Immunomodulatory Effects of Polysaccharides from Porphyra haitanensis in Hydrocortisone-Induced Immunocompromised Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of PHP
2.3. Animal Experiment
2.4. Hematological Analysis, Peripheral Blood T Lymphocyte Subset Assay, and Organ Index
2.5. Detection of Phagocytic Activity
2.6. Spleen T Lymphocyte Proliferation Assay
2.7. Delayed-Type Hypersensitivity (DTH), Serum Hemolysin Response Test and Antibody-Producing Cells Detection
2.8. Carbon Clearance Assay
2.9. Serum Cytokines and Immunoglobulins Determination
2.10. Quantification of Acid Phosphatase (ACP) and Lactate Dehydrogenase (LDH) Activities
2.11. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PHP
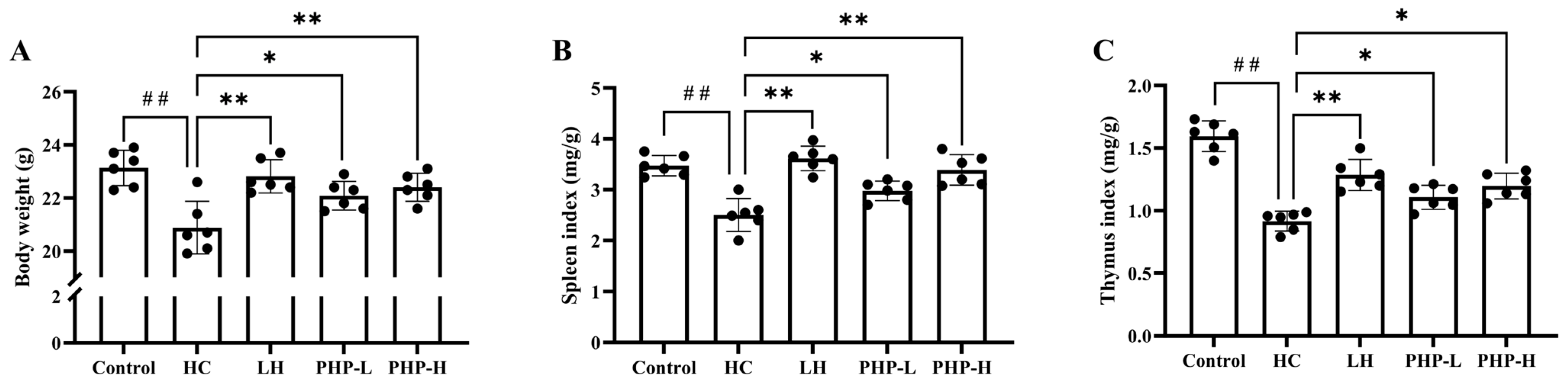
3.2. Effect of PHP on Body Weight, Immune Organ Indexes, and Hematological Parameters
3.3. Effect of PHP on Macrophage Function
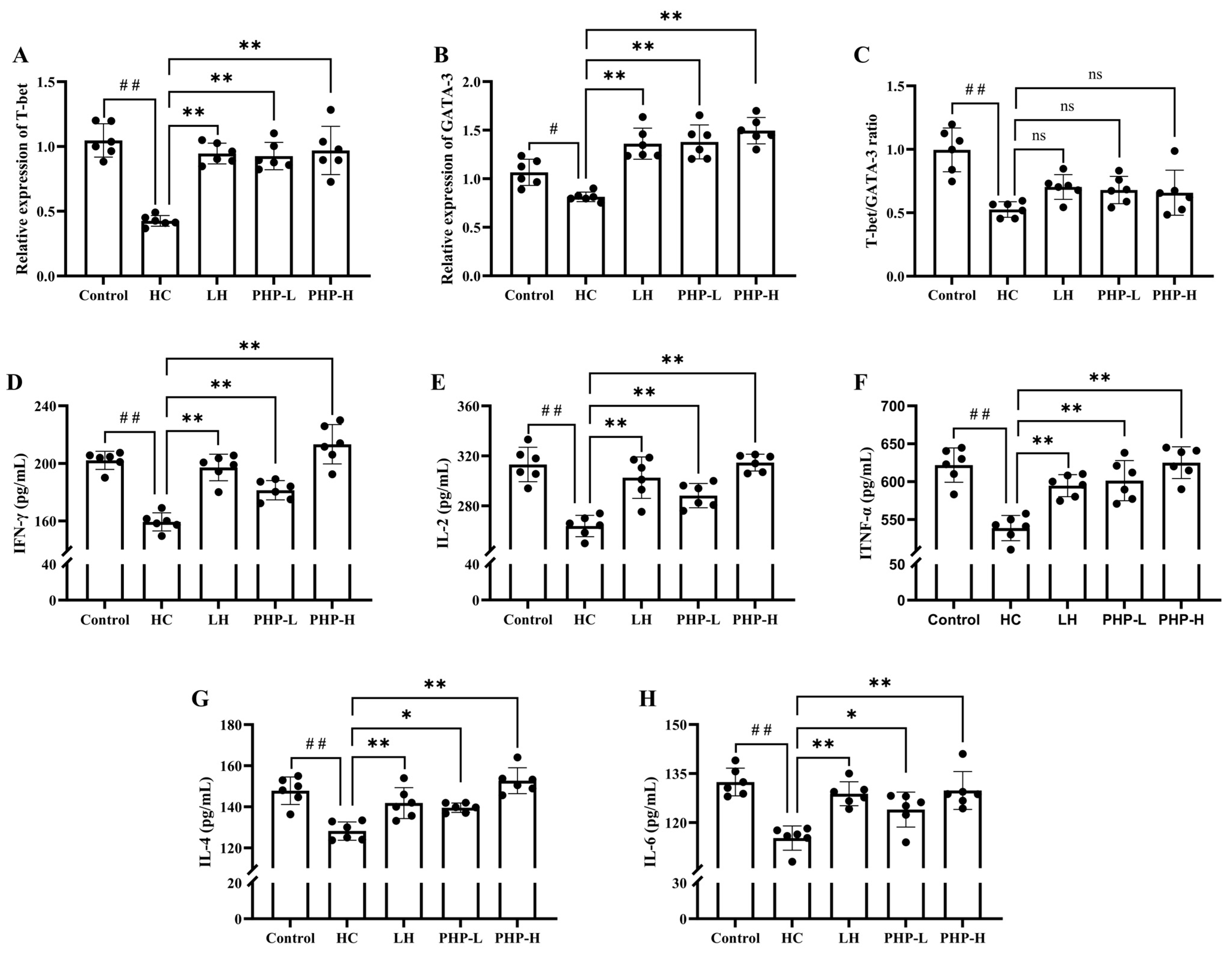
3.4. Effect of PHP on Cellular Immune Response
3.5. Effect of PHP on mRNA Levels of T-Bet and GATA3 and Secretion of Serum Cytokines
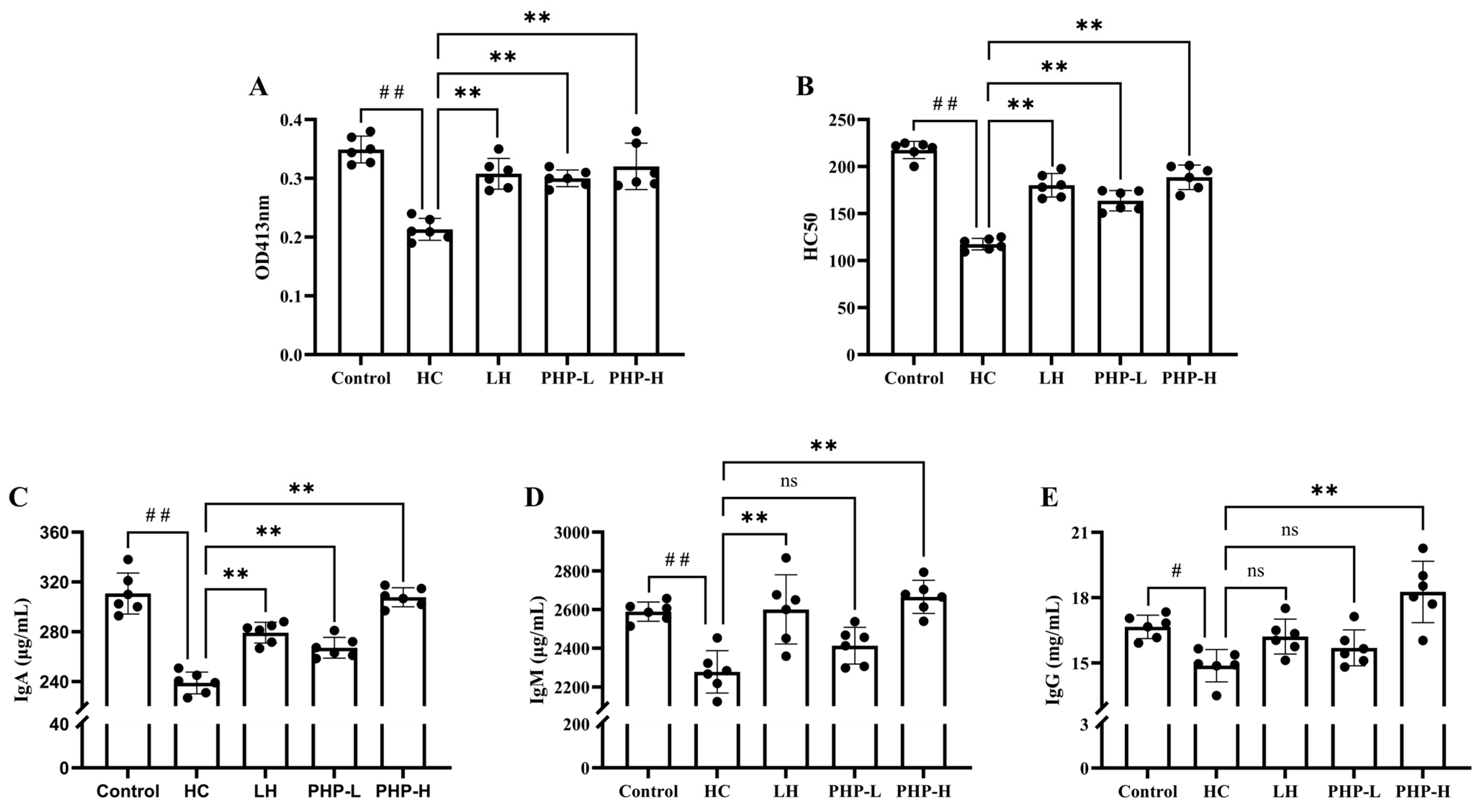
3.6. Effect of PHP on the Humoral Immune Response
3.7. Effect of PHP on the TLR-4/NF-κB Pathway
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Méndez López, L.F.; González Llerena, J.L.; Vázquez Rodríguez, J.A.; Medellín Guerrero, A.B.; González Martínez, B.E.; Solís Pérez, E.; López-Cabanillas Lomelí, M. Dietary Modulation of the Immune System. Nutrients 2024, 16, 4363. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Rout, L.; Ki, J.-S. Immunomodulatory and Anti-Inflammatory and Anticancer Activities of Porphyran, a Sulfated Galactan. Carbohydr. Polym. 2023, 301, 120326. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Qian, Y.; Wang, C.; Xie, M.; Huang, J.; Wang, Y. Two Polysaccharides from Porphyra Modulate Immune Homeostasis by NF-κB-Dependent Immunocyte Differentiation. Food Funct. 2019, 10, 2083–2093. [Google Scholar] [CrossRef]
- Shi, C.; Pan, T.; Cao, M.; Liu, Q.; Zhang, L.; Liu, G. Suppression of Th2 Immune Responses by the Sulfated Polysaccharide from Porphyra haitanensis in Tropomyosin-Sensitized Mice. Int. Immunopharmacol. 2015, 24, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Xu, S.; Li, L.; Pan, T.; Shi, C.; Liu, H.; Cao, M.; Su, W.; Liu, G. In Vitro and in Vivo Immunomodulatory Activity of Sulfated Polysaccharide from Porphyra haitanensis. Carbohydr. Polym. 2017, 165, 189–196. [Google Scholar] [CrossRef]
- Bhatia, S.; Rathee, P.; Sharma, K.; Chaugule, B.B.; Kar, N.; Bera, T. Immuno-Modulation Effect of Sulphated Polysaccharide (Porphyran) from Porphyra vietnamensis. Int. J. Biol. Macromol. 2013, 57, 50–56. [Google Scholar] [CrossRef]
- Yoshizawa, Y.; Enomoto, A.; Todoh, H.; Ametani, A.; Kaminogawa, S. Activation of Murine Macrophages by Polysaccharide Fractions from Marine Algae (Porphyra yezoensis). Biosci. Biotechnol. Biochem. 1993, 57, 1862–1866. [Google Scholar] [CrossRef]
- Huo, Y.-F.; Li, Y.-T.; Xia, W.; Wang, C.; Xie, Y.-Y.; Wang, Y.-B.; Zhou, T.; Fu, L.-L. Degraded Polysaccharides from Porphyra haitanensis: Purification, Physico-Chemical Properties, Antioxidant and Immunomodulatory Activities. Glycoconj. J. 2021, 38, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gu, X.; Jiang, Y.; Zhu, W.; Yao, L.; Liu, Z.; Gao, H.; Wang, L. Antagonistic Effect of Laver, Pyropia yezonensis and P. haitanensis, on Subchronic Lead Poisoning in Rats. Biol. Trace Elem. Res. 2018, 181, 296–303. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Liu, K.; Chen, W.; Zhong, S.; Tan, K. Recent Progress in Porphyra haitanensis Polysaccharides: Extraction, Purification, Structural Insights, and Their Impact on Gastrointestinal Health and Oxidative Stress Management. Food Chem. X 2024, 22, 101414. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed—Porphyra haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Dang, T.; Fang, J.; Deng, Y.; Liu, Q.; Dai, W.; Sun, J.; Wang, L.; Liu, Y.; Sun, T.; et al. Preparation, Structural Characterization, and Bioactivity of PHPD-IV-4 Derived from Porphyra haitanensis. Food Chem. 2020, 329, 127042. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, S.; Wang, X.; Zhang, M.; Du, C.; Zhou, M.; Zhang, X.; Zhao, C.; Yang, J.; Song, Q.; et al. Immunostimulatory and Immunoadjuvant Capacities of Soluble Rhamnan-Type Ulva Oligosaccharides. Algal Res. 2024, 82, 103614. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Liu, B.; Yu, Y. Optimization of Pine Nut (Pinus koraiensis) Meal Protein Peptides on Immunocompetence in Innate and Adaptive Immunity Response Aspects. Food Agric. Immunol. 2017, 28, 109–120. [Google Scholar] [CrossRef]
- Meng, M.; Wang, H.; Li, Z.; Guo, M.; Hou, L. Protective Effects of Polysaccharides from Cordyceps gunnii Mycelia against Cyclophosphamide-Induced Immunosuppression to TLR4/TRAF6/NF-κB Signalling in BALB/c Mice. Food Funct. 2019, 10, 3262–3271. [Google Scholar] [CrossRef]
- Ilangkovan, M.; Jantan, I.; Bukhari, S.N.A. Phyllanthin from Phyllanthus amarus Inhibits Cellular and Humoral Immune Responses in Balb/C Mice. Phytomedicine 2016, 23, 1441–1450. [Google Scholar] [CrossRef]
- Li, Q.; Chen, G.; Chen, H.; Zhang, W.; Ding, Y.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Se-Enriched G. Frondosa Polysaccharide Protects against Immunosuppression in Cyclophosphamide-Induced Mice via MAPKs Signal Transduction Pathway. Carbohydr. Polym. 2018, 196, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, Z.; Li, L.; Li, Y.; Zhao, H.; Jiang, X. Immunomodulatory Activity of R-Phycoerythrin from Porphyra haitanensis via TLR4/NF-κB-Dependent Immunocyte Differentiation. Food Funct. 2020, 11, 2173–2185. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Morris, G.A.; Xie, J. Sulfated Polysaccharides: Immunomodulation and Signaling Mechanisms. Trends Food Sci. Technol. 2019, 92, 1–11. [Google Scholar] [CrossRef]
- Yuan, L.; Zhong, Z.-C.; Liu, Y.; Quan, H.; Lu, Y.-Z.; Zhang, E.-H.; Cai, H.; Li, L.-Q.; Lan, X.-Z. Structures and Immunomodulatory Activity of One Galactose- and Arabinose-Rich Polysaccharide from Sambucus adnata. Int. J. Biol. Macromol. 2022, 207, 730–740. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, M.; Zhang, Y.; Deng, F.; Luo, J.; Wang, N.; Liu, M.; Ao, L.; Fang, Q.; Wang, Q.; et al. Artesunate Protects Immunosuppression Mice Induced by Glucocorticoids via Enhancing Pro-Inflammatory Cytokines Release and Bacterial Clearance. Eur. J. Pharmacol. 2021, 890, 173630. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.; Zhang, Y.; Li, Y.; Sun, J. Immunomodulatory and Antioxidant Effects of Pomegranate Peel Polysaccharides on Immunosuppressed Mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, C.; Xie, H.; Wang, L.; Hu, J. Effect of Gan Cao (Glycyrrhiza uralensis Fisch) Polysaccharide on Growth Performance, Immune Function, and Gut Microflora of Broiler Chickens. Poult. Sci. 2022, 101, 102068. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Huang, R.; Li, S.; Xie, R.; Qian, B.; Zhang, Z.; Li, L.; Wang, B.; Tian, C.; Yang, J.; et al. Ginsenoside Rh2 Reverses Cyclophosphamide-Induced Immune Deficiency by Regulating Fatty Acid Metabolism. J. Leukoc. Biol. 2019, 106, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ahn, S.; Rhee, H.-I.; Lee, D.-C. Coptis Chinensis Franch. Extract up-Regulate Type I Helper T-Cell Cytokine through MAPK Activation in MOLT-4 T Cell. J. Ethnopharmacol. 2016, 189, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Chen, Y.; Tan, L.; Fan, Q.; Zhong, M.; Wu, H. Effects of Different Anesthetic Methods on Immune Function and Oxidative Stress in Patients Undergoing Laparoscopic Herniorrhaphy. Wideochir Inne Tech. Maloinwazyjne 2021, 16, 329–335. [Google Scholar] [CrossRef]
- Long, Y.; Ji, H.; Yang, J.; Ji, H.; Dai, K.; Ding, W.; Zheng, G.; Yu, J. Immunoregulatory Effects of Codonopsis pilosula Polysaccharide Modified Selenium Nanoparticles on H22 Tumor-Bearing Mice. Foods 2024, 13, 4073. [Google Scholar] [CrossRef]
- Jie, D.; Gao, T.; Shan, Z.; Song, J.; Zhang, M.; Kurskaya, O.; Sharshov, K.; Wei, L.; Bi, H. Immunostimulating Effect of Polysaccharides Isolated from Ma-Nuo-Xi Decoction in Cyclophosphamide-Immunosuppressed Mice. Int. J. Biol. Macromol. 2020, 146, 45–52. [Google Scholar] [CrossRef]
- Yu, Q.; Nie, S.-P.; Wang, J.-Q.; Liu, X.-Z.; Yin, P.-F.; Huang, D.-F.; Li, W.-J.; Gong, D.-M.; Xie, M.-Y. Chemoprotective Effects of Ganoderma atrum Polysaccharide in Cyclophosphamide-Induced Mice. Int. J. Biol. Macromol. 2014, 64, 395–401. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, H.-C.; He, S.-B.; Zhang, X.-F.; Ling, Y.-H.; Li, X.-Y.; Zhang, H.; Hou, D.-D. The Immunoenhancement Effects of Sea Buckthorn Pulp Oil in Cyclophosphamide-Induced Immunosuppressed Mice. Food Funct. 2021, 12, 7954–7963. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Haque, M.A.; Arsyad, L. Magnoflorine from Tinospora crispa Upregulates Innate and Adaptive Immune Responses in Balb/c Mice. Int. Immunopharmacol. 2022, 111, 109081. [Google Scholar] [CrossRef] [PubMed]
- Okoye, A.A.; Picker, L.J. CD 4+ T-cell Depletion in HIV Infection: Mechanisms of Immunological Failure. Immunol. Rev. 2013, 254, 54–64. [Google Scholar] [CrossRef]
- Guerriero, J.L. Macrophages. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 342, pp. 73–93. ISBN 978-0-12-815381-9. [Google Scholar]
- Islam, M.; Kumar, K.; Sevak, J.K.; Jindal, A.; Vyas, A.K.; Ramakrishna, G.; Kottilil, S.; Sharma, M.K.; Sarin, S.K.; Trehanpati, N. Immune Drivers of HBsAg Loss in HBeAg-Negative CHB Patients after Stopping Nucleotide Analog and Administration of Peg-IFN. Hepatol. Commun. 2023, 7, e0098. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.-T.; Yue, N.; Li, A.-M.; Yu, S.-H.; Zhao, D.-P.; Zhu, Y.-L.; Wang, C.; Zhang, J.-J.; Wang, L.-Y. Immunomodulatory Effects of Lepidium meyenii Walp. Polysaccharides on an Immunosuppression Model Induced by Cyclophosphamide. J. Immunol. Res. 2022, 2022, 1210890. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Cai, Z.; Jin, G.; Ahn, D.U.; Huang, X. Immunomodulatory Effects of Chicken Soups Prepared with the Native Cage-Free Chickens and the Commercial Caged Broilers. Poult. Sci. 2022, 101, 102053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.-L.; Zhang, S.; Kong, D.-J.; Yang, R.-N.; Cao, L.; Wang, J.-X.; Yoshida, S.; Song, Z.-L.; Liu, T.; et al. Metronomic Capecitabine Inhibits Liver Transplant Rejection in Rats by Triggering Recipients’ T Cell Ferroptosis. World J. Gastroenterol. 2023, 29, 3084–3102. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Ye, S.; Hong, X.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G. Immunoenhancement Effects of Pentadecapeptide Derived from Cyclina sinensis on Immune-Deficient Mice Induced by Cyclophosphamide. J. Funct. Foods 2019, 60, 103408. [Google Scholar] [CrossRef]
- Huang, J.; Pang, M.; Li, G.; Wang, N.; Jin, L.; Zhang, Y. Alleviation of Cyclophosphamide-Induced Immunosuppression in Mice by Naturally Acetylated Hemicellulose from Bamboo Shavings. Food Agric. Immunol. 2017, 28, 328–342. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.; Chao, T.; Peng, J.; Wang, C.; Chen, K. Ginsenoside Re Inhibits Myocardial Fibrosis by Regulating miR-489/Myd88/NF-κB Pathway. J. Ginseng Res. 2023, 47, 218–227. [Google Scholar] [CrossRef]
- Li, B.; Yao, B.-C.; Chen, Q.-L.; Song, Y.-Q.; Jiang, N.; Zhao, L.-L.; Guo, Z.-G. The Protective Role and Mechanism of Liriodendrin in Rats with Myocardial Infarction. J. Thorac. Dis. 2022, 14, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Meng, M.; Han, L.; Cheng, D.; Cao, X.; Wang, C. Structural Characterization and Immunomodulatory Activity of Grifola frondosa Polysaccharide via Toll-like Receptor 4-Mitogen-Activated Protein Kinases-Nuclear Factor κB Pathways. Food Funct. 2016, 7, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Yang, Y.-H.; Liang, Y.-C.; Chiu, C.-J.; Chu, K.-H.; Chou, H.-N.; Chiang, B.-L. A Novel Phycobiliprotein Alleviates Allergic Airway Inflammation by Modulating Immune Responses. Am. J. Respir. Crit. Care Med. 2011, 183, 15–25. [Google Scholar] [CrossRef] [PubMed]



| Genes | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| β-Actin | TGTCCACCTTCCAGCAGATGT | AGCTCAGTAACAGTCCGCCTAGA |
| T-Bet | CCTCAATACCCGCCCAAGATG | CATGGGCAGAGTTCGCATGG |
| GATA-3 | CAGGCAGGGAGTGTGTGAAC | GCATTGCAAAGGTAGTGCCC |
| Groups | RBC (1013/L) | WBC (109/L) | Hb (g/L) | PLT (1011/L) | LYMPH (109/L) |
|---|---|---|---|---|---|
| Control | 1.01 ± 0.10 | 3.64 ± 0.19 | 157.07 ± 2.32 | 8.81 ± 0.31 | 2.56 ± 0.24 |
| HC | 0.92 ± 0.08 ## | 2.33 ± 0.36 ## | 140.62 ± 3.55 # | 7.55 ± 0.11 ## | 1.05 ± 0.23 ## |
| LH | 1.05 ± 0.17 ** | 2.98 ± 0.12 * | 151.50 ± 4.24 * | 8.42 ± 0.09 * | 1.42 ± 0.31 * |
| PHP-L | 1.02 ± 0.14 ** | 3.09 ± 0.17 ** | 151.00 ± 3.16 * | 8.19 ± 0.18 | 1.73 ± 0.15 ** |
| PHP-H | 1.08 ± 0.23 ** | 3.73 ± 0.26 ** | 158.83 ± 5.21 ** | 9.17 ± 0.24 ** | 2.13 ± 0.21 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Wang, C.; Zong, W.; Shen, Z.; Wang, P. Immunomodulatory Effects of Polysaccharides from Porphyra haitanensis in Hydrocortisone-Induced Immunocompromised Mice. Foods 2025, 14, 1018. https://doi.org/10.3390/foods14061018
Du C, Wang C, Zong W, Shen Z, Wang P. Immunomodulatory Effects of Polysaccharides from Porphyra haitanensis in Hydrocortisone-Induced Immunocompromised Mice. Foods. 2025; 14(6):1018. https://doi.org/10.3390/foods14061018
Chicago/Turabian StyleDu, Chunying, Chun Wang, Wenwen Zong, Zhaopeng Shen, and Peng Wang. 2025. "Immunomodulatory Effects of Polysaccharides from Porphyra haitanensis in Hydrocortisone-Induced Immunocompromised Mice" Foods 14, no. 6: 1018. https://doi.org/10.3390/foods14061018
APA StyleDu, C., Wang, C., Zong, W., Shen, Z., & Wang, P. (2025). Immunomodulatory Effects of Polysaccharides from Porphyra haitanensis in Hydrocortisone-Induced Immunocompromised Mice. Foods, 14(6), 1018. https://doi.org/10.3390/foods14061018






