Impact of Oil Temperature and Splashing Frequency on Chili Oil Flavor: Volatilomics and Lipidomics
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Materials and Reagents
2.2. Sample Preparation
2.3. GC-IMS Analysis of Volatile Compounds in Chili Oil
2.3.1. GC Conditions for GC-IMS
2.3.2. IMS Conditions
2.4. PLS-DA and VIP Value Analysis
2.5. GC-MS Analysis of Volatile Compounds in Chili Oil
2.5.1. GC Conditions
2.5.2. MS Conditions
2.5.3. Qualitative and Quantitative Analyses
2.6. Calculation of Relative Odor Activity Value
2.7. E-Nose Analysis
2.8. Determination of Fatty Acids
2.8.1. Fatty Acid Methyl Esterification Treatment
2.8.2. GC Analysis
2.9. Lipidomics Analysis of Chili Oil
2.10. Kyoto Encyclopaedia of Genes and Genomes (KEGG) Analysis of Differential Lipids
2.11. Statistical Analysis
3. Results and Discussion
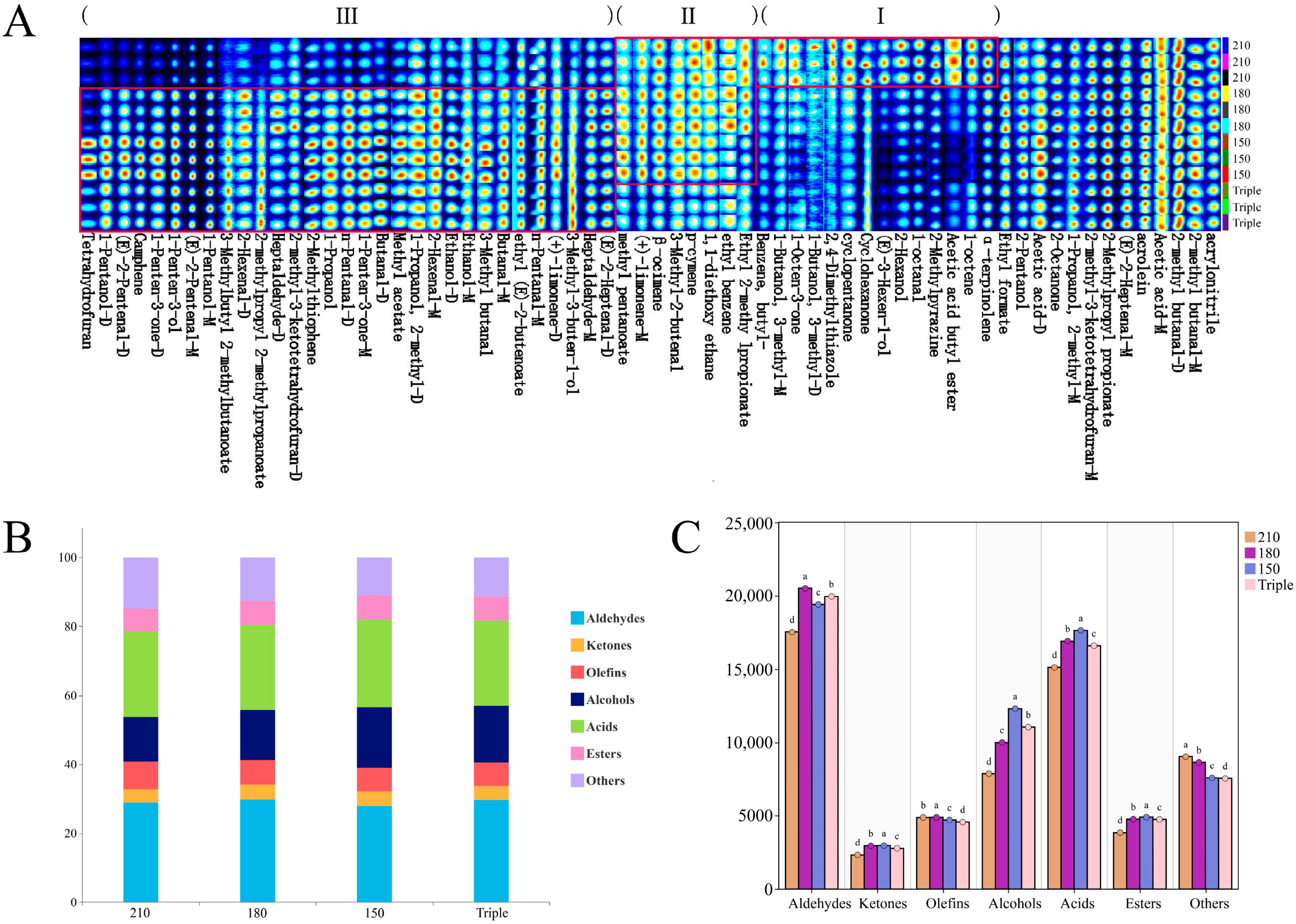
3.1. Volatile Profile of Chili Oil Identified by GC-IMS
3.2. Volatile Profile of Chili Oil Identified by GC-MS
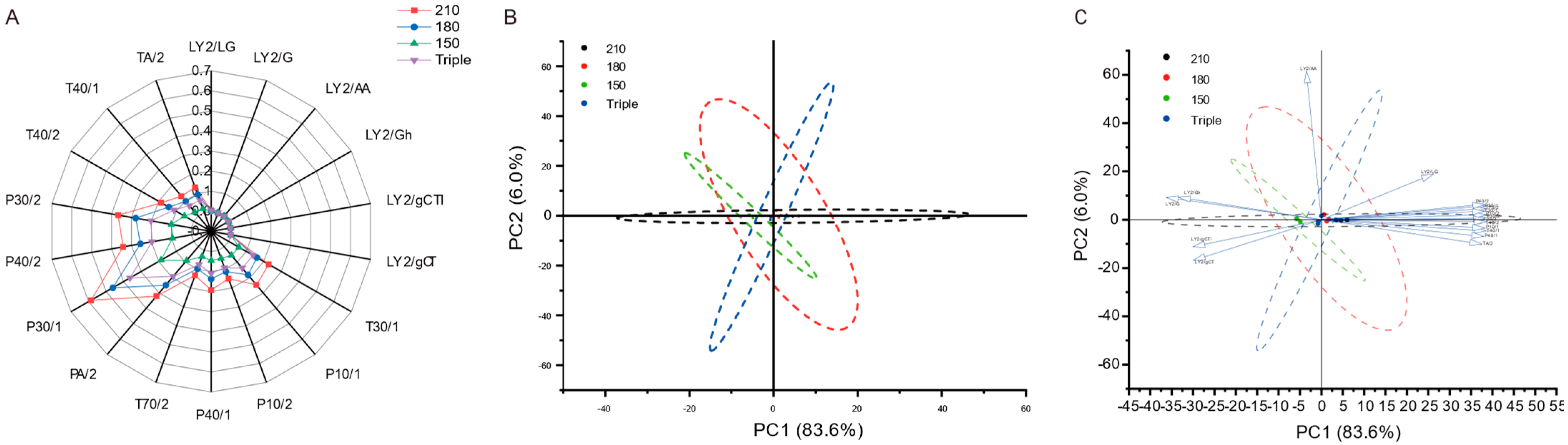
3.3. E-Nose Analysis
3.4. Key Volatile Flavor Compounds of Chili Oil Analysis
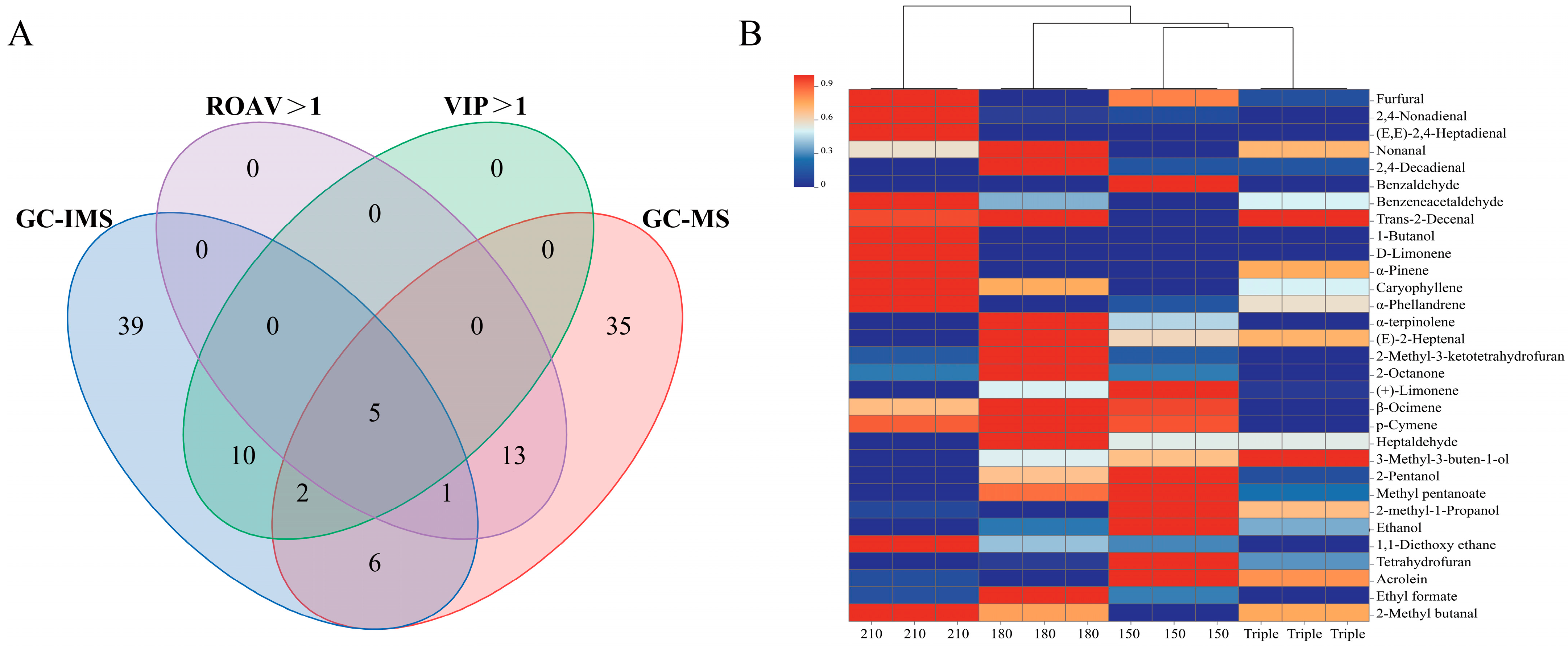
3.4.1. VIP Analysis
3.4.2. Analysis of ROAV
3.4.3. Identification of Key Volatile Flavor Compounds by VIP and ROAV
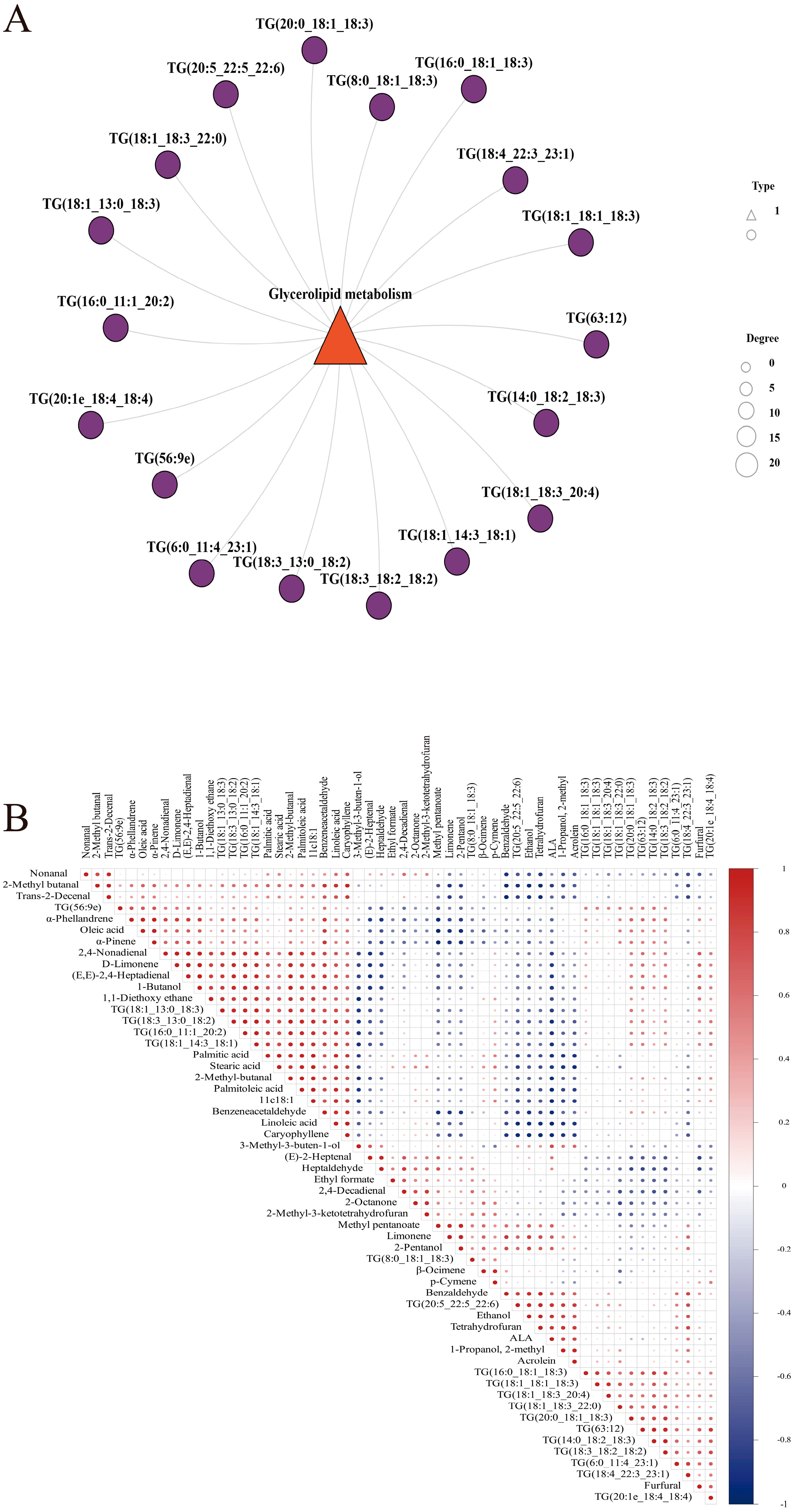
3.5. Free Fatty Acid and Lipidomics Analyses of Chili Oil
3.6. Correlation Analysis of Differential Lipids with Key Flavor Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koç, G.C. The effect of different drying techniques and microwave finish drying on the powder properties of the red pepper powder (Capsicum annuum L.). J. Food Sci. Technol. Mysore 2020, 57, 4576–4587. [Google Scholar] [CrossRef]
- Jang, Y.K.; Jung, E.S.; Lee, H.A.; Choi, D.; Lee, C.H. Metabolomic Characterization of Hot Pepper (Capsicum annuum “CM334”) during Fruit Development. J. Agric. Food Chem. 2015, 63, 9452–9460. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, B.; Zhao, W.; Lao, F.; Chen, F.; Liao, X.; Wu, J. Shifts in autochthonous microbial diversity and volatile metabolites during the fermentation of chili pepper (Capsicum frutescens L.). Food Chem. 2021, 335, 127512. [Google Scholar] [CrossRef] [PubMed]
- Pu, B.; Teah, M.; Phau, I. Hot Chili Peppers, Tears and Sweat: How Experiencing Sichuan Cuisine will Influence Intention to Visit City of Origin. Sustainability 2019, 11, 3561. [Google Scholar] [CrossRef]
- Li, D.; Chu, B.; Li, B.; Wang, X.; Chen, X.; Gu, Q. The difference analysis of physicochemical indexes and volatile flavor compounds of chili oil prepared from different varieties of chili pepper. Food Res. Int. 2024, 190, 114657. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Zheng, G.; Sun, J.; Zhou, H.; Lijun, Y. Analysis of flavor compounds in chili oil by head-space solid-phase micro-extraction gas chromatography mass spectrometry. Mod. Food Sci. Technol. 2017, 33, 276–284. [Google Scholar] [CrossRef]
- Yang, F.; Deng, F.; Yuan, H.; Jin, L.; Jia, H. Study on physicochemical properties and flavor components of chili oil from different geographical origins based on gas chromatography-ion mobility spectrometry(GC-IMS) combined with multivariate statistical method. J. Nucl. Agric. Sci. 2023, 37, 1393–1402. [Google Scholar] [CrossRef]
- Tao, X.; Wang, Y.; Yin, S.; Liu, C.; Liu, J.; Zhang, Q.; Liu, D.; Zhou, P. Effects of different types of cooking oil on flavor characteristics of chili oil. Food Ferment. Ind. 2024, 50, 341–352. [Google Scholar] [CrossRef]
- Yang, F.; Deng, F.; Jia, H.; Yuan, H.; Yao, K. Study on the effects of granularity of paprika on physicochemicalproperties and volatile flavor compounds of chili oil. Food Mach. 2023, 39, 157–165. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, C.; Jiang, H.; Wu, H.; Qiao, M.; Yang, F. Study on effect of chili particle size on the quality and flavor of chili oil. China Condiment 2023, 48, 63–66,74. [Google Scholar] [CrossRef]
- Sun, H.; Ni, H.; Yang, Y.; Wu, L.; Cai, H.N.; Xiao, A.F.; Chen, F. Investigation of sunlight-induced deterioration of aroma of pummelo (Citrus maxima) essential oil. J. Agric. Food Chem. 2014, 62, 11818–11830. [Google Scholar] [CrossRef]
- Sun, J.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y.; Zhang, H. Effects of Storage Conditions on the Flavor Stability of Fried Pepper (Zanthoxylum bungeanum) Oil. Foods 2021, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, S.; Grauwet, T.; Kebede, B.T.; Hendrickx, M.; Van Loey, A. Study of chemical changes in pasteurised orange juice during shelf-life: A fingerprinting-kinetics evaluation of the volatile fraction. Food Res. Int. 2015, 75, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Huang, L.; Zhang, F.; Wen, Y.; Zhang, Q.; Yu, M. Effect of Oil Temperature on the Flavor of Chilli Oil by GC-IMS and Sensory Evaluation. J. Chin. Inst. Food Sci. Technol. 2021, 21, 328–335. [Google Scholar] [CrossRef]
- Lin, S.; Ma, W.; He, X.; Fu, G.; Zhong, J.; Peng, H.; Wan, Y. Effect of oil temperatures on the flavor and spiciness of chili oil. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2023, 44, 25–32. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, L.; Ma, F.; Zhang, W.; Wang, X.; Zhang, Q.; Luo, D.; Ma, H.; Li, P. Determination of free steroidal compounds in vegetable oils by comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry. Food Chem. 2018, 245, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, X.; Li, Y.; Yao, D. Study on processing technology of chili flavor beef tallow andits volatile compounds analysis. Food Mach. 2021, 37, 149–154,214. [Google Scholar] [CrossRef]
- Xu, L.; Sun, P.; Yu, X.; Qu, Q.; Zhang, Z. Optimization of processing conditions for five-spice condiment oil based on electronic nose analysis. Food Sci. 2014, 35, 308–313. [Google Scholar] [CrossRef]
- Mishra, S.; Firdaus, M.A.; Patel, M.; Pandey, G. A study on the effect of repeated heating on the physicochemical and antioxidant properties of cooking oils used by fried food vendors of Lucknow city. Discov. Food 2023, 3, 7. [Google Scholar] [CrossRef]
- Liu, M.; Hu, L.; Deng, N.; Cai, Y.; Li, H.; Zhang, B.; Wang, J. Effects of different hot-air drying methods on the dynamic changes in color, nutrient and aroma quality of three chili pepper (Capsicum annuum L.) varieties. Food Chem. X 2024, 22, 101262. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Ammerlaan, B.; van den Berg, M.; van Duynhoven, J.; Jacobs, D.; Mumm, R.; Hall, R.D. Comparison of volatile trapping techniques for the comprehensive analysis of food flavourings by Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2020, 1624, 461191. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, J.; Xu, H.; Li, M. Effect of oil temperature on the quality of chili oil. China Condiment 2022, 47, 124–127,132. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Q.; Fu, X.; Wu, Y.; Qi, Z.; Lu, G.; Ning, J. Fatty acid degradation driven by heat during ripening contributes to the formation of the “Keemun aroma”. Food Chem. 2024, 451, 139458. [Google Scholar] [CrossRef]
- Guo, D.; Wan, P.; Liu, J.; Chen, D.-W. Use of egg yolk phospholipids to boost the generation of the key odorants as well as maintain a lower level of acrylamide for vacuum fried French fries. Food Control 2021, 121, 107592. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Xie, J.; Xiao, Q.; Cheng, J.; Chen, F.; Wang, S.; Sun, B. Formation mechanism of aroma compounds in a glutathione-glucose reaction with fat or oxidized fat. Food Chem. 2019, 270, 436–444. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, X.-H.; Zhang, Y.-Y.; Nie, C.; Zhou, D.; Qin, L. Mechanism of salt effect on flavor formation in lightly-salted large yellow croaker by integrated multiple intelligent sensory and untargeted lipidomics analyses. Food Chem. 2024, 435, 137542. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, L.; Liu, K.; Wang, J.; Gao, R.; Zhao, Y.; Bai, F.; Li, Y.; Wu, J.; Zeng, M.; et al. Flavor formation analysis based on sensory profiles and lipidomics of unrinsed mixed sturgeon surimi gels. Food Chem. X 2023, 17, 100534. [Google Scholar] [CrossRef]
- Song, G.; Zeng, M.; Chen, S.; Lyu, Z.; Jiang, N.; Wang, D.; Yuan, T.; Li, L.; Mei, G.; Shen, Q.; et al. Exploring molecular mechanisms underlying changes in lipid fingerprinting of salmon (Salmo salar) during air frying integrating machine learning-guided REIMS and lipidomics analysis. Food Chem. 2024, 460, 140770. [Google Scholar] [CrossRef]
- Cao, Q.; Fan, X.; Xu, J.; Shi, Z.; Wang, W.; Wang, Z.; Sun, Y.; Xia, Q.; Zhou, C.; Pan, D. Insights into the molecular mechanisms of lipid metabolism of air-dried goose on the formation of flavor substances by co-inoculation of lactic acid bacteria and staphylococcus based on GC-MS and lipidomics. Food Chem. 2025, 463, 141388. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, S.; Feng, Y.; Jiang, Y.; Yuan, H.; Shan, X.; Zhang, Q.; Niu, L.; Wang, S.; Zhou, Q.; et al. Seasonal variation in non-volatile flavor substances of fresh tea leaves (Camellia sinensis) by integrated lipidomics and metabolomics using UHPLC-Q-Exactive mass spectrometry. Food Chem. 2025, 462, 140986. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wan, P.; Liu, J.; Yao, J.; Chen, D.-W. Investigation on the changes of carotenoids and capsaicinoids in chili oil at different frying temperature by using 1H NMR. Curr. Res. Food Sci. 2023, 6, 100411. [Google Scholar] [CrossRef]
- Yang, F.; Yuan, H.; Jia, H.; Deng, F.; Wang, Z. Effects of chili varieties on physicochemical properties and flavor compounds of chili oil based on GC-IMS combined with multivariate statistical methods. Food Ferment. Ind. 2023, 49, 319–328. [Google Scholar] [CrossRef]
- Xiong, Y.; Baozhu, W.; Tianyang, W.; Lian, Y.; Yuwen, Y.; Huachang, W.; Jing, D. Effect of NaCl concentration on flavor profile of aromatic yeast YC14 using headspace solid-phase microextraction-gas chromatography-mass spectrometry, gas chromatography-ion mobility spectrometry, and E-nose. Food Ferment. Ind. 2024, 50, 282–289. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Qin, S.; Li, J.; Tang, D.; Xi, B. GC-IMS and multivariate analyses of volatile organic components in different Chinese breeds of chickens. Heliyon 2024, 10, e29664. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, Y.; Saleh, A.S.M.; Yang, X.; Ma, J.; Gao, Z.; Zhang, D.; Li, W.; Wang, Z. Characterization of aroma profiles of Chinese four most famous traditional red-cooked chickens using GC–MS, GC-IMS, and E-nose. Food Res. Int. 2023, 173, 113335. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Wang, Q.; Yang, L.; Karrar, E.; Jin, Q.; Zhang, H.; Wu, G.; Wang, X. Capsaicinoids and volatile flavor compounds profile of Sichuan hotpot as affected by cultivar of chili peppers during processing. Food Res. Int. 2023, 165, 112476. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, S.; Jung, M. Transforming roasted sesame oil into oil-impregnated powder: Avant-garde plating technique in molecular cooking - Mechanisms, physicochemical properties, and quantitative release of odorant-active compounds. LWT 2024, 191, 115654. [Google Scholar] [CrossRef]
- Ren, H.; Peng, D.; Liu, M.; Wang, Y.; Li, Z.; Zhao, H.; Zheng, Y.; Liu, Y.; Feng, X. Dynamic changes in chemical components, volatile profile and antioxidant properties of Xanthoceras sorbifolium leaf tea during manufacturing process. Food Chem. 2025, 468, 142409. [Google Scholar] [CrossRef]
- Ping, C.; Liu, Y.; Bi, J.; Cai, X.; Li, X.; Qiao, M. Identification of characteristic flavor quality of ceramic-pot sealed meat after reheating based on HS-GC-IMS, bionic sensory combined chemometrics. Food Chem. X 2024, 23, 101640. [Google Scholar] [CrossRef]
- Feng, S.; Li, P.; Li, Y.; Xiao, X.; Chen, X.; Leng, H.; Liang, H.; Zhou, L.; Chen, T.; Ding, C. Volatile profiles and characteristic odorants in camellia seeds with different heat pretreatments. Food Chem. 2025, 468, 142497. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Dong, L.; Zhong, S.; Jing, H.; Deng, Z.; Wen, Q.; Li, J. Chemical composition of Camellia chekiangoleosa Hu. seeds during ripening and evaluations of seed oils quality. Ind. Crops Prod. 2022, 177, 114499. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pang, Y.; Shen, G.; Bai, B.; Yang, Y.; Zeng, M. Identification and selection of volatile compounds derived from lipid oxidation as indicators for quality deterioration of frozen white meat and red meat using HS-SPME-GC–MS combined with OPLS-DA. Food Chem. 2025, 463, 141112. [Google Scholar] [CrossRef]
- Dong, M.; Zhou, Z.; Wang, B.; Zhang, Y.; Huang, X.; Qin, L. Investigating the quality discrepancy between different salmon and tracing the key lipid precursors of roasted flavor. Food Chem. 2025, 463, 141452. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wu, D.; Nie, J.; Li, X.; Guo, Y.; Huang, Q. Unlocking aroma in three types of vinasse fish by sensomics approach. Food Chem. 2024, 460, 140496. [Google Scholar] [CrossRef]
- Wang, J.; Che, J.; Wang, X.-S.; Qin, L.; Huang, X.-H. Tea marinating-induced improvement of quality in roasted chicken: The potential relationship between tea, flavor, and hazardous substances. Food Chem. X 2024, 24, 102033. [Google Scholar] [CrossRef]
- Giri, A.; Khummueng, W.; Mercier, F.; Kondjoyan, N.; Tournayre, P.; Meurillon, M.; Ratel, J.; Engel, E. Relevance of two-dimensional gas chromatography and high resolution olfactometry for the parallel determination of heat-induced toxicants and odorants in cooked food. J. Chromatogr. A 2015, 1388, 217–226. [Google Scholar] [CrossRef]
- Du, W.; Zhao, M.; Zhen, D.; Tan, J.; Wang, T.; Xie, J. Key aroma compounds in Chinese fried food of youtiao. Flavour. Fragr. J. 2020, 35, 88–98. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, C.; Wang, C.; Chen, H.; Liu, Y.; Li, S.; Lin, D.; Wu, D.; Qin, W. Evaluation of the non-aldehyde volatile compounds formed during deep-fat frying process. Food Chem. 2018, 243, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Hu, J.; Wang, L.; Yi, Y.; Deng, J.; He, L. Analysis on the effect of oil temperature on volatile compounds of Jiaoma paste based on SPME-GC-MS. China Condiment 2022, 47, 182–188. [Google Scholar] [CrossRef]
- Yao, W.; Cai, Y.; Liu, D.; Chen, Y.; Li, J.; Zhang, M.; Chen, N.; Zhang, H. Analysis of flavor formation during production of Dezhou braised chicken using headspace-gas chromatography-ion mobility spec-trometry (HS-GC-IMS). Food Sci. 2022, 370, 130989. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhong, M.; Feng, L.; Huo, Y.; Pan, L. Evaluation of flavor characteristics in tartary buckwheat (Fagopyrum tataricum) by E-nose, GC-IMS, and HS-SPME-GC-MS: Influence of different roasting temperatures. LWT 2024, 191, 115672. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Parker, J.K. 8—Thermal generation or aroma. In Flavour Development, Analysis and Perception in Food and Beverages; Parker, J.K., Elmore, J.S., Methven, L., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 151–185. [Google Scholar]
- Dignac, M.F.; Houot, S.; Derenne, S. How the polarity of the separation column may influence the characterization of compost organic matter by pyrolysis-GC/MS. J. Anal. Appl. Pyrolysis 2006, 75, 128–139. [Google Scholar] [CrossRef]
- Liu, D.; Bai, L.; Feng, X.; Chen, Y.P.; Zhang, D.; Yao, W.; Zhang, H.; Chen, G.; Liu, Y. Characterization of Jinhua ham aroma profiles in specific to aging time by gas chromatography-ion mobility spectrometry (GC-IMS). Meat Sci. 2020, 168, 108178. [Google Scholar] [CrossRef]
- Yin, J.; Wu, M.; Lin, R.; Li, X.; Ding, H.; Han, L.; Yang, W.; Song, X.; Li, W.; Qu, H.; et al. Application and development trends of gas chromatography–ion mobility spectrometry for traditional Chinese medicine, clinical, food and environmental analysis. Microchem. J. 2021, 168, 106527. [Google Scholar] [CrossRef]
- Wei, S.; Wei, L.; Xie, B.; Li, J.; Lyu, J.; Wang, S.; Khan, M.A.; Xiao, X.; Yu, J. Characterization of volatile profile from different coriander (Coriandrum sativum L.) varieties via HS-SPME/GC–MS combined with E-nose analyzed by chemometrics. Food Chem. 2024, 457, 140128. [Google Scholar] [CrossRef] [PubMed]
- Ping, C.; Deng, X.; Guo, Z.; Luo, W.; Li, X.; Xin, S. Characterizing the flavor profiles of Linjiangsi broad bean (Vicia faba L.) paste using bionic sensory and multivariate statistics analyses based on ripening time and fermentation environment. Food Chem. X 2024, 23, 101677. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yu, Y.; Wang, Z.; Akhtar, K.H.; Saleh, A.S.M.; Li, W.; Zhang, D. Insights into flavor formation of braised chicken: Based on E-nose, GC–MS, GC-IMS, and UPLC-Q-Exactive-MS/MS. Food Chem. 2024, 448, 138972. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Zhu, L. Tea quality evaluation by applying E-nose combined with chemometrics methods. J. Food Sci. Technol. 2021, 58, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zeng, Y.; Bai, Y.; Zhang, L. Analysis of key volatile flavour compounds in chilli oil by odoraetivity value combined with gas chromatography-olfactometry. Food Ferment. Ind. 2024, 50, 286–292. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.; Qiu, X.; Yang, S.; Chen, F.; Ni, H.; Li, Q. Comparison of potent odorants in raw and cooked mildly salted large yellow croaker using odor-active value calculation and omission test: Understanding the role of cooking method. Food Chem. 2023, 402, 134015. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Chen, J.; Liu, D.; Ye, X.; Cheng, H. Chemometric Identification of Volatile Components in Essential Oils Extracted from Sweet Orange Peels by Different Methods. Food Sci. 2024, 45, 155–163. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Jia, H.; Xu, C.; Yuan, H. Comparison of the flavor qualities of chili oils prepared from different types of vegetable oil. Mod. Food Sci. Technol. 2024, 40, 338–350. [Google Scholar] [CrossRef]
- Multari, S.; Marsol-Vall, A.; Heponiemi, P.; Suomela, J.-P.; Yang, B. Changes in the volatile profile, fatty acid composition and other markers of lipid oxidation of six different vegetable oils during short-term deep-frying. Food Res. Int. 2019, 122, 318–329. [Google Scholar] [CrossRef]
- Dong, T.; Tian, Z.; Wang, S.; Sun, J.; Chen, H.; Wang, S.; Sun, B. Identification of key off-flavor compounds during storage of fried pepper (Zanthoxylum bungeanum Maxim.) oils by sensory-directed flavor analysis and partial least squares regression (PLSR). J. Food Compos. Anal. 2024, 131, 106268. [Google Scholar] [CrossRef]
- Zhang, K.; Hao, R.; Wang, S.; Zhang, Z.; Li, D.; Li, X.; Zhao, B.; Zhang, S.; Zhao, Y.; Chen, X. Correlation of lipid hydrolysis, oxidation, and molecular transformation with volatile compound revolution in pork during postmortem wet-aging process. Food Chem. 2025, 470, 142656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, Y.; Xia, W.; Yu, D.; Wang, B.; Xu, J. Insight into the role of lipids in odor changes of frozen grass carp (Ctenopharyngodon idella) based on lipidomics and GC–MS analysis: Impact of freeze-thaw cycles and heat treatment. Food Chem. 2024, 459, 140436. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Huang, Y.; Li, X.; Luo, M.; Lin, H.; Tang, H.; Jiang, H.; Fu, Q.; Yuan, Y. Investigation of the physicochemical properties and the chemical components of chili rapeseed oil via different preparation oil temperatures. LWT 2024, 204, 116432. [Google Scholar] [CrossRef]
- Al-Dalali, S.; Li, C.; Xu, B. Insight into the effect of frozen storage on the changes in volatile aldehydes and alcohols of marinated roasted beef meat: Potential mechanisms of their formation. Food Chem. 2022, 385, 132629. [Google Scholar] [CrossRef]
- Wei, X.; Wei, Y.; Xue, J.; Zhang, X.; Shao, X. Effect of High-Temperature Heating on Fatty Acid Composition and Physicochemical Properties of Peony Seed Oil. Food Sci. 2018, 39, 15–20. [Google Scholar] [CrossRef]
- Zheng, Z.; Tian, M.; Liao, G.; Chen, G.; Zhong, Y.; Yang, Y.; Wang, G. Evaluation of the effects of compound curing agents on the lipid profiles and volatile flavors in Nuodeng ham based on lipidomics and GC-IMS analysis. Food Res. Int. 2024, 176, 113810. [Google Scholar] [CrossRef]
- Ujong, A.E.; Emelike, N.J.T.; Owuno, F.; Okiyi, P.N. Effect of frying cycles on the physical, chemical and antioxidant properties of selected plant oils during deep-fat frying of potato chips. Food Chem. Adv. 2023, 3, 100338. [Google Scholar] [CrossRef]
- Pan, L.; Xu, W.; Gao, Y.; Ouyang, H.; Liu, X.; Wang, P.; Yu, X.; Xie, T.; Li, S. Exploring the lipid oxidation mechanisms during pumpkin seed kernels storage based on lipidomics: From phenomena, substances, and metabolic mechanisms. Food Chem. 2024, 455, 139808. [Google Scholar] [CrossRef]
- Gao, C.; Li, Q.; Wen, H.; Zhou, Y. Lipidomics analysis reveals the effects of Schizochytrium sp. supplementation on the lipid composition of Tan sheep meat. Food Chem. 2025, 463, 141089. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Wang, J.; Zhang, X.; Leng, Y.; Wang, T.; Liu, W.; Wei, C. Investigation of the oxidation rules and oxidative stability of seabuckthorn fruit oil during storage based on lipidomics and metabolomics. Food Chem. 2025, 476, 143238. [Google Scholar] [CrossRef]
- Yu, X.; Li, B.; Ouyang, H.; Xu, W.; Zhang, R.; Fu, X.; Gao, S.; Li, S. Exploring the oxidative rancidity mechanism and changes in volatile flavors of watermelon seed kernels based on lipidomics. Food Chem. X 2024, 21, 101108. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Hui, T.; Ma, J. Generation of key aroma compounds in Beijing roasted duck induced via Maillard reaction and lipid pyrolysis reaction. Food Res. Int. 2020, 136, 109328. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Li, L.; Feng, J.; Dai, Z.; Huang, Y.-W.; Shen, Q. Zwitterionic hydrophilic interaction solid-phase extraction and multi-dimensional mass spectrometry for shotgun lipidomic study of Hypophthalmichthys nobilis. Food Chem. 2017, 216, 347–354. [Google Scholar] [CrossRef]
- Yuan, B.; Chen, Y.; Zhou, Q.; Deng, Q. Advances in the study of volatile flavor substances in flaxseed oil. Food Sci. Technol. 2023, 44, 290–298. [Google Scholar] [CrossRef]


| Count | Compounds | CAS | RI | Rt [Sec] | Dt [A.U.] | Signal Strength of Chili Oil Samples | |||
|---|---|---|---|---|---|---|---|---|---|
| 210 | 180 | 150 | Triple | ||||||
| 1 | 2-Methylpyrazine | C109080 | 1305.5 | 1049.813 | 1.07746 | 4101.05 ± 367.12 a | 3075.07 ± 86.75 b | 1964.86 ± 72.03 d | 2460.33 ± 122.13 c |
| 2 | Cyclohexanone | C108941 | 1304.8 | 1047.828 | 1.15969 | 777.05 ± 98.56 a | 718.52 ± 9.08 ab | 645.73 ± 11.46 b | 729.92 ± 9.03 a |
| 3 | (E)-2-Heptenal-M | C18829555 | 1324.1 | 1105.601 | 1.25768 | 2319.61 ± 48.9 c | 2678.96 ± 27.96 a | 2546.42 ± 33.28 b | 2570.76 ± 27.33 b |
| 4 | (E)-2-Heptenal-D | C18829555 | 1322.4 | 1100.43 | 1.66918 | 393.67 ± 14.83 d | 525.6 ± 12.54 a | 461.44 ± 11.33 c | 498.45 ± 10.83 b |
| 5 | 2-methyl-3-ketotetrahydrofuran-M | C3188009 | 1289.8 | 1001.287 | 1.07903 | 3161.42 ± 162.83 b | 3469.44 ± 39.28 a | 3126 ± 14.86 b | 3022.21 ± 80.18 b |
| 6 | 2-methyl-3-ketotetrahydrofuran-D | C3188009 | 1293.4 | 1013.221 | 1.42265 | 420.72 ± 9.81 c | 527.24 ± 31.32 a | 459.64 ± 11.06 b | 482.63 ± 23.9 b |
| 7 | 2-Octanone | C111137 | 1290.3 | 1002.98 | 1.32266 | 675.48 ± 34.54 b | 816.75 ± 68.04 a | 676.65 ± 28.62 b | 623.96 ± 41.22 b |
| 8 | (E)-3-Hexen-1-ol | C928972 | 1304.9 | 1048.192 | 1.23114 | 431.53 ± 68.5 a | 228.16 ± 22.76 b | 116.58 ± 3.36 c | 172.71 ± 15.69 bc |
| 9 | 2-Hexanol | C626937 | 1281.1 | 972.99 | 1.28301 | 348.04 ± 23.95 a | 251.03 ± 11.37 b | 189.81 ± 9.03 c | 231.89 ± 7.49 b |
| 10 | 1-octanal | C124130 | 1280.5 | 971.2 | 1.39002 | 529.88 ± 24.48 a | 346.03 ± 13.12 b | 200.91 ± 10.30 d | 310.3 ± 14.86 c |
| 11 | 1-Octen-3-one | C4312996 | 1305 | 1048.361 | 1.27791 | 197.65 ± 16.12 a | 164.8 ± 22.17 b | 122.2 ± 3.79 c | 158.67 ± 13.87 b |
| 12 | 2,4-Dimethylthiazole | C541582 | 1267.8 | 929.822 | 1.0936 | 225.04 ± 9.04 a | 179.02 ± 8.17 b | 136.38 ± 7.58 c | 132.09 ± 10.47 c |
| 13 | 1-Pentanol-M | C71410 | 1256.6 | 893.263 | 1.25483 | 2146.95 ± 57.86 d | 3207.4 ± 28.49 c | 4003.41 ± 29.69 a | 3697.2 ± 54.22 b |
| 14 | 1-Pentanol-D | C71410 | 1256 | 891.335 | 1.51987 | 128.38 ± 3.33 d | 342.34 ± 10.71 c | 573.7 ± 13.00 a | 508.72 ± 10.79 b |
| 15 | 3-Methylbutyl 2-methylbutanoate | C27625350 | 1256.1 | 891.875 | 1.41854 | 178.3 ± 20.68 d | 284.31 ± 10.74 c | 374.39 ± 3.36 b | 395.03 ± 8.29 a |
| 16 | 2-Hexenal-M | C505577 | 1217.9 | 767.392 | 1.18068 | 556.03 ± 49.77 c | 822.58 ± 16.29 b | 863.82 ± 11.55 ab | 893.17 ± 11.87 a |
| 17 | 2-Hexenal-D | C505577 | 1217.8 | 767.294 | 1.51277 | 67.64 ± 3.01 d | 130.25 ± 4.51 a | 120.99 ± 1.06 b | 109.57 ± 2.54 c |
| 18 | 1-Butanol, 3-methyl-M | C123513 | 1207.9 | 734.99 | 1.24247 | 815.02 ± 113.44 a | 598.9 ± 24.5 b | 589.12 ± 14.8 b | 530.23 ± 24.31 b |
| 19 | (+)-limonene-M | C138863 | 1200.7 | 711.651 | 1.2158 | 161.75 ± 3.09 b | 202.93 ± 7.12 a | 203.42 ± 2.95 a | 152.16 ± 3.46 c |
| 20 | (+)-limonene-D | C138863 | 1200.5 | 710.778 | 1.30166 | 574.11 ± 15.52 d | 676.87 ± 9.56 b | 817.6 ± 7.96 a | 594.21 ± 11.09 c |
| 21 | β-ocimene | C13877913 | 1193.3 | 687.467 | 1.21933 | 360.9 ± 12.29 b | 390.48 ± 11.77 a | 385.86 ± 8.83 a | 295.66 ± 1.27 c |
| 22 | p-cymene | C99876 | 1193.1 | 686.726 | 1.30079 | 248.4 ± 16.86 a | 253.6 ± 5.95 a | 249.63 ± 1.58 a | 197.17 ± 4.85 b |
| 23 | 1-Butanol, 3-methyl-D | C123513 | 1206.9 | 731.873 | 1.48993 | 270.01 ± 38.97 a | 243.64 ± 6.20 ab | 246.05 ± 16.54 ab | 229.81 ± 6.46 b |
| 24 | Heptaldehyde-M | C111717 | 1185.8 | 668.993 | 1.34474 | 1045.7 ± 15.34 c | 1431.67 ± 43.3 a | 1252.81 ± 20.03 b | 1254.03 ± 13.79 b |
| 25 | Heptaldehyde-D | C111717 | 1185.8 | 669.016 | 1.69011 | 98.14 ± 7.73 c | 170.15 ± 15.97 b | 133.1 ± 0.75 b | 133.02 ± 1.30 a |
| 26 | 3-Methyl-3-buten-1-ol | C763326 | 1182.4 | 661.423 | 1.16731 | 243.11 ± 17.13 c | 299.67 ± 13.13 b | 317.9 ± 20.63 a | 353.33 ± 10.08 a |
| 27 | ethyl (E)-2-butenoate | C623701 | 1139.8 | 563.984 | 1.18187 | 434.99 ± 20.05 d | 552.28 ± 17.38 b | 586.16 ± 8.86 a | 598.6 ± 13.65 c |
| 28 | (E)-2-Pentenal-M | C1576870 | 1129.9 | 541.439 | 1.10678 | 1071.6 ± 26.25 d | 1972.47 ± 11.75 b | 2258.29 ± 7.77 a | 1840.85 ± 11.87 c |
| 29 | (E)-2-Pentenal-D | C1576870 | 1129.2 | 539.777 | 1.35191 | 141.19 ± 5.39 ab | 457.17 ± 9.18 ab | 610.72 ± 9.14 b | 436.73 ± 6.72 a |
| 30 | 2-Pentanol | C6032297 | 1115.7 | 509.044 | 1.21348 | 187.26 ± 0.73 d | 220.61 ± 2.10 b | 237.07 ± 8.70 a | 193.01 ± 2.87 c |
| 31 | methyl pentanoate | C624248 | 1104.7 | 483.858 | 1.21608 | 85.68 ± 6.73 a | 131.25 ± 3.56 b | 137.84 ± 2.4 d | 98.38 ± 1.09 c |
| 32 | cyclopentanone | C120923 | 1100.7 | 474.81 | 1.09289 | 95.72 ± 2.38 c | 82.93 ± 1.62 d | 64.25 ± 2.12 a | 71.48 ± 0.58 b |
| 33 | 1-Propanol, 2-methyl-M | C78831 | 1094.1 | 462.814 | 1.17339 | 499.31 ± 21.58 c | 442.21 ± 7.62 b | 548.64 ± 5.37 a | 528.97 ± 7.44 b |
| 34 | 2-Methylpropyl propionate | C540421 | 1087.1 | 454.347 | 1.27644 | 2253.63 ± 25.34 a | 2847.17 ± 11.47 c | 2915.57 ± 12.06 d | 2765.34 ± 25.77 b |
| 35 | 1-Propanol, 2-methyl-D | C78831 | 1087.5 | 454.838 | 1.36941 | 183.51 ± 5.44 d | 229.49 ± 3.72 c | 248.04 ± 7.63 a | 228.19 ± 2.66 b |
| 36 | Acetic acid butyl ester | C123864 | 1059.7 | 420.914 | 1.24241 | 93.78 ± 8.96 d | 35.99 ± 1.4 c | 22.55 ± 1.04 a | 44.75 ± 1.66 b |
| 37 | Camphene | C79925 | 1044.6 | 402.469 | 1.19839 | 180.7 ± 7.37 d | 358.9 ± 3.33 c | 578.71 ± 9.07 a | 422.28 ± 6.84 b |
| 38 | 2-Methylthiophene | C554143 | 1044.9 | 402.86 | 1.037 | 784.11 ± 29.35 c | 1028.88 ± 0.68 b | 1266.67 ± 22.29 a | 1062.96 ± 3.50 a |
| 39 | 1-Propanol | C71238 | 1034.3 | 389.908 | 1.1132 | 82.79 ± 6.15 d | 141.97 ± 1.51 c | 201.64 ± 3.12 a | 174.81 ± 7.93 b |
| 40 | 2-methylpropyl 2-methylpropanoate | C97858 | 1044.3 | 402.142 | 1.30628 | 34.65 ± 2.52 d | 61.81 ± 6.82 c | 82.42 ± 5.95 b | 89.8 ± 6.51 a |
| 41 | 1-Penten-3-one-M | C1629589 | 1024.5 | 377.974 | 1.08163 | 467.39 ± 8.62 d | 818.54 ± 6.91 c | 989.66 ± 6.15 a | 844.12 ± 17.09 b |
| 42 | 1-Penten-3-one-D | C1629589 | 1024 | 377.394 | 1.30766 | 116.49 ± 6.85 c | 344.56 ± 4.24 b | 465.44 ± 7.35 a | 339.51 ± 2.01 b |
| 43 | n-Pentanal-M | C110623 | 990.5 | 340.009 | 1.196 | 1249.3 ± 38.17 b | 1248.54 ± 8.91 b | 1317.04 ± 11.52 a | 1254.42 ± 8.37 b |
| 44 | n-Pentanal-D | C110623 | 989.9 | 339.661 | 1.42194 | 858.62 ± 31.03 d | 2117.34 ± 28.65 b | 2272.33 ± 10.31 a | 1927.94 ± 28.04 c |
| 45 | Ethanol-M | C64175 | 935.4 | 308.331 | 1.04246 | 932.89 ± 45.48 c | 1012.08 ± 29.09 b | 1215.26 ± 28.15 a | 1005.31 ± 1.74 b |
| 46 | Ethanol-D | C64175 | 929.9 | 305.223 | 1.13009 | 624.97 ± 54.34 d | 767.03 ± 14.06 c | 1183.95 ± 16.76 a | 859.8 ± 5 b |
| 47 | 3-Methyl butanal | C590863 | 926.4 | 303.177 | 1.17748 | 176.05 ± 9.74 d | 197.59 ± 5.33 c | 253.62 ± 1.26 a | 229.49 ± 1.32 b |
| 48 | 1,1-diethoxy ethane | C105577 | 890.6 | 282.623 | 1.04629 | 330.27 ± 34.81 a | 266.04 ± 12.44 b | 253.46 ± 6.52 b | 221.97 ± 4.4 c |
| 49 | Butanal-M | C123728 | 886.6 | 280.325 | 1.12814 | 446.44 ± 25.33 c | 502.39 ± 8.38 b | 550.23 ± 3.28 a | 533.93 ± 5.27 a |
| 50 | Butanal-D | C123728 | 886.1 | 280.014 | 1.28234 | 127.97 ± 8.91 d | 249.64 ± 7.41 c | 308.59 ± 1.83 a | 282.97 ± 4.32 b |
| 51 | Tetrahydrofuran | C109999 | 866.1 | 268.521 | 1.06058 | 96.39 ± 6.69 d | 109.87 ± 2.91 c | 387.22 ± 7.59 a | 189.19 ± 2.03 b |
| 52 | Methyl acetate | C79209 | 854.2 | 261.688 | 1.02974 | 103.78 ± 2.6 d | 155.97 ± 3.00 c | 223.36 ± 1.38 a | 173.43 ± 1.36 b |
| 53 | acrolein | C107028 | 836.7 | 251.653 | 1.06861 | 1098.11 ± 9.63 c | 1085.76 ± 18.94 c | 1193.93 ± 6.97 a | 1172.34 ± 5.6 b |
| 54 | Ethyl formate | C109944 | 817.1 | 240.333 | 1.08588 | 300.26 ± 43.52 b | 365.94 ± 6.05 a | 311.61 ± 10.30 b | 290.63 ± 15.18 b |
| 55 | 1-octene | C111660 | 865.6 | 268.246 | 1.17378 | 189.96 ± 15.52 a | 101.87 ± 3.27 c | 80.84 ± 0.94 d | 127.26 ± 1.75 b |
| 56 | Ethyl 2-methy lpropionate | C97621 | 950.8 | 317.19 | 1.18463 | 366.46 ± 15.02 a | 335.49 ± 6.7 b | 250.16 ± 1.01 d | 294.98 ± 3.63 c |
| 57 | α-terpinolene | C586629 | 1280.9 | 972.566 | 1.21701 | 2461.53 ± 19.48 a | 1944.87 ± 16.09 b | 1410.12 ± 11.20 d | 1751.1 ± 9.10 c |
| 58 | 3-Methyl-2-butenal | C107868 | 1202.7 | 717.938 | 1.09149 | 78.43 ± 1.32 b | 80.77 ± 2.26 b | 88.24 ± 2.92 a | 82.28 ± 3.96 b |
| 59 | Acetic acid-M | C64197 | 1505 | 1648.066 | 1.05986 | 8419.01 ± 235.96 a | 8657.26 ± 38.71 a | 8059.46 ± 44.68 b | 8567.82 ± 208.41 a |
| 60 | Acetic acid-D | C64197 | 1503.7 | 1644.151 | 1.16857 | 6709.72 ± 617.07 c | 8242.27 ± 254.43 b | 9570.86 ± 72.06 a | 8011.48 ± 446.27 b |
| 61 | 1-Penten-3-ol | C616251 | 1157.2 | 603.728 | 0.9416 | 981.2 ± 29.22 d | 2018.38 ± 35.05 c | 2639.41 ± 19.07 a | 2339.27 ± 21.39 b |
| 62 | 2-methyl butanal-D | C96173 | 920.1 | 299.568 | 1.40038 | 7707.64 ± 60.23 a | 7201.29 ± 36.32 b | 5617.37 ± 34.45 c | 7155.32 ± 32.07 b |
| 63 | 2-methyl butanal-M | C96173 | 912.2 | 294.997 | 1.16278 | 176.26 ± 9.09 d | 235.59 ± 3.92 c | 331.1 ± 4.38 a | 255.76 ± 4.01 b |
| No. | Compounds | CAS | Content (μg/kg) | |||
|---|---|---|---|---|---|---|
| 210 | 180 | 150 | Triple | |||
| Aldehydes | ||||||
| A1 | 2-Methyl-butanal | 96-17-3 | 260.50 ± 10.81 a | 90.50 ± 14.04 b | 20.25 ± 5.05 c | ND |
| A2 | 2,2-Dimethyl-propanal | 630-19-3 | 30.40 ± 4.09 b | ND | ND | 40.41 ± 5.05 a |
| A3 | (E)-2-Methyl-2-butenal | 497-03-0 | 10.65 ± 2.05 | ND | ND | ND |
| A4 | (E)-2-Pentenal | 1576-87-0 | 40.52 ± 8.09 a | ND | 20.52 ± 4.05 b | ND |
| A5 | Hexanal | 66-25-1 | 10.32 ± 3.03 | ND | ND | ND |
| A6 | Furfural | 98-01-1 | 70.55 ± 12.07 a | ND | 60.25 ± 8.10 a | 10.53 ± 1.04 b |
| A7 | (Z)-3-Hexenal | 6789-80-6 | 90.47 ± 15.09 a | 10.25 ± 2.04 c | 10.52 ± 2.08 c | 50.48 ± 6.07 b |
| A8 | Heptanal | 111-71-7 | 10.69 ± 3.12 b | 40.35 ± 8.05 a | ND | 10.58 ± 3.04 b |
| A9 | (E)-2-Heptenal | 18829-55-5 | 20.14 ± 5.06 a | 10.72 ± 3.03 b | ND | ND |
| A10 | 2,4-Nonadienal | 6750-03-4 | 60.15 ± 10.05 a | 10.35 ± 2.04 b | 10.75 ± 2.02 b | 10.53 ± 1.02 b |
| A11 | (E,E)-2,4-Heptadienal | 4313-03-5 | 20.85 ± 6.04 | ND | ND | ND |
| A12 | Octanal | 124-13-0 | 20.75 ± 5.01 b | 30.81 ± 5.06 a | ND | ND |
| A13 | Nonanal | 124-19-6 | 20.45 ± 2.02 c | 40.52 ± 6.45 a | ND | 30.85 ± 6.04 b |
| A14 | 2-Hexenal | 505-57-7 | 130.24 ± 15.10 b | 40.85 ± 7.03 c | ND | 490.96 ± 12.44 a |
| A15 | 2-methyl-Pentanal | 123-15-9 | 10.63 ± 3.04 b | 40.48 ± 6.06 a | ND | 10.63 ± 2.03 b |
| A16 | 2-methyl-2-Butenal | 107-86-8 | 80.75 ± 12.11 a | 90.78 ± 10.18 a | 90.85 ± 9.16 a | 10.85 ± 2.04 b |
| A17 | 2,4-Decadienal | 2363-88-4 | ND | 60 ± 12.09 a | 10.39 ± 2.04 b | 10.85 ± 1.03 b |
| A18 | Benzaldehyde | 100-52-7 | ND | ND | 10.35 ± 1.03 | ND |
| A19 | Benzeneacetaldehyde | 122-78-1 | 20.25 ± 4.04 a | 10.24 ± 4.04 b | ND | 10 ± 1.02 b |
| A20 | Trans-2-Decenal | 3913-81-3 | 150.47 ± 10.21 a | 160.36 ± 10.30 a | ND | 160.59 ± 10.28 a |
| Total | 1050.41 ± 117.94 a | 630.25 ± 95.01 c | 230.68 ± 33.53 d | 840.65 ± 54.10 b | ||
| Ketones | ||||||
| B1 | 2-Butanone | 78-93-3 | 10.14 ± 1.04 d | 190.58 ± 20.23 b | 20.52 ± 6.05 c | 390.53 ± 15.42 a |
| B2 | 2,2-Dimethyl-3-heptanone | 19078-97-8 | 220.74 ± 18.31 a | 180.28 ± 15.25 b | ND | ND |
| B3 | 2,5-Dimethyl-3-hexanone | 1888-57-9 | 60.41 ± 8.09 a | ND | 10.52 ± 2.02 b | ND |
| B4 | 2-Methyl-4-heptanone | 626-33-5 | 1060.52 ± 21.22 a | 20.75 ± 7.04 c | 20.41 ± 5.06 c | 90.53 ± 10.14 b |
| B5 | 2,4-Dimethyl-3-pentanone | 565-80-0 | 190.89 ± 15.24 a | 20.24 ± 7.04 c | 70.52 ± 10.12 b | 10.85 ± 2.03 d |
| B6 | 5-Methyl-3-hexanone | 623-56-3 | 10.14 ± 1.02 a | ND | ND | 10.53 ± 1.05 a |
| B7 | 3-Heptanone | 106-35-4 | 50.72 ± 4.12 a | ND | 40.52 ± 3.08 b | 10.53 ± 1.04 c |
| B8 | 4-Methyl-3-heptanone | 6137-11-7 | 50.53 ± 6.14 a | ND | 40.52 ± 5.13 b | 10.56 ± 2.04 c |
| B9 | 5-Nonanone | 502-56-7 | 40.71 ± 5.14 b | 90.24 ± 7.18 a | 10.32 ± 1.04 c | ND |
| Total | 1690.15 ± 80.32 a | 500.28 ± 49.70 b | 210.21 ± 32.50 c | 520.23 ± 31.72 b | ||
| Esters | ||||||
| C1 | Propanoic acid, butyl ester | 590-01-2 | 30.52 ± 4.04 b | ND | 70.56 ± 6.15 a | 20.85 ± 3.06 c |
| C2 | Butanoic acid, methyl ester | 623-42-7 | 150.41 ± 10.31 | ND | ND | ND |
| C3 | 2-Butenoic acid, 3-methyl-, hexyl ester | 17627-41-7 | 10.62 ± 1.04 | ND | ND | ND |
| C4 | Propanoic acid, 2-methyl-, 2-methylpropyl ester | 97-85-8 | 10.25 ± 1.08 | ND | ND | ND |
| Total | 200.18 ± 16.47 a | ND | 70.53 ± 6.15 b | 20.32 ± 3.06 c | ||
| Alcohols | ||||||
| D1 | Ethanol | 64-17-5 | 10.41 ± 1.04 a | ND | ND | 10.53 ± 1.05 a |
| D2 | 2-methyl-1-Butanol | 137-32-6 | 190.72 ± 15.34 a | ND | 80.33 ± 8.23 b | ND |
| D3 | 3-Penten-2-ol | 1569-50-2 | 40.41 ± 3.08 a | ND | 10.53 ± 1.04 b | ND |
| D4 | 1-Butanol | 71-36-3 | 10.25 ± 1.06 | ND | ND | ND |
| D5 | 1-Pentanol | 71-41-0 | 10.71 ± 1.15 a | ND | ND | 10.11 ± 1.05 a |
| D6 | Methanethiol | 74-93-1 | 40.52 ± 4.08 a | 20.34 ± 2.06 b | ND | 10.52 ± 1.14 c |
| Total | 300.28 ± 25.75 a | 20.74 ± 2.06 d | 90.35 ± 9.27 b | 30.62 ± 2.19 c | ||
| Olefins | ||||||
| E1 | 2,4-Dimethyl- heptane | 2213-23-2 | 180.75 ± 10.33 a | 70.21 ± 5.24 c | ND | 90.85 ± 8.23 b |
| E2 | 3,4,5-Trimethyl- heptane | 20278-89-1 | 60.73 ± 5.14 a | 30.26 ± 3.13 b | ND | ND |
| E3 | 2,4,6-Trimethyl- heptane | 2613-61-8 | 80.70 ± 4.19 b | 100.34 ± 10.24 a | ND | ND |
| E4 | 1,1-Dimethoxy-2-butene | 21962-24-3 | 80.50 ± 6.18 | ND | ND | ND |
| E5 | D-Limonene | 5989-27-5 | 20.42 ± 3.08 | ND | ND | ND |
| E6 | 2,2-Dimethyl-3-Hexene | 3123-93-1 | 40.46 ± 4.14 b | 10.26 ± 1.04 c | 10.42 ± 1.05 c | 60.22 ± 5.18 a |
| E7 | 1,3-Octadiene | 1002-33-1 | 10.70 ± 1.04 | ND | ND | ND |
| E8 | Limonene | 138-86-3 | 10.42 ± 1.03 b | ND | ND | 1060 ± 22.04 a |
| E9 | α-Pinene | 80-56-8 | 30.46 ± 4.14 a | 10.46 ± 1.03 c | 10.53 ± 1.02 c | 20.55 ± 2.06 b |
| E10 | Camphene | 79-92-5 | ND | 10.36 ± 1.04 b | 30.88 ± 1.11 a | ND |
| E11 | Caryophyllene | 87-44-5 | 20.48 ± 3.05 a | 20.15 ± 2.04 a | 10.98 ± 1.03 b | 10.66 ± 0.09 b |
| E12 | α-Phellandrene | 99-83-2 | 30.85 ± 2.07 a | 10.25 ± 1.02 c | 10.78 ± 1.14 c | 20.23 ± 2.07 b |
| E13 | α-terpinolene | 586-62-9 | ND | 30.86 ± 2.12 a | 10.36 ± 1.09 b | ND |
| E14 | β-Ocimene | 13877-91-3 | 20.74 ± 2.06 b | 20.74 ± 2.04 b | 40.99 ± 4.13 a | 10.36 ± 1.15 c |
| Total | 580.41 ± 46.45 b | 310.86 ± 28.94 c | 120.55 ± 10.57 d | 1270.56 ± 32.59 a | ||
| Ethers | ||||||
| F1 | n-Butyl ether | 142-96-1 | ND | 10.75 ± 1.04 b | 20.78 ± 2.06 a | ND |
| F2 | Estragole | 140-67-0 | 150.41 ± 12.35 a | ND | 20.89 ± 3.06 b | ND |
| Total | 150.42 ± 12.35 a | 10.75 ± 1.04 c | 40.56 ± 5.12 b | ND | ||
| Acids | ||||||
| G1 | Acetic acid | 64-19-7 | 140.85 ± 10.34 a | 30.38 ± 2.19 b | 30.75 ± 2.10 b | 30.78 ± 2.08 b |
| G2 | Propanoic acid | 79-09-4 | 390.86 ± 14.80 a | 220.53 ± 10.34 b | 100.88 ± 10.30 c | 10.89 ± 1.04 d |
| G3 | Pentanoic acid | 109-52-4 | 10.96 ± 1.26 | ND | ND | ND |
| Total | 540.46 ± 26.40 a | 250.58 ± 12.53 b | 130.56 ± 12.40 c | 40.74 ± 3.12 d | ||
| Other compounds | ||||||
| H1 | Anethole | 4180-23-8 | 10.48 ± 2.04 | ND | ND | ND |
| H2 | 2-Propyl-furan | 4229-91-8 | 450.47 ± 11.00 a | 10.52 ± 1.25 b | 10.45 ± 1.14 b | 450.66 ± 11.02 a |
| H3 | Trans-Bergamotene | 13474-59-4 | 70.86 ± 6.15 | ND | ND | ND |
| H4 | p-Cymene | 99-87-6 | 10.18 ± 1.44 c | ND | 20.44 ± 2.16 b | 30.65 ± 4.10 a |
| Total | 540.25 ± 20.63 a | 10.25 ± 1.25 d | 30.47 ± 3.30 c | 480.53 ± 15.12 b | ||
| No. | Compounds | CAS | Threshold (In Oil) (mg/kg) | ROAV | Odor | |||
|---|---|---|---|---|---|---|---|---|
| 210 | 180 | 150 | Triple | |||||
| 1 | 2-methyl-butanal | 96-17-3 | 0.023 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 84.47 ± 1.74 b | - | malt |
| 2 | (E,E)-2,4-Heptadienal | 4313-03-5 | 0.01 | 15.00 ± 1.25 | - | - | - | fatty |
| 3 | Furfural | 98-01-1 | 3.00 | 0.20 ± 0.05 b | - | 1.62 ± 0.04 a | 0.05 ± 0.02 c | bread, almond, sweet popcorn |
| 4 | (E)-2-Heptenal | 18829-55-5 | 0.013 | 15.38 ± 1.36 a | 5.77 ± 0.07 b | - | - | fatty |
| 5 | 2,4-Nonadienal | 6750-03-4 | 0.005 | 105.00 ± 2.85 b | 15.00 ± 0.24 d | 194.29 ± 2.56 a | 21.51 ± 1.02 c | fatty, flower, green |
| 6 | Nonanal | 124-19-6 | 0.150 | 1.33 ± 0.15 c | 2.33 ± 0.04 b | - | 3.58 ± 0.25 a | fatty, citrus, green |
| 7 | 2,4-Decadienal | 2363-88-4 | 0.135 | - | 3.89 ± 0.06 b | 5.40 ± 0.25 a | 1.19 ± 0.24 c | citrus, chicken |
| 8 | Benzaldehyde | 100-52-7 | 0.060 | - | - | 12.14 ± 0.15 | - | nutty |
| 9 | Benzeneacetaldehyde | 122-78-1 | 0.022 | 9.09 ± 0.16 b | 3.41 ± 0.05 c | - | 9.78 ± 0.26 a | chocolate, cocoa |
| 10 | Trans-2-Decenal | 3913-81-3 | 3.220 | 130.00 ± 1.82 c | 137.50 ± 1.25 b | - | 295.70 ± 3.25 a | fatty, mushroom |
| 11 | D-Limonene | 5989-27-5 | 0.034 | 4.41 ± 0.52 | - | - | - | citrus, mint |
| 12 | Limonene | 138-86-3 | 0.200 | 0.38 ± 0.08 b | - | - | 100.00 ± 0.00 a | lemon, orange |
| 13 | α-Pinene | 80-56-8 | 0.006 | 41.67 ± 1.89 c | 8.33 ± 0.25 d | 80.95 ± 2.56 a | 71.68 ± 1.58 b | pine, turpentine |
| 14 | Caryophyllene | 87-44-5 | 0.390 | 0.45 ± 0.07 c | 0.38 ± 0.02 d | 1.87 ± 0.05 a | 0.69 ± 0.02 b | wood, spice |
| 15 | α-Phellandrene | 99-83-2 | 0.040 | 5.63 ± 0.45 c | 1.25 ± 0.04 d | 18.21 ± 0.69 a | 8.06 ± 0.35 b | turpentine, mint, spice |
| 16 | α-terpinolene | 586-62-9 | 0.200 | - | 1.13 ± 0.09 b | 4.86 ± 0.21 a | - | pine |
| 17 | β-Ocimene | 13877-91-3 | 0.034 | 5.15 ± 0.22 d | 5.88 ± 0.15 c | 100.00 ± 0.00 a | 7.91 ± 0.78 b | citrus, wood, green |
| 18 | p-Cymene | 99-87-6 | 0.120 | 1.04 ± 0.06 c | - | 14.17 ± 0.74 a | 4.03 ± 0.35 b | musty |
| 19 | 1-Butanol | 71-36-3 | 0.038 | 1.97 ± 0.07 | - | - | - | fruit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, X.; Su, S.; Yao, Z.; Zhu, Z.; Chen, X.; Lao, F.; Li, X. Impact of Oil Temperature and Splashing Frequency on Chili Oil Flavor: Volatilomics and Lipidomics. Foods 2025, 14, 1006. https://doi.org/10.3390/foods14061006
Li X, Liu X, Su S, Yao Z, Zhu Z, Chen X, Lao F, Li X. Impact of Oil Temperature and Splashing Frequency on Chili Oil Flavor: Volatilomics and Lipidomics. Foods. 2025; 14(6):1006. https://doi.org/10.3390/foods14061006
Chicago/Turabian StyleLi, Xiaoping, Xiaopeng Liu, Shiting Su, Zhao Yao, Zhenhua Zhu, Xingyou Chen, Fei Lao, and Xiang Li. 2025. "Impact of Oil Temperature and Splashing Frequency on Chili Oil Flavor: Volatilomics and Lipidomics" Foods 14, no. 6: 1006. https://doi.org/10.3390/foods14061006
APA StyleLi, X., Liu, X., Su, S., Yao, Z., Zhu, Z., Chen, X., Lao, F., & Li, X. (2025). Impact of Oil Temperature and Splashing Frequency on Chili Oil Flavor: Volatilomics and Lipidomics. Foods, 14(6), 1006. https://doi.org/10.3390/foods14061006








