A Novel Polysaccharide Purified from Tricholoma matsutake: Structural Characterization and In Vitro Immunological Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
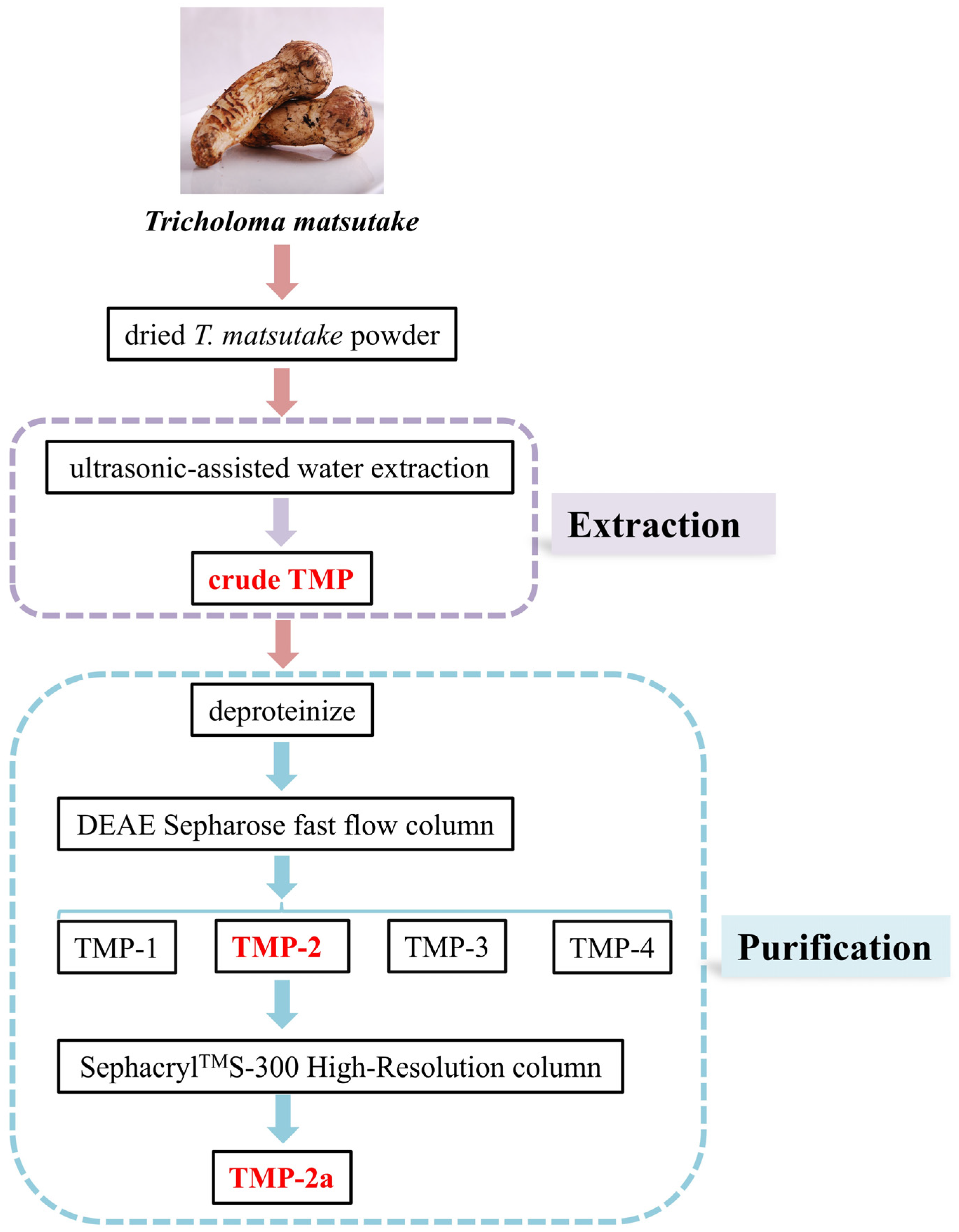
2.2. Preparation of Crude TMP
2.3. Isolation and Purification of TMP
2.4. The Monosaccharide Composition of TMP-2a
2.5. The Molecular Weight Determination of TMP-2a
2.6. The Fourier Transform Infrared Spectroscopy (FT-IR) Detection of TMP-2a
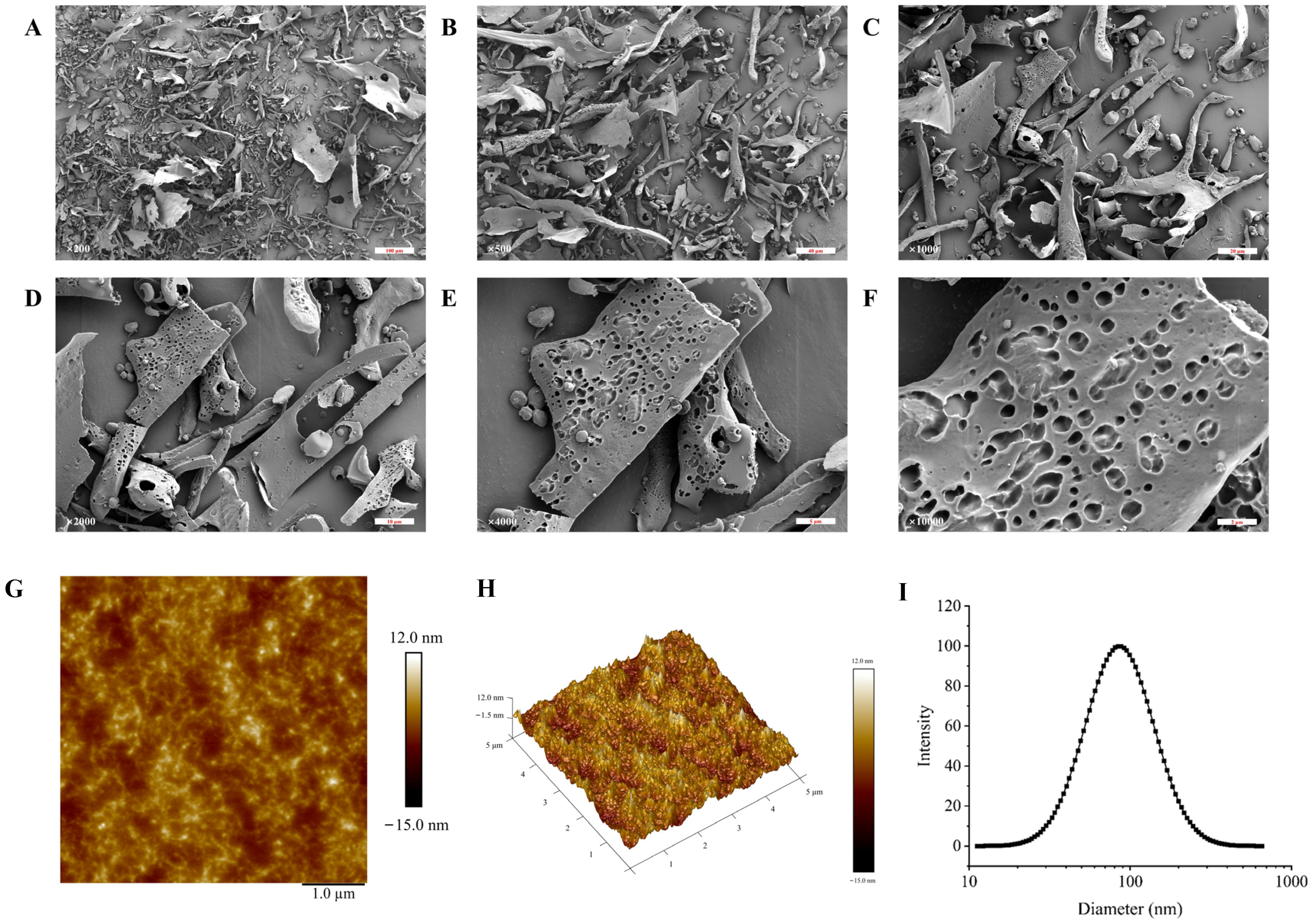
2.7. The Scanning Electron Microscope (SEM) Detection of TMP-2a
2.8. The Atomic Force Microscopy (AFM) Detection of TMP-2a
2.9. The Particle Size and ζ-Potential Determination of TMP-2a
2.10. The Methylation Detection of TMP-2a
2.11. The Nuclear Magnetic Resonance (NMR) Detection of TMP-2a
2.12. In Vitro Immunological Activity
2.12.1. Cell Culture
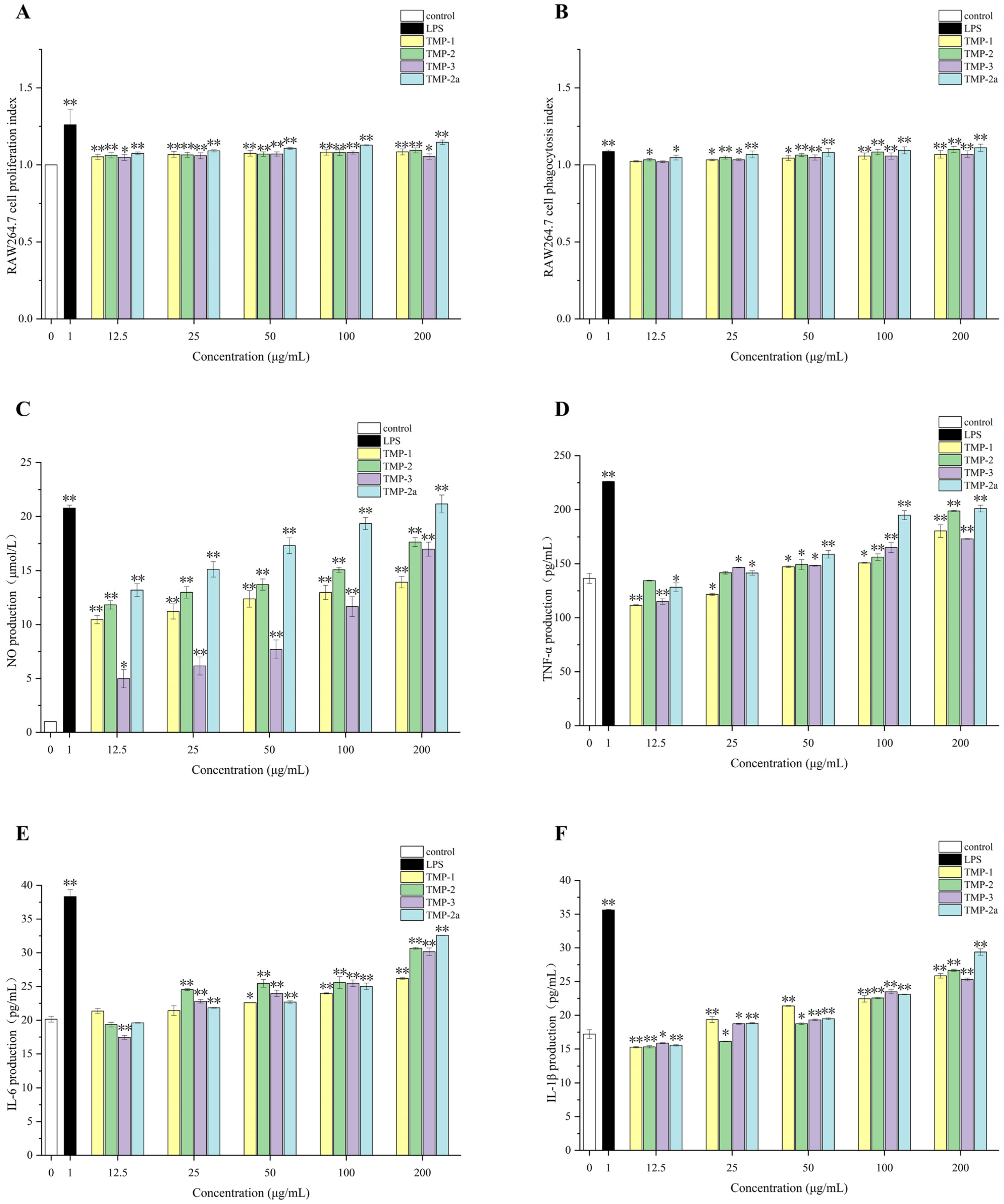
2.12.2. Effect of TMP on Proliferation of RAW264.7 Cells
2.12.3. Phagocytic Activity of TMP on RAW264.7 Cells
2.12.4. Effect of TMP on NO Release from RAW264.7 Cells
2.12.5. Effect of TMP on Cytokines (TNF-α,IL-6,IL-1β) of RAW264.7 Cells
2.13. Statistical Analysis
3. Results and Discussion
3.1. Purification of TMP-2a by SephacrylTMS-300 High-Resolution Column
3.2. The Analysis of Monosaccharide Composition of TMP-2a
3.3. The Determination of Molecular Weight of TMP-2a
3.4. The FT-IR Spectral Analysis of TMP-2a
3.5. The Characterization of the Apparent Structure of TMP-2a
3.6. The Methylation Analysis of TMP-2a
3.7. The NMR Analysis of TMP-2a
3.8. In Vitro Immune Activity of TMP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, I.H.; Choi, H.K.; Kim, Y.S. Comparison of umami-taste active components in the pileus and stipe of pine-mushrooms (Tricholoma matsutake Sing.) of different grades. Food Chem. 2010, 118, 804–807. [Google Scholar] [CrossRef]
- Yang, S.; Ren, X.D.; Sheng, J.X.; Lu, J.H.; Li, T.T.; Tang, F.R.; Wang, Y.F.; Meng, L.J.; Meng, Q.F.; Teng, L.R. Preparation and the antitumor activity in vitro of polysaccharides from Tricholoma matsutake. World. J. Microbiol. Biotechnol. 2010, 26, 497–503. [Google Scholar] [CrossRef]
- Ishihara, Y.; Iijima, H.; Yagi, Y.; Matsunaga, K. Enhanced recovery of NK cell activity in mice under restraint stress by the administration of a biological response modifier derived from the mycelia of the basidiomycete Tricholoma matsutake. Stress 2003, 6, 141–148. [Google Scholar] [CrossRef] [PubMed]
- You, L.J.; Gao, Q.; Feng, M.Y.; Yang, B.; Ren, J.Y.; Gu, L.J.; Cui, C.; Zhao, M.M. Structural characterisation of polysaccharides from Tricholoma matsutake and their antioxidant and antitumour activities. Food Chem. 2013, 138, 2242–2249. [Google Scholar] [CrossRef]
- Yin, X.L.; You, Q.H.; Su, X.Y. A comparison study on extraction of polysaccharides from Tricholoma matsutake by response surface methodology. Carbohydr. Polym. 2014, 102, 419–422. [Google Scholar] [CrossRef]
- Chen, Y.; Du, X.J.; Zhang, Y.; Liu, X.H.; Wang, X.D. Ultrasound extraction optimization, structural features, and antioxidant activity of polysaccharides from Tricholoma matsutake. J. Zhejiang Univ-Sci. B 2017, 18, 674–684. [Google Scholar] [CrossRef]
- Liang, S.M.; Guo, Q.; Ge, C.R.; Xiao, Z.C. Optimization Extraction, Monosaccharide Composition and Antioxidant Activity in Vitro of Polysaccharide from Tricholoma matsutake. J. Yunnan Agric. Univ. (Nat. Sci.) 2024, 39, 108–118. [Google Scholar] [CrossRef]
- Ding, X.; Tang, J.; Cao, M.; Guo, C.X.; Zhang, X.; Zhong, J.; Zhang, J.; Sun, Q.; Feng, S.; Yang, Z.R.; et al. Structure elucidation and antioxidant activity of a novel polysaccharide isolated from Tricholoma matsutake. Int. J. Biol. Macromol. 2010, 47, 271–275. [Google Scholar] [CrossRef]
- Yang, H.R.; Chen, L.H.; Zeng, Y.J. Structure, Antioxidant Activity and In Vitro Hypoglycemic Activity of a Polysaccharide Purified from Tricholoma matsutake. Foods 2021, 10, 2184. [Google Scholar] [CrossRef]
- Cheng, H.; Jia, Y.; Wang, L.; Liu, X.Y.; Liu, G.R.; Li, L.; He, C.F. Isolation and structural elucidation of a novel homogenous polysaccharide from Tricholoma matsutake. Nat. Prod. Res. 2015, 30, 58–64. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Ma, W.J.; Chen, X.F.; Wang, B.; Lou, W.J.; Chen, X.; Hua, J.L.; Sun, Y.J.; Zhao, Y.; Peng, T. Characterization, antioxidativity, and anti-carcinoma activity of exopolysaccharide extract from Rhodotorula mucilaginosa CICC 33013. Carbohydr. Polym. 2017, 181, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Qiu, Y.J.; Duan, Y.Q.; He, Y.Q.; Xiang, H.; Sun, W.X.; Zhang, H.H.; Ma, H.L. Characterization, antioxidant, antineoplastic and immune activities of selenium modified Sagittaria sagittifolia L. polysaccharides. Food Res. Int. 2022, 153, 110913. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, J.H.; Ren, G.F.; Zhang, Y.H.; Tan, X.Y.; Yang, L.N. Punicalagin Prevents Inflammation in LPS-Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef]
- Li, J.E.; Nie, S.P.; Xie, M.Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Dai, Z.Q.; Su, D.; Zhang, Y.; Sun, Y.; Hu, B.; Ye, H.; Jabbar, S.; Zeng, X.X. Immunomodulatory activity in vitro and in vivo of verbascose from mung beans (Phaseolus aureus). J. Agric. Food Chem. 2014, 62, 10727–10735. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Yang, F.C.; Chang, J.S. Extraction of polysaccharides from edible mushrooms: Emerging technologies and recent advances. Carbohydr. Polym. 2021, 251, 117006. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Huang, R.J.; Li, S.; Jiang, L.; Shao, L.H.; Zhang, Q.; Shan, C.H. Polysaccharides from sea buckthorn-Ultrasound-assisted enzymatic extraction, purification, structural characterization, and antioxidant activity analysis. Food Chem. X 2025, 10, 102265. [Google Scholar] [CrossRef]
- Li, Y.X.; Ren, M.J.; Yan, H.; Luo, L.; Fang, X.; He, L.; Kang, W.Y.; Wu, M.Y.; Liu, H.Y. Purification, structural characterization, and immunomodulatory activity of two polysaccharides from Portulaca oleracea L. Int. J. Biol. Macromol. 2024, 230, 130508. [Google Scholar] [CrossRef]
- Chi, Y.; Li, Y.; Zhang, G.; Gao, Y.; Ye, H.; Gao, J.; Wang, P. Effect of extraction techniques on properties of polysaccharides from Enteromorpha prolifera and their applicability in iron chelation. Carbohydr. Polym. 2018, 181, 616–623. [Google Scholar] [CrossRef]
- Li, Q.; Wang, W.; Zhu, Y.; Chen, Y.; Zhang, W.J.; Yu, P.; Mao, G.H.; Zhao, T.; Feng, W.W.; Yang, L.Q.; et al. Structural elucidation and antioxidant activity of a novel Se-polysaccharide from Se-enriched Grifola frondosa. Carbohydr. Polym. 2017, 161, 42–52. [Google Scholar] [CrossRef]
- Tang, W.; Liu, C.C.; Liu, J.J.; Hu, L.Y.; Huang, Y.S.; Yuan, L.; Liu, F.W.; Pan, S.J.; Chen, S.P.; Bian, S.G.; et al. Purification of polysaccharide from Lentinus edodes water extract by membrane separation and its chemical composition and structure characterization. Food Hydrocoll. 2020, 105, 105851. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.H.; Qiao, Y.; Wang, X.H.; Zhang, Q.; Zhao, W.; Huang, L. Purification, characterization and anticancer activities of exopolysaccharide produced by Rhodococcus erythropolis HX-2. Int. J. Biol. Macromol. 2019, 12, 228. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.S.; Dong, X.D.; Ma, R.J.; Ji, H.Y.; Yu, J.; Liu, A.J. Characterization of a polysaccharide from Polygala tenuifolia Willd. with immune activity via activation of MAPKs pathway. Bioorg. Chem. 2023, 130, 106214. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lei, Z.X.; Zhao, M.M.; Wu, C.Z.; Wang, L.B.; Xu, Y.Q. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crops Prod. 2020, 147, 112249. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yang, X.; Zhang, J.X.; Liang, L.; Miao, F.; Ji, T.; Ye, Z.Q.; Chu, M.; Ren, J.Y.; Xu, X. Synthesis, stability and anti-fatigue activity of selenium nanoparticles stabilized by Lycium barbarum polysaccharides. Int. J. Biol. Macromol. 2021, 179, 418–428. [Google Scholar] [CrossRef]
- Ru, Y.; Chen, X.; Wang, J.; Guo, L.H.; Lin, Z.Y.; Peng, X.; Qiu, B.; Wong, W.L. Structural characterization, hypoglycemic effects and mechanism of a novel polysaccharide from Tetrastigma hemsleyanum Diels et Gilg. Int. J. Biol. Macromol. 2019, 123, 775–783. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, R.A.; Matulewicz, M.C.; Ciancia, M. NMR spectroscopy for structural elucidation of sulfated polysaccharides from red seaweeds. Int. J. Biol. Macromol. 2022, 199, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shen, W.; Zhou, Y.R.; Ma, L.M.; Xu, D.Y.; Ding, J.L.; He, L.Y.; Shen, B.Y.; Zhou, C.L. Characterization of a polysaccharide from Eupolyphaga sinensis Walker and its effective antitumor activity via lymphocyte activation. Int. J. Biol. Macromol. 2020, 162, 31–42. [Google Scholar] [CrossRef]
- Ghosh, K.; Chandra, K.; Roy, S.K.; Mondal, S.; Maiti, D.; Das, D.; Ojha, A.K.; Islam, S.S. Structural investigation of a polysaccharide (Fr. I) isolated from the aqueous extract of an edible mushroom, Volvariella diplasia. Carbohydr. Res. 2008, 343, 1071–1078. [Google Scholar] [CrossRef]
- Shi, H.; Bi, S.X.; Li, H.; Li, J.H.; Li, C.L.; Yu, R.M.; Song, L.Y.; Zhu, J.H. Purification and characterization of a novel mixed-linkage α, β-d-glucan from Arca subcrenata and its immunoregulatory activity. Int. J. Biol. Macromol. 2021, 182, 207–216. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wu, J.H.; Sun, B.G.; Zhao, M.M.; Sun, W.Z.; Sun, J.Y.; Huang, M.Q. Isolation, purification, structure characterization of a novel glucan from Huangshui, a byproduct of Chinese Baijiu, and its immunomodulatory activity in LPS-stimulated THP-1 cells. Int. J. Biol. Macromol. 2020, 161, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Cao, J.J.; Zhang, B.; Chen, H.Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227–236. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Huang, G.L.; Chen, G.Y. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chem. 2021, 361, 130089. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Khatua, S.; Simal-Gandara, J.; Acharya, K. Understanding immune-modulatory efficacy in vitro. Chem. Biol. Interact. 2022, 352, 109776. [Google Scholar] [CrossRef]
- Li, X.J.; Zhou, J.J.; Liu, C.R.; Xiong, Q.R.; Duan, H.W.; Cheung, P.C.K. Stable and Biocompatible Mushroom β-Glucan Modified Gold Nanorods for Cancer Photothermal Therapy. J. Agric. Food Chem. 2017, 65, 9529–9536. [Google Scholar] [CrossRef]
- Klaus, A.; Kozarski, M.; Nikšić, M.; Jakovljević, D.; Todorović, N.; Stefanoska, I.; Van Griensven, L.J.L.D. The edible mushroom Laetiporus sulphureus as potential source of natural antioxidants. Int. J. Food Sci. Nutr. 2013, 64, 73–81. [Google Scholar] [CrossRef]
- Bi, H.T.; Han, H.; Li, Z.H.; Ni, W.H.; Chen, Y.; Zhu, J.J.; Gao, T.T.; Hao, M.; Zhou, Y.F. A water-soluble polysaccharide from the fruit bodies of Bulgaria inquinans (Fries) and its anti-Malarial activity. Evid.-Based Complement. Altern. 2011, 2011, 973460. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.K.; Dey, B.; Maity, K.K.; Patra, S.; Mandal, S.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Structural characterization of an immunoenhancing heteroglycan isolated from an aqueous extract of an edible mushroom, Lentinus squarrosulus (Mont.) Singer. Carbohydr. Res. 2010, 345, 2542–2549. [Google Scholar] [CrossRef]
- Li, Y.M.; Zhong, R.F.; Chen, J.; Luo, Z.G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Y.; Zhang, H.H.; Zhang, J.X.; Wen, C.T.; Zhou, J.; Yao, H.; He, Y.Q.; Ma, H.L.; Duan, Y.Q. Optimization, characterization, rheological study and immune activities of polysaccharide from Sagittaria sagittifolia L. Carbohydr. Polym. 2020, 246, 116595. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Huang, R.M.; Wen, P.; Song, Y.; He, B.L.; Tan, J.L.; Hao, H.L.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef]
- Liu, S.J.; Boeck, D.M.; van Dam, H.V.; Dijke, T.P. Regulation of the TGF-β pathway by deubiquitinases in cancer. Int. J. Biochem. Cell B 2016, 76, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Liu, H.Q.; Li, S.Y.; Sun, H.; He, X.M.; Huang, Y.; Long, H. Transcriptome Analysis Reveals Possible Immunomodulatory Activity Mechanism of Chlorella sp. Exopolysaccharides on RAW264.7 Macrophages. Mar. Drugs. 2021, 19, 217. [Google Scholar] [CrossRef]
- Hou, Y.L.; Ding, X.; Hou, W.R.; Zhong, J.; Zhu, H.Q.; Ma, B.X.; Xu, T.; Li, J.H. Anti-microorganism, anti-tumor, and immune activities of a novel polysaccharide isolated from Tricholoma matsutake. Pharmacogn. Mag. 2013, 9, 244–249. [Google Scholar] [CrossRef]
- Ruthesa, A.C.; Smiderlea, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef] [PubMed]
- Maity, P.; Pattanayak, S.; Maity, S.; Nandi, A.K.; Sen, I.K.; Behera, B.; Maiti, T.K.; Mallick, P.; Sikdar, S.R.; Islam, S.S. A partially methylated mannogalactan from hybrid mushroom Pfle 1p: Purification, structural characterization, and study of immunoactivation. Carbohydr. Res. 2014, 395, 1–8. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Dey, B.; Maity, K.K.; Patra, S.; Mandal, S.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Heteroglycan from an alkaline extract of a somatic hybrid mushroom (PfloVv1aFB) of Pleurotus florida and Volvariella volvacea: Structural characterization and study of immunoenhancing properties. Carbohydr. Res. 2012, 354, 110–115. [Google Scholar] [CrossRef]
- Yelithao, K.; Surayot, U.; Lee, C.; Palanisamy, S.; Prabhu, N.M.; Lee, J.; You, S. Studies on structural properties and immune-enhancing activities of glycomannans from Schizophyllum commune. Carbohydr. Polym. 2019, 218, 37–45. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2020, 227, 115314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.M.; Liu, X.Z.; Guo, Y.; Qiang, W.; Peng, D.Y.; Cao, L. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr. Polym. 2010, 81, 784–789. [Google Scholar] [CrossRef]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Zhang, T.; Xin, Y.; Huang, X.J.; Yin, J.Y.; Nie, S.P. Comprehensive evaluation of alkali-extracted polysaccharides from Agrocybe cylindracea: Comparison on structural characterization. Carbohydr. Polym. 2021, 255, 117502. [Google Scholar] [CrossRef]
- Mueller, A.; Raptis, J.; Rice, P.J.; Kalbfleisch, J.H.; Stout, R.D.; Ensley, H.E.; Browder, W.; Williams, D.L. The influence of glucan polymer structure and solution conformation on binding to (1→3)-β-D-glucan receptors in a human monocyte-like cell line. Glycobiology 2000, 10, 339–346. [Google Scholar] [CrossRef]




| Item | TMP-2a |
|---|---|
| Monosaccharide composition (%) | |
| Fucose | 8.1 |
| Galactose | 22.6 |
| Glucose | 56.8 |
| Mannose | 12.5 |
| Relative molecular weight | |
| Retention time (min) | 41.02 |
| Mw (Da) | 27,749 |
| Mn (Da) | 20,896 |
| Particle size and ζ-potential determination | |
| Particle size (nm) | 97.13 ± 0.64 |
| PdI | 0.24 ± 0.03 |
| ζ-potential (mV) | −8.30 ± 0.45 |
| Retention Time | Methylated Sugar | Mass Fragments (m/z) | Molar Ratio | Type of Linkage |
|---|---|---|---|---|
| 11.926 | 2,3,4-Me3-Fucp | 43, 59, 72, 89, 101, 115, 117, 131, 175 | 0.011 | Fucp-(1→ |
| 16.277 | 2,3,4,6-Me4-Glcp | 43, 71, 87, 101, 117, 129, 145, 161, 205 | 0.203 | Glcp-(1→ |
| 20.908 | 2,4,6-Me3-Glcp | 43, 87, 99, 101, 117, 129, 161, 173, 233 | 0.167 | →3)-Glcp-(1→ |
| 21.405 | 2,3,6-Me3-Glcp | 43, 87, 99, 101, 113, 117, 129, 131, 161, 173, 233 | 0.047 | →4)-Glcp-(1→ |
| 22.622 | 2,3,4-Me3-Glcp | 43, 87, 99, 101, 117, 129, 161, 189, 233 | 0.314 | →6)-Glcp-(1→ |
| 24.307 | 2,3,4-Me3-Galp | 43, 87, 99, 101, 117, 129, 161, 189, 233 | 0.046 | →6)-Galp-(1→ |
| 27.708 | 2,4-Me2-Glcp | 43, 87, 117, 129, 159, 189, 233 | 0.141 | →3,6)-Glcp-(1→ |
| 29.256 | 3,4–Me2-Manp | 43, 87, 99, 129, 189 | 0.073 | →2,6)-Manp-(1→ |
| Code | Glycosyl Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6a, b/C6 | ||
| A | →6)-β-D-Glcp-(1→ | 4.43 | 3.24 | 3.4 | 3.56 | 3.43 | 3.77, 4.13 |
| 102.99 | 72.99 | 69.47 | 74.88 | 73.05 | 68.76 | ||
| B | β-D-Glcp-(1→ | 4.41 | 3.24 | 3.4 | 3.56 | 3.46 | 3.63 |
| 102.99 | 75.81 | 69.47 | 74.81 | 72.97 | 60.71 | ||
| C | →3)-β-D-Glcp-(1→ | 4.72 | 3.37 | 3.66 | 3.42 | 3.39 | 3.88 |
| 101.67 | 75.58 | 84.45 | 69.64 | 75.91 | 61.12 | ||
| D | →3,6)-β-D-Glcp-(1→ | 4.64 | 3.29 | 3.67 | 3.33 | 3.63 | 3.43 |
| 102.65 | 73.01 | 84.33 | 75.81 | 74.49 | 68.58 | ||
| E | →2,6)-α-D-Manp-(1→ | 5.05 | 3.86 | 3.98 | 3.7 | 3.78 | 3.83 |
| 98.24 | 77.06 | 69.35 | 72.58 | 70.44 | 69.04 | ||
| F | →4)-β-D-Glcp-(1→ | 4.46 | 3.44 | 3.57 | 3.73 | 3.52 | 3.54 |
| 102.95 | 73.17 | 72.85 | 78.08 | 76.36 | 62.47 | ||
| G | →6)-α-D-Galp-(1→ | 4.92 | 3.78 | 4.12 | 3.55 | 4.02 | 3.52 |
| 98.39 | 71.53 | 68.35 | 69.58 | 70.29 | 66.65 | ||
| H | α-L-Fucp-(1→ | 4.97 | 3.7 | 3.96 | ND | ND | 1.13 |
| 101.58 | 71.84 | 70.6 | ND | ND | 15.71 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Guo, Q.; Li, J.; Zhao, P.; Ge, C.; Li, S.; Xiao, Z. A Novel Polysaccharide Purified from Tricholoma matsutake: Structural Characterization and In Vitro Immunological Activity. Foods 2025, 14, 1031. https://doi.org/10.3390/foods14061031
Liang S, Guo Q, Li J, Zhao P, Ge C, Li S, Xiao Z. A Novel Polysaccharide Purified from Tricholoma matsutake: Structural Characterization and In Vitro Immunological Activity. Foods. 2025; 14(6):1031. https://doi.org/10.3390/foods14061031
Chicago/Turabian StyleLiang, Shuangmin, Qi Guo, Jun Li, Ping Zhao, Changrong Ge, Shijun Li, and Zhichao Xiao. 2025. "A Novel Polysaccharide Purified from Tricholoma matsutake: Structural Characterization and In Vitro Immunological Activity" Foods 14, no. 6: 1031. https://doi.org/10.3390/foods14061031
APA StyleLiang, S., Guo, Q., Li, J., Zhao, P., Ge, C., Li, S., & Xiao, Z. (2025). A Novel Polysaccharide Purified from Tricholoma matsutake: Structural Characterization and In Vitro Immunological Activity. Foods, 14(6), 1031. https://doi.org/10.3390/foods14061031







