Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Soy Protein Hydrolysate and Determination of Hydrolysis Degree
2.3. Characterization of Structure of Soy Protein Hydrolysate
2.3.1. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Measurements
2.3.2. UV Spectrometry Measurements
2.3.3. Fluorescence Spectrometry Measurements
2.3.4. Free Sulfhydryl Content Measurements
2.3.5. Surface Hydrophobicity Measurements
2.3.6. Fourier Transform Infrared (FT-IR) Spectral Measurements
2.4. Characterization of Functional Properties of Soy Protein Hydrolysate
2.4.1. Solubility Measurements
2.4.2. Emulsification and Foaming Properties Measurements
2.4.3. Antioxidant Measurements
2.5. Preparation of Soy Milk Powder
2.5.1. Soy Milk Powder Solubility Measurements
2.5.2. Scanning Electron Microscopy (SEM) Measurements
2.5.3. Electronic Tongue Evaluation Measurements
2.6. Statistical Analysis
3. Results and Discussion
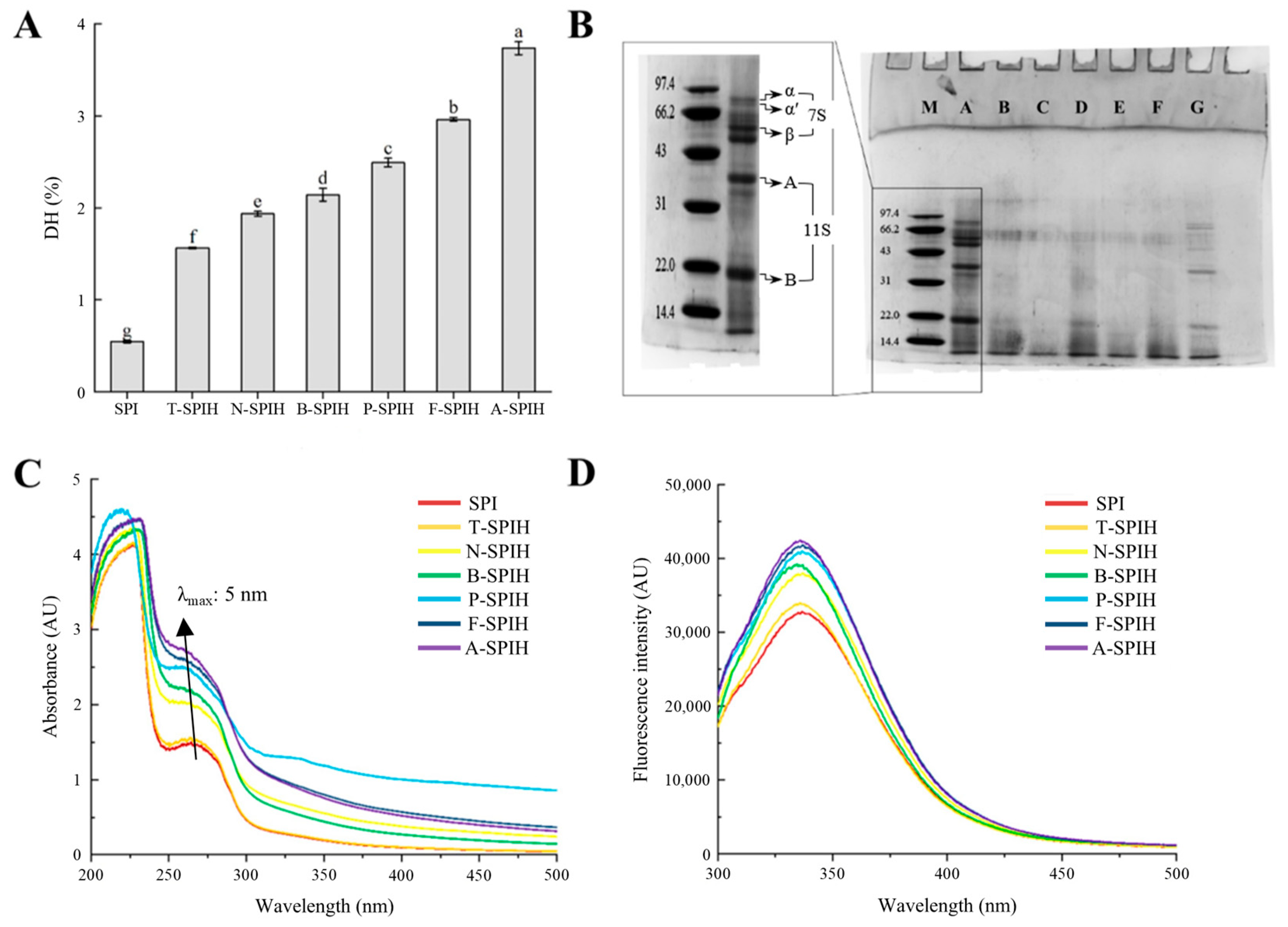
3.1. DH and SDS-PAGE Analysis
3.2. UV Absorption and Fluorescence Spectrum Analysis
3.3. Free Sulfhydryl Group Content and Surface Hydrophobicity Analysis
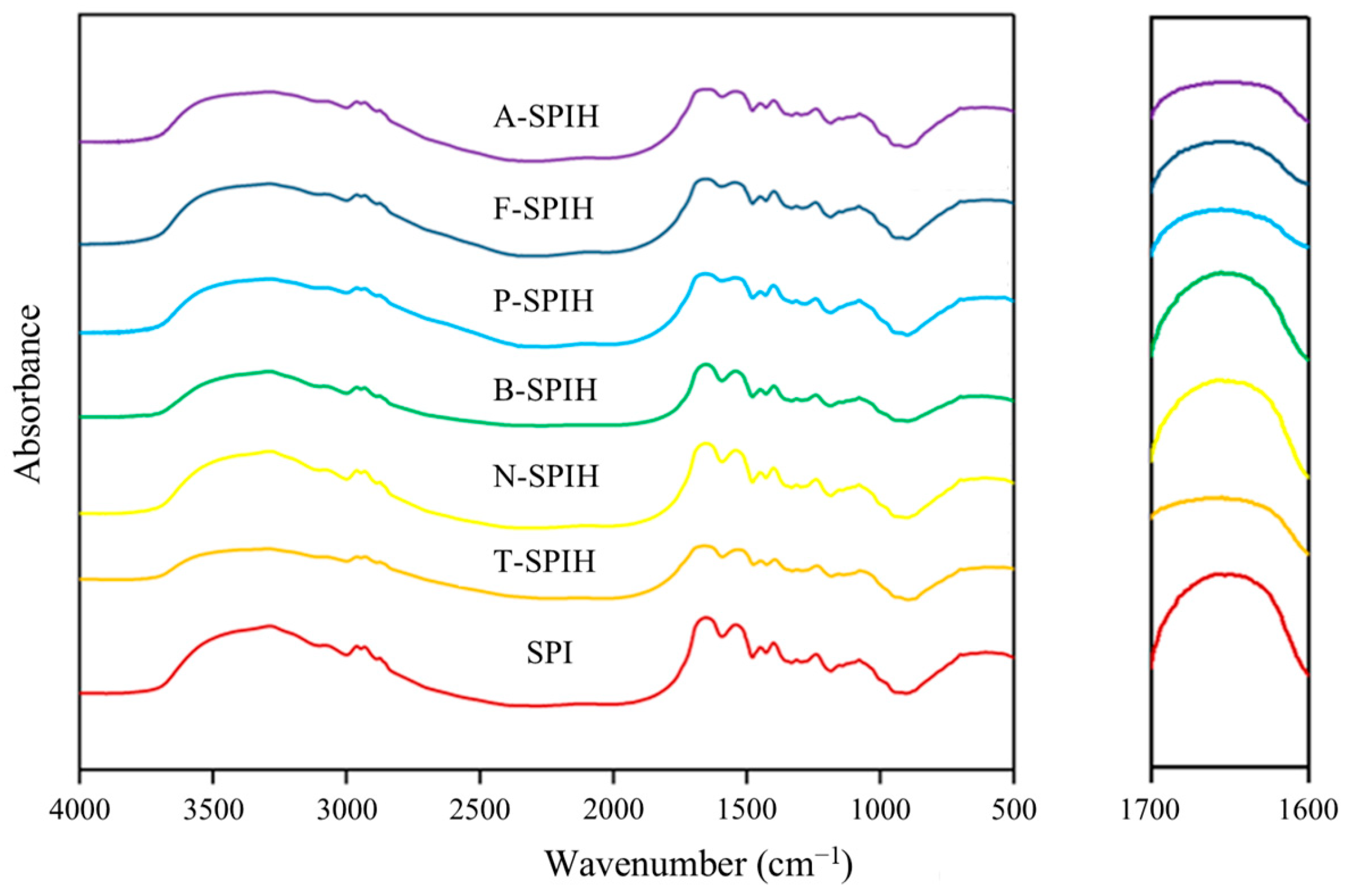
3.4. Secondary Structure Analysis
3.5. Solubility Analysis
3.6. Emulsification and Foaming Properties Analysis
3.7. Antioxidant Properties Analysis
3.8. Solubility Evaluation of Soy Milk Powder Analysis
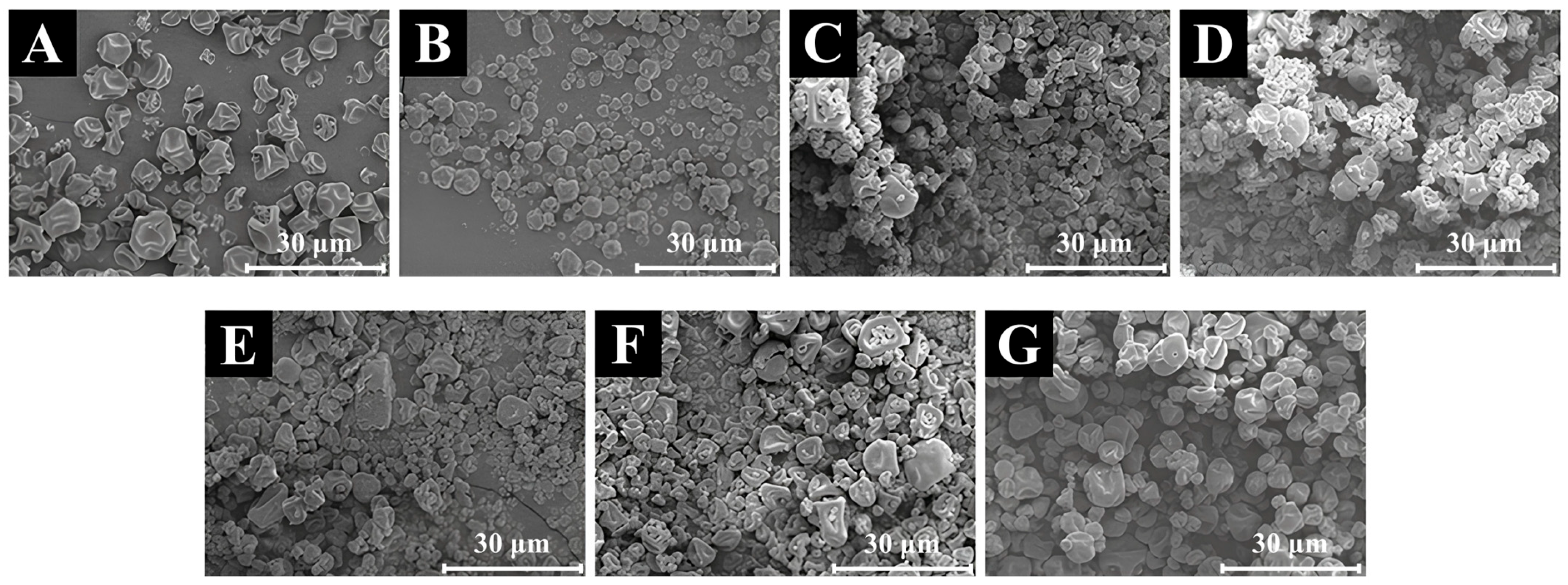
3.9. SEM Analysis
3.10. Evaluation of Soy Milk Powder Electronic Tongue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sui, X.; Zhang, T.; Zhang, X.; Jiang, L. High-Moisture Extrusion of Plant Proteins: Fundamentals of Texturization and Applications. Annu. Rev. Food Sci. Technol. 2024, 15, 125–149. [Google Scholar] [CrossRef]
- Giri, S.K.; Mangaraj, S. Processing Influences on Composition and Quality Attributes of Soymilk and its Powder. Food Eng. Rev. 2012, 4, 149–164. [Google Scholar] [CrossRef]
- Bhandari, B.; Zisu, B. Effect of Ultrasound Treatment on the Evolution of Solubility of Milk Protein Concentrate Powder, Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; pp. 1383–1401. [Google Scholar]
- Han, H.; Choi, J.K.; Park, J.; Im, H.C.; Han, J.H.; Huh, M.H.; Lee, Y.-B. Recent innovations in processing technologies for im-provement of nutritional quality of soymilk. CyTA J. Food 2021, 19, 287–303. [Google Scholar] [CrossRef]
- Teng, H.; Mi, Y.; Cao, H.; Chen, L. Enzymatic acylation of raspberry anthocyanin: Evaluations on its stability and oxidative stress prevention. Food Chem. 2022, 372, 130766. [Google Scholar] [CrossRef]
- Tian, R.; Feng, J.; Huang, G.; Tian, B.; Zhang, Y.; Jiang, L.; Sui, X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochemistry 2020, 68, 105202. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, X.; Gouda, M.; Cai, Z.; Jin, Y. Effect of enzymatic hydrolysis on the solubility of egg yolk powder from the changes in structure and functional properties. LWT 2019, 110, 214–222. [Google Scholar] [CrossRef]
- De Castro, R.J.S.; Sato, H.H. Antioxidant activities and functional properties of soy protein isolate hydrolysates obtained using microbial proteases. Int. J. Food Sci. Technol. 2014, 49, 317–328. [Google Scholar] [CrossRef]
- Shu, G.; Huang, J.; Bao, C.; Meng, J.; Chen, H.; Cao, J. Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Converting Enzyme-Inhibitory Activity in Goat and Cow Milk. Biomolecules 2018, 8, 101. [Google Scholar] [CrossRef]
- Lamsal, B.P.; Jung, S.; Johnson, L.A. Rheological properties of soy protein hydrolysates obtained from limited enzymatic hy-drolysis. LWT Food Sci. Technol. 2007, 40, 1215–1223. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Schweiggert-Weisz, U.; Eisner, P. Soy protein hydrolysates fermentation: Effect of debittering and degradation of major soy allergens. LWT 2016, 71, 202–212. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Sussmann, D.; Schweiggert-Weisz, U.; Eisner, P. Enzymatic treatment of soy protein isolates: Effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci. Nutr. 2015, 4, 11–23. [Google Scholar] [CrossRef]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Wen, J.; Jin, H.; Wang, L.; Zhang, Y.; Jiang, L.; Sui, X. Fabrication and characterization of high internal phase Pickering emulsions based on pH-mediated soy protein-epigallocatechin-3-gallate hydrophobic and hydrophilic nano-stabilizer. LWT 2023, 179, 114638. [Google Scholar] [CrossRef]
- Weng, Z.; Sun, L.; Wang, F.; Sui, X.; Fang, Y.; Tang, X.; Shen, X. Assessment the flavor of soybean meal hydrolyzed with Alcalase enzyme under different hydrolysis conditions by E-nose, E-tongue and HS-SPME-GC–MS. Food Chem. X 2021, 12, 100141. [Google Scholar] [CrossRef]
- Rivera-Jiménez, J.; Berraquero-García, C.; Pérez-Gálvez, R.; García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Peptides and protein hydrolysates exhibiting anti-inflammatory activity: Sources, structural features and modulation mechanisms. Food Funct. 2022, 13, 12510–12540. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-converting enzyme inhibitory activity of chickpea and pea protein hydrolysates. Food Res. Int. 2010, 43, 1642–1649. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, X.; Zhang, X.; Liu, H.; Ao, Q. Amino acid, structure and antioxidant properties of Haematococcus pluvialis protein hydrolysates produced by different proteases. Int. J. Food Sci. Technol. 2021, 56, 185–195. [Google Scholar] [CrossRef]
- Wei, C.-K.; Thakur, K.; Liu, D.-H.; Zhang, J.-G.; Wei, Z.-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chem. 2018, 263, 186–193. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Zhao, J.; Xiong, Y.L.; McNear, D.H. Changes in structural characteristics of antioxidative soy protein hydrolysates resulting from scavenging of hydroxyl radicals. J. Food Sci. 2013, 78, C152–C159. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.; Salami, M.; Mohammadian, M.; Emam-Djomeh, Z.; Jahanbani, R.; Moosavi-Movahedi, A.A. Physicochemical and bio-functional properties of walnut proteins as affected by trypsin-mediated hydrolysis. Food Biosci. 2020, 36, 100611. [Google Scholar] [CrossRef]
- Lan, T.; Dong, Y.; Zheng, M.; Jiang, L.; Zhang, Y.; Sui, X. Complexation between soy peptides and epigallocatechin-3-gallate (EGCG): Formation mechanism and morphological characterization. LWT 2020, 134, 109990. [Google Scholar] [CrossRef]
- Sponton, O.E.; Perez, A.A.; Carrara, C.; Santiago, L.G. Effect of limited enzymatic hydrolysis on linoleic acid binding properties of β-lactoglobulin. Food Chem. 2014, 146, 577–582. [Google Scholar] [CrossRef]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J. Cereal Sci. 2019, 85, 168–174. [Google Scholar] [CrossRef]
- Omar, A.; Gao, Y.; Wubulikasimu, A.; Arken, A.; Aisa, H.A.; Yili, A. Effects of trypsin-induced limited hydrolysis on the structural, functional, and bioactive properties of sericin. RSC Adv. 2021, 11, 25431–25440. [Google Scholar] [CrossRef]
- Sharafodin, H.; Soltanizadeh, N. Potential application of DBD Plasma Technique for modifying structural and physicochemical properties of Soy Protein Isolate. Food Hydrocoll. 2022, 122, 107077. [Google Scholar] [CrossRef]
- Tang, Y.; Stone, A.; Wang, Y.; Jafarian, Z.; Zhou, L.; Kimmel, J.; House, J.; Tanaka, T.; Nickerson, M. Effects of enzyme treatments on the functionality of commercial pea and pea blended protein ingredients. Food Biosci. 2023, 53, 102838. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emul-sifying properties of a lentil protein isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Xiong, Y.L.; Xia, X. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010, 118, 403–410. [Google Scholar] [CrossRef]
- Hu, H.; Li-Chan, E.C.; Wan, L.; Tian, M.; Pan, S. The effect of high intensity ultrasonic pre-treatment on the properties of soybean protein isolate gel induced by calcium sulfate. Food Hydrocoll. 2013, 32, 303–311. [Google Scholar] [CrossRef]
- Ottesen, M.; Johansen, J.; Svendsen, I. Subtilisin: Stability Properties and Secondary Binding Sites. In Structure–Function Relationships of Proteolytic Enzymes; Desnuelle, P., Neurath, H., Ottesen, M., Eds.; Academic Press: Cambridge, MA, USA, 1970; pp. 175–187. [Google Scholar]
- Huang, G.; Zhang, Q.; Wang, X.; Zhang, Y.; Sui, X. Secondary Structural Analysis Using Fourier Transform Infrared and Circular Dichroism Spectroscopy. In Plant-Based Proteins: Production, Physicochemical, Functional, and Sensory Properties; Li, Y., Ed.; Springer: New York, NY, USA, 2025; pp. 265–277. [Google Scholar]
- Zhang, D.; Ge, X.; Jiao, Y.; Liu, Y. Quality analysis of steamed beef with black tea and the mechanism of action of main active ingredients of black tea on myofibrillar protein. Food Chem. 2024, 441, 137997. [Google Scholar] [CrossRef]
- Shivu, B.; Seshadri, S.; Li, J.; Oberg, K.A.; Uversky, V.N.; Fink, A.L. Distinct β-Sheet Structure in Protein Aggregates Determined by ATR–FTIR Spectroscopy. Biochemistry 2013, 52, 5176–5183. [Google Scholar] [CrossRef] [PubMed]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymatic hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011, 124, 1354–1362. [Google Scholar] [CrossRef]
- Grossmann, L.; McClements, D.J. Current insights into protein solubility: A review of its importance for alternative proteins. Food Hydrocoll. 2023, 137, 108416. [Google Scholar] [CrossRef]
- Islam, Z.-U.; Mir, N.A.; Gani, A. Effect of controlled enzymatic treatment on the physicochemical, structural and functional properties of high-intensity ultrasound treated album (Chenopodium album) protein. Food Hydrocoll. 2023, 144, 108940. [Google Scholar] [CrossRef]
- Mune, M.A.M.; Sogi, D.S. Emulsifying and foaming properties of protein concentrates prepared from cowpea and bambara bean using different drying methods. Int. J. Food Prop. 2016, 19, 371–384. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic hydrolysis of rice dreg protein: Effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef]
- Razavizadeh, R.S.; Farmani, J.; Motamedzadegan, A. Enzyme-assisted extraction of chicken skin protein hydrolysates and fat: Degree of hydrolysis affects the physicochemical and functional properties. J. Am. Oil Chem. Soc. 2022, 99, 621–632. [Google Scholar] [CrossRef]
- Mao, X.; Hua, Y. Composition, Structure and Functional Properties of Protein Concentrates and Isolates Produced from Walnut (Juglans regia L.). Int. J. Mol. Sci. 2012, 13, 1561–1581. [Google Scholar] [CrossRef]
- Matsumiya, K.; Murray, B.S. Soybean protein isolate gel particles as foaming and emulsifying agents. Food Hydrocoll. 2016, 60, 206–215. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Z.; Yang, M.; Zhang, Y.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Micronization using combined alkaline protease hy-drolysis and high-speed shearing homogenization for improving the functional properties of soy protein isolates. Bioresour. Bioprocess. 2022, 9, 77. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.; Chen, W.; Wang, J.; Wang, S. Influence of ultrasound-assisted ionic liquid pretreatments on the functional properties of soy protein hydrolysates. Ultrason. Sonochemistry 2021, 73, 105546. [Google Scholar] [CrossRef]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Mersha, Z.; Lynch, P.; Almutaimi, A. Effect of enzymatic hydrolysis using endo-and exo-proteases on secondary structure, functional, and antioxidant properties of chickpea protein hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- Taylor, M.; Richardson, T. Antioxidant Activity of Skim Milk: Effect of Heat and Resultant Sulfhydryl Groups. J. Dairy Sci. 1980, 63, 1783–1795. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; Barac, M.; Gökmen, V. Effects of isolation, enzymatic hydrolysis, heating, hydratation and Maillard reaction on the antioxidant capacity of cereal and legume proteins. Food Res. Int. 2012, 49, 1–6. [Google Scholar] [CrossRef]
- Intarasirisawat, R.; Benjakul, S.; Visessanguan, W.; Wu, J. Antioxidative and functional properties of protein hydrolysate from defatted skipjack (Katsuwonous pelamis) roe. Food Chem. 2012, 135, 3039–3048. [Google Scholar] [CrossRef]
- Oussaief, O.; Jrad, Z.; Adt, I.; Khorchani, T.; El-Hatmi, H. Dromedary Milk Protein Hydrolysates Show Enhanced Antioxidant and Functional Properties. Food Technol. Biotechnol. 2020, 58, 147–158. [Google Scholar] [CrossRef]
- Correa, A.P.F.; Daroit, D.J.; Coelho, J.; Meira, S.M.M.; Lopes, F.C.; Segalin, J.; Risso, P.H.; Brandelli, A. Antioxidant, antihyper-tensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. J. Sci. Food Agric. 2011, 91, 2247–2254. [Google Scholar]
- Zhao, G.; Liu, Y.; Zhao, M.; Ren, J.; Yang, B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem. 2011, 127, 1438–1443. [Google Scholar] [CrossRef]
- Gu, L.; Jiao, H.; McClements, D.J.; Ji, M.; Li, J.; Chang, C.; Dong, S.; Su, Y.; Yang, Y. Improvement of egg yolk powder properties through enzymatic hydrolysis and subcritical fluid extraction. LWT 2021, 150, 112075. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Li, Y.; Tong, X.; Regenstein, J.M.; Huang, Y.; Ma, W.; Sami, R.; Qi, B.; Jiang, L. Effect of the condition of spray-drying on the properties of the polypeptide-rich powders from enzyme-assisted aqueous extraction processing. Dry. Technol. 2019, 37, 2105–2115. [Google Scholar] [CrossRef]
- Deng, X.; Lin, H.; Ahmed, I.; Sui, J. Isolation and identification of the umami peptides from Trachinotus ovatus hydrolysate by consecutive chromatography and Nano-HPLC-MS/MS. LWT 2021, 141, 110887. [Google Scholar] [CrossRef]
- Romli, S.R.; Murad, M. Influence of fresh pineapple intake on human taste detection and recognition thresholds of basic taste stimulants. J. Sens. Stud. 2022, 37, e12729. [Google Scholar] [CrossRef]
- Wu, J.; Huang, P.; Feng, Y.; Cui, C.; Xu, J.; Li, L. Enhancing Kokumi Sensation and Reducing Bitterness in Acid-Hydrolyzed Vegetable Proteins through Lactate and Thermal Processing. J. Agric. Food Chem. 2023, 71, 19694–19704. [Google Scholar] [CrossRef]



| Samples | Surface Hydrophobicity/H0 | Free Sulfhydryl Group Content/(μmoL/g) | Relative Content of Secondary Structure/% | |||
|---|---|---|---|---|---|---|
| α-Helix | β-Sheet | β-Turn | Random Coil | |||
| SPI | 38,916.77 ± 384.57 a | 3.46 ± 0.05 e | 21.82 ± 0.04 a | 40.78 ± 0.20 a | 19.08 ± 0.02 f | 18.33 ± 0.03 f |
| T-SPIH | 14,519.33 ± 729.55 f | 3.78 ± 0.03 d | 21.19 ± 0.02 b | 40.52 ± 0.20 ab | 19.79 ± 0.18 e | 18.51 ± 0.01 e |
| N-SPIH | 20,299.16 ± 260.15 e | 3.87 ± 0.03 d | 18.64 ± 0.03 c | 40.22 ± 0.09 b | 20.44 ± 0.18 d | 20.72 ± 0.03 d |
| B-SPIH | 26,565.35 ± 401.54 d | 5.28 ± 0.03 c | 17.09 ± 0.01 d | 38.12 ± 0.09 c | 23.95 ± 0.07 c | 20.84 ± 0.07 d |
| P-SPIH | 29,415.68 ± 1097.15 c | 5.59 ± 0.04 b | 17.20 ± 0.01 d | 37.36 ± 0.15 d | 24.15 ± 0.09 c | 21.28 ± 0.18 c |
| F-SPIH | 30,365.79 ± 1555.24 c | 5.68 ± 0.07 b | 16.79 ± 0.07 e | 36.02 ± 0.14 e | 25.28 ± 0.10 b | 21.90 ± 0.10 b |
| A-SPIH | 34,403.75 ± 576.85 b | 7.37 ± 0.07 a | 15.11 ± 0.17 f | 34.51 ± 0.21 f | 26.66 ± 0.02 a | 23.73 ± 0.09 a |
| Samples | Solubility/% | EAI/(m2/g) | ESI/% | FC/% | FS/% | DPPH/% | ABTS/% |
|---|---|---|---|---|---|---|---|
| SPI | 6.89 ± 1.18 e | 14.88 ± 1.32 d | 37.77 ± 5.02 a | 1.58 ± 0.05 c | 0.50 ± 0.09 cd | 21.08 ± 0.27 f | 43.46 ± 0.46 e |
| T-SPIH | 9.45 ± 0.94 d | 17.88 ± 0.90 d | 20.35 ± 0.61 b | 1.63 ± 0.09 bc | 0.97 ± 0.03 a | 40.63 ± 0.79 e | 67.16 ± 1.61 d |
| N-SPIH | 13.39 ± 0.68 c | 31.35 ± 5.83 c | 15.82 ± 1.27 bc | 1.65 ± 0.10 bc | 0.72 ± 0.02 b | 45.50 ± 0.97 d | 67.61 ± 1.10 d |
| B-SPIH | 15.55 ± 0.91 bc | 42.43 ± 7.45 c | 14.34 ± 0.71 c | 1.79 ± 0.11 abc | 0.67 ± 0.04 b | 47.62 ± 0.25 c | 71.43 ± 0.74 c |
| P-SPIH | 17.06 ± 1.20 b | 59.71 ± 3.28 b | 13.95 ± 0.45 c | 1.80 ± 0.17 abc | 0.62 ± 0.06 bc | 47.77 ± 0.62 c | 82.14 ± 1.44 b |
| F-SPIH | 17.09 ± 0.22 b | 78.54 ± 5.66 a | 13.14 ± 1.26 c | 1.87 ± 0.11 ab | 0.37 ± 0.04 e | 49.81 ± 0.17 b | 85.71 ± 0.40 a |
| A-SPIH | 19.99 ± 0.86 a | 87.01 ± 4.03 a | 13.38 ± 1.16 c | 1.97 ± 0.10 a | 0.40 ± 0.03 de | 51.57 ± 0.17 a | 83.44 ± 1.68 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chang, B.; Huang, G.; Wang, D.; Gao, Y.; Fan, Z.; Sun, H.; Sui, X. Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties. Foods 2025, 14, 906. https://doi.org/10.3390/foods14050906
Li Q, Chang B, Huang G, Wang D, Gao Y, Fan Z, Sun H, Sui X. Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties. Foods. 2025; 14(5):906. https://doi.org/10.3390/foods14050906
Chicago/Turabian StyleLi, Qian, Baoyue Chang, Guo Huang, Di Wang, Yue Gao, Zhijun Fan, Hongbo Sun, and Xiaonan Sui. 2025. "Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties" Foods 14, no. 5: 906. https://doi.org/10.3390/foods14050906
APA StyleLi, Q., Chang, B., Huang, G., Wang, D., Gao, Y., Fan, Z., Sun, H., & Sui, X. (2025). Differential Enzymatic Hydrolysis: A Study on Its Impact on Soy Protein Structure, Function, and Soy Milk Powder Properties. Foods, 14(5), 906. https://doi.org/10.3390/foods14050906






