Studies on the Efficient Extraction of Ovotransferrin and the Effect of Heating Treatment on Its Structure and Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of H-Ovotransferrin
2.3. Extraction Yield of H-OVT
2.4. Single Factor Experiments
2.5. Response Surface Experimental Design
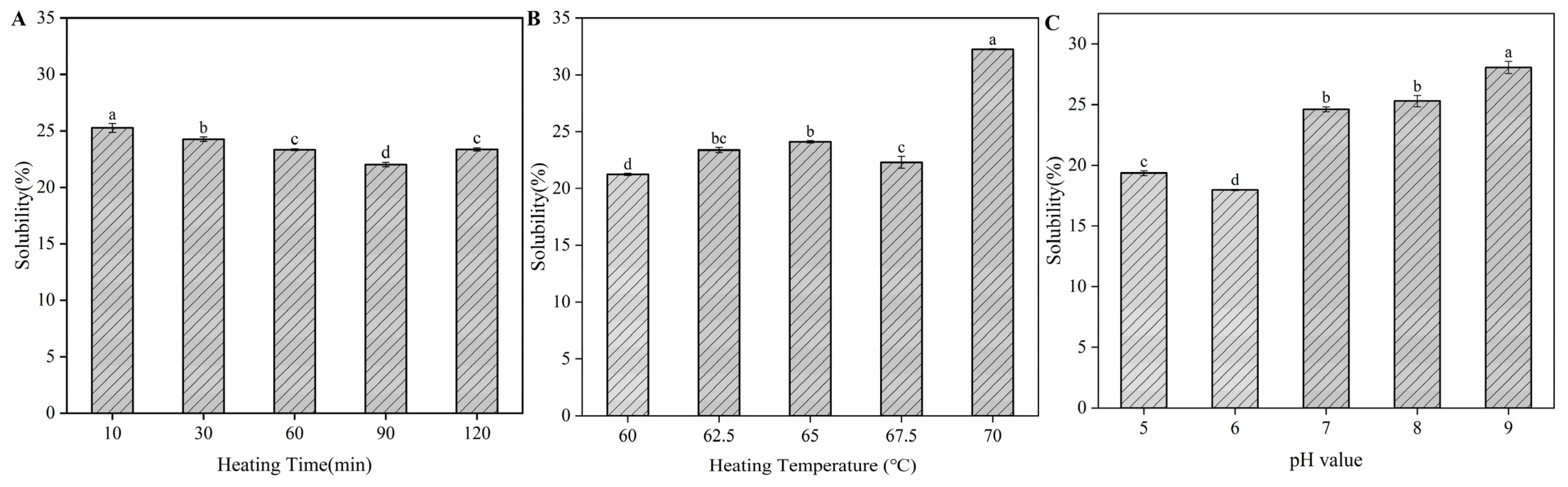
2.6. Determination of Solubility
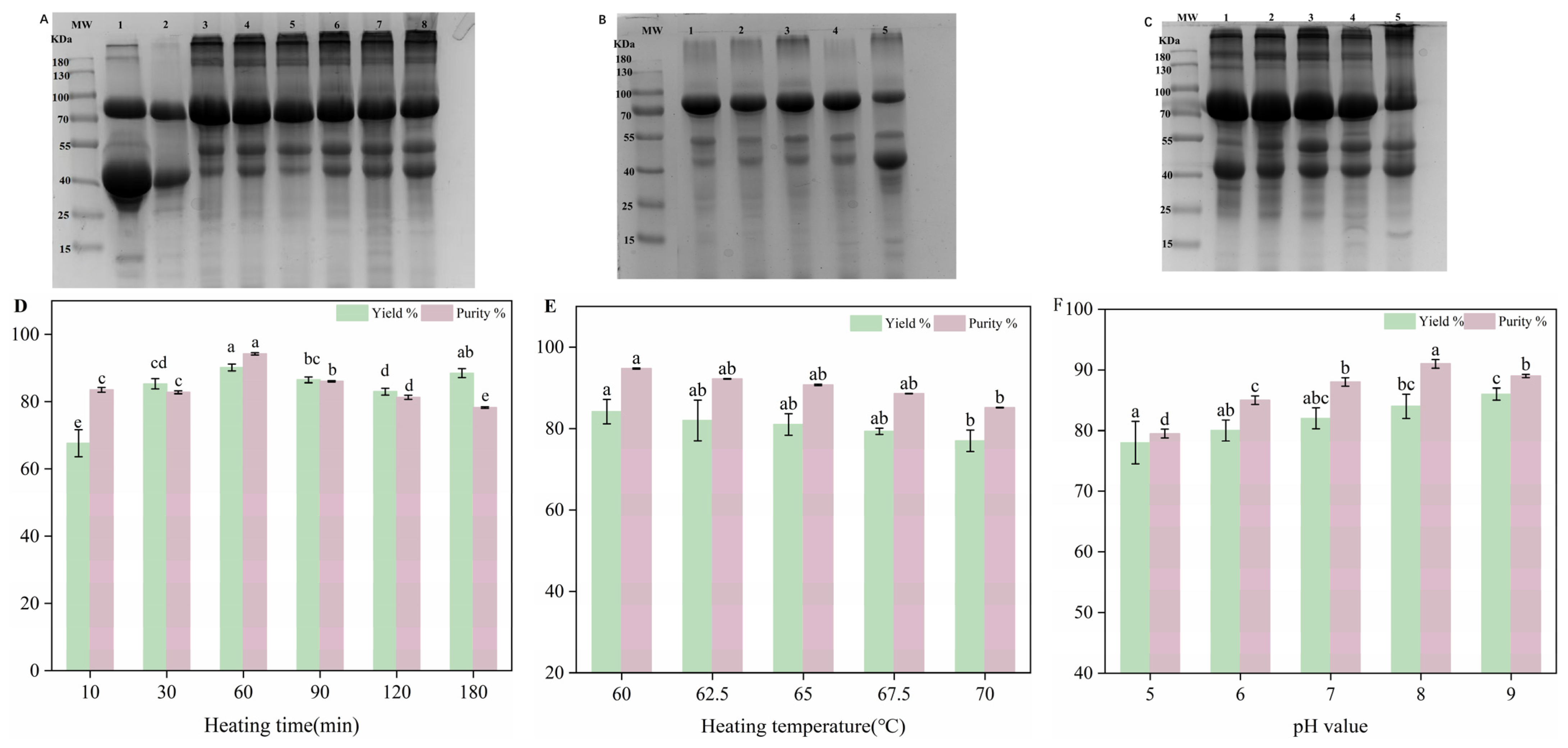
2.7. SDS-PAGE
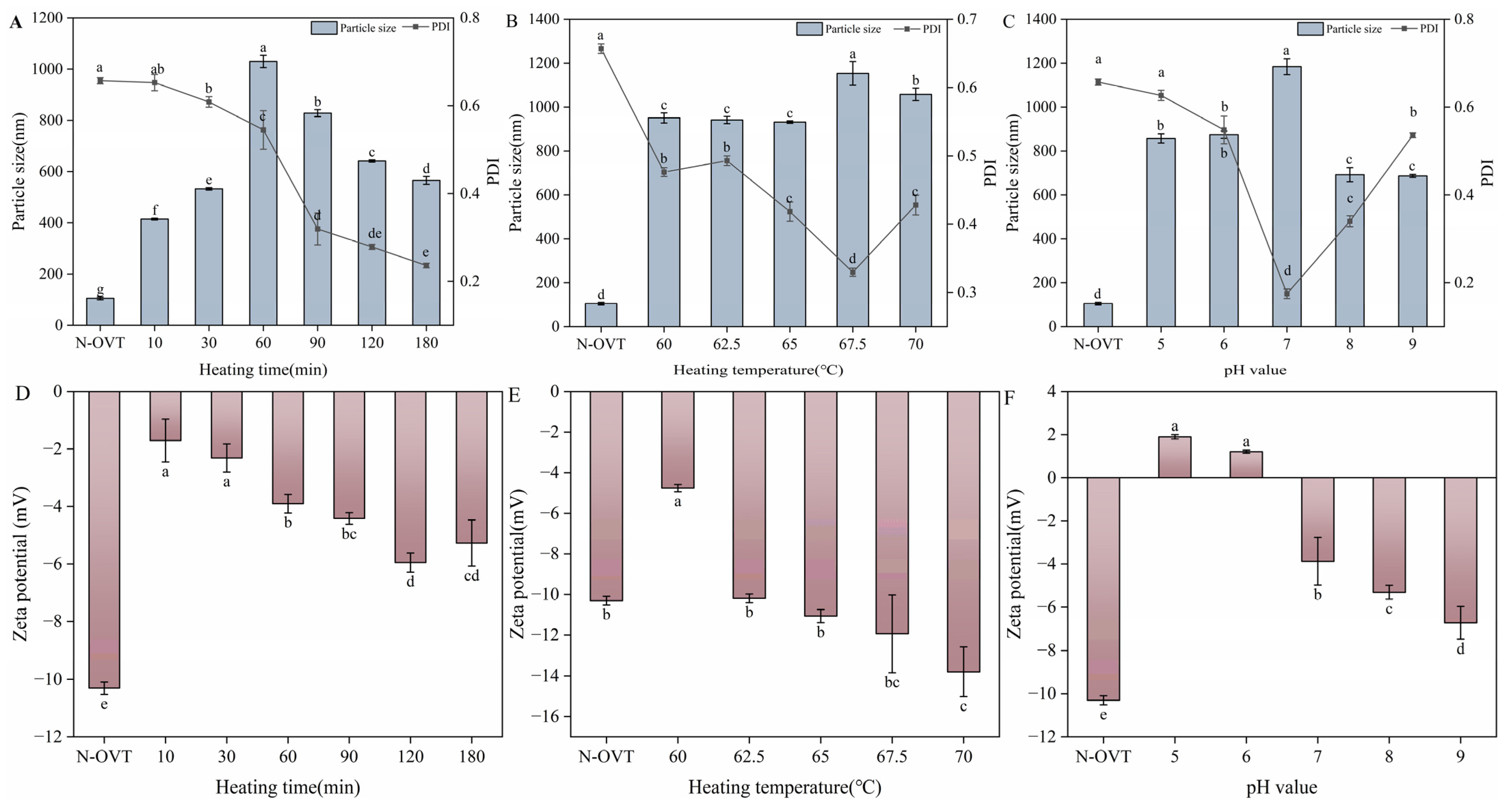
2.8. Particle Size, Zeta Potential and PDI Measurements
2.9. Fourier-Transform Infrared Spectroscopy
2.10. Biological Activity of H-OVT
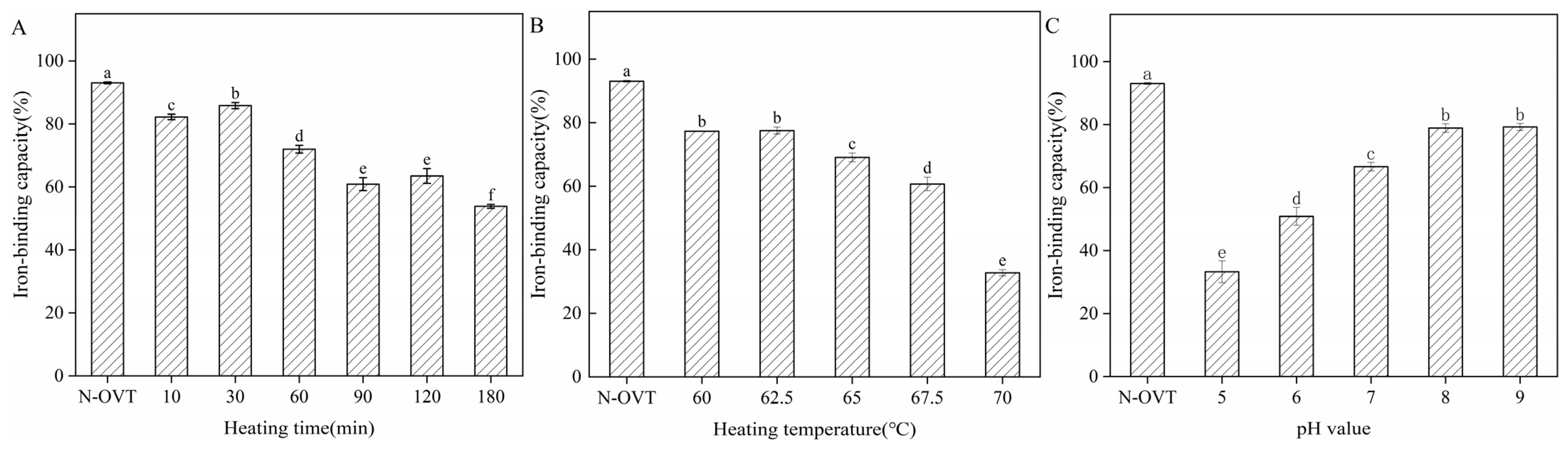
2.10.1. Determination of Iron-Binding Capacity
2.10.2. Determination of Antioxidant Capacity
Hydroxyl Radical Scavenging Ability
DPPH• Scavenging Activity
ABTS+ Scavenging Activity
2.11. Statistical Analysis
3. Results
3.1. Effect of Three Factors on Purity of H-OVT
3.2. Effect of Three Factors on Yield of H-OVT
3.3. Response Surface Optimization of OVT Conditions for Heat Treatment Extraction
3.4. Model Analysis of Variance Results
3.5. Confirmation and Verification of the Optimal Conditions of Response Surface
3.6. Protein Solubility of H-OVT
3.7. Particle Size, Zeta Potential and PDI of H-OVT
3.8. Fourier-Transform Infrared Spectroscopy of H-OVT
3.9. Iron-Binding Capacity of H-OVT
3.10. Antioxidant Capacity of H-OVT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Legros, J.; Jan, S.; Bonnassie, S.; Gautier, M.; Croguennec, T.; Pezennec, S.; Cochet, M.-F.; Nau, F.; Andrews, S.C.; Baron, F. The Role of Ovotransferrin in Egg-White Antimicrobial Activity: A Review. Foods 2021, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-Derived Tri-Peptide IRW Exerts Antihypertensive Effects in Spontaneously Hypertensive Rats. PLoS ONE 2013, 8, e82829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, X.; Ma, B.; Fu, X.; Ren, H.; Ma, M. Ultrasonic pretreatment enhanced the glycation of ovotransferrin and improved its antibacterial activity. Food Chem. 2021, 346, 128905. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Cheng, Y.; Zhu, J.; Huang, Q. Genipin-crosslinked ovotransferrin particle-stabilized Pickering emulsions as delivery vehicles for hesperidin. Food Hydrocoll. 2019, 94, 561–573. [Google Scholar] [CrossRef]
- Wei, Z.; Huang, Q. Development of high internal phase Pickering emulsions stabilised by ovotransferrin-gum arabic particles as curcumin delivery vehicles. Int. J. Food Sci. Technol. 2020, 55, 1891–1899. [Google Scholar] [CrossRef]
- Zhu, G.; Luo, J.; Du, H.; Jiang, Y.; Tu, Y.; Yao, Y.; Xu, M. Ovotransferrin enhances intestinal immune response in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2018, 120, 1–9. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Hoq, M.I.; Aoki, T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int. J. Biol. Macromol. 2007, 41, 631–640. [Google Scholar] [CrossRef]
- Abeyrathne, E.; Lee, H.Y.; Ahn, U. Separation of ovotransferrin and ovomucoid from chicken egg white. Poult. Sci. 2014, 93, 1010–1017. [Google Scholar] [CrossRef]
- Tankrathok, A.; Daduang, S.; Patramanon, R.; Araki, T.; Thammasirirak, S. Purification Process for the Preparation and Characterizations of Hen Egg White Ovalbumin, Lysozyme, Ovotransferrin, and Ovomucoid. Prep. Biochem. Biotechnol. 2009, 39, 380–399. [Google Scholar] [CrossRef]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of β-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E. Effect of heat-treatment on the physico-chemical properties of egg white proteins: A kinetic study. J. Food Eng. 2006, 75, 316–326. [Google Scholar] [CrossRef]
- Ai, M.M.; Zhang, Z.; Fan, H.; Cao, Y.Y.; Jiang, A.M. High-intensity ultrasound together with heat treatment improves the oil-in-water emulsion stability of egg white protein peptides. Food Hydrocoll. 2021, 111, 106256. [Google Scholar] [CrossRef]
- Minmin, A.; Quan, Z.; Nan, X.; Shanguang, G.; Yuanyuan, C.; Hong, F.; Ziting, L.; Ledan, Z.; Shuchang, L.; Jiaoli, L.; et al. Enhancement of gel characteristics of NaOH-induced duck egg white gel by adding Ca(OH)2 with/without heating. Food Hydrocoll. 2020, 103, 105654. [Google Scholar] [CrossRef]
- Hayes, M. Measuring Protein Content in Food: An Overview of Methods. Foods 2020, 9, 10. [Google Scholar] [CrossRef]
- Qing, M.; Zang, J.; Liu, Y.; Chi, Y.; Chi, Y. Mechanistic study on the decline of foaming characteristics of egg white under heat stress: Emphasizing apparent phenomena, structure, and intermolecular interactions. Int. J. Biol. Macromol. 2024, 281, 136446. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, W.Y.; Zhang, Q.X.; Tu, J.; Wu, L.Y. The impact of structural properties on the absorption of hen egg-white ovotransferrin with or without Fe3+ at the air/oil-water interface. J. Food Eng. 2025, 386, 112287. [Google Scholar] [CrossRef]
- Tang, T.; Lv, Y.; Su, Y.; Li, J.; Gu, L.; Yang, Y.; Chang, C. The differential non-covalent binding of epicatechin and chlorogenic acid to ovotransferrin and the enhancing efficiency of immunomodulatory activity. Int. J. Biol. Macromol. 2024, 259, 129298. [Google Scholar] [CrossRef]
- Farrell, H.M.; Wickham, E.D.; Unruh, J.J.; Qi, P.X.; Hoagland, P.D. Secondary structural studies of bovine caseins: Temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization. Food Hydrocoll. 2001, 15, 341–354. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Zhu, D.-Y.; Thakur, K.; Wang, C.-H.; Wang, H.; Ren, Y.-F.; Zhang, J.-G.; Wei, Z.-J. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int. J. Biol. Macromol. 2018, 111, 92–101. [Google Scholar] [CrossRef]
- Kuang, J.; Hamon, P.; Lechevalier, V.; Saurel, R. Thermal Behavior of Pea and Egg White Protein Mixtures. Foods 2023, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 2007, 28, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S.; Wujing, W.; Yong, W.; Hongbing, C.; Ping, T.; Jinyan, G. Pasteurization induced protein interaction decreased the potential allergenicity of ovalbumin and ovomucoid in egg white. J. Sci. Food Agric. 2022, 102, 6835–6847. [Google Scholar] [CrossRef]
- Chang, K.; Jiang, W.; Liu, J. Effect of subcritical water treatment on the structure and foaming properties of egg white protein. Food Hydrocoll. 2022, 124, 107241. [Google Scholar] [CrossRef]
- Kitabatake, N.; Ishida, A.; Doi, E. Physicochemical and functional-properties of hen ovalbumin dephosphorylated by acid-phosphatase. Agric. Biol. Chem. 1988, 52, 967–973. [Google Scholar] [CrossRef][Green Version]
- Mine, Y.; Noutomi, T.; Haga, N. Thermally induced changes in egg white proteins. J. Agric. Food Chem. 1990, 38, 2122–2125. [Google Scholar] [CrossRef]
- Weiyi, X.; Shenghua, H.; Ying, M.; Yixin, Z.; Qiming, L.; Lifeng, W.; Rongchun, W. Effect of pH on the formation of serum heat-induced protein aggregates in heated yak milk. Int. J. Dairy Technol. 2015, 68, 342–348. [Google Scholar] [CrossRef]
- Abdallah, F.B.; Chahine, J.M.E. Transferrins-Hen ovo-transferrin, interaction with bicarbonate and iron uptake. Eur. J. Biochem. 1998, 258, 1022–1031. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Ramel, J.V.; Santiago, L.G. Protein nanovehicles produced from egg white. Part 1: Effect of pH and heat treatment time on particle size and binding capacity. Food Hydrocoll. 2017, 73, 67–73. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Momen, S.; Mohammadian, M.; Salami, M.; Moosavi-Movahedi, A.A. Effect of free radical-induced aggregation on physicochemical and interface-related functionality of egg white protein. Food Hydrocoll. 2019, 87, 734–746. [Google Scholar] [CrossRef]
- Pan, F.G.; Wu, X.L.; Gong, L.L.; Xu, H.J.; Yuan, Y.X.; Lu, J.M.; Zhang, T.; Liu, J.B.; Shang, X.M. Dextran sulfate acting as a chaperone-like component on inhibition of amorphous aggregation and enhancing thermal stability of ovotransferrin. Food Chem. 2024, 445, 138720. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Malik, M.; Singh Saini, C. Heat treatment of sunflower protein isolates near isoelectric point: Effect on rheological and structural properties. Food Chem. 2019, 276, 554–561. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Wang, B.; Zhao, B.T.; Meng, Y.C.; Chen, J.; Yang, F.M. Effect of Hydrothermal Treatment on the Structure and Functional Properties of Quinoa Protein Isolate. Foods 2022, 11, 2954. [Google Scholar] [CrossRef]
- Kher, A.; Udabage, P.; McKinnon, I.; McNaughton, D.; Augustin, M.A. FTIR investigation of spray-dried milk protein concentrate powders. Vib. Spectrosc. 2007, 44, 375–381. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Xu, D.; Sheng, B.; Qin, D.; Chen, J.; Li, B.; Zhang, X. Application of ultrasound pretreatment and glycation in regulating the heat-induced amyloid-like aggregation of β-lactoglobulin. Food Hydrocoll. 2018, 80, 122–129. [Google Scholar] [CrossRef]
- Momen, S.; Salami, M.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A.A. The techno-functional properties of camel whey protein compared to bovine whey protein for fabrication a model high protein emulsion. LWT 2019, 101, 543–550. [Google Scholar] [CrossRef]
- Giansanti, F.; Massucci, M.T.; Giardi, M.F.; Nozza, F.; Pulsinelli, E.; Nicolini, C.; Botti, D.; Antonini, G. Antiviral activity of ovotransferrin derived peptides. Biochem. Biophys. Res. Commun. 2005, 331, 69–73. [Google Scholar] [CrossRef]
- Pakdaman, R.; ElHageChahine, J.M. A mechanism for iron uptake by transferrin. Eur. J. Biochem. 1996, 236, 922–931. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, D.; Kumar, R.; Kumar, R. Electrostatic effects control the stability and iron release kinetics of ovotransferrin. JBIC J. Biol. Inorg. Chem. 2014, 19, 1009–1024. [Google Scholar] [CrossRef]
- Chung, T.D.Y.; Raymond, K.N. Lactoferrin: The role of conformational changes in its iron binding and release. J. Am. Chem. Soc. 1993, 115, 6765–6768. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Antioxidant activity of protein extracts from heat-treated or thermally processed chickpeas and white beans. Food Chem. 2007, 103, 301–312. [Google Scholar] [CrossRef]
- De la Fuente, M.A.; Singh, H.; Hemar, Y. Recent advances in the characterisation of heat-induced aggregates and intermediates of whey proteins. Trends Food Sci. Technol. 2002, 13, 262–274. [Google Scholar] [CrossRef]
- You, S.-J.; Udenigwe, C.C.; Aluko, R.E.; Wu, J. Multifunctional peptides from egg white lysozyme. Food Res. Int. 2010, 43, 848–855. [Google Scholar] [CrossRef]
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; Barać, M.; Gökmen, V. Effects of isolation, enzymatic hydrolysis, heating, hydratation and Maillard reaction on the antioxidant capacity of cereal and legume proteins. Food Res. Int. 2012, 49, 1–6. [Google Scholar] [CrossRef]
- Jae Hoon, L.; Sun Hee, M.; Hyun Suk, K.; Eunju, P.; Dong Uk, A.; Hyun-Dong, P. Antioxidant and anticancer effects of functional peptides from ovotransferrin hydrolysates. J. Sci. Food Agric. 2017, 97, 4857–4864. [Google Scholar] [CrossRef]




| Level | Factor | ||
|---|---|---|---|
| A Time (min) | B Temperature (°C) | C pH | |
| 1 | 30 | 60 | 6 |
| 2 | 60 | 62.5 | 7 |
| 3 | 90 | 65 | 8 |
| Run | Factors | Yield (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | Time (min) | Temperature (°C) | pH | ||
| 1 | −1 | −1 | 0 | 30 | 60 | 8 | 85.64 |
| 2 | 1 | −1 | 0 | 90 | 60 | 8 | 85.98 |
| 3 | −1 | 1 | 0 | 30 | 65 | 8 | 83.72 |
| 4 | 1 | 1 | 0 | 90 | 65 | 8 | 85.50 |
| 5 | −1 | 0 | −1 | 30 | 62.5 | 7 | 82.16 |
| 6 | 1 | 0 | −1 | 90 | 62.5 | 7 | 83.49 |
| 7 | −1 | 0 | 1 | 30 | 62.5 | 9 | 83.61 |
| 8 | 1 | 0 | 1 | 90 | 62.5 | 9 | 84.38 |
| 9 | 0 | −1 | −1 | 60 | 60 | 7 | 88.33 |
| 10 | 0 | 1 | −1 | 60 | 65 | 7 | 88.17 |
| 11 | 0 | −1 | 1 | 60 | 60 | 9 | 88.80 |
| 12 | 0 | 1 | 1 | 60 | 65 | 9 | 89.81 |
| 13 | 0 | 0 | 0 | 60 | 62.5 | 8 | 92.10 |
| 14 | 0 | 0 | 0 | 60 | 62.5 | 8 | 92.27 |
| 15 | 0 | 0 | 0 | 60 | 62.5 | 8 | 92.66 |
| 16 | 0 | 0 | 0 | 60 | 62.5 | 8 | 92.34 |
| 17 | 0 | 0 | 0 | 60 | 62.5 | 8 | 91.82 |
| Source | Sum of Squares | df | Mean Square | F | p | Significant |
|---|---|---|---|---|---|---|
| Model | 211.12 | 9 | 23.46 | 95.54 | <0.0001 | Significant |
| A-A | 2.23 | 1 | 2.23 | 9.07 | 0.0196 | |
| B-B | 0.3 | 1 | 0.3 | 1.24 | 0.3024 | |
| C-C | 2.46 | 1 | 2.46 | 10.04 | 0.0158 | |
| AB | 0.52 | 1 | 0.52 | 2.11 | 0.1895 | |
| AC | 0.078 | 1 | 0.078 | 0.32 | 0.5897 | |
| BC | 0.34 | 1 | 0.34 | 1.37 | 0.2801 | |
| A2 | 161.20 | 1 | 161.20 | 656.56 | <0.0001 | |
| B2 | 2.85 | 1 | 2.85 | 11.60 | 0.0113 | |
| C2 | 28.96 | 1 | 28.96 | 117.94 | <0.0001 | |
| Residual | 1.72 | 7 | 0.25 | 4.41 | 0.0930 | Not significant |
| Lack of Fit | 1.32 | 3 | 0.44 | |||
| Pure Error | 0.4 | 4 | 0.1 | |||
| Cor Total | 212.83 | 16 | ||||
| R2 = 0.9904 | R2adj = 0.9781 | CV = 0.73 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Zhao, Q.; Chang, C.; Li, J.; Guo, L.; Hu, S.; Huang, Z.; Gu, L.; Yang, Y. Studies on the Efficient Extraction of Ovotransferrin and the Effect of Heating Treatment on Its Structure and Activity. Foods 2025, 14, 905. https://doi.org/10.3390/foods14050905
Su Y, Zhao Q, Chang C, Li J, Guo L, Hu S, Huang Z, Gu L, Yang Y. Studies on the Efficient Extraction of Ovotransferrin and the Effect of Heating Treatment on Its Structure and Activity. Foods. 2025; 14(5):905. https://doi.org/10.3390/foods14050905
Chicago/Turabian StyleSu, Yujie, Qianwen Zhao, Cuihua Chang, Junhua Li, Lulu Guo, Shende Hu, Zijian Huang, Luping Gu, and Yanjun Yang. 2025. "Studies on the Efficient Extraction of Ovotransferrin and the Effect of Heating Treatment on Its Structure and Activity" Foods 14, no. 5: 905. https://doi.org/10.3390/foods14050905
APA StyleSu, Y., Zhao, Q., Chang, C., Li, J., Guo, L., Hu, S., Huang, Z., Gu, L., & Yang, Y. (2025). Studies on the Efficient Extraction of Ovotransferrin and the Effect of Heating Treatment on Its Structure and Activity. Foods, 14(5), 905. https://doi.org/10.3390/foods14050905









