structure, antioxidant activity and immune activity of polysaccharides from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2024, 261, 129591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Yu, Y.; Liang, Y.Z.; Chen, X.Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide Degradation by the Intestinal Microbiota and Its Influence on Human Health and Disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Gao, Y.; Su, Y.; Li, J.; Ren, W.C.; Wang, Q.H.; Kuang, H.X. Probiotics with anti-type 2 diabetes mellitus properties: Targets of polysaccharides from traditional Chinese medicine. Chin. J. Nat. Medicines. 2022, 20, 641–655. [Google Scholar] [CrossRef]
- Wei, X.X.; Li, N.; Wu, X.Y.; Cao, G.D.; Qiao, H.P.; Wang, J.; Hao, R.R. The preventive effect of Glycyrrhiza polysaccharide on lipopolysaccharide-induced acute colitis in mice by modulating gut microbial communities. Int. J. Biol. Macromol. 2023, 239, 124199. [Google Scholar] [CrossRef]
- Song, W.D.; Wang, Y.Y.; Li, G.C.; Xue, S.N.; Zhang, G.L.; Dang, Y.Y.; Wang, H.B. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276814. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Cheng, H.; Wang, H.; Tan, Y.; Feng, W.; Peng, C. Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review. Food Res. Int. 2022, 160, 111653. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, C.; Che, Y.; Zhang, T.; Dai, C.; Nguyen, A.D.; Duan, K.; Huang, Y.; Li, N.; Zhou, H.; et al. Effects of Glycyrrhiza Polysaccharides on Chickens’ Intestinal Health and Homeostasis. Front. Vet. Sci. 2022, 9, 891429–891442. [Google Scholar] [CrossRef]
- Qiao, Y.; Guo, Y.; Zhang, W.; Guo, W.; Oleksandr, K.; Bozhko, N.; Wang, Z.; Liu, C. Effects of Compound Polysaccharides Derived from Astragalus and Glycyrrhiza on Growth Performance, Meat Quality and Antioxidant Function of Broilers Based on Serum Metabolomics and Cecal Microbiota. Antioxidants 2022, 11, 1872–1894. [Google Scholar] [CrossRef]
- Meng, M.; Sun, Y.; Qi, Y.L.; Xu, J.; Sun, J.G.; Bai, Y.H.; Han, L.R.; Han, R.; Hou, L.H.; Sun, H.Q. Structural characterization and induction of tumor cell apoptosis of polysaccharide from purple sweet potato (Ipomoea batatas (L.) Lam). Int. J. Biol. Macromol. 2023, 235, 123799. [Google Scholar] [CrossRef]
- Sun, S.J.; Deng, P.; Peng, C.E.; Ji, H.Y.; Mao, L.F.; Peng, L.Z. Extraction, Structure and Immunoregulatory Activity of Low Molecular Weight Polysaccharide from Dendrodium officinale. Polymers 2022, 14, 2899. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Zhang, Y.W.; Song, J.P.; Li, Y.Q.; Zhou, L.Y.; Xu, H.T.; Wu, K.Z.; Gao, J.; Zhao, M.M.; Zheng, Y. Bergamot polysaccharides relieve DSS-induced ulcerative colitis via regulating the gut microbiota and metabolites. Int. J. Biol. Macromol. 2023, 253, 127335. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Dong, Y.; Xie, Y.; Xiao, Y.X.; Ke, C.; Shi, K.; Zhou, Z.S.; Tu, J.Y.; Qu, L.H.; Liu, Y.J. Atractylodes lancea Rhizome Polysaccharide Alleviates Immunosuppression and Intestinal Mucosal Injury in Mice Treated with Cyclophosphamide. J. Agric. Food Chem. 2023, 71, 17112–17129. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Ye, Q.J.; Wang, A.J.; Chen, Y. Paeoniae Decoction restores intestinal barrier dysfunction by promoting the interaction between ILC3 and gut flora. Phytomedicine 2024, 132, 155873–155886. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Q.Y.; Wang, W.Z.; Chu, S.; Liu, X.X.; Liu, Y.J.; Tan, C.; Zhu, F.; Deng, S.J.; Dong, Y.L.; et al. Compound sophorae decoction enhances intestinal barrier function of dextran sodium sulfate induced colitis via regulating notch signaling pathway in mice. Biomed. Pharmacother. 2021, 133, 133–146. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, M.L.; Feng, Y.R.; Cai, S.H.; Luo, Z.C.; Shan, J.J.; Di, L.Q. Modulation of cellular metabolism and alleviation of bacterial dysbiosis by Aconiti lateralis radix praeparata in non-small cell lung cancer treatment. Phytomedicine 2024, 126, 155099–155111. [Google Scholar] [CrossRef]
- Shan, J.J.; Peng, L.X.; Qian, W.J.; Xie, T.; Kang, A.; Gao, B.; Di, L.Q. Integrated Serum and Fecal Metabolomics Study of Collagen-Induced Arthritis Rats and the Therapeutic Effects of the Zushima Tablet. Front. Pharmacol. 2018, 9, 891. [Google Scholar] [CrossRef]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S. Occludin: A Novel Integral Membrane Protein Localizing at Tight Junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Herb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Khatib, K.; Guo, S.H.; Ye, D.M.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Strukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gan, X.A.; Qin, B.Y.; Chen, J.; Zhao, Y.L.; Qiu, B.Y.; Chen, W.H.; Yu, Y.; Shi, S.S.; Li, T.Z.; et al. Structural characteristics of steamed Polygonatum cyrtonema polysaccharide and its bioactivity on colitis via improving the intestinal barrier and modifying the gut microbiota. Carbohydr. Polym. 2024, 327, 121669. [Google Scholar] [CrossRef]
- Xie, N.N.; Wu, C.Y.; Ge, Q.; Zhou, J.; Long, F.; Mao, Q.; Li, S.L.; Shen, H. Structure-specific antitumor effects and potential gut microbiota-involved mechanisms of ginseng polysaccharides on B16F10 melanoma-bearing mice. Food Funct. 2023, 14, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, P.; Toc, D.A.; Colosi, I.A.; Costache, C.; Ruospo, G.; Berar, G.; Galbau, S.G.; Ghilea, A.C.; Botan, A.; Pana, A.G.; et al. An Overview of the Factors Involved in Biofilm Production by the Enterococcus Genus. Int. J. Mol. Sci. 2023, 24, 11577. [Google Scholar] [CrossRef]
- Sangiorgio, G.; Calvo, M.; Migliorisi, G.; Campanile, F.; Stefani, S. The Impact of Enterococcus spp. in the Immunocompromised Host: A Comprehensive Review. Pathogens 2024, 13, 409. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.H.; Zheng, D.; Zeng, X.F.; Zhou, Z.X.; Han, L.T.; Huang, P.; Zhang, F.Y.; Wang, W.S.; Cheng, X.; et al. Serum Metabolomics Combined With 16S rRNA Gene Sequencing to Analyze the Changes of Intestinal Flora in Rats With MI and the Intervention Effect of Fuling-Guizhi. Nat. Prod. Commun. 2023, 18, 1–13. [Google Scholar] [CrossRef]
- Gu, W.C.; Zhang, L.K.; Han, T.; Huang, H.L.; Chen, J. Dynamic Changes in Gut Microbiome of Ulcerative Colitis: Initial Study from Animal Model. J. Inflamm. Res. 2022, 15, 2631–2647. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Guo, Z.J.; Li, X.M.; Chen, P.; Li, Y.; Zhang, J.B.; Mao, C.Q.; Ji, D.; Su, L.L.; Gao, B.; et al. Effects of wine processed Polygonatum polysaccharides on immunomodulatory effects and intestinal microecology in mice. Qual. Assur. Saf. Crops Foods 2023, 15, 161–174. [Google Scholar]
- Hu, Q.; Wu, C.Y.; Yu, J.T.; Luo, J.M.; Peng, X.C. Angelica sinensis polysaccharide improves rheumatoid arthritis by modifying the expression of intestinal Cldn5, Slit3 and Rgs18 through gut microbiota. Int. J. Biol. Macromol. 2022, 209, 153–161. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Tu, S.Y.; Ji, X.W.; Wu, J.N.; Meng, J.X.; Gao, J.S.; Shao, X.; Shi, S.; Wang, G.; Qiu, J.J.; et al. Dubosiella newyorkensis modulates immune tolerance in colitis via the L-lysine-activated AhR-IDO1-Kyn pathway. Nat. Commun. 2024, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Sang, R.X.; Feng, G.L.; Feng, Y.X.; Zhang, R.; Yan, X.B. Microbiological and metabolic pathways analysing the mechanisms of alfalfa polysaccharide and sulfated alfalfa polysaccharide in alleviating obesity. Int. J. Biol. Macromol. 2024, 263, 130334. [Google Scholar] [CrossRef] [PubMed]
- Beutheu, S.; Ghouzali, I.; Galas, L.; Déchelotte, P.; Coëffier, M. Glutamine and arginine improve permeability and tight junction protein expression in methotrexate-treated Caco-2 cells. Clin. Nutr. 2013, 32, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.J.; Liu, N.; Wu, X.H.; Wang, G.Y.; Lin, L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018, 97, 2675–2683. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.C.; Shan, A.S.; Han, Y.; Jin, Y.C.; Fang, H.T.; Zhao, Y.; Shen, J.L.; Zhou, C.H.; Li, C.J.; et al. Dietary glutamine supplementation enhances expression of ZO-1 and occludin and promotes intestinal development in Min piglets. Acta Agric. Scand. Sect. A Anim. Sci. 2017, 67, 15–21. [Google Scholar] [CrossRef]
- Wang, B.; Wu, G.Y.; Zhou, Z.G.; Dai, Z.L.; Sun, Y.L.; Ji, Y.; Li, W.; Wang, W.W.; Liu, C.; Han, F.; et al. Glutamine and intestinal barrier function. Amino Acids 2015, 47, 2143–2154. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.Y.; Zhu, W.Y. L-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids 2013, 45, 501–512. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Li, X.H.; Hu, S.X.; Shen, X.D.; Zhang, R.A.; Liu, C.G.; Xiao, L.; Lin, J.J.; Huang, L.; He, W.T.; Wang, X.Y.; et al. Multiomics reveals microbial metabolites as key actors in intestinal fibrosis in Crohn’s disease. EMBO Mol. Med. 2024, 16, 2427–2449. [Google Scholar] [CrossRef]
- Ji, P.; Li, C.C.; Wei, Y.M.; Hua, Y.L.; Yao, W.L.; Wu, F.L.; Zhang, X.S.; Yuan, Z.W.; Zhao, N.S.; Zhang, Y.H.; et al. A new method providing complementary explanation of the blood-enriching function and mechanism of unprocessed Angelica sinensis and its four kinds of processed products based on tissue-integrated metabolomics and confirmatory analysis. Biomed. Chromatogr. 2022, 36, e5252. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Zhang, T.; Li, W.L.; Zhang, M.G.; Zhao, L.W.; Wang, N.X.; Zhang, X.W.; Zhang, B.B. Dietary supplementation with Macleaya cordata extract alleviates intestinal injury in broiler chickens challenged with lipopolysaccharide by regulating gut microbiota and plasma metabolites. Front. Immunol. 2024, 15, 1414869. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Pirola, C.J. Alanine and aspartate aminotransferase and glutamine-cycling pathway: Their roles in pathogenesis of metabolic syndrome. World J. Gastroenterol. 2012, 18, 3775–3781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Higgins, C.B.; Tica, S.; Adams, J.A.; Sun, J.M.; Kelly, S.C.; Zong, X.Y.; Dietzen, D.J.; Pietka, T.; Ballentine, S.J.; et al. Hierarchical tricarboxylic acid cycle regulation by hepatocyte arginase 2 links the urea cycle to oxidative metabolism. Cell Metab. 2024, 36, 2069–2085. [Google Scholar] [CrossRef]
- Li, H.S.; Fang, Q.Y.; Nie, Q.X.; Hu, J.L.; Yang, C.; Huang, T.; Li, H.; Nie, S.P. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Li, M.; Su, J.; Wu, J.H.; Zhao, D.; Huang, M.Q.; Lu, Y.P.; Zheng, J.; Zheng, F.P.; Sun, B.G.; Liang, H.Y. The Regulatory Effect of Huangshui Polysaccharides on Intestinal Microbiota and Metabolites during In Vitro Fermentation. J. Agric. Food Chem. 2024, 72, 5222–5236. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Deng, D.W.; Pan, C.; Ren, J.; Wei, T.F.; Wu, Z.M.; Zhang, B.; Li, S.; Yin, P.Y.; Shang, D. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front. Immunol. 2022, 13, 951137. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, C.C.; Cai, R.Z.; Xin, L.J.; Li, Y.S.; Ke, L.X.; Ye, W.; Ouyang, T.; Liang, J.H.; Wu, R.H.; et al. NMR and MS reveal characteristic metabolome atlas and optimize esophageal squamous cell carcinoma early detection. Nat. Commun. 2024, 15, 2463. [Google Scholar] [CrossRef]
- Cong, S.; Wang, L.R.; Meng, Y.; Cai, X.L.; Zhang, C.X.; Gu, Y.Q.; Ma, X.M.; Luo, L. Saussurea involucrata oral liquid regulates gut microbiota and serum metabolism during alleviation of collagen-induced arthritis in rats. Phytother. Res. 2023, 37, 1242–1259. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; LI, Y.; Gu, L.T.; Sun, H.H.; Liu, M.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.Z.; Jiang, D.L.; Yu, K.; Yu, W.W.; Chi, Z.X.; Chen, S.; Li, M.B.; Yang, D.H.; Wang, Z.; et al. Epithelial Gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol. 2022, 7, eabk2092. [Google Scholar] [CrossRef] [PubMed]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. -Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, W.N.; Douangpanya, J.; Mu, S.R.; Jaeckel, P.; Zhang, M.; Maxwell, J.R.; Rottman, J.B.; Labitzke, K.; Willee, A.; Beckmann, H.; et al. Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses. PLoS ONE 2017, 12, e0180190. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080. [Google Scholar] [CrossRef]
- Yan, J.L.; Pan, Y.B.; Shao, W.M.; Wang, C.P.; Wang, R.N.; He, Y.Q.; Zhang, M.; Wang, Y.S.; Li, T.Z.M.; Wang, Z.F.; et al. Beneficial effect of the short-chain fatty acid propionate on vascular calcification through intestinal microbiota remodelling. Microbiome 2022, 10, 195–224. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef]
- Morris, S.M. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef]
- Guo, J.W.; Yu, J.; Peng, F.; Li, J.Z.; Tan, Z.R.; Chen, Y.; Rao, T.; Wang, Y.C.; Peng, J.B.; Zhou, H.H. In vitro and in vivo analysis of metabolites involved in the TCA cycle and glutamine metabolism associated with cisplatin resistance in human lung cancer. Expert Rev. Proteom. 2021, 18, 233–240. [Google Scholar] [CrossRef]
- Fu, X.D.; Liu, Z.M.; Zhu, C.L.; Mou, H.J.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jhun, J.; Woo, J.S.; Lee, K.H.; Hwang, S.H.; Moon, J.; Park, G.; Choi, S.S.; Kim, S.J.; Jung, Y.J.; et al. Gut microbiome-derived butyrate inhibits the immunosuppressive factors PD-L1 and IL-10 in tumor-associated macrophages in gastric cancer. Gut Microbes 2024, 16, 2300846. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Wang, D.; Zhou, S.B.; Zhou, T. Hypoglycemic Effects of Gynura divaricata (L.) DC Polysaccharide and Action Mechanisms via Modulation of Gut Microbiota in Diabetic Mice. J. Agric. Food Chem. 2024, 72, 9893–9905. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, H.; Xu, N.; Li, M.N.; Wang, L.; Ge, H.F.; Xie, Z.W.; Li, D.X.; Wang, H.Y. Large Yellow Tea Polysaccharide Alleviates HFD-Induced Intestinal Homeostasis Dysbiosis via Modulating Gut Barrier Integrity, Immune Responses, and the Gut Microbiome. J. Agric. Food Chem. 2024, 72, 7230–7243. [Google Scholar] [CrossRef]
- Li, G.F.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.Y.; Gao, X.; Zhu, R.X.; Li, Z.T.; Li, M.S.; Liu, Z.J. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef]
- Yip, W.; Hughes, M.R.; Li, Y.C.; Cait, A.; Hirst, M.; Mohn, W.W.; McNagny, K.M. Butyrate Shapes Immune Cell Fate and Function in Allergic Asthma. Front. Immunol. 2021, 12, 628453. [Google Scholar] [CrossRef]
- Ma, J.J.; Wu, W.Y.; Liao, J.; Liu, L.; Wang, Q.; Xiao, G.S.; Liu, H.F. Preparation of Dendrobium officinale Polysaccharide by Lactic Acid Bacterium Fermentation and Its Protective Mechanism against Alcoholic Liver Damage in Mice. J. Agric. Food Chem. 2024, 72, 17633–17648. [Google Scholar] [CrossRef]

| Rt (min) | lg MW | MW (Da) |
|---|---|---|
| 8.25 | 6.67 | 4.67 × 106 |
| 8.78 | 6.34 | 2.19 × 106 |
| 9.41 | 5.94 | 8.71 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Sun, J.; Xie, W.; Xue, S.; Li, X.; Guo, J.; Shan, J.; Peng, G.; Zheng, Y. Immunomodulation of Glycyrrhiza Polysaccharides In Vivo Based on Microbiome and Metabolomics Approaches. Foods 2025, 14, 874. https://doi.org/10.3390/foods14050874
Wu Y, Sun J, Xie W, Xue S, Li X, Guo J, Shan J, Peng G, Zheng Y. Immunomodulation of Glycyrrhiza Polysaccharides In Vivo Based on Microbiome and Metabolomics Approaches. Foods. 2025; 14(5):874. https://doi.org/10.3390/foods14050874
Chicago/Turabian StyleWu, Yixuan, Jie Sun, Wenjie Xie, Simin Xue, Xinli Li, Jianming Guo, Jinjun Shan, Guoping Peng, and Yunfeng Zheng. 2025. "Immunomodulation of Glycyrrhiza Polysaccharides In Vivo Based on Microbiome and Metabolomics Approaches" Foods 14, no. 5: 874. https://doi.org/10.3390/foods14050874
APA StyleWu, Y., Sun, J., Xie, W., Xue, S., Li, X., Guo, J., Shan, J., Peng, G., & Zheng, Y. (2025). Immunomodulation of Glycyrrhiza Polysaccharides In Vivo Based on Microbiome and Metabolomics Approaches. Foods, 14(5), 874. https://doi.org/10.3390/foods14050874







