Physicochemical, Volatile Compound Profile, Antioxidant, and Cytotoxic Activities of Northeastern Thai Ethnic Ready-to-Serve Food Pastes Jaew Hon and Gang Om: A Comparative Study of Laboratory and Industrial Production Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Production Process
2.2. Industrial Production Process
2.3. Physicochemical Analysis
2.4. Antioxidant Activity and Bioactive Content
2.4.1. Sample Preparation and Extraction
2.4.2. 2,2-Diphenyl-1-picryl hydrazyl (DPPH) Radical Scavenging Activity
2.4.3. Ferric Reducing Antioxidant Power (FRAP)
2.4.4. Total Phenolic Content (TPC)
2.4.5. Total Flavonoid Content (TFC)
2.5. GC-MS Analysis of Volatile Compounds
2.6. Cancer Cell Cultivation and Cytotoxicity Determination Using MTT Assay
2.7. Antiprolifeative Activity Using Clonogenic Formation Assay
2.8. Apoptosis-Related Gene Expression Using Real-Time Polymerase Chain Reaction
2.9. Protein Expression by Western Blot Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Attributes of the Four Pastes in Laboratory and Industrial Production
3.2. Antioxidant Activity and Bioactive Content Analysis
3.3. Volatile Compound Profile
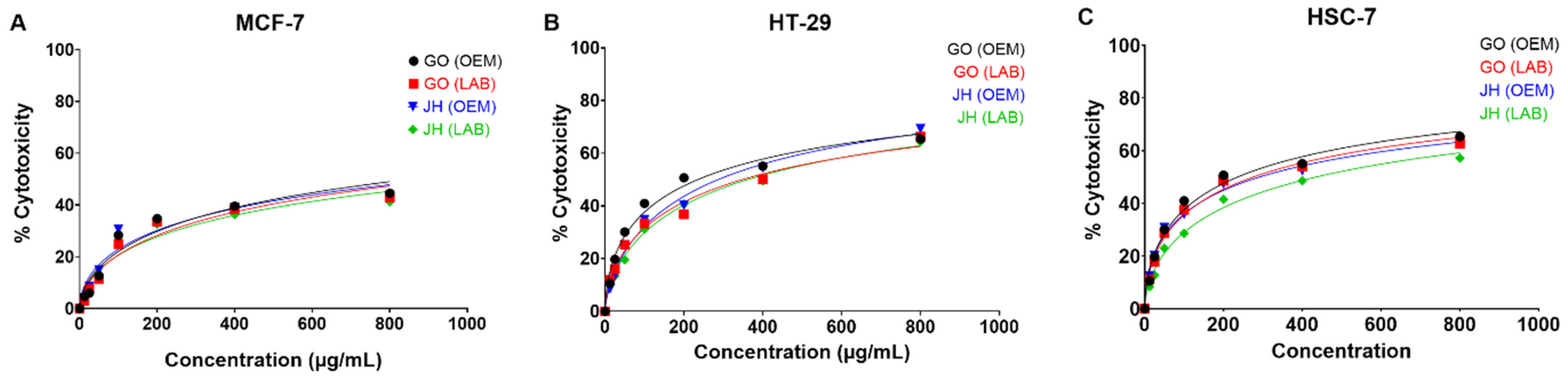
3.4. Cytotoxicity and Antiproliferative Assessment
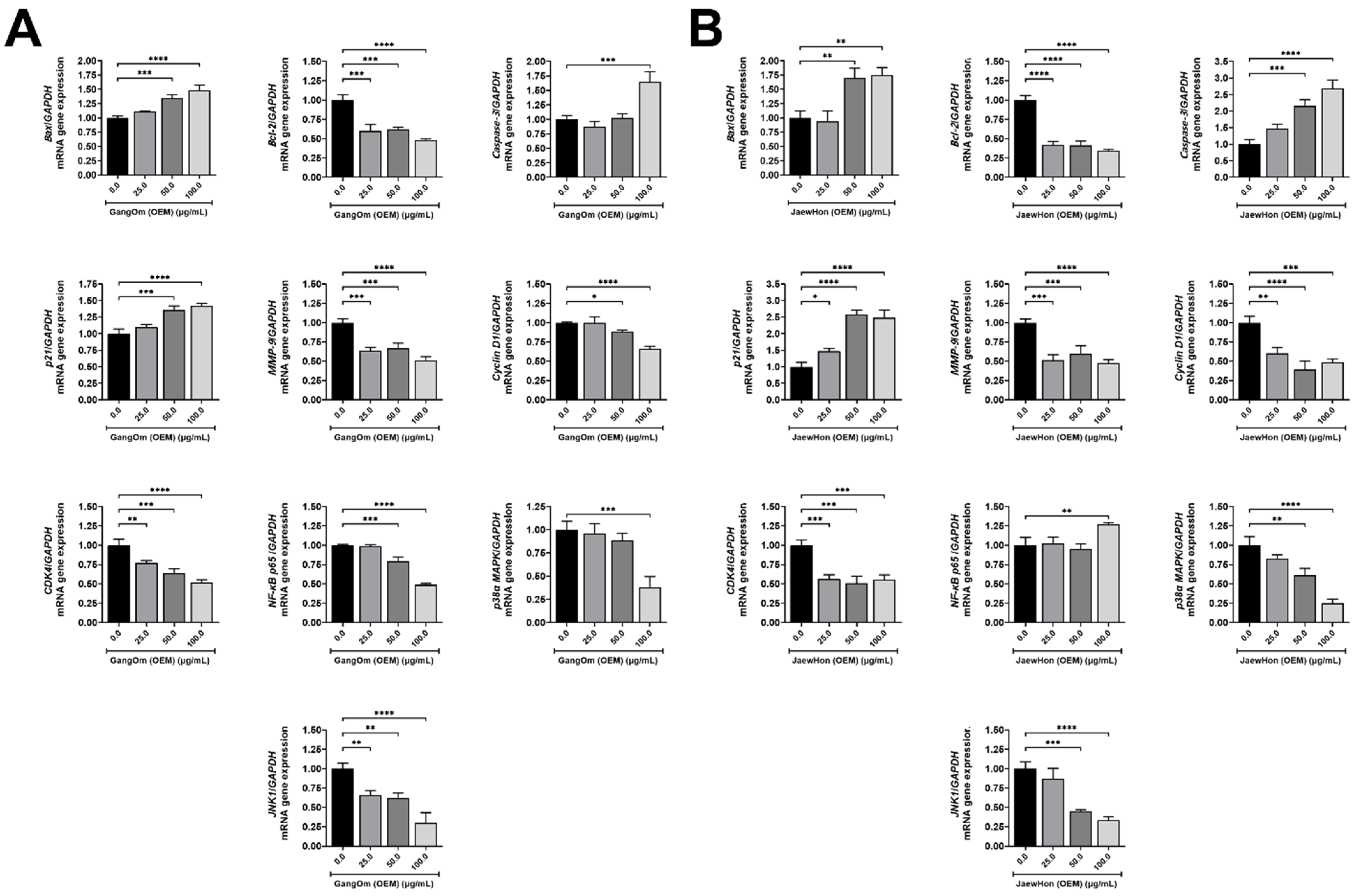
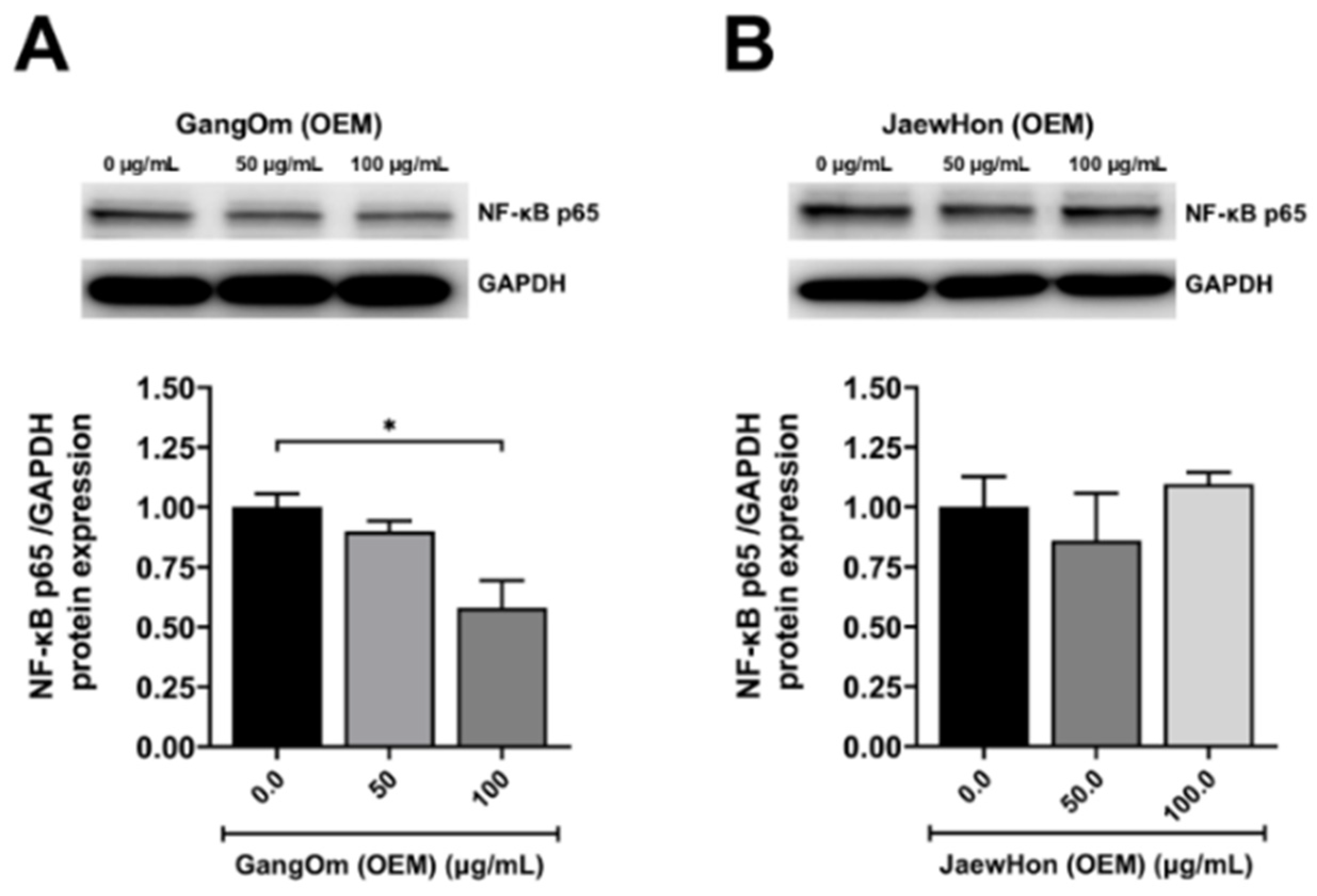
3.5. Gene and Protein Expression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandhanaphalin, M. Isan cuisine. J. Thai Food Cult. 2021, 1, 1–7. [Google Scholar]
- Sawangsai, T.; Sawangsai, R. Wisdom ethnic foods to the health of Thai-Cambodian, Thai-Kui, and Thai-Lao in northeastern Thailand. Res. Dev. J. Loei Rajabhat Univ. 2017, 11, 84–91. [Google Scholar]
- Buathong, R.; Duangsrisai, S. Plant ingredients in Thai food: A well-rounded diet for natural bioactive associated with medicinal properties. PeerJ 2023, 11, e14568. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Whiteside, S. Effect of retort processing, containers, and motion types on digestibility of black beans (Phaseolus vulgaris L.) starch. Int. J. Food Sci. Technol. 2023, 59, 916–924. [Google Scholar] [CrossRef]
- Benjakul, S.; Chantakun, K.; Karnjanapratum, S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J. Food Sci. Technol. 2018, 55, 3779–3791. [Google Scholar] [CrossRef]
- Priyanka, D.; Sindhoora, S.; Perupogu, V.; Kulkarni, S.G.; Nagarajan, S. Influence of thermal processing on the volatile constituents of muskmelon puree. J. Food Sci. Technol. 2015, 52, 3111–3116. [Google Scholar] [CrossRef][Green Version]
- Sattayakhom, A.; Songsamoe, S.; Yusakul, G.; Kalarat, K.; Matan, N.; Koomhin, P. Effects of Thai local ingredient odorants, Litsea cubeba and garlic essential oils, on brainwaves and moods. Molecules 2021, 26, 2939. [Google Scholar] [CrossRef]
- Khanthapok, P.; Sukrong, S. Anti-aging and health benefits from Thai food: Protective effects of bioactive compounds on the free radical theory of aging. J. Food Health Bioenviron. Sci. 2019, 12, 54–67. [Google Scholar]
- Siripongvutikorn, S.; Pumethakul, K.; Yupanqui, C.T.; Seechamnanturakit, V.; Detarun, P.; Utaipan, T.; Sirinupong, N.; Chansuwan, W.; Wittaya, T.; Samakradhamrongthai, R.S. Phytochemical profiling and antioxidant activities of the most favored ready-to-use Thai curries, Pad-Ka-Proa (spicy basil leaves) and Massaman. Foods 2024, 13, 582. [Google Scholar] [CrossRef]
- Paisarnsombat, S.; Kheerajit, C.; Rampai, N.; Sompong, N. Using media for public relations of Thai food to the global market in China. Int. J. Eng. Sci. Technol. 2021, 3, 119–125. [Google Scholar] [CrossRef]
- Cheenkaew, Y.; Panpipat, W.; Chaijan, M. Southern-style Pad Thai sauce: From traditional culinary treat to convenience food in retortable pouches. PLoS ONE 2020, 15, e0233391. [Google Scholar] [CrossRef]
- Bepary, R.H.; Wadikar, D.D.; Vasudish, C.R.; Semwal, A.D.; Sharma, G.K. Ranking-based formula optimization, quality investigation, and real-time shelf-life prediction of ready-to-eat ricebean (Vigna umbellata) curry. J. Food Sci. Technol. 2022, 59, 4390–4404. [Google Scholar] [CrossRef] [PubMed]
- Weeragul, K.; Nantaragsa, N.; Siripanwattana, C.; Gaowmanee, T.; Poonnakasem, N. Bioactive compounds and nutrition of Thai sauces in retort pouch and physicochemical properties kinetics during storage. J. Curr. Sci. Technol. 2024, 14, 28. [Google Scholar] [CrossRef]
- Saengha, W.; Karirat, T.; Buranrat, B.; Matra, K.; Deeseenthum, S.; Katisart, T.; Luang-In, V. Cold plasma treatment on mustard green seeds and its effect on growth, isothiocyanates, antioxidant activity, and anticancer activity of microgreens. Int. J. Agric. Biol. 2021, 25, 667–676. [Google Scholar] [CrossRef]
- Phuseerit, O.; Wrigley, C.; Siriamornpun, S. Bioactives and antioxidant activities of marigold flower juices and the effect of different storage times. Sci. Technol. Eng. J. 2021, 7, 58–73. [Google Scholar]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Saengha, W.; Karirat, T.; Pitisin, N.; Plangklang, S.; Butkhup, L.; Udomwong, P.; Ma, N.L.; Konsue, A.; Chanthaket, P.; Katisart, T.; et al. Exploring the Bioactive Potential of Calostoma insigne, an Endangered Culinary Puffball Mushroom, from Northeastern Thailand. Foods 2024, 13, 113. [Google Scholar] [CrossRef]
- Awuah, G.B.; Ramaswamy, H.S.; Economides, A. Thermal processing and quality: Principles and overview. Chem. Eng. Process. Process Intensif. 2007, 46, 584–602. [Google Scholar] [CrossRef]
- Wangcharoen, W.; Morasuk, W. Antioxidant capacity and phenolic content of some Thai culinary plants. Maejo Int. J. Sci. Technol. 2007, 1, 100–106. [Google Scholar]
- Ounjaijean, S.; Chachiyo, S.; Kulprachakarn, K.; Boonyapranai, K.; Srichairatanakool, S.; Rerkasem, K. Antioxidant and anti-inflammatory protective properties of Thai shallot (Allium ascalonicum cv. Chiangmai) juice on human vascular endothelial cell lines (EA.hy926). Walailak J. Sci. Technol. 2019, 16, 175–184. [Google Scholar] [CrossRef]
- Ratseewo, J.; Tangkhawanit, E.; Meeso, N.; Kaewseejan, N.; Siriamornpun, S. Changes in antioxidant properties and volatile compounds of kaffir lime leaf as affected by cooking processes. Int. Food Res. J. 2016, 23, 188–196. [Google Scholar]
- Ahmed, M.; Eun, J.B. Flavonoids in fruits and vegetables after thermal and nonthermal processing: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 3159–3188. [Google Scholar] [CrossRef]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of Processing on Phenolic Antioxidants of Fruits, Vegetables, and Grains—A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar] [CrossRef]
- Jacob, K.; Garcia-Alonso, F.J.; Ros, G.; Periago, M.J. Stability of carotenoids, phenolic compounds, ascorbic acid, and antioxidant capacity of tomatoes during thermal processing. Arch. Latinoam. Nutr. 2010, 60, 192–198. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Huang, R.; Wu, W.; Shen, S.; Fan, J.; Chang, Y.; Chen, S.; Ye, X. Evaluation of colorimetric methods for quantification of citrus flavonoids to avoid misuse. Anal. Methods 2018, 10, 2575–2587. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Atzeri, A.; Dessì, M.A.; Falconieri, D.; Porcedda, S. Extraction and separation of volatile and fixed oils from seeds of Myristica fragrans by supercritical CO2: Chemical composition and cytotoxic activity on Caco-2 cancer cells. J. Food Sci. 2012, 77, C448–C453. [Google Scholar] [CrossRef] [PubMed]
- Janatová, A.; Doskočil, I.; Božik, M.; Fraňková, A.; Tlustoš, P.; Klouček, P. The chemical composition of ethanolic extracts from six genotypes of medical cannabis (Cannabis sativa L.) and their selective cytotoxic activity. Chem. Biol. Interact. 2022, 353, 109800. [Google Scholar] [CrossRef] [PubMed]
- Bălănescu, F.; Botezatu, A.V.; Marques, F.; Busuioc, A.; Marincaş, O.; Vînătoru, C.; Cârâc, G.; Furdui, B.; Dinica, R.M. Bridging the chemical profile and biological activities of a new variety of Agastache foeniculum (Pursh) Kuntze extracts and essential oil. Int. J. Mol. Sci. 2023, 24, 828. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Li, S.; Hua, C.; Gao, H.; Ning, D.; Li, C.; Zhang, C.; Jiang, F. Chemical Composition, Antitumor Properties, and Mechanism of the Essential Oil from Plagiomnium acutum T. Kop. Int. J. Mol. Sci. 2022, 23, 14790. [Google Scholar] [CrossRef]
- Vuko, E.; Radman, S.; Jerković, I.; Kamenjarin, J.; Vrkić, I.; Fredotović, Ž. A plant worthy of further study: Volatile and non-volatile compounds of Portenschlagiella ramosissima (Port.) Tutin and its biological activity. Pharmaceuticals 2022, 15, 1454. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Ray, A.; Sahoo, A.; Das, P.K.; Kamila, P.K.; Kar, S.K.; Nayak, S.; Panda, P.C. Anti-proliferative activity of Piper trioicum leaf essential oil based on phytoconstituent analysis, molecular docking, and in silico ADMET approaches. Comb. Chem. High Throughput Screen. 2023, 26, 183–190. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant, and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef]
- Kumar, A.; Yt, K.; Mishra, A.K.; Singh, M.; Singh, H.; Kaushik, N.; Mishra, A. Potential role of benzoic acid and its synthetic derivatives to alleviate cancer: An up-to-date review. Curr. Drug Discov. Technol. 2025, 22, e15701638311865. [Google Scholar] [CrossRef]
- Gupta, J.; Ahuja, A.; Gupta, R. Green approaches for cancer management: An effective tool for healthcare. Anticancer Agents Med. Chem. 2022, 22, 101–114. [Google Scholar] [CrossRef]
- Caponio, G.R.; Cofano, M.; Lippolis, T.; Gigante, I.; De Nunzio, V.; Difonzo, G.; Noviello, M.; Tarricone, L.; Gambacorta, G.; Giannelli, G.; et al. Anti-proliferative and pro-apoptotic effects of digested Aglianico grape pomace extract in human colorectal cancer cells. Molecules 2022, 27, 6791. [Google Scholar] [CrossRef]
- Das, R.; Mehta, D.K.; Dhanawat, M. Medicinal plants in cancer treatment: Contribution of nuclear factor-kappa B (NF-kB) inhibitors. Mini Rev. Med. Chem. 2022, 22, 1938–1962. [Google Scholar] [CrossRef]
- Shahabi Nejad, F.; Karami, H.; Darvish, M. Triggering of endoplasmic reticulum stress by tannic acid inhibits the proliferation and migration of colorectal cancer cells. Asian Pac. J. Cancer Prev. 2023, 24, 2705–2711. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
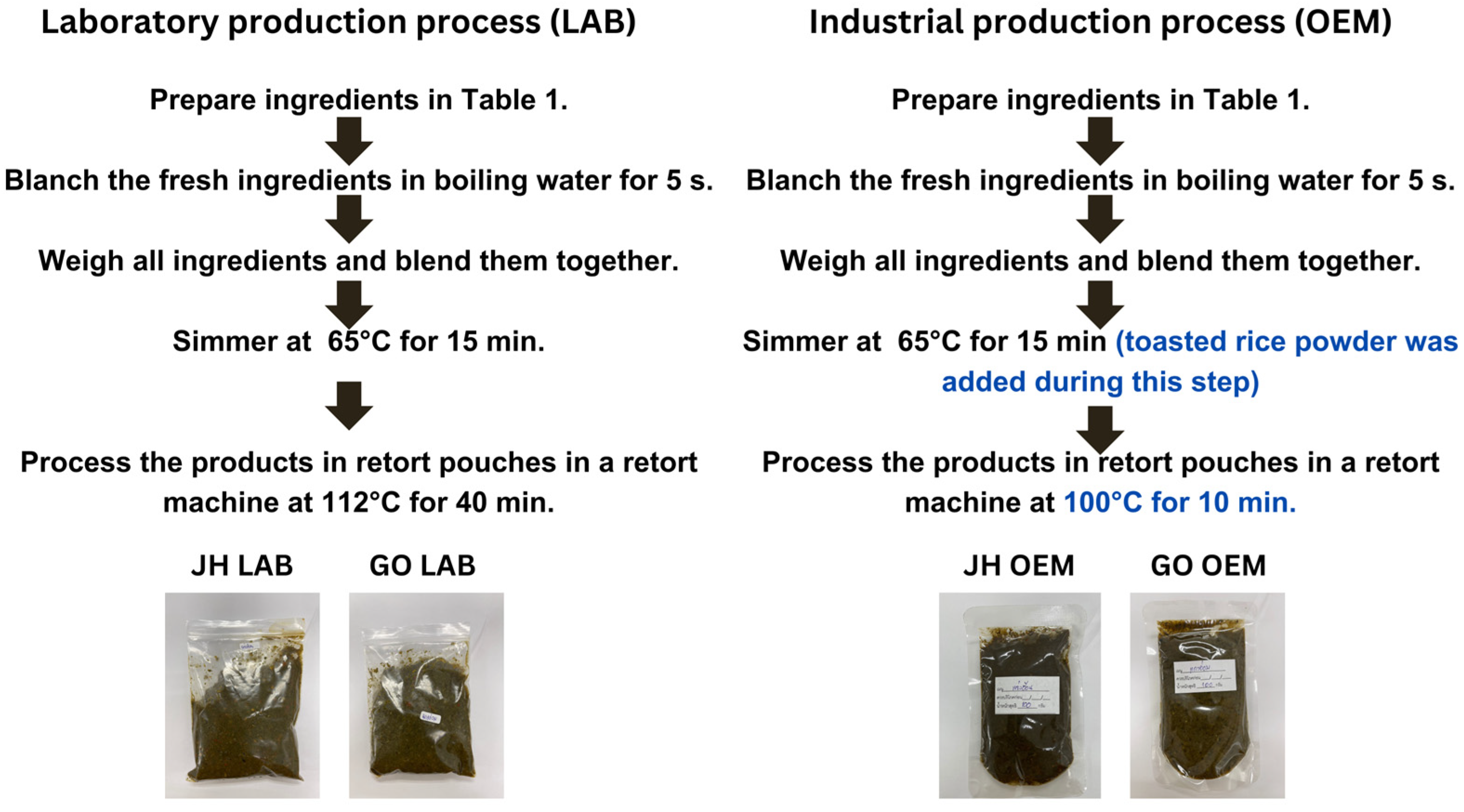
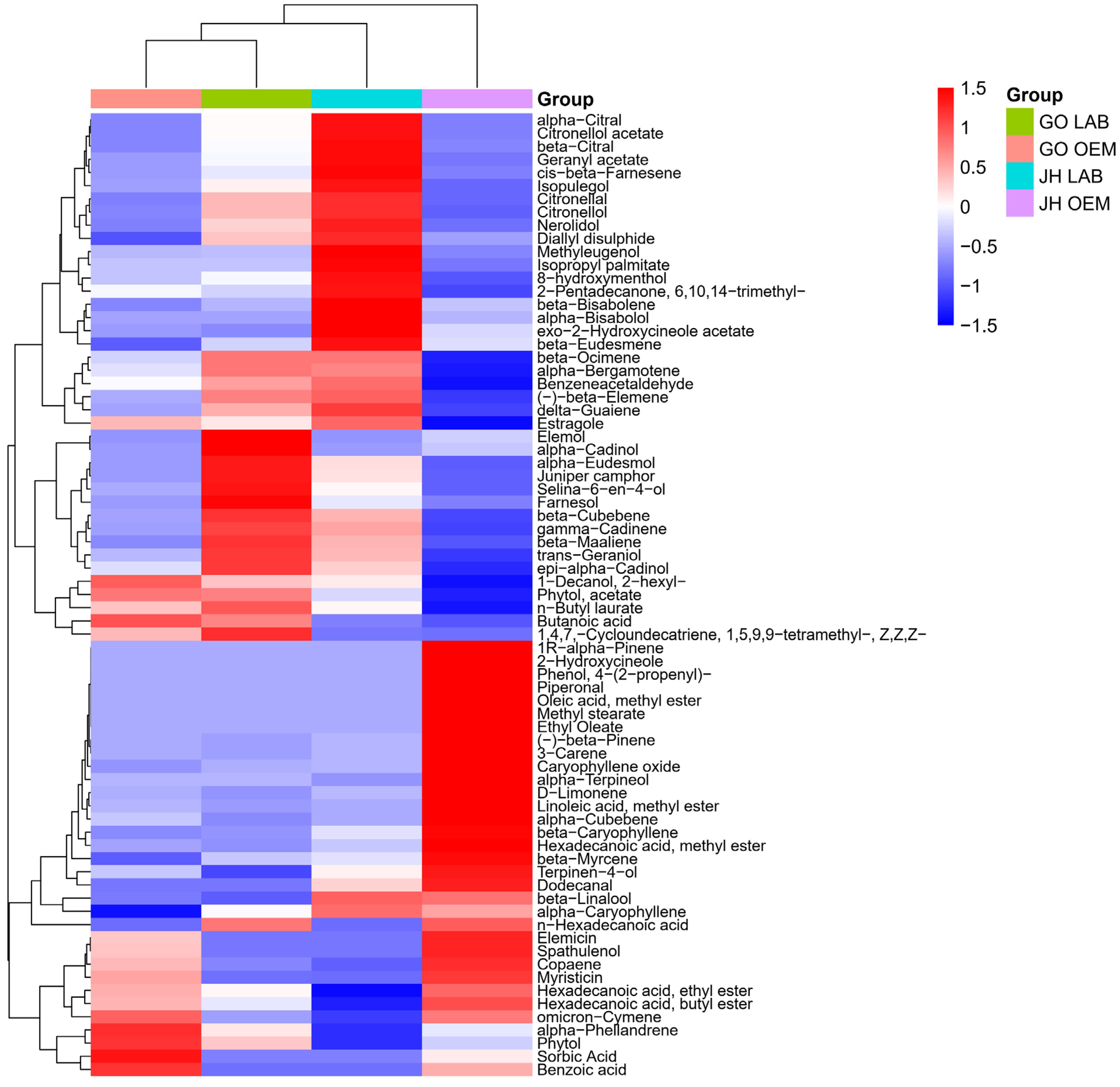

| No. | Ingredients | JH OEM | JH LAB | GO OEM | GO LAB |
|---|---|---|---|---|---|
| 1 | Water | 0 | 39 | 35.9 | 43 |
| 2 | Fermented fish sauce | 3.92 | 2 | 9.5 | 8 |
| 3 | Shallots | 4.7 | 4 | 9.24 | 8 |
| 4 | Lemongrass | 5.15 | 5 | 8.16 | 7 |
| 5 | Toasted rice powder | 1.93 | 2 | 7.36 | 6 |
| 6 | Sweet basil leaves | 0 | 0 | 5.23 | 5 |
| 7 | Garlic | 3.95 | 4 | 2.15 | 2 |
| 8 | Chili powder | 2.13 | 2 | 0 | 0 |
| 9 | Chilli | 0 | 0 | 3.45 | 2.5 |
| 10 | Galangal | 1.65 | 2 | 0 | 0 |
| 11 | Wild betel leaves | 4.5 | 5 | 2.15 | 5 |
| 12 | Salt | 1.96 | 4 | 1.89 | 1.5 |
| 13 | Kaffir lime leaves | 0.8 | 1 | 0.6 | 1 |
| 14 | Chicken seasoning powder | 4.5 | 0 | 0.8 | 1 |
| 15 | Dill | 0 | 0 | 7.76 | 5 |
| 16 | Hoary basil | 4.5 | 5 | 5.23 | 5 |
| 17 | Braised soup | 42.93 | 10 | 0 | 0 |
| 18 | Coconut sugar | 1.9 | 2 | 0 | 0 |
| 19 | Tamarind juice | 1.9 | 2 | 0 | 0 |
| 20 | Black pepper | 4.5 | 1 | 0 | 0 |
| 21 | Sawtooth coriander | 4.5 | 5 | 0 | 0 |
| 22 | Vietnamese coriander | 4.5 | 5 | 0 | 0 |
| 23 | Fish sauce | 0 | 0 | 0.5 | 0 |
| 24 | Potassium sorbate INS 202 | 0.04 | 0 | 0.04 | 0 |
| 25 | Sodium benzoate INS 211 | 0.04 | 0 | 0.04 | 0 |
| Total | 100 | 100 | 100 | 100 |
| Gene | Gene Role | Primer | Sequence (5′-3′) | Size (bp) |
|---|---|---|---|---|
| GAPDH | Housekeeping gene | Forward | GGATTTGGTCGTATTGGGCG | 115 |
| Reverse | TCCCGTTCTCAGCCATGTAG | |||
| Apoptotic pathway | ||||
| Bax | Promote apoptosis | Forward | GAGCAGCCCAGAGGCG | 276 |
| Reverse | AGCTGCCACTCGGAAAAAGA | |||
| Bcl-2 | Inhibit apoptosis | Forward | ATGTGTGTGGAGAGCGTCAA | 135 |
| Reverse | ATCACCAAGTGCACCTACCC | |||
| Caspase-3 | Execute apoptosis via cleavage | Forward | GTGCTATTGTGAGGCGGTTG | 271 |
| Reverse | GTTTCCCTGAGGTTTGCTGC | |||
| p38α MAPK | Regulate apoptosis via stress signaling | Forward | AACAGGATGCCAAGCCATGA | 229 |
| Reverse | CATAAGGATCGGCCACTGGT | |||
| JNK1 | Activate apoptosis via stress response | Forward | CTCTCCTTTAGGTGCAGCAGT | 102 |
| Reverse | GAGGCCAAAGTCGGATCTGT | |||
| Cell cycle regulation pathway | ||||
| p21 | Cell cycle arrest | Forward | CCCAACGCACCGAATAGTTAC | 167 |
| Reverse | GAAAACTCCCCAGGAAGCCT | |||
| Cyclin D1 | Drive cell cycle | Forward | GCTGTAGTGGGGTTCTAGGC | 297 |
| Reverse | AGCGTATCGTAGGAGTGGGA | |||
| CDK4 | Regulate cell cycle | Forward | GTATGGGGCCGTAGGAACC | 113 |
| Reverse | AGGCAGAGATTCGCTTGTGT | |||
| Inflammation and survival pathway | ||||
| NF-κB p65 | Mediate inflammation, apoptosis resistance | Forward | CTGCACTGTGGGGTCACAT | 114 |
| Reverse | GGACACTTGAATCAGCAGGC | |||
| Matrix remodeling and tumor progression pathway | ||||
| MMP-9 | Matrix, remodeling, Apoptosis regulation | Forward | TATGACATCCTGCAGTGCCC | 111 |
| Reverse | TTGTATCCGGCAAACTGGCT | |||
| Sample | pH | TDS (°Brix) | aw | L* | a* | b* |
|---|---|---|---|---|---|---|
| GO OEM | 5.28 ± 0.04 a | 7.83 ± 0.15 c | 0.94 ± 0.00 a | 31.43 ± 0.56 a | 1.03 ± 0.13 c | 7.90 ± 0.20 a |
| GO LAB | 5.21 ± 0.01 b | 6.67 ± 0.31 d | 0.92 ± 0.00 b | 30.67 ± 0.27 b | 1.03 ± 0.08 c | 8.33 ± 0.27 a |
| JH OEM | 5.13 ± 0.01 c | 11.57 ± 0.21 a | 0.91 ± 0.00 c | 29.57 ± 0.21 c | 1.82 ± 0.13 a | 8.39 ± 0.66 a |
| JH LAB | 4.92 ± 0.02 d | 10.73 ± 0.25 b | 0.90 ± 0.00 d | 29.68 ± 0.14 c | 1.46 ± 0.06 b | 8.66 ± 0.42 a |
| Ingredient | DPPH (mg AAE/100 g DW) | FRAP (mg FeSO4/100 g DW) | TPC (mg GAE/100 g DW) | TFC (mg QE/100 g DW) |
|---|---|---|---|---|
| Dill | 95.95 ± 2.88 f | 592.19 ± 4.65 b | 287.03 ± 0.13 c | 213.9 ± 6.96 e |
| Vietnamese coriander | 33.18 ± 0.08 g | 424.46 ± 6.29 e | 171.14 ± 0.18 f | 118.01 ± 1.26 g |
| Hoary basil | 148.9 ± 6.58 d | 606.84 ± 6.93 b | 252.51 ± 1.64 d | 333.68 ± 1.51 b |
| Sawtooth coriander | 18.65 ± 1.36 h | 420.77 ± 1.09 e | 50.29 ± 0.17 i | 82.74 ± 1.31 g |
| Wild betel leaves | 125.25 ± 4.97 e | 556.56 ± 11.82 c | 230.26 ± 0.21 e | 364.87 ± 4.38 a |
| Chili | 1419.51 ± 54.38 a | 855.43 ± 28.01 a | 1313.92 ± 17.28 a | 237.73.41 ± 1.08 d |
| Kaffir lime leaves | 558.5 ± 23.33 b | 520.46 ± 45.94 c | 123.61 ± 0.14 h | 205.84 ± 9.89 e |
| Garlic | 129.54 ± 1.68 e | 152.12 ± 10.37 g | 129.55 ± 0.47 g | 118.58 ± 0.69 f |
| Shallot | 264.13 ± 0.01 c | 486.06 ± 5.61 d | 381.87 ± 18.16 b | 86.53 ± 2.32 g |
| Sweet basil leaves | 101.76 ± 6.28 f | 385.42 ± 1.15 f | 286.53 ± 0.26 c | 274.37 ± 3.99 c |
| Sample | DPPH (mg AAE/100 g) | FRAP (mg FeSO4/100 g) | TPC (mg GAE/100 g) | TFC (mg QE/100 g) |
|---|---|---|---|---|
| GO OEM | 82.76 ± 3.54 b | 247.43 ± 4.24 c | 430.49 ± 14.21 a | 345.57 ± 5.30 a |
| GO LAB | 49.51 ± 0.10 c | 115.22 ± 0.4 d | 259.81 ± 1.94 c | 124.69 ± 5.21 c |
| JH OEM | 84.41 ± 0.70 b | 809.55 ± 6.79 a | 352.85 ± 8.17 b | 191.44 ± 1.40 b |
| JH LAB | 96.25 ± 1.32 a | 742.5 ± 4.91 b | 433.50 ± 0.10 a | 89.67 ± 6.64 d |
| Compound | CAS Number | % Relative Abundance | |||
|---|---|---|---|---|---|
| JH OEM | JH LAB | GO OEM | GO LAB | ||
| 64-19-7 | 0.42 | 0.45 | 0.71 | 0.83 |
| 107-92-6 | 0.27 | 0.39 | 1.33 | 1.18 |
| 7785-70-8 | 0.37 | nd | nd | nd |
| 18172-67-3 | 0.98 | 0.12 | 0.09 | 0.07 |
| 123-35-3 | 0.25 | 0.15 | 0.10 | 0.14 |
| 99-83-2 | 0.48 | 0.19 | 0.83 | 0.55 |
| 13466-78-9 | 2.64 | 0.32 | 0.25 | 0.19 |
| 527-84-4 | 0.19 | 0.06 | 0.20 | 0.10 |
| 5989-27-5 | 2.45 | 0.69 | 0.66 | 0.50 |
| 122-78-1 | 0.04 | 0.12 | 0.09 | 0.11 |
| 13877-91-3 | 0.06 | 0.14 | 0.10 | 0.14 |
| 2179-57-9 | 0.05 | 0.13 | 0.03 | 0.09 |
| 126-90-9 | 0.45 | 0.46 | 0.27 | 0.25 |
| 110-44-1 | 7.52 | 0.00 | 18.81 | 0.00 |
| 89-79-2 | 0.19 | 0.61 | 0.25 | 0.37 |
| 106-23-0 | 0.27 | 1.34 | 0.34 | 0.93 |
| 562-74-3 | 0.25 | 0.15 | 0.12 | 0.06 |
| 98-55-5 | 0.48 | 0.12 | 0.15 | 0.15 |
| 140-67-0 | 4.23 | 6.67 | 6.15 | 5.89 |
| 65-85-0 | 3.83 | nd | 5.90 | nd |
| 106-22-9 | 0.56 | 1.15 | 0.62 | 0.92 |
| 5392-40-5 | 0.11 | 0.95 | 0.10 | 0.38 |
| 106-24-1 | 0.27 | 1.08 | 0.66 | 1.47 |
| 97-53-0 | 0.13 | nd | nd | nd |
| 141-27-5 | 0.16 | 1.62 | 0.18 | 0.68 |
| 470-67-7 | 0.35 | nd | nd | nd |
| 120-57-0 | 0.47 | nd | nd | nd |
| 16409-46-4 | 0.57 | 2.28 | 0.23 | 0.14 |
| 17699-14-8 | 0.31 | 0.20 | 0.21 | 0.19 |
| 150-84-5 | 0.66 | 1.96 | 0.67 | 1.12 |
| 1197-01-9 | 0.89 | 5.77 | 2.19 | 2.79 |
| 3856-25-5 | 1.79 | 0.80 | 1.41 | 0.90 |
| 105-87-3 | 0.49 | 2.29 | 0.65 | 1.09 |
| 515-13-9 | 0.80 | 1.68 | 1.08 | 1.60 |
| 93-15-2 | 0.19 | 0.69 | 0.26 | 0.27 |
| 112-54-9 | 0.94 | 0.47 | nd | nd |
| 87-44-5 | 17.31 | 8.58 | 5.89 | 6.31 |
| 17699-05-7 | 1.06 | 3.38 | 2.38 | 3.48 |
| 6753-98-6 | 1.53 | 1.60 | 1.13 | 1.42 |
| 18794-84-8 | 0.52 | 2.82 | 0.68 | 1.17 |
| 13744-14-8 | 0.61 | 1.27 | 0.85 | 1.59 |
| 20307-84-0 | 0.69 | 1.20 | 0.45 | 0.67 |
| 473-04-1 | 0.19 | 0.69 | 0.30 | 0.96 |
| 3691-12-1 | 0.22 | 0.76 | 0.36 | 0.60 |
| 495-61-4 | 0.47 | 1.09 | 0.35 | 0.44 |
| 39029-41-9 | 0.41 | 1.85 | 0.88 | 2.36 |
| 607-91-0 | 24.50 | 3.99 | 18.09 | 4.27 |
| 489-41-8 | 0.26 | 0.28 | 1.11 | 1.67 |
| 639-99-6 | 0.32 | 0.26 | 0.26 | 0.64 |
| 487-11-6 | 1.31 | nd | 0.71 | nd |
| 7212-44-4 | 0.76 | 1.72 | 0.80 | 1.24 |
| 1139-30-6 | 1.46 | 0.44 | 0.35 | 0.43 |
| 473-15-4 | 0.24 | 0.58 | 0.35 | 0.92 |
| 23123-36-6 | 1.35 | 7.99 | 4.28 | 17.02 |
| 6750-60-3 | 3.76 | nd | 2.09 | nd |
| 481-34-5 | 0.77 | 2.20 | 1.75 | 3.00 |
| 481-34-5 | 0.50 | nd | nd | 3.82 |
| 515-69-5 | 0.15 | 2.61 | nd | nd |
| 464-45-9 | 0.09 | 0.97 | 0.36 | 1.95 |
| 4602-84-0 | 0.10 | 0.33 | 0.16 | 0.94 |
| 2425-77-6 | 0.63 | 1.72 | 2.34 | 1.90 |
| 7541-49-3 | 0.20 | 0.81 | 1.39 | 1.36 |
| 1937-63-1 | 0.06 | 0.18 | 0.11 | 0.10 |
| 112-39-0 | 1.27 | 0.24 | 0.13 | 0.05 |
| 57-10-3 | 0.11 | nd | nd | 0.10 |
| 123-95-5 | 0.09 | 0.49 | 0.58 | 0.76 |
| 628-97-7 | 0.11 | 0.00 | 0.09 | 0.07 |
| 142-91-6 | 0.04 | 0.09 | 0.05 | 0.05 |
| 112-63-0 | 0.74 | 0.03 | 0.05 | nd |
| 112-62-9 | 0.53 | nd | nd | nd |
| 150-86-7 | 0.21 | 0.06 | 0.45 | 0.31 |
| 112-61-8 | 0.06 | nd | nd | nd |
| 111-62-6 | 0.04 | nd | nd | nd |
| 5923-95-1 | 0.08 | 0.04 | 0.07 | 0.06 |
| Sample | MCF-7 | HT-29 | HSC-7 | MCF-7 | HT-29 | HSC-7 |
|---|---|---|---|---|---|---|
| Emax (%) | Emax (%) | Emax (%) | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | |
| GO OEM | 44.51 ± 0.19 aC | 71.88 ± 0.17 aA | 65.41 ± 0.18 aB | 857.17 ± 3.33 aC | 276.10 ± 1.08 aB | 235.13 ± 1.71 aA |
| GO LAB | 42.91 ± 0.00 cC | 66.32 ± 0.20 cA | 62.77 ± 0.18 bB | 960.30 ± 3.10 cC | 348.33 ± 0.99 cA | 274.90 ± 1.54 bB |
| JH OEM | 43.17 ± 0.11 bC | 69.34 ± 0.44 bA | 62.47 ± 0.10 bB | 938.00 ± 1.56 bB | 286.77 ± 2.17 bA | 290.53 ± 2.45 cA |
| JH LAB | 41.21 ± 0.19 dC | 64.40 ± 0.20 dA | 57.22 ± 0.36 cB | 1123.00 ± 2.00 dC | 361.73 ± 2.20 dA | 433.60 ± 2.17 dB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luang-In, V.; Saengha, W.; Karirat, T.; Promjamorn, P.; Seephua, N.; Bunyatratchata, A.; Inchuen, S.; Banlue, K.; Suwannarong, S.; Siriamornpun, S. Physicochemical, Volatile Compound Profile, Antioxidant, and Cytotoxic Activities of Northeastern Thai Ethnic Ready-to-Serve Food Pastes Jaew Hon and Gang Om: A Comparative Study of Laboratory and Industrial Production Processes. Foods 2025, 14, 876. https://doi.org/10.3390/foods14050876
Luang-In V, Saengha W, Karirat T, Promjamorn P, Seephua N, Bunyatratchata A, Inchuen S, Banlue K, Suwannarong S, Siriamornpun S. Physicochemical, Volatile Compound Profile, Antioxidant, and Cytotoxic Activities of Northeastern Thai Ethnic Ready-to-Serve Food Pastes Jaew Hon and Gang Om: A Comparative Study of Laboratory and Industrial Production Processes. Foods. 2025; 14(5):876. https://doi.org/10.3390/foods14050876
Chicago/Turabian StyleLuang-In, Vijitra, Worachot Saengha, Thipphiya Karirat, Piyathida Promjamorn, Nidthaya Seephua, Apichaya Bunyatratchata, Sudathip Inchuen, Kriangsak Banlue, Sarinthorn Suwannarong, and Sirithon Siriamornpun. 2025. "Physicochemical, Volatile Compound Profile, Antioxidant, and Cytotoxic Activities of Northeastern Thai Ethnic Ready-to-Serve Food Pastes Jaew Hon and Gang Om: A Comparative Study of Laboratory and Industrial Production Processes" Foods 14, no. 5: 876. https://doi.org/10.3390/foods14050876
APA StyleLuang-In, V., Saengha, W., Karirat, T., Promjamorn, P., Seephua, N., Bunyatratchata, A., Inchuen, S., Banlue, K., Suwannarong, S., & Siriamornpun, S. (2025). Physicochemical, Volatile Compound Profile, Antioxidant, and Cytotoxic Activities of Northeastern Thai Ethnic Ready-to-Serve Food Pastes Jaew Hon and Gang Om: A Comparative Study of Laboratory and Industrial Production Processes. Foods, 14(5), 876. https://doi.org/10.3390/foods14050876







