Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Preparation of Plant Materials for Analyses
2.3. Macronutrient Analysis
2.4. Micronutrient Analysis
2.5. Extraction of Phytochemicals from Powders of Hemp Seed-Based Foods and Hemp By-Products
2.6. Extraction of Phytochemicals from Hemp Cream Product
2.7. Extraction of Phytochemicals from Hemp Oil Product
2.8. Plant Metabolite Analysis by LC-MS/MS
2.9. Statistical Analysis
3. Results and Discussion
3.1. Macronutrients
3.1.1. Protein Content and Reference Nutrient Intakes Provided for the Hemp Seed-Based Samples
3.1.2. Dietary Fiber
Comparison of Dietary Fiber Content of Hemp Seed-Based Samples with Other Sources of Dietary Fiber
Effect of Food Processing on Dietary Fiber Composition of Hemp Seed-Based Samples and Their Potential Health Benefits
3.1.3. Total Fat Content and Fatty Acid Composition
Fatty Acid Composition and Recommended Dietary Allowance Provided by Hemp Seed-Based Samples
Omega-6/Omega-3 Ratios of Hemp Seed-Based Samples and Their Potential Health Benefits
3.2. Mineral Content and the Reference Nutrient Intakes Provided by Hemp Seed-Based Samples
3.3. Phytochemical Composition
3.3.1. Free and Bound Forms of Phytochemicals from Hemp Seed-Based Samples
3.3.2. Potential Health Benefits of Phytochemicals Identified in Hemp Seed-Based Samples and the Relevance of Guiding Possible Applications Within the Food Industry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADI | Average daily intake |
| FAMEs | Fatty acid methyl esters |
| GC | Gas chromatography |
| GLP-1 | Glucagon-like peptide 1 |
| HCl | Hydrochloric acid |
| ICP-MS | Inductively couple plasma mass spectrometry |
| IS1 | Internal standard 1 |
| IS2 | Internal standard 2 |
| LC-MS/MS | Liquid chromatography-mass spectrometry |
| NSPs | Non-starch polysaccharides |
| PCA | Principal component analysis |
| RNI | Reference nutrient intake |
| ω-6/ω-3 | Omega-6/omega-3 |
References
- If the World Adopted a Plant-Based Diet We Would Reduce Global Agricultural Land Use from 4 to 1 Billion Hectares. Available online: https://ourworldindata.org/land-use-diets (accessed on 4 December 2023).
- Chen, Y.; Pinegar, L.; Immonen, J.; Powell, K.M. Conversion of food waste to renewable energy: A techno-economic and environmental assessment. J. Clean. Prod. 2023, 385, 135741. [Google Scholar] [CrossRef]
- Neogi, S.; Sharma, V.; Khan, N.; Chaurasia, D.; Ahmad, A.; Chauhan, S.; Singh, A.; You, S.; Pandey, A.; Bhargava, P.C. Sustainable biochar: A facile strategy for soil and environmental restoration, energy generation, mitigation of global climate change and circular bioeconomy. Chemosphere 2022, 293, 133474. [Google Scholar] [CrossRef] [PubMed]
- World Resources Report, Creating a Sustainable Food Future. Available online: https://wriorg.s3.amazonaws.com/s3fs-public/creating-sustainable-food-future_2.pdf (accessed on 19 September 2023).
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Hörtenhuber, S.J.; Seiringer, M.; Theurl, M.C.; Größbacher, V.; Piringer, G.; Kral, I.; Zollitsch, W.J. Implementing an appropriate metric for the assessment of greenhouse gas emissions from livestock production: A national case study. Animal 2022, 16, 100638. [Google Scholar] [CrossRef]
- Munialo, C.D.; Vriesekoop, F. Plant-based foods as meat and fat substitutes. Food Sci. Nutr. 2023, 11, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red meat consumption, cardiovascular diseases, and diabetes: A systematic review and meta-analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, Y.; Cai, L.; Jiang, S.; Li, C. Reconsidering Meat Intake and Human Health: A Review of Current Research. Mol. Nutr. Food Res. 2022, 66, 2101066. [Google Scholar] [CrossRef]
- Chang, R.; Javed, Z.; Taha, M.; Yahya, T.; Valero-Elizondo, J.; Brandt, E.J.; Cainzos-Achirica, M.; Mahajan, S.; Ali, H.; Nasir, K. Food insecurity and cardiovascular disease: Current trends and future directions. Am. J. Prev. Cardiol. 2021, 9, 100303. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin protein positively affects plasma LDL cholesterol and LDL:HDL cholesterol ratio in hypercholesterolemic adults after four weeks of supplementation: A randomized, controlled crossover study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Neacsu, M.; Vaughan, N.J.; Multari, S.; Haljas, E.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; Fyfe, C.; Anderson, S.; Horgan, G.; et al. Hemp and buckwheat are valuable sources of dietary amino acids, beneficially modulating gastrointestinal hormones and promoting satiety in healthy volunteers. Eur. J. Nutr. 2022, 61, 1057–1072. [Google Scholar] [CrossRef]
- Hwang, H.; Truong, V.; Jeong, W. A comparative study of nutrient compositions, phytochemicals, and antioxidant activities of two dehulled hemp seed cultivars harvested from different regions in the Republic of Korea. Food Biosci. 2025, 64, 105752. [Google Scholar] [CrossRef]
- Rbah, Y.; Taaifi, Y.; Allay, A.; Belhaj, K.; Melhaoui, R.; Houmy, N.; Ben Moumen, A.; Azeroual, E.; Addi, M.; Mansouri, F.; et al. A Comprehensive Exploration of the Fatty Acids Profile, Cholesterol, and Tocopherols Levels in Liver from Laying Hens Fed Diets Containing Nonindustrial Hemp Seed. Scientifica 2024, 2024, 8848436. [Google Scholar] [CrossRef] [PubMed]
- Taaifi, Y.; Benmoumen, A.; Belhaj, K.; Aazza, S.; Abid, M.; Azeroual, E.; Elamrani, A.; Mansouri, F.; Serghini Caid, H. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 2021, 56, 5931–5947. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Žydelis, R.; Ruzgas, R.; Bakšinskaitė, A.; Tilvikienė, V. The Seeds of Industrial Hemp (Cannabis sativa L.) a Source of Minerals and Biologically Active Compounds. J. Nat. Fibers 2022, 19, 13025–13039. [Google Scholar] [CrossRef]
- Rajasekhar, K.; Fausto, S.; Byron, S.; Frank, C.; Chris, B.; Rodney, C.; Eric, W. Characterization of the Nutritional and Safety Properties of Hemp Seed Cake as Animal Feed Ingredient. Int. J. Livest. Prod. 2021, 12, 53–63. [Google Scholar] [CrossRef]
- Floares, D.; Cocan, I.; Hotea, I.; Panda, A.; Radulov, I. Exploring the physicochemical properties of recovered industrial hemp seed (Cannabis sativa L.) cake waste. Res. J. Agric. Sci. 2024, 56, 1–8. [Google Scholar]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M. Characterization of Byproducts Originating from Hemp Oil Processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef]
- Cabral, E.M.; Zhu, X.; Garcia-Vaquero, M.; Pérez-Vila, S.; Tang, J.; Gómez-Mascaraque, L.G.; Poojary, M.M.; Curtin, J.; Tiwari, B.K. Recovery of Protein from Industrial Hemp Waste (Cannabis sativa L.) Using High-Pressure Processing and Ultrasound Technologies. Foods 2023, 12, 2883. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Babini, E.; Gianotti, A. Prebiotic potential and bioactive volatiles of hemp byproduct fermented by lactobacilli. LWT 2021, 151, 112201. [Google Scholar] [CrossRef]
- Food Waste and Its Links to Greenhouse Gases and Climate Change. Available online: https://www.usda.gov/media/blog/2022/01/24/food-waste-and-its-links-greenhouse-gases-and-climate-change#:~:text=EPA%20estimated%20that%20each%20year,42%20coal%2Dfired%20power%20plants (accessed on 26 April 2024).
- Semwogerere, F.; Katiyatiya, C.L.F.; Chikwanha, O.C.; Marufu, M.C.; Mapiye, C. Bioavailability and Bioefficacy of Hemp By-Products in Ruminant Meat Production and Preservation: A Review. Front. Vet. Sci. 2020, 7, 572906. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Burkitt, M.J.; Scobbie, L.; Chesson, A. Radical formation and coupling of hydroxycinnamic acids containing 1,2-dihydroxy substituents. Bioorganic Chem. 2003, 31, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Forrester, A.R.; Chesson, A.; Burkitt, M.J. Oxidative Coupling during Lignin Polymerization Is Determined by Unpaired Electron Delocalization within Parent Phenylpropanoid Radicals. Arch. Biochem. Biophys. 1996, 332, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Industrial Hemp Licensing: Factsheet. Available online: https://www.gov.uk/government/publications/industrial-hemp-licensing-guidance/industrial-hemp-licensing-factsheet (accessed on 18 February 2025).
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/3/Y5022E/Y5022E00.htm (accessed on 20 September 2023).
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), 33–50. [Google Scholar]
- Anderson, S. Soxtec: Its Principles and Applications. In Oil Extraction and Analysis: Critical Issues and Competitive Studies; Luthria, D.L., Ed.; AOCS: New York, NY, USA, 2004; pp. 11–24. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Nechev, J.; Christie, W.W.; Robaina, R.; de Diego, F.; Popov, S.; Stefanov, K. Chemical composition of the sponge Hymeniacidon sanguinea from the Canary Islands. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 365–374. [Google Scholar] [CrossRef]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and Phytochemical Content of High-Protein Crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef]
- Neacsu, M.; McMonagle, J.; Fletcher, R.J.; Scobbie, L.; Duncan, G.J.; Cantlay, L.; de Roos, B.; Duthie, G.G.; Russell, W.R. Bound phytophenols from ready-to-eat cereals: Comparison with other plant-based foods. Food Chem. 2013, 141, 2880–2886. [Google Scholar] [CrossRef]
- Pirisi, F.M.; Cabras, P.; Cao, C.F.; Migliorini, M.; Muggelli, M. Phenolic compounds in virgin olive oil. 2. Reappraisal of the extraction, HPLC separation, and quantification procedures. J. Agric. Food Chem. 2000, 48, 1191–1196. [Google Scholar] [CrossRef]
- Protein in the Diet. Available online: https://www.nutrition.org.uk/healthy-sustainable-diets/protein/?level=Health%20professional (accessed on 27 July 2023).
- Sharma, P.; Kotari, S.L. Barley: Impact of processing on physicochemical and thermal properties-A review. Food Rev. Int. 2017, 33, 359–381. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- Miranda, C.G.; Speranza, P.; Kurozawa, L.E.; Kawazoe Sato, A.C. Lentil protein: Impact of different extraction methods on structural and functional properties. Heliyon 2022, 8, e11775. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Rosell, C.M.; Castellari, M. Pea protein ingredients: A mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021, 110, 729–742. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Li, A.; Liu, R.H.; Gao, X.; Li, D.; Kou, X.; Xue, Z. Nutritional constituent and health benefits of chickpea (Cicer arietinum L.): A review. Food Res. Int. 2021, 150, 110790. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, R.; Yuan, W. Composition and secondary structure of proteins isolated from six different quinoa varieties from China. J. Cereal Sci. 2020, 95, 103036. [Google Scholar] [CrossRef]
- Elhardallou, S. Functional Properties of Cowpea (Vigna ungiculata L. Walp), and Lupin (Lupinus termis) Flour and Protein Isolates. J. Nutr. Food Sci. 2013, 3, 234. [Google Scholar] [CrossRef]
- Biodiversity and Nutrition (a Common Path). Available online: https://www.fao.org/fileadmin/templates/food_composition/documents/upload/Interodocumento.pdf (accessed on 11 April 2024).
- Teterycz, D.; Sobota, A.; Przygodzka, D.; Łysakowska, P. Hemp seed (Cannabis sativa L.) enriched pasta: Physicochemical properties and quality evaluation. PLoS ONE 2021, 16, e0248790. [Google Scholar] [CrossRef]
- Ermişer, D.; Yalçın, E. Dietary fibre, protein profile and technological characteristics of durum spaghetti enriched with refined/whole grain hull-less barley flour. J. Cereal Sci. 2021, 102, 103315. [Google Scholar] [CrossRef]
- Carbohydrates and Health. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 18 June 2023).
- Mitmesser, S.; Combs, M. Chapter 23 Prebiotics: Inulin and Other Oligosaccharides. In The Microbiota in Gastrointestinal Pathophysiology; Floch, M.H., Ringel, Y., Walker, W.A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 201–208. [Google Scholar]
- Gavande, P.V.; Goyal, A.; Fontes, C.M.G.A. Chapter 1—Carbohydrates and Carbohydrate-Active enZymes (CAZyme): An overview. In Glycoside Hydrolases; Goyal, A., Sharma, K., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 1–23. [Google Scholar]
- Schupfer, E.; Pak, S.C.; Wang, S.; Micalos, P.S.; Jeffries, T.; Ooi, S.L.; Golombick, T.; Harris, G.; El-Omar, E. The effects and benefits of arabinoxylans on human gut microbiota—A narrative review. Food Biosci. 2021, 43, 101267. [Google Scholar] [CrossRef]
- Smith, M.M.; Melrose, J. Xylan Prebiotics and the Gut Microbiome Promote Health and Wellbeing: Potential Novel Roles for Pentosan Polysulfate. Pharmaceuticals 2022, 15, 1151. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, X.; Li, S.; Qin, W.; Huang, Z.; Luo, Y.; Li, H.; Wu, D.; Zhang, Q.; Zhao, Y.; et al. Possible beneficial effects of xyloglucan from its degradation by gut microbiota. Trends Food Sci. Technol. 2020, 97, 65–75. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.S.; de Boeck, G.; Becker, K. Dietary roles of non-starch polysaccharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef] [PubMed]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Day, L. Lipid Chemistry. In Encyclopedia of Food Grains; Wrigley, C., Corke, H., Seetharaman, K., Faubion, J., Eds.; Academic Press: Oxford, UK, 2016; pp. 248–256. [Google Scholar]
- Rosentrater, K.A.; Evers, A.D. Chapter 4—Chemical components and nutrition. In Kent’s Technology of Cereals; Rosentrater, K.A., Evers, A.D., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 267–368. [Google Scholar]
- Chung, O.K.; Ohm, J.; Ram, M.S.; Park, S.; Howitt, C.A. Chapter 10—Wheat Lipids. In Wheat; Khan, K., Shewry, P.R., Eds.; AACC International Press: St. Paul, MN, USA, 2009; pp. 363–399. [Google Scholar]
- Innis, S.M.; Nelson, C.M. Dietary triacyglycerols rich in sn-2 palmitate alter post-prandial lipoprotein and unesterified fatty acids in term infants. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Technical Summary, Stearic Acid. Available online: https://www.beefresearch.org/resources/human-nutrition/white-papers/stearic-acid#:~:text=Dietary%20stearic%20acid%20intake%20accounts,years%20of%20age%20and%20older (accessed on 10 July 2023).
- Saturated Fats and Health: SACN Report. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/814995/SACN_report_on_saturated_fat_and_health.pdf (accessed on 10 July 2023).
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Crawford, M.A. Fatty-acid ratios in free-living and domestic animals: Possible implications for atheroma. Lancet 1968, 291, 1329–1333. [Google Scholar] [CrossRef]
- Hunter, J.E. Omega-3 Fatty Acids from Vegetable Oils. In Dietary Ω3 and Ω6 Fatty Acids: Biological Effects and Nutritional Essentiality; Galli, C., Simopoulos, A.P., Eds.; Springer: Boston, MA, USA, 1989; pp. 43–55. [Google Scholar]
- Laitinen, L.A.; Laitinen, A.; Haahtela, T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am. Rev. Respir. Dis. 1993, 147, 697–704. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Genetic Variation and Evolutionary Aspects of Diet. In Antioxidant Status, Diet, Nutrition, and Health; Papas, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 64–89. [Google Scholar]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Dietary Reference Values a Guide. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/743790/Dietary_Reference_Values_-_A_Guide__1991_.pdf (accessed on 25 July 2023).
- Micronutrient Deficiency. Available online: https://ourworldindata.org/micronutrient-deficiency#:~:text=Prevalence%20rates%20are%20typically%20highest,between%2015%20and%2020%20percent (accessed on 27 September 2023).
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Torija-Isasa, M.E.; Sánchez-Mata, M.d.C. Mineral elements and related antinutrients, in whole and hulled hemp (Cannabis sativa L.) seeds. J. Food Compos. Anal. 2022, 109, 104516. [Google Scholar] [CrossRef]
- Dahdouh, S.; Grande, F.; Espinosa, S.N.; Vincent, A.; Gibson, R.; Bailey, K.; King, J.; Rittenschober, D.; Charrondière, U.R. Development of the FAO/INFOODS/IZINCG Global Food Composition Database for Phytate. J. Food Compos. Anal. 2019, 78, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; Vaughan, N.; Raikos, V.; Multari, S.; Duncan, G.J.; Duthie, G.G.; Russell, W.R. Phytochemical profile of commercially available food plant powders: Their potential role in healthier food reformulations. Food Chem. 2015, 179, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, M.; De Lima Sampaio, S.; Hayes, H.E.; Duncan, G.J.; Vaughan, N.J.; Russell, W.R.; Raikos, V. Nutritional Content, Phytochemical Profiling, and Physical Properties of Buckwheat (Fagopyrum esculentum) Seeds for Promotion of Dietary and Food Ingredient Biodiversity. Crops 2022, 2, 21. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Duncan, G.J.; Morris, A.; Scobbie, L.; Henderson, D.; Morrice, P.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation. Appl. Sci. 2023, 13, 5781. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012, 134, 1991–1998. [Google Scholar] [CrossRef]
- Švarc-Gajić, J.; Rodrigues, F.; Moreira, M.M.; Delerue-Matos, C.; Morais, S.; Dorosh, O.; Silva, A.M.; Bassani, A.; Dzedik, V.; Spigno, G. Chemical composition and bioactivity of oilseed cake extracts obtained by subcritical and modified subcritical water. Bioresour. Bioprocess. 2022, 9, 114. [Google Scholar] [CrossRef]
- Frankowski, J.; Przybylska-Balcerek, A.; Graczyk, M.; Niedziela, G.; Sieracka, D.; Stuper-Szablewska, K. The Effect of Mineral Fertilization on the Content of Bioactive Compounds in Hemp Seeds and Oil. Molecules 2023, 28, 4870. [Google Scholar] [CrossRef]
- Capcanari, T.; Covaliov, E.; Negoița, C.; Siminiuc, R.; Chirsanova, A.; Reșitca, V.; Țurcanu, D. Hemp Seed Cake Flour as a Source of Proteins, Minerals and Polyphenols and Its Impact on the Nutritional, Sensorial and Technological Quality of Bread. Foods 2023, 12, 4327. [Google Scholar] [CrossRef]
- Benkirane, C.; Mansouri, F.; Ben Moumen, A.; Taaifi, Y.; Melhaoui, R.; Caid, H.S.; Fauconnier, M.; Elamrani, A.; Abid, M. Phenolic profiles of non-industrial hemp (Cannabis sativa L.) seed varieties collected from four different Moroccan regions. Int. J. Food Sci. Technol. 2023, 58, 1367–1381. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef]
- McLusky, S.R.; Bennett, M.H.; Beale, M.; Lewis, M.J.; Gaskin, P.; Mansfield, J. Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J. 1999, 17, 523–534. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Gao, Y.; Zhan, J.; You, Y.; Huang, W. Gentisic acid prevents diet-induced obesity in mice by accelerating the thermogenesis of brown adipose tissue. Food Funct. 2021, 12, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Hossin, A.Y.; Inafuku, M.; Takara, K.; Nugara, R.N.; Oku, H. Syringin: A Phenylpropanoid Glycoside Compound in Cirsium brevicaule A. GRAY Root Modulates Adipogenesis. Molecules 2021, 26, 1531. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Ju, M.; Majumder, R.; Shukla, S.; Huh, Y.S.; Na, M.; Lee, S.H.; Han, Y. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci. Rep. 2018, 8, 9216. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Um, J.; Hong, S. The beneficial effect of vanillic acid on ulcerative colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, J.; Tan, L.; Li, Z.; Gao, P.; He, S.; Wang, Q.; Tang, D.; Wang, C.; Wang, F.; et al. (-)-Syringaresinol attenuates ulcerative colitis by improving intestinal epithelial barrier function and inhibiting inflammatory responses. Phytomedicine 2024, 124, 155292. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, S.; Shin, H.; Kang, S.; Kim, J.; Ko, H.; Kim, S. p-Coumaric acid modulates glucose and lipid metabolism via AMP-activated protein kinase in L6 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
- Janicke, B.; Önning, G.; Oredsson, S.M. Differential Effects of Ferulic Acid and p-Coumaric Acid on S Phase Distribution and Length of S Phase in the Human Colonic Cell Line Caco-2. J. Agric. Food Chem. 2005, 53, 6658–6665. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Amoli, P.I.; Hadidi, M.; Hasiri, Z.; Rouhafza, A.; Jelyani, A.Z.; Hadian, Z.; Khaneghah, A.M.; Lorenzo, J.M. Incorporation of Low Molecular Weight Chitosan in a Low-Fat Beef Burger: Assessment of Technological Quality and Oxidative Stability. Foods 2021, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef] [PubMed]

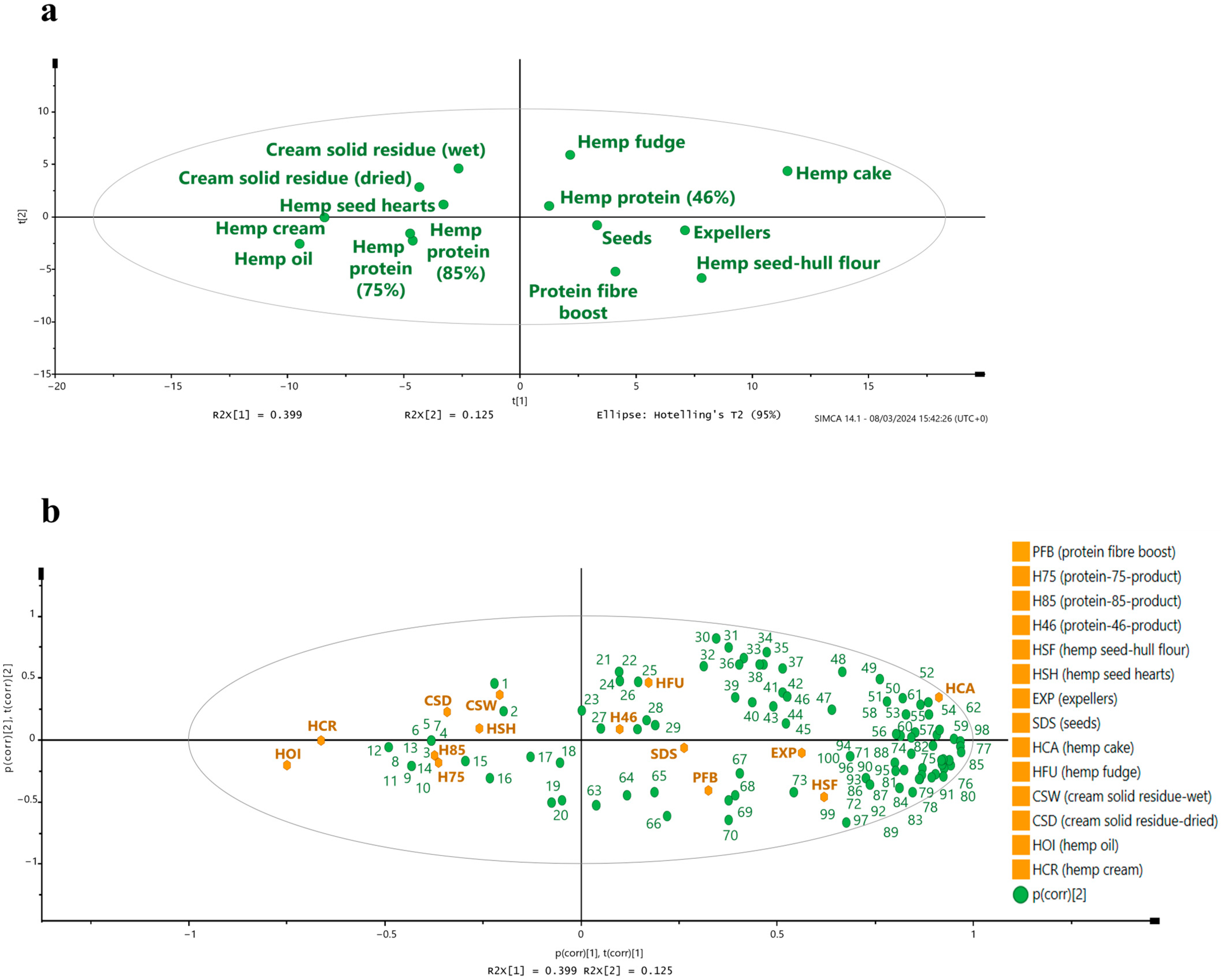
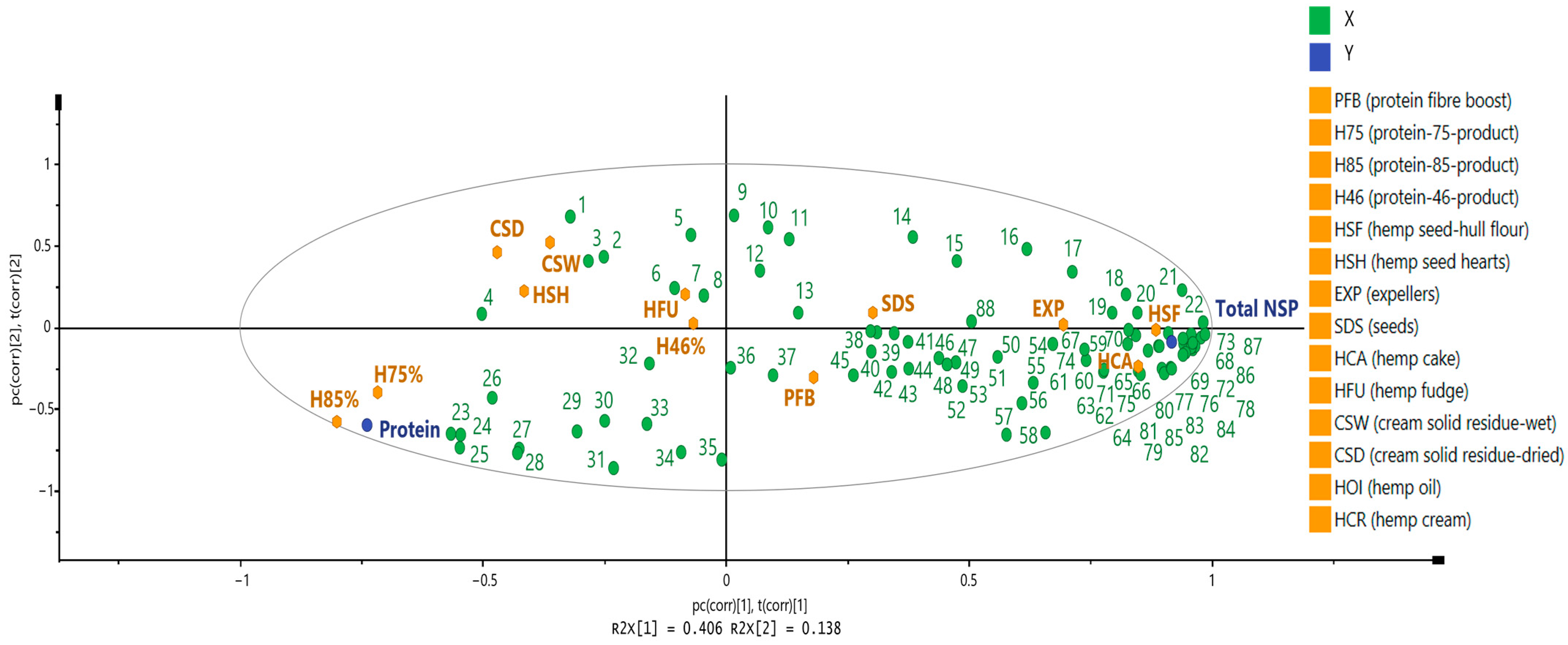
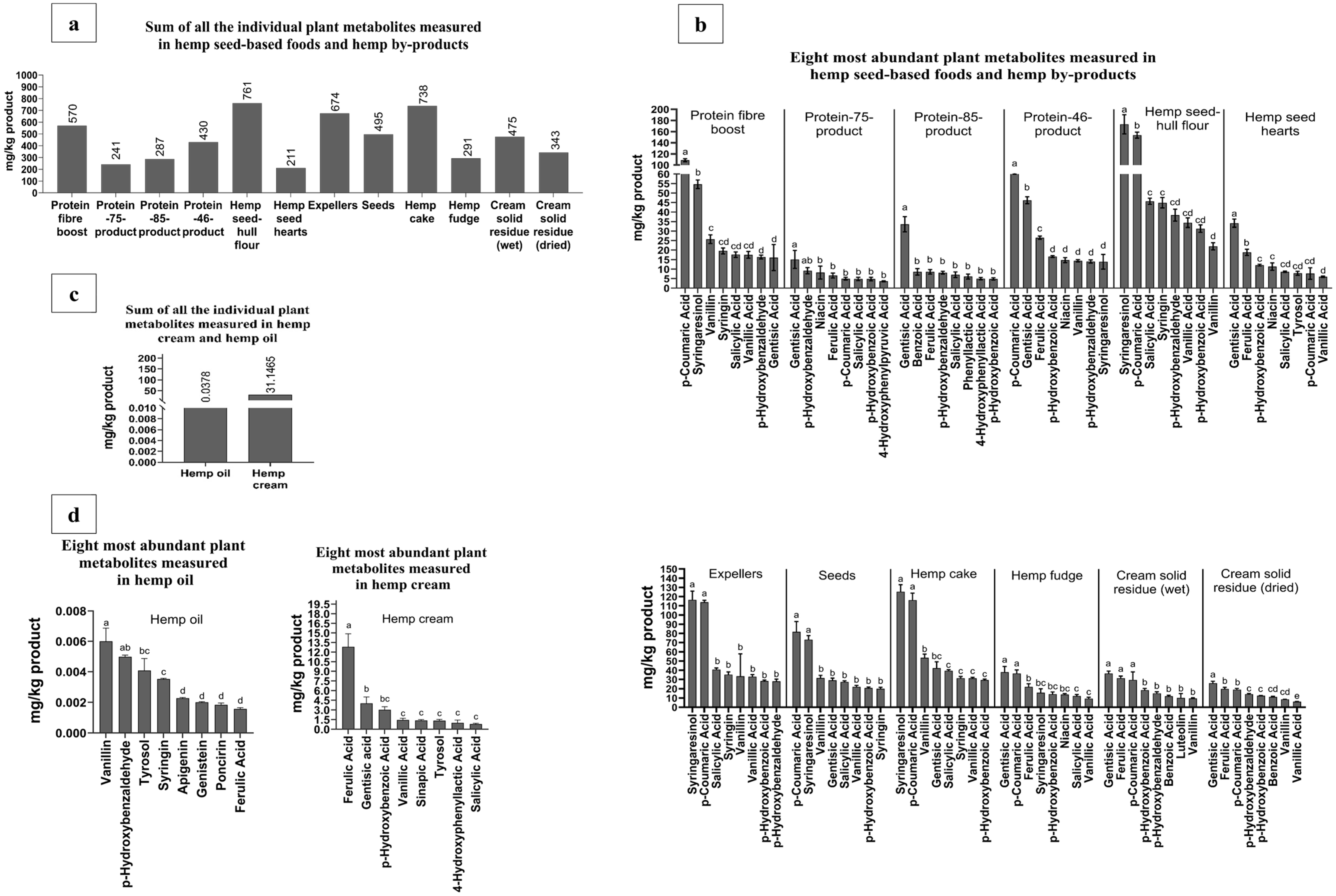
| Sample Type | Macronutrient Content (% Dry Weight) | |||||
|---|---|---|---|---|---|---|
| Protein | Total Fat | Soluble NSPs | Insoluble NSPs | Ash | Moisture Content | |
| Protein fiber boost | 38.15 ± 0.13 d | 16.62 ± 0.19 f | 0.73 ± 0.19 a | 19.20 ± 0.66 d | 5.92 ± 0.03 c | 8.33 ± 0.08 cd |
| Protein-75-product | 74.50 ± 0.29 b | 10.91 ± 0.18 h | 0.33 ± 0.04 b | 4.27 ± 0.05 h | 11.74 ± 0.03 a | 6.29 ± 0.01 f |
| Protein-85-product | 93.01 ± 0.18 a | 3.24 ± 0.18 j | 0.43 ± 0.11 b | 3.91 ± 0.06 h | 7.76 ± 0.32 b | 4.72 ± 0.09 g |
| Protein-46-product | 55.62 ± 0.14 c | 13.44 ± 0.35 g | 0.62 ± 0.19 ab | 13.41 ± 0.46 f | 7.30 ± 0.02 b | 7.33 ± 0.08 e |
| Hemp seed-hull flour | 13.57 ± 0.10 i | 8.52 ± 0.13 i | 0.49 ± 0.03 ab | 39.80 ± 0.07 a | 2.33 ± 0.02 g | 8.42 ± 0.02 c |
| Hemp seed hearts | 35.87 ± 0.42 e | 45.51 ± 0.60 c | 0.45 ± 0.10 ab | 3.60 ± 0.08 h | 4.45 ± 0.02 e | 6.01 ± 0.04 f |
| Expellers | 25.13 ± 0.19 h | 10.10 ± 0.59 hi | 0.50 ± 0.02 ab | 31.60 ± 0.81 b | 3.58 ± 0.03 f | 7.87 ± 0.04 d |
| Seeds | 26.67 ± 0.19 g | 30.93 ± 1.60 e | 0.48 ± 0.02 ab | 17.50 ± 0.65 e | 3.41 ± 0.02 f | 8.47 ± 0.11 c |
| Hemp cake | 34.44 ± 0.19 f | 10.76 ± 0.18 h | 0.45 ± 0.07 ab | 24.20 ± 0.17 c | 4.38 ± 0.01 e | 9.70 ± 0.05 b |
| Hemp fudge | 37.66 ± 0.37 d | 45.10 ± 1.12 c | 0.40 ± 0.05 b | 4.81 ± 0.09 h | 5.04 ± 0.04 d | 5.12 ± 0.23 g |
| Cream solid residue (wet) | 35.60 ± 0.29 e | 41.34 ± 0.39 d | 0.40 ± 0.01 b | 8.13 ± 0.29 g | 4.34 ± 0.46 e | 2.85 ± 0.05 h |
| Cream solid residue (dried) | 35.91 ± 0.04 e | 42.22 ± 0.23 d | 0.40 ± 0.07 b | 8.63 ± 0.17 g | 4.36 ± 0.12 e | 2.88 ± 0.05 h |
| Hemp oil | n/q | 93.80 ± 0.89 a | n/q | n/q | n/q | n/q |
| Hemp cream | n/q | 63.10 ± 0.83 b | n/q | n/q | 0.20 ± 0.01 h | 19.40 ± 0.52 a |
| Sample Type | NSP Type | Monosaccharide Content of NSP (% DM) | Total NSPs (% DM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rhamnose | Fucose | Arabinose | Xylose | Mannose | Galactose | Glucose | Uronic acid | |||
| Protein fiber boost | Soluble | 0.03 ± 0.03 c | 0.04 ± 0.01 bc | 0.06 ± 0.03 bc | 0.07 ± 0.02 bc | 0.26 ± 0.02 a | 0.07 ± 0.02 bc | 0.05 ± 0.00 bc | 0.12 ± 0.04 b | 0.73 |
| Insoluble | 0.38 ± 0.00 e | 0.10 ± 0.01 e | 1.38 ± 0.03 d | 7.07 ± 0.16 a | 0.46 ± 0.02 e | 0.71 ± 0.02 de | 5.94 ± 0.66 b | 3.15 ± 0.19 c | 19.20 | |
| Protein-75-product | Soluble | n/d | 0.02 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.01 b | 0.22 ± 0.01 a | n/d | 0.034 ± 0.00 b | 0.02 ± 0.04 b | 0.33 |
| Insoluble | 0.12 ± 0.00 e | 0.04 ± 0.00 f | 0.88 ± 0.00 b | 0.53 ± 0.00 c | 0.26 ± 0.00 d | 0.23 ± 0.00 d | 1.20 ± 0.00 a | 0.88 ± 0.05 b | 4.27 | |
| Protein-85-product | Soluble | n/d | 0.02 ± 0.01 b | 0.01 ± 0.00 b | 0.01 ± 0.01 b | 0.21 ± 0.01 a | 0.01 ± 0.01 b | 0.03 ± 0.01 b | 0.09 ± 0.08 b | 0.43 |
| Insoluble | 0.12 ± 0.01 e | 0.04 ± 0.00 e | 0.56 ± 0.04 c | 0.45 ± 0.04 c | 0.26 ± 0.01 d | 0.24 ± 0.01 d | 1.28 ± 0.07 a | 0.91 ± 0.07 b | 3.91 | |
| Protein-46-product | Soluble | 0.04 ± 0.02 b | 0.04 ± 0.01 b | 0.05 ± 0.02 b | 0.06 ± 0.03 b | 0.26 ± 0.03 a | 0.06 ± 0.02 b | 0.06 ± 0.04 b | 0.02 ± 0.00 b | 0.62 |
| Insoluble | 0.33 ± 0.03 fg | 0.11 ± 0.02 g | 1.63 ± 0.03 d | 3.02 ± 0.04 b | 0.47 ± 0.02 ef | 0.63 ± 0.03 e | 4.74 ± 0.27 a | 2.42 ± 0.05 c | 13.41 | |
| Hemp seed-hull flour | Soluble | 0.01 ± 0.01 c | 0.04 ± 0.01 b | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.24 ± 0.01 a | 0.03 ± 0.00 b | 0.04 ± 0.01 b | 0.02 ± 0.00 bc | 0.49 |
| Insoluble | 0.53 ± 0.00 e | 0.07 ± 0.00 f | 0.73 ± 0.00 d | 23.90 ± 0.09 a | 0.54 ± 0.01 e | 0.69 ± 0.01 d | 9.27 ± 0.01 b | 4.11 ± 0.10 c | 39.80 | |
| Hemp seed hearts | Soluble | 0.01 ± 0.01 b | 0.03 ± 0.00 b | 0.03 ± 0.01 b | 0.06 ± 0.06 b | 0.22 ± 0.01 a | 0.03 ± 0.01 b | 0.04 ± 0.01 b | 0.01 ± 0.00 b | 0.45 |
| Insoluble | 0.10 ± 0.00 e | 0.03 ± 0.00 f | 0.74 ± 0.02 b | 0.61 ± 0.03 c | 0.24 ± 0.01 d | 0.19 ± 0.01 d | 1.00 ± 0.02 a | 0.73 ± 0.01 b | 3.60 | |
| Expellers | Soluble | 0.02 ± 0.02 b | 0.04 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.01 b | 0.24 ± 0.01 a | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.50 |
| Insoluble | 0.46 ± 0.00 de | 0.07 ± 0.00 e | 0.91 ± 0.00 d | 18.60 ± 0.28 a | 0.48 ± 0.02 de | 0.64 ± 0.01 de | 6.85 ± 0.59 b | 3.52 ± 0.13 c | 31.60 | |
| Seeds | Soluble | n/d | 0.03 ± 0.00 b | 0.03 ± 0.03 b | 0.02 ± 0.01 b | 0.25 ± 0.01 a | 0.04 ± 0.00 b | 0.04 ± 0.00 b | 0.04 ± 0.01 b | 0.48 |
| Insoluble | 0.28 ± 0.00 e | 0.05 ± 0.00 e | 0.80 ± 0.01 d | 9.64 ± 0.30 a | 0.37 ± 0.01 e | 0.45 ± 0.02 de | 3.80 ± 0.28 b | 2.10 ± 0.04 c | 17.50 | |
| Hemp cake | Soluble | n/d | 0.04 ± 0.02 b | 0.03 ± 0.01 b | 0.02 ± 0.01 b | 0.25 ± 0.01 a | 0.02 ± 0.02 b | 0.02 ± 0.00 b | 0.04 ± 0.01 b | 0.45 |
| Insoluble | 0.38 ± 0.01 e | 0.07 ± 0.00 f | 1.05 ± 0.01 d | 13.30 ± 0.18 a | 0.45 ± 0.02 e | 0.59 ± 0.01 e | 5.41 ± 0.05 b | 2.90 ± 0.10 c | 24.20 | |
| Hemp fudge | Soluble | n/d | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.01 ± 0.01 b | 0.22 ± 0.04 a | 0.01 ± 0.01 b | 0.03 ± 0.00 b | 0.03 ± 0.01 b | 0.40 |
| Insoluble | 0.12 ± 0.02 fg | 0.03 ± 0.00 g | 0.57 ± 0.01 d | 1.22 ± 0.06 b | 0.25 ± 0.03 e | 0.19 ± 0.00 ef | 1.56 ± 0.07 a | 0.76 ± 0.01 c | 4.81 | |
| Cream solid residue (wet) | Soluble | n/d | 0.03 ± 0.00 bc | 0.02 ± 0.00 c | 0.01 ± 0.00 c | 0.21 ± 0.01 a | 0.01 ± 0.01 c | 0.03 ± 0.00 c | 0.05 ± 0.01 b | 0.40 |
| Insoluble | 0.22 ± 0.00 cd | 0.06 ± 0.00 d | 1.38 ± 0.01 b | 2.06 ± 0.08 a | 0.32 ± 0.01 c | 0.40 ± 0.00 c | 1.81 ± 0.21 a | 1.83 ± 0.10 a | 8.13 | |
| Cream solid residue (dried) | Soluble | n/d | 0.04 ± 0.02 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.24 ± 0.04 a | 0.01 ± 0.01 b | 0.03 ± 0.00 b | 0.02 ± 0.01 b | 0.40 |
| Insoluble | 5.01 ± 0.01 e | 0.24 ± 0.00 f | 0.07 ± 0.03 c | 1.46 ± 0.09 ab | 2.02 ± 0.00 de | 0.34 ± 0.01 d | 0.42 ± 0.06 a | 2.11 ± 0.03 b | 8.63 | |
| Sample Type | Fatty Acid Methyl Esters % from Total Fat | Total Fat (%) | Ratio ω-6/ω-3 (Omega-6/Omega-3) | |||||
|---|---|---|---|---|---|---|---|---|
| Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | Gamma-Linolenic Acid | Alpha-Linolenic Acid | |||
| Protein fiber boost | 8.98 ± 0.04 b | 3.04 ± 0.03 efg | 14.19 ± 0.04 fg | 59.74 ± 0.09 bcd | 3.06 ± 0.00 ab | 15.80 ± 0.02 b | 16.62 ± 0.19 f | 3.97 |
| Protein-75-product | 7.40 ± 0.09 d | 3.33 ± 0.07 cd | 15.73 ± 0.43 cd | 57.82 ± 1.31 def | 2.63 ± 0.15 d | 16.31 ± 0.46 b | 10.91 ± 0.18 h | 3.71 |
| Protein-85-product | 10.41 ± 1.60 a | 4.24 ± 0.17 a | 16.94 ± 0.85 ab | 53.80 ± 2.02 h | 2.23 ± 0.15 e | 12.07 ± 0.66 e | 3.24 ± 0.18 j | 4.64 |
| Protein-46-product | 9.07 ± 0.10 ab | 3.61 ± 0.03 b | 15.73 ± 0.14 cd | 59.32 ± 0.05 bcd | 2.63 ± 0.01 d | 13.38 ± 0.13 d | 13.44 ± 0.35 g | 4.63 |
| Hemp seed-hull flour | 9.03 ± 0.12 ab | 3.18 ± 0.07 def | 15.70 ± 0.10 cd | 60.10 ± 0.03 bc | 2.71 ± 0.01 cd | 14.30 ± 0.19 c | 8.52 ± 0.13 i | 4.39 |
| Hemp seed hearts | 7.08 ± 0.06 de | 3.04 ± 0.03 efg | 15.10 ± 0.03 def | 59.38 ± 0.08 bcd | 2.70 ± 0.01 cd | 15.85 ± 0.03 b | 45.51 ± 0.60 c | 3.92 |
| Expellers | 8.86 ± 0.41 bc | 3.35 ± 0.01 bcd | 15.79 ± 0.35 cd | 59.29 ± 0.62 bcd | 2.65 ± 0.05 d | 14.10 ± 0.15 c | 10.10 ± 0.59 hi | 4.14 |
| Seeds | 7.46 ± 0.02 cd | 3.08 ± 0.03 efg | 15.40 ± 0.03 cde | 61.10 ± 0.10 b | 2.74 ± 0.01 cd | 16.10 ± 0.05 b | 30.93 ± 1.60 e | 3.96 |
| Hemp cake | 9.09 ± 0.18 ab | 3.51 ± 0.09 bc | 16.10 ± 0.08 bc | 60.71 ± 0.36 b | 2.62 ± 0.03 d | 14.86 ± 0.23 c | 10.76 ± 0.18 h | 4.27 |
| Hemp fudge | 7.11 ± 0.07 de | 2.98 ± 0.08 fg | 14.57 ± 0.06 efg | 58.57 ± 0.30 cde | 2.71 ± 0.02 cd | 15.10 ± 0.10 b | 45.10 ± 1.12 c | 3.83 |
| Cream solid residue (wet) | 7.97 ± 0.09 bcd | 3.28 ± 0.02 cde | 14.14 ± 0.36 fg | 55.50 ± 0.37 gh | 2.91 ± 0.01 bc | 14.38 ± 0.04 c | 41.34 ± 0.39 d | 4.06 |
| Cream solid residue (dried) | 7.81 ± 0.18 bcd | 2.91 ± 0.17 gh | 13.87 ± 0.21 g | 56.47 ± 0.17 efg | 2.57 ± 0.06 d | 14.69 ± 0.09 c | 42.22 ± 0.23 d | 4.02 |
| Hemp oil | 5.93 ± 0.36 e | 2.67 ± 0.02 h | 12.91 ± 0.21 h | 55.10 ± 0.54 fg | 3.05 ± 0.20 ab | 16.25 ± 0.25 b | 93.80 ± 0.89 a | 3.63 |
| Hemp cream | 7.71 ± 0.07 bcd | 3.45 ± 0.04 bc | 17.35 ± 0.07 a | 69.53 ± 0.45 a | 3.26 ± 0.02 ª | 18.85 ± 0.14 a | 63.10 ± 0.83 b | 3.86 |
| Sample Type | Microelement (mg/100 g Dry Weight) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | P | Mn | Fe | Cu | Zn | |
| Protein fiber boost | 0.08 ± 0.10 c | 1166.81 ± 17.10 b | 373.84 ± 5.88 a | 628.39 ± 8.48 c | 1296.89 ± 14.41 d | 19.17 ± 0.19 b | 23.68 ± 0.39 b | 1.73 ± 0.01 a | 8.13 ± 0.08 ef |
| Protein-75-product | 17.29 ± 0.17 a | 980.95 ± 15.10 d | 280.34 ± 3.11 b | 1581.36 ± 5.74 a | 3096.66 ± 115 a | 24.29 ± 0.20 a | 36.20 ± 0.18 a | 0.99 ± 0.01 fgh | 27.62 ± 0.28 a |
| Protein-85-product | 7.17 ± 0.04 b | 135.04 ± 1.82 h | 166.64 ± 3.22 e | 401.69 ± 7.42 fg | 1922.26 ± 28.36 b | 13.23 ± 0.76 c | 34.62 ± 0.59 a | 0.81 ± 0.02 h | 24.72 ± 0.36 b |
| Protein-46-product | n/d | 1431.00 ± 49.08 a | 282.25 ± 11.10 b | 818.60 ± 28.06 b | 1740.12 ± 54.10 c | 19.15 ± 0.37 b | 22.30 ± 0.43 b | 1.75 ± 0.03 a | 12.72 ± 0.36 c |
| Hemp seed- hull flour | n/d | 456.27 ± 36.37 g | 201.63 ± 19.37 cd | 143.50 ± 18.40 h | 220.31 ± 19.85 h | 9.38 ± 0.90 de | 10.52 ± 1.51 f | 0.91 ± 0.07 gh | 1.71 ± 0.21 h |
| Hemp seed hearts | 0.56 ± 0.98 c | 870.54 ± 19.72 de | 108.42 ± 2.92 f | 509.06 ± 9.57 de | 1081.56 ± 37.51 e | 7.55 ± 0.15 f | 10.75 ± 0.17 ef | 1.12 ± 0.03 ef | 8.42 ± 0.20 e |
| Expellers | n/d | 757.80 ± 31.61 ef | 208.21 ± 11.33 c | 332.05 ± 19.89 g | 642.00 ± 28.82 g | 10.90 ± 0.51 d | 10.22 ± 0.62 f | 1.29 ± 0.05 de | 4.79 ± 0.34 g |
| Seeds | n/d | 676.69 ± 13.45 f | 168.04 ± 9.94 de | 348.39 ± 10.29 g | 694.94 ± 13.74 g | 9.38 ± 0.47 de | 9.22 ± 0.27 f | 1.07 ± 0.04 fg | 5.23 ± 0.24 g |
| Hemp cake | 0.09 ± 0.15 c | 914.22 ± 22.36 d | 232.66 ± 6.27 c | 450.24 ± 22.34 ef | 886.53 ± 12.34 f | 13.43 ± 0.88 c | 12.76 ± 0.56 de | 1.48 ± 0.06 bc | 6.91 ± 0.37 f |
| hemp fudge | n/d | 985.16 ± 106 cd | 268.84 ± 28.21 b | 607.67 ± 58.92 c | 1267.16 ± 135 d | 8.94 ± 0.89 ef | 15.59 ± 1.45 c | 1.41 ± 0.13 cd | 9.24 ± 0.93 e |
| Cream solid residue (wet) | 7.54 ± 0.16 b | 1190.85 ± 5.04 b | 141.52 ± 3.04 ef | 519.30 ± 4.31 de | 1213.96 ± 14.66 de | 8.84 ± 0.08 ef | 14.48 ± 0.14 cd | 1.58 ± 0.01 abc | 11.10 ± 0.16 d |
| Cream solid residue (dried) | 6.64 ± 0.47 b | 1116.09 ± 97.15 bc | 159.85 ± 13.29 e | 562.20 ± 44.65 cd | 1292.29 ± 113 d | 10.12 ± 0.89 de | 16.39 ± 1.34 c | 1.60 ± 0.11 ab | 12.13 ± 1.06 cd |
| Hemp oil | 0.22 ± 0.18 c | 0.79 ± 0.18 i | 1.63 ± 0.85 g | 0.07 ± 0.01 i | n/d | 0.01 ± 0.00 g | 0.07 ± 0.01 g | 0.01 ± 0.00 i | 0.02 ± 0.04 i |
| Hemp cream | 0.81 ± 0.16 c | 105.64 ± 8.14 hi | 2.40 ± 0.07 g | 8.22 ± 0.47 i | 45.10 ± 3.94 hi | 0.05 ± 0.00 g | 0.21 ± 0.01 g | 0.16 ± 0.01 i | 0.04 ± 0.02 i |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Sanchez, R.; Hayward, N.J.; Henderson, D.; Duncan, G.J.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs. Foods 2025, 14, 875. https://doi.org/10.3390/foods14050875
Ramos-Sanchez R, Hayward NJ, Henderson D, Duncan GJ, Russell WR, Duncan SH, Neacsu M. Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs. Foods. 2025; 14(5):875. https://doi.org/10.3390/foods14050875
Chicago/Turabian StyleRamos-Sanchez, Ricardo, Nicholas J. Hayward, Donna Henderson, Gary J. Duncan, Wendy R. Russell, Sylvia H. Duncan, and Madalina Neacsu. 2025. "Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs" Foods 14, no. 5: 875. https://doi.org/10.3390/foods14050875
APA StyleRamos-Sanchez, R., Hayward, N. J., Henderson, D., Duncan, G. J., Russell, W. R., Duncan, S. H., & Neacsu, M. (2025). Hemp Seed-Based Foods and Processing By-Products Are Sustainable Rich Sources of Nutrients and Plant Metabolites Supporting Dietary Biodiversity, Health, and Nutritional Needs. Foods, 14(5), 875. https://doi.org/10.3390/foods14050875








