Characteristics of Wheat Noodle “Kitanokaori” Using Weakly Acidic Hard Water in Terms of Functional Qualities, Such as Inhibiting Postprandial Abrupt Increase in Blood Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of the Moisture Contents of Six Kinds of Wheat Flour
2.3. Analysis of Phosphorus Contents of Six Kinds of Wheat Flour
2.4. Preparation of Wheat Starch
2.5. Iodine Absorption Spectrum
2.6. Pasting Properties
2.7. Preparation of Wheat Noodles
2.8. Measurement of Physical Properties of Various Boiled Noodles Using Weakly Acidic Hard Water or Purified Water
2.9. Measurement of Color Difference of Various Boiled Flour Noodles Using Weakly Acidic Hard Water or Purified Water
2.10. Analysis of Calcium Contents, RS (Resistant Starch), and FD (Dietary Fiber) of Various Boiled Flour Noodles
2.11. Noodle Making for Feed
2.12. Animal Feed Test and Diets
2.13. Statistical Analyses
3. Results and Discussion
3.1. Moisture and Phosphorus Contents of Six Kinds of Wheat Flour
3.2. Iodine Absorption Spectrum for the Survey of Wheat Starch Microstructure
3.3. Pasting Properties of Six Kinds of Wheat Flours in Purified Water or in Weakly Acidic Hard Water with an RVA
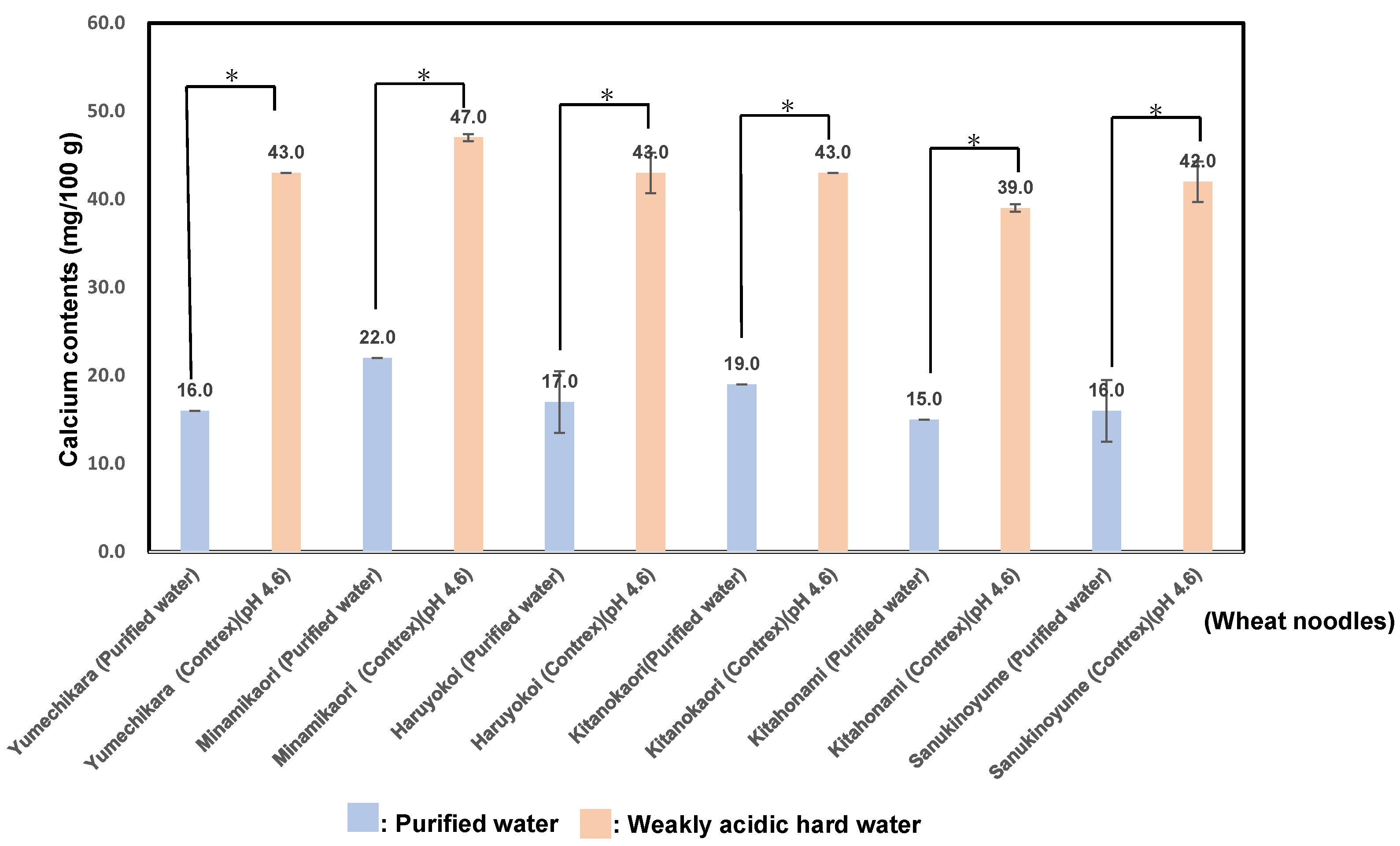
3.4. Calcium Contents in Six Kinds of Wheat Flour Noodles Using Weakly Acidic Hard Water (pH 4.6) or Purified Water
3.5. Textural Properties of Six Kinds of Domestic Wheat Flour Noodles Using Weakly Acidic Hard Water (pH 4.6) or Purified Water
3.6. Improvement of the Color of Six Kinds of Wheat Noodles Using Weakly Acidic Hard Water, Contrex (pH 4.6)
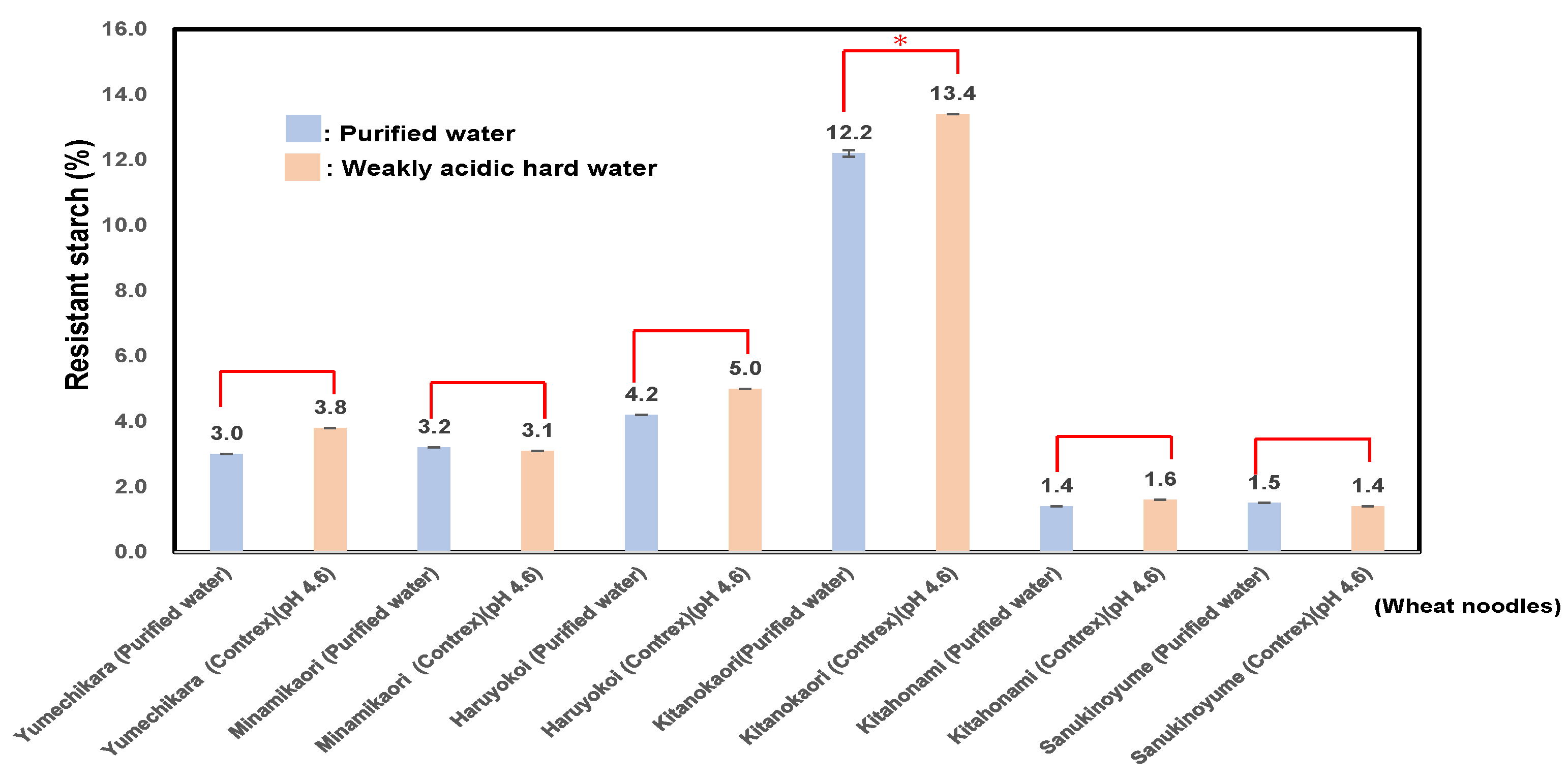
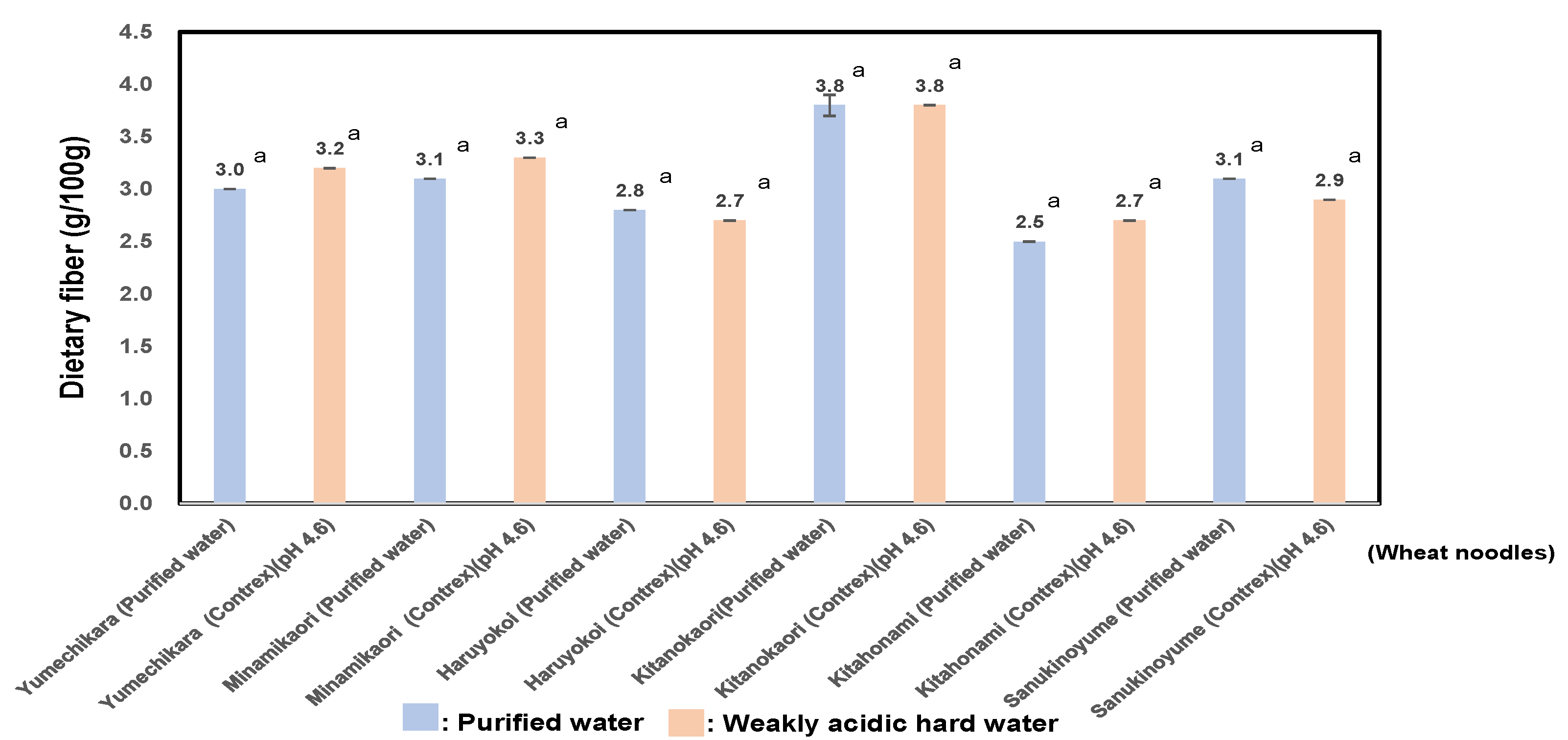
3.7. Measurement of Bio-Functional Properties of RS (Resistant Starch) and Dietary Fiber of Six Kinds of Wheat Flour Noodles Using Weakly Acidic Hard Water or Purified Water
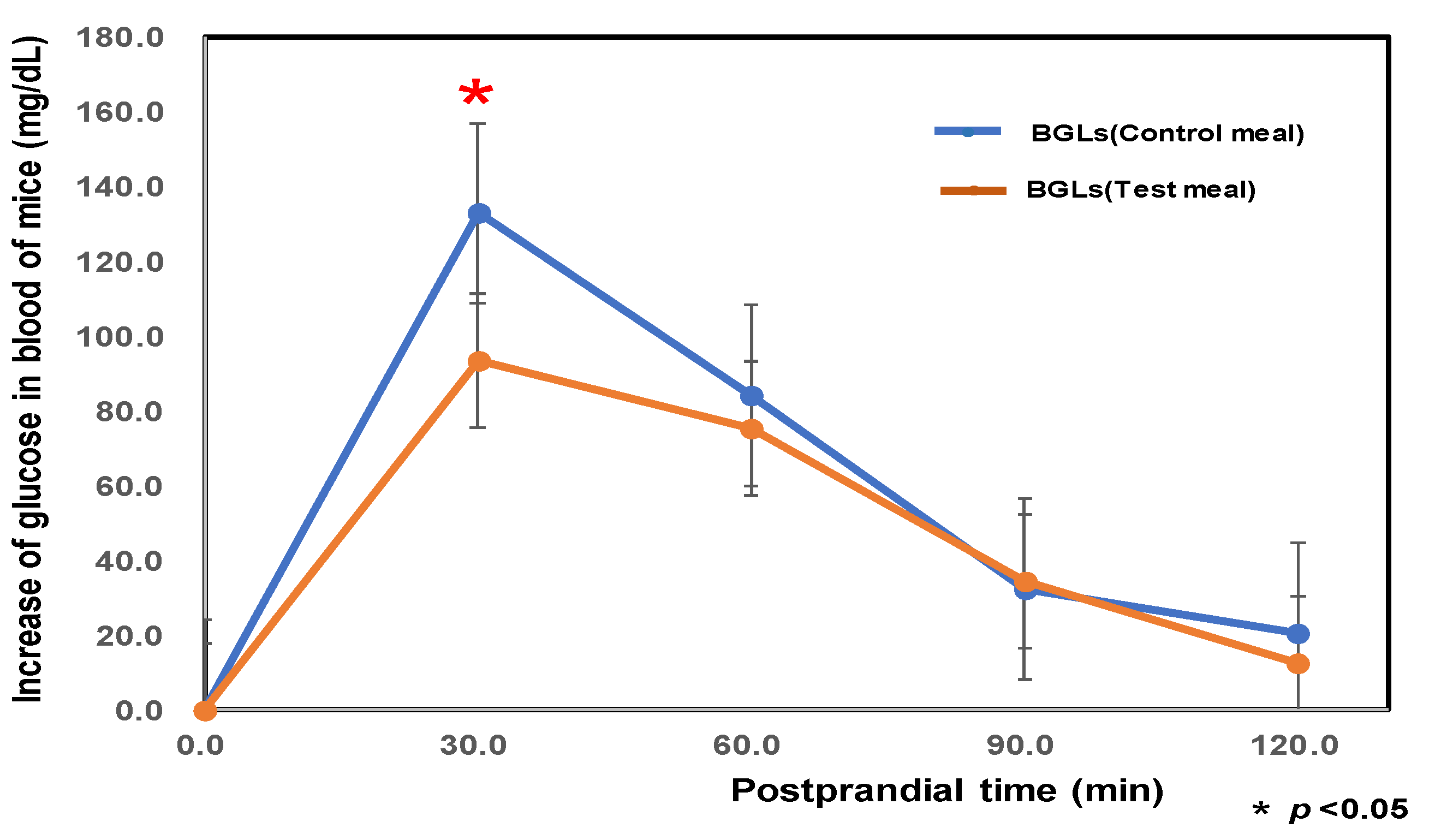
3.8. Determination of the Initial BGL of Aged Mice Kept for 8 Weeks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- The IDF (International Diabetes Federation) Diabetes Atlas 10th. Available online: https://diabetesatlas.org/date/en/ (accessed on 7 February 2024).
- ADI (Alzheimer’s Disease International) Report. 2015. Available online: https://www.alzint.org/resource/world-alzheimerreport-2015/ (accessed on 5 February 2024).
- Nakamura, S.; Ikeuchi, T.; Araki, A.; Kasuga, K.; Watanabe, K.; Hirayama, M.; Ito, M.; Ohtsubo, K. Possibility for prevention of type 2 diabetes mellitus and dementia using three kinds of brown rice blends after high-pressure treatment. Foods 2022, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, M.; Takano, H.; Osakabe, N.; Yasuda, A.; Inoue, K.; Yanagisawa, R.; Ohwatari, T.; Uematsu, H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition 2007, 23, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef]
- Vestergaard, P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—A meta-analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Khoo, J.C.; Miller, E.; Barnett, J.; Witztum, J.L.; Steinberg, D. Low density lipoprotein rich in oleic acid is protected against oxidative modification: Implications for dietary prevention of atherosclerosis. Proc. Natl. Acad. Sci. USA 1990, 87, 3894–3898. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Cooney, M.J.; Tu, A.H.; Chang, K.Y.; Cao, J.; Ando, A.; An, G.J.; Melia, M.; de Juan, E., Jr. Normalization of retinal vascular permeability in experimental diabetes with genistein. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2110–2114. [Google Scholar]
- Villegas, R.; Gao, Y.T.; Dai, Q.; Yang, G.; Cai, H.; Li, H.; Zheng, W.; Shu, X.O. Dietary calcium and magnesium intakes and the risk of type 2 diabetes. The Shanghai women’s health study. Am. J. Clin. Nutr. 2009, 89, 1059–1067. [Google Scholar] [CrossRef]
- Liu, S.; Choi, H.K.; Ford, E.; Song, Y.; Klevak, A.; Buring, J.; Manson, J.E. A prospective study of dairy intake and the risk of type 2 diabetes in women. Diabetes Care 2006, 29, 1579–1584. [Google Scholar] [CrossRef]
- Carson, G.R.; Edwards, N.M. Chapter 4: Criteria of wheat and flour quality. In WHEAT: Chemistry and Technology; Scientific Societies: St. Paul, MN, USA, 2009; pp. 97–118. [Google Scholar]
- Oda, S.; Schofield, J.D. Characterisation of friabilin polypeptides. J. Cereal Sci. 1997, 26, 29–36. [Google Scholar] [CrossRef]
- Morris, C. Puroindolines: The molecular genetic basis of wheat grain hardness. Plant Mol. Biol. 2002, 48, 633–647. [Google Scholar] [CrossRef]
- Silano, V.; Pocchiari, F.; Kasarda, D.D. Physical characterization of alpha-amylase inhibitors from wheat. Biochim. Biophys. Acta 1973, 317, 139. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Miyazaki, T.; Murayama, R.; Kodama, T.; Kitamura, I.; Inoue, S. Wheat album with amylase-inhibitory activity suppresses glycemic rise after rice loading in Human subjects. Jpn. Soc. Nutr. Food Sci. 1999, 52, 285–291. [Google Scholar] [CrossRef]
- Piironen, V.; Lampi, A.M.; Ekholm, P. Chapter 7: Micronutrients and phytochemicals in wheat grain. In WHEAT: Chemistry and Technology; Scientific Societies: St. Paul, MN, USA, 2009; pp. 179–222. [Google Scholar]
- Kim, J.; Kweon, M. Quality and noodle-making performance of wheat flour with varied gluten strengths altered by addition of various arabinoxylans. J. Food Sci. 2024, 89, 7494–7508. [Google Scholar] [CrossRef]
- Cormick, G.; Romero, I.B.; Puchulu, M.B.; Perez, S.M.; Sosa, M.; Garitta, L.; Elizagoyen, E.; Gugole, M.F.; Belizán, J.M.; Matamoros, N.; et al. A simulation study to improve calcium intake through wheat flour fortification. Public Health Nutr. 2024, 27, e159. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Hofmeyr, G.J.; Cormick, G.; Garcia-Casal, M.N.; Peña-Rosas, J.P.; Betrán, A.P. Current calcium fortification experiences. Ann. N. Y. Acad. Sci. 2021, 1484, 55–73. [Google Scholar] [CrossRef]
- Michel, S.; Bayram, M. Kinetics of chemical and color changes in wheat and water during atmospheric cooking as affected by the acidity, hardness, and iron content of water. J. Food Sci. 2024, 89, 4758–4770. [Google Scholar] [CrossRef]
- Bronder, K.L.; Zimmerman, S.L.; van den Wijngaart, A.; Codling, K.; Johns, K.A.G.; Pachón, H. Instant noodles made with fortified wheat flour to improve micronutrient intake in Asia: A review of simulation, nutrient retention and sensory studies. Asia Pac. J. Clin. Nutr. 2017, 26, 191–201. [Google Scholar] [CrossRef]
- Kanadhia, K.C.; Shri Ramavataram, D.V.S.S.; Nilakhe, S.P.D.; Patel, S. A study of water hardness and the prevalence of hypomagnesaemia and hypocalcaemia in healthy subjects of Surat district (Gujarat). Magnes Res. 2014, 27, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.S.; Van Delft, W. Changes in the mineral composition of food as a result of cooking in "hard" and "soft" waters. Arch. Env. Health 1981, 36, 33–35. [Google Scholar] [CrossRef]
- Lee, C.-L. The advantages of deep ocean water for the development of functional fermentation food. Appl. Microbiol. Biotechnol. 2015, 99, 2523–2531. [Google Scholar] [CrossRef]
- Shih, M.K.; Hsu, Q.Y.; Liou, B.K.; Peng, Y.H.; Hou, C.Y. Deep Ocean Water Concentrate Changes Physicochemical Characteristics, the Profile of Volatile Components and Consumer Acceptance for Taiwanese Rice Shochu. Foods 2020, 9, 1806. [Google Scholar] [CrossRef]
- Martínez-Martín, I.; Hernández-Jiménez, M.; Revilla, I.; Vivar-Quintana, A.M. Prediction of Mineral Composition in Wheat Flours Fortified with Lentil Flour Using NIR Technology. Sensors 2023, 23, 1491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Na Guo, X.; Zhu, K.X. Effect of phytic acid on the appearance of yellow alkaline noodles: Color and dark spots. J. Cereal Sci. 2024, 16, 103853. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Qian, H.; Zhang, H.; Li, Y.; Wu, G.; Qi, X. The effects of phosphate salts on the pasting, mixing and noodle-making performance of wheat flour. Food Chem. 2019, 283, 353–358. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Li, T.; Dam, R.M.; Willett, W.C. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006, 29, 650–656. [Google Scholar] [CrossRef]
- Yamanaka, C.; Aoe, S. The effects of dietary calcium levels on pancreatic function in KK Mice. J. Jpn. Soc. Nutr. Food Sci. 2018, 71, 75–82. [Google Scholar] [CrossRef]
- Nakamura, S.; Hasegawa, M.; Kobayashi, Y.; Komata, C.; Katsura, J.; Maruyama, Y.; Ohtsubo, K. Palatability and bio-functionality of chalky grains generated by high-temperature ripening and development of formulae for estimating the degree of damage using a rapid visco analyzer of Japonica unpolished rice. Foods 2022, 11, 3422. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ohtsubo, K. Effects of hard water boiling on chalky rice in term of texture improvement and Ca fortification. Foods 2023, 12, 2510. [Google Scholar] [CrossRef]
- Kömürcü, T.C. Usage of primitive wheat (Triticum monococcum and Triticum dicoccum) flour and whole egg in noodle production. Food Sci. Technol. Int. 2023, 29, 5. [Google Scholar] [CrossRef]
- Zi, Y.; Cheng, D.; Li, H.; Guo, J.; Ju, W.; Wang, C.; Humphreys, D.G.; Liu, A.; Cao, X.; Liu, C.; et al. Effects of the different waxy proteins on starch biosynthesis, starch physicochemical properties and Chinese noodle quality in wheat. Mol. Breed. 2022, 42, 23. [Google Scholar] [CrossRef]
- Inokuma, T.; Vrinten, P.; Shimbata, T.; Sunohara, A.; Fujita, M.; Nakamura, K.; Ishikawa, N.; Takata, K.; Kiribuchi-Otobe, C.; Nakamura, T. Longer Bread Shelf-Life and Improved Noodle-Making Properties Imparted by a Novel Wheat Genotype Are Stable in Different Genetic Backgrounds. J. Agric. Food Chem. 2021, 69, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Hai-Teng Li, H.T.; Li, Z.; Fox, G.P.; Gidley, M.J.; Dhital, S. Protein-starch matrix plays a key role in enzymic digestion of high-amylose wheat noodle. Food Chem. 2021, 336, 127719. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Imai, S.; Sato, T.; Kaneko, Y.; Otsubo, H. Quality characteristics of soft wheats and their use in Japan. I. Methods of assessing wheat suitability for Japanese products. Cereal Chem. 1976, 53, 988–997. [Google Scholar]
- Toyokawa, H.; Rubenthaler, G.L.; Powers, J.R.; Schanus, E.G. Japanese noodle qualities. I. Flour components. Cereal Chem. 1989, 66, 382–386. [Google Scholar]
- Pulliainen, T.K.; Wallin, H.C. Determination of total phosphorus in foods by colorimetric measurement of phosphorus as molybdenum blue after dry-ashing: NMKL interlaboratory study. J. AOAC Int. 1994, 77, 1557–1561. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sawada, S.; Onogaki, I. Effects of quality and quantity of alkali solution on the properties of rice starch. Denpun. Kagaku. 1981, 28, 241–244. [Google Scholar]
- Juliano, B.O.; Onate, L.M.; Mundo, A.M. A simplified assay for milled rice amylose. Food Technol. 1965, 19, 1006–1011. [Google Scholar]
- Nakamura, S.; Satoh, H.; Ohtsubo, K. Development of formulae for estimating amylose content, amylopectin chain length distribution, and resistant starch content based on the iodine absorption curve of rice starch. Biosci. Biotechnol. Biochem. 2015, 79, 443–455. [Google Scholar] [CrossRef]
- Toyoshima, H.; Okadome, H.; Ohtsubo, K.; Suto, M.; Horisue, N.; Inatsu, O.; Narizuka, A.; Aizaki, M.; Inouchi, N.; Fuwa, H. Cooperative test on the small-scale rapid method for the gelatinization properties test of rice flours with a rapid visco analyser. Nippon. Shokuhin Kogakukaishi 1997, 44, 579–584. [Google Scholar] [CrossRef]
- Nakamura, S.; Katsura, J.; Kato, K.; Ohtsubo, K. Development of formulae for estimating amylose content and resistant starch content based on the pasting properties measured by RVA of Japonica polished rice and starch. Biosci. Biotechnol. Biochem. 2016, 2, 329–340. [Google Scholar] [CrossRef]
- Okadome, H.; Toyoshima, H.; Sudo, M.; Ando, I.; Numaguchi, K.; Ohtsubo, K. Palatability evaluation for Japonica rice grains based on multiple physical measurements of individual cooked rice grain. J. Jpn. Soc. Food Sci. Technol. 1998, 45, 398–407. (In Japanese) [Google Scholar] [CrossRef]
- McCleary, B.M.; Monaghan, D.A. Measurement of resistant starch. J. AOAC Int. 2002, 85, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Satoh, H.; Ohtsubo, K. Characteristics of pregelatinized ae mutant rice flours prepared by boiling after preroasting. J. Agric. Food Chem. 2011, 59, 10665–10676. [Google Scholar] [CrossRef]
- Nagao, S. Wheat Science, 6th ed.; Asakura Bookstore: Tokyo, Japan, 2006; p. 49. [Google Scholar]
- Piironen, V.; Lampi, A.M.; Ekholm, P.; Salmenkallio-Marttila, M.; Liukkonen, K.H. Chapter 7. Minerals and trace elements. In WHEAT: Chemistry and Technology; Scieentific Societies: St. Paul, MN, USA, 2009; pp. 193–196. [Google Scholar]
- Balint, A.F.; Kovacs, G.; Erdei, L.; Sutka, J. Comparison of the Cu, Zn, Fe, Ca and Mg contents of the grains of wild, ancient and cultivated wheat species. Cereal Res. Commun. 2001, 29, 375–382. [Google Scholar] [CrossRef]
- Akman, Z.; Kara, B. Genotypic variations for mineral content at different growth stages in wheat (Triticum aestivum L.). Cereal Res. Commun. 2003, 31, 459–466. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Engelsen, S.B. Starch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Hizukuri, S.; Takeda, Y.; Matsubayashi, T. The effect of phosphorus in starch granules on raw starch digestion by bacterial alpha-amylase. J. Jpn. Soc. Starch Sci. 1979, 26, 112–116. [Google Scholar] [CrossRef]
- Borkowska-Burnecka, J.; Leśniewicz, A.; Zyrnicki, W. Comparison of pneumatic and ultrasonic nebulizations in inductively coupled plasma atomic emission spectrometry–matrix effects and plasma parameters. Spectrochim. Acta B 2006, 61, 579–587. [Google Scholar] [CrossRef]
- Zeng, M.; Morris, C.F.; Batey, I.L.; Wrigley, C.W. Sources of variation for starch gelatinization, pasting, and gelation properties in wheat. Cereal Chem. 1997, 74, 63. [Google Scholar] [CrossRef]
- Medcalf, D.G.; Gilles, K.A. Wheat starches 1. Comparison of physicochemical properties. Cereal Chem. 1965, 42, 558–568. [Google Scholar]
- Inouchi, N.; Hibiu, H.; Horibata, T.; Fuwa, H.; Itami, T. Structure and properties of endosperm starches from cultivated rice of Asia and other countries. J. Appl. Glycosci. 2005, 52, 239–246. [Google Scholar] [CrossRef]
- Hirano, H.; Sano, Y. Enhancement of Wx gene expression and the accumulation of amylose in response to cool temperature during seed development in rice. Plant Cell Physiol. 1998, 39, 807–812. [Google Scholar] [CrossRef]
- Yamamori, M.; Fufita, S.; Hayakawa, K.; Matsuki, J.; Yasui, T. Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. T. Heor. Appl. Genet. 2000, 101, 21–29. [Google Scholar] [CrossRef]
- Takeda, Y.; Hizukuri, S.; Juliano, B.O. Structures of rice amylopectins with low and high affinities for iodine. Carbohydr. Res. 1987, 168, 79–88. [Google Scholar] [CrossRef]
- Duffus, C.M.; Murdoch, S.M. Variation in starch granule size distribution and amylose content during wheat endosperm development. Cereal Chem. 1979, 56, 427–429. [Google Scholar]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef]
- Igarashi, T.; Yanagihara, T.; Kanda, H.; Kawamoto, K.; Masaki, K. Development of new eating quality evaluation method based on iodine adsorption multispectral analysis of rice flour. J. Crop Sci. 2009, 78, 66–73. [Google Scholar]
- Nakamura, S.; Yamaguchi, H.; Benitani, Y.; Ohtsubo, K. Development of a novel formula for estimating the amylose content of starch using Japonica milled rice flours based on the iodine absorption curve. Biosci. Biotechnol. Biochem. 2020, 84, 2347–2359. [Google Scholar] [CrossRef]
- Appels, R. Insights into noodle quality attributes in wheat. Chem. Aust. 1998, 65, 4–7. [Google Scholar]
- Nakamura, S.; Katsura, J.; Maruyama, Y.; Ohtsubo, K. Relationship between fatty acid composition and starch properties of 30 japonica rice cultivars. Cereal Chem. 2018, 96, 228–242. [Google Scholar] [CrossRef]
- Takeda, T.; Hizukuri, S. Location of phosphate groups in potato amylopectin. Carbohydr. Res. 1982, 102, 321–327. [Google Scholar] [CrossRef]
- Wren, J.J.; Merryfield, D.S. Firmly-bound’ lysolecithin of wheat starch. J. Sci. Food Agric. 1970, 21, 254. [Google Scholar] [CrossRef]
- Fujino, Y. Complex Lipid in Food. Food Hyg. Saf. Sci. 1972, 13, 257–271. [Google Scholar] [CrossRef]
- Kim, H.O.; Hill, R.D. Physical characteristics of wheat starch granule gelatinization in the presence of cyclohepta-amylose. Cereal Chem. 1984, 61, 432–435. [Google Scholar]
- Lin Jane, J. Current understanding on starch granule structures. J. Appl. Glycosci. 2006, 53, 205–213. [Google Scholar] [CrossRef]
- Kaneko, K.; Ota, K.; Sumino, T.; Maeda, Y. Effect of anions on binding between calcium and pectic substance. J. Nutr. Sci. Vitaminol. 1989, 42, 391–395. [Google Scholar]
- Kainuma, K.; Yamamoto, K.; Suzuki, S.; Takaya, K.; Fuwa, H. Studies on structure and physico-chemical properties of starch. Part IV. Structural, chemical and rheological properties of air classified small-and large granule potato starch. J. Jpn. Soc. Starch Sci. 1978, 25, 3–11. [Google Scholar] [CrossRef]
- Kainuma, K.; Miyamoto, S.; Yoshioka, S.; Suzuki, S. Studies on structure and physico-chemical properties of starch. J. Jpn. Soc. Starch Sci. 1976, 23, 59–66. [Google Scholar] [CrossRef]
- Nakamura, S.; Satoh, A.; Aizawa, M.; Ohtsubo, K. Characteristics of physicochemical properties of chalky grains of Japonica rice generated by high temperature during ripening. Foods 2022, 11, 97. [Google Scholar] [CrossRef]
- Nakamura, S.; Katsura, J.; Maruyama, Y.; Ohtsubo, K. Evaluation of hardness and retrogradation of cooked rice based on its pasting properties using a novel RVA testing. Foods 2021, 10, 987. [Google Scholar] [CrossRef]
- Nakamura, S.; Katsura, J.; Suda, A.; Maruyama, Y.; Ohtsubo, K. Effects of binding between Ca in hard water and phosphorus in amylopectin on the qualities of boiled rice and rice noodles prepared by soaking and boiling in hard water. Foods 2024, 13, 2094. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Takasu, A.; Yamada, E.; Tsukamoto, Y.; Furukawa, Y. Characterization of solubilization of insoluble calcium and magnesium with various kinds of vinegar. Nippon Shokuhin Kagaku Kogakukaishi 1998, 45, 655–662. [Google Scholar] [CrossRef]
- Southgate, D.A.T. Minerals trace elements, and potential hazards. Am. J. Clin. Nutr. 1987, 45, 1256–1266. [Google Scholar] [CrossRef]
- Yanaka, M.; Takata, K.; Funatsuki, W.; Ishikawa, N.; Takahashi, T. Effects of the composition of glutenin subunits controlled by the Glu-A1 and Glu-D1 and protein content on the noodle quality in Japanese soft wheat. Jpn. J. Crop Sci. 2017, 86, 169–176. [Google Scholar] [CrossRef]
- Oda, M.; Yasuda, Y.; Okazaki, S.; Yamauchi, Y.; Yokohama, Y. A method of flour quality assessment for Japanese noodles. Cereal Chem. 1980, 57, 253–254. [Google Scholar]
- Crosbie, G.B.; Lambe, W.J.; Tsutsui, H.; Gilmour, R.F. Further evaluation of the flour swelling volume test for identifying wheats potentially suitable for Japanese noodles. J. Cereal Sci. 1992, 15, 271–280. [Google Scholar] [CrossRef]
- Crosbie, G.B. The relationship between starch swelling properties, paste viscosity and boiled noodle quality in wheat flours. J. Cereal Sci. 1991, 13, 145–150. [Google Scholar] [CrossRef]
- Noda, T. The preparation and food applications of divalent cation-substituted potato starch. J. Biorheol. 2021, 35, 2–9. [Google Scholar] [CrossRef]
- Noda, T.; Takigawa, S.; Matsuura-Endo, C.; Ishiguro, K.; Nagasawa, K.; Jinno, M. Properties of Calcium-fortified potato starch prepared by immersion in natural mineral water and its food application. J. Appl. Glycosci. 2015, 62, 159–164. [Google Scholar] [CrossRef]
- KeeBaik, B.; Czuchajowska, Z.; Pomeranz, Y. Discoloration of dough for oriental noodles. Cereal Chem. 1995, 72, 198–205. [Google Scholar]
- Paznocht, L.; Kotikova, Z.; Orsak, M.; Lachman, J. Carotenoid changes of colored-grain wheat flours during bun-making. Food Chem. 2019, 277, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Hou, G. Oriental noodles. Adv. Food Nutr. 2001, 43, 141–193. [Google Scholar]
- Ito, M.; Ohta, K.; Nishio, Z.; Tabiki, T.; Hashimoto, N.; Funatsuki, W.; Miura, H.; Yamauchi, H. Quality evaluation of yellow alkaline noodles made from the KItanokaori wheat cultivar. Food Sci. Technol. Res. 2007, 13, 253–260. [Google Scholar] [CrossRef]
- Sajilate, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch: A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Nagahata, Y.; Kobayashi, I.; Goto, M.; Nakaura, Y.; Inouchi, N. The formation of resistant starch during acid hydrolysis of high-amylose corn starch. J. Appl. Glycosci. 2013, 60, 123–130. [Google Scholar] [CrossRef]
- Shimada, R.; Yoshimura, M. Effect of resistant starch type 4 from different starch sources on the physical properties and palatability of bread. J. Biorheol. 2021, 35, 10–17. [Google Scholar] [CrossRef]
- Tabiki, T.; Takata, K.; Nishio, Z.; Kuwabara, T.; Ozeki, S.; Tabaya, S.; Iriki, N.; Yamauchi, H.; Ichinose, Y. “Kitanokaori”: A new winter wheat variety. Res. Bull. Nalt. Agric. Res. Cent. Hokkaido Reg. 2006, 185, 1–12. [Google Scholar]
- Rouau, X.; Moreau, D. Modification of some physicochemical properties of wheat flour pentosans by an enzyme complex recommended for baking. Cereal Chem. 1993, 70, 626–632. [Google Scholar]
- Michniewicz, J.; Biliaderis, G.G.; Bushuk, W. Effect of added pentosans on some properties of wheat bread. Food Res. Technol. 2001, 212, 374–376. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hino, A.; Yamaguchi, Y.; Kai, T. Isolation and characterization of pentosans from some species of wheat flour. Bull. Nakamura Gakuen Univ. 1999, 31, 157–162. [Google Scholar]
- Shogren, M.D.; Hashimoto, S.; Pomeranz, Y. Cereal pentosans: Their estimation and significance. II. Pentosans and breadmaking characteristics of hard red winter wheat flours. Cereal Chem. 1987, 64, 35–38. [Google Scholar]
- Shibuya, N.; Misaki, A. Structure of hemicellulose isolated from rice endosperm cell wall: Mode of linkages and sequences in xyloglucan, β-glucan and arabinoxylan. Agric. Biol. Chem. 1978, 42, 2267–2274. [Google Scholar]
- Ogata, M.; Iwasaki, N.; Uchigata, Y. Osteoporosis as a Complication of Diabetes; Society of Tokyo Women’s Medical University: Tokyo, Japan, 2017; pp. E142–E147. [Google Scholar]
- Farlay, D.; Armas, L.A.G.; Gineys, E.; Akhter, M.P.; Recker, R.R.; Boivin, G. Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus. J. Bone Miner Res. 2016, 31, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castano-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal comolications of inadequate glucose control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
| Phosphorus | |
| contents | |
| (mg/100 g) | |
| Yumechikara | 132.0 ± 2.6 a |
| Minaminokaori | 109.0 ± 1.5 c |
| Haruyokoi | 126.0 ± 3.2 b |
| Kitanokaori | 127.0 ± 3.0 b |
| Kitahonami | 92.0 ± 2.1 d |
| Sanukinoyume | 75.0 ± 1.8 e |
| Cultivars | AAC | λmax | Aλmax | λmax/ | Fb3 |
|---|---|---|---|---|---|
| (%) | Aλmax | (DP ≧ 37) (%) | |||
| Yumechikara | 25.6 ± 0.5 c | 598.0 ± 2.8 a | 0.440 ± 0.003 c | 1359.1 ± 2.3 a | 18.9 ± 0.1 b |
| Minamnoikaori | 28.0 ± 0.6 a | 601.0 ± 2.8 a | 0.469 ± 0.004 a | 1282.8 ± 3.6 d | 20.2 ± 0.2 a |
| Haruyokoi | 27.2 ± 0.1 b | 599.0 ± 1.4 a | 0.461 ± 0.001 a | 1300.8 ± 5.1 c | 19.8 ± 0.0 a |
| Kitanokaori | 25.9 ± 0.3 c | 600.0 ± 0.0 a | 0.441 ± 0.004 c | 1360.6 ± 13.1 a | 18.9 ± 0.2 b |
| Kitahonami | 26.7 ± 0.3 b | 601.5 ± 0.7 a | 0.450 ± 0.005 b | 1338.2 ± 16.3 b | 19.3 ± 0.2 b |
| Sanukinoyume | 26.6 ± 0.7 b | 600.0 ± 2.8 a | 0.450 ± 0.006 b | 1333.4 ± 10.5 b | 19.3 ± 0.3 b |
| Max. vis | Mini. vis | BD | Fin. vis | SB | |
|---|---|---|---|---|---|
| (cP) | (cP) | (cP) | (cP) | (cP) | |
| Yumechikara (Purified water) | 2868.0 ± 25.5 a | 1263.5 ± 10.6 a | 1604.5 ± 14.8 b | 2609.5 ± 7.8 a | −258.5 ± 17.7 a |
| Yumechikara (Contrex) (pH 4.6) | 3165.0 ± 227.7 a | 1206.5 ± 46.0 b | 1958.5 ± 181.7 a | 2528.0 ± 113.1 a | −637.0 ± 114.6 b |
| Minamikaori (Purified water) | 1781.5 ± 36.1 b | 844.5 ± 26.2 a | 937.0 ± 9.9 a | 1991.0 ± 62.2 b | −209.5 ± 26.2 a |
| Minamikaori (Contrex) (pH 4.6) | 2098.0 ± 4.2 a | 1173.0 ± 5.7 b | 925.0 ± 9.9 a | 2369.0 ± 5.7 a | −271.0 ± 1.4 b |
| Haruyokoi (Purified water) | 3139.0 ± 7.1 b | 1304.0 ± 17.0 a | 1835.0 ± 9.9 b | 2662.0 ± 25.5 a | −477.0 ± 18.4 a |
| Haruyokoi (Contrex) (pH 4.6) | 3230.5 ± 2.1 a | 1181.5 ± 13.4 b | 2049.0 ± 15.6 a | 2486.5 ± 16.3 b | −744.0 ± 18.4 b |
| Kitanokaori (Purified water) | 2772.0 ± 38.2 b | 1214.5 ± 10.6 a | 1557.5 ± 27.6 b | 2589.5 ±23.3 a | −182.5 ± 14.8 a |
| Kitanokaori (Contrex) (pH 4.6) | 3091.0 ± 26.9 a | 1115.0 ± 7.1 b | 1976.0 ± 19.8 a | 2513.5 ± 13.4 b | −577.5 ± 13.4 b |
| Kitahonami (Purified water) | 3177.0 ± 19.8 b | 1381.5 ± 4.9 a | 1795.5 ± 14.8 b | 2834.5 ± 6.4 a | −342.5 ± 13.4 a |
| Kitahonami (Contrex) (pH 4.6) | 3642.5 ± 244.0 a | 1386.5 ± 47.4 a | 2256.0 ± 196.6 a | 2856.0 ± 93.3 a | −786.5 ± 150.6 b |
| Sanukinoyume (Purified water) | 4068.0 ± 9.9 b | 1580.5 ± 19.1 a | 2487.5 ± 9.2 b | 3163.0 ± 32.5 a | −905.0 ± 22.6 a |
| Sanukinoyume (Contrex) (pH 4.6) | 4107.0 ± 1.4 a | 868.8 ± 0.4 b | 2706.0 ± 14.1 a | 2905.5 ± 2.1 b | −1201.5 ± 3.5 b |
| Pt | Cons | Set/Cons | Max/Min | Max/Fin | |
| (°C) | (°C) | ||||
| Yumechikara (Purified water) | 50.5 ± 0.3 b | 1346.0 ± 2.8 a | −0.19 ± 0.01 a | 2.27 ± 0.00 b | 1.10 ± 0.01 b |
| Yumechikara (Contrex) (pH 4.6) | 59.7 ± 0.5 a | 1321.5 ± 67.2 a | −0.48 ± 0.06 b | 2.62 ± 0.09 a | 1.25 ± 0.03 a |
| Minamikaori (Purified water) | 53.5 ± 4.7 a | 1146.5 ± 36.1 b | −0.18 ± 0.02 a | 2.11 ± 0.02 a | 0.89 ± 0.01 a |
| Minamikaori (Contrex)(pH 4.6) | 59.5 ± 0.6 a | 1196.0 ± 11.3 a | −0.23 ± 0.00 b | 1.79 ± 0.01 b | 0.89 ± 0.00 a |
| Haruyokoi (Purified water) | 50.7 ± 0.0 b | 1358.0 ± 8.5 a | −0.35 ± 0.02 a | 2.41 ± 0.03 b | 1.18 ± 0.01 b |
| Haruyokoi (Contrex) (pH 4.6) | 59.5 ± 0.3 a | 1305.0 ± 2.8 b | −0.57 ± 0.02 b | 2.73 ± 0.03 a | 1.30 ± 0.01 a |
| Kitanokaori (Purified water) | 55.7 ± 0.1 a | 1375.0 ± 12.7 a | −0.13 ± 0.01 a | 2.28 ± 0.01 b | 1.07 ± 0.01 b |
| Kitanokaori (Contrex) (pH 4.6) | 58.0 ± 1.3 b | 1398.5 ± 6.4 a | −0.41 ± 0.01 b | 2.77 ± 0.01 a | 1.23 ± 0.00 a |
| Kitahonami (Purified water) | 51.2 ± 0.9 b | 1453.0 ± 1.4 a | −0.24 ± 0.01 a | 2.30 ± 0.01 b | 1.12 ± 0.00 b |
| Kitahonami (Contrex) (pH 4.6) | 59.7 ± 0.6 a | 1469.5 ± 46.0 a | −0.53 ± 0.09 b | 2.63 ± 0.09 a | 1.27 ± 0.04 a |
| Sanukinoyume (Purified water) | 50.2 ± 0.0 b | 1582.5 ± 13.4 a | −0.57 ± 0.02 a | 2.57 ± 0.02 b | 1.29 ± 0.01 b |
| Sanukinoyume (Contrex) (pH 4.6) | 59.4 ± 0.6 a | 1504.5 ± 10.6 b | −0.80 ± 0.00 b | 2.93 ± 0.03 a | 1.41 ± 0.00 a |
| DF | RS | P | Ca | Tende | Pliab | Tough | Britt | Hard | Max. vis | Mini. vis | BD | Fin. vis | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dietary fiber | 1.00 | ||||||||||||
| RS | 0.82 ** | 1.00 | |||||||||||
| P | 0.38 | 0.58 * | 1.00 | ||||||||||
| Ca | 0.15 | 0.11 | 0.06 | 1.00 | |||||||||
| Tenderness | −0.14 | −0.38 | −0.78 ** | −0.35 | 1.00 | ||||||||
| Pliability | −0.51 | −0.71 ** | −0.39 | 0.04 | 0.08 | 1.00 | |||||||
| Toughness | 0.02 | −0.32 | −0.57 | −0.32 | 0.91 ** | 0.05 | 1.00 | ||||||
| Brittleness | −0.30 | −0.19 | −0.37 | 0.18 | −0.06 | 0.01 | −0.28 | 1.00 | |||||
| Hardness | 0.18 | −0.04 | 0.44 | 0.04 | −0.24 | 0.29 | 0.13 | −0.69 * | 1.00 | ||||
| Max. vis | −0.30 | −0.23 | −0.52 | 0.04 | 0.57 | −0.25 | 0.39 | 0.39 | −0.63 * | 1.00 | |||
| Mini. vis | −0.35 | −0.39 | −0.57 | −0.20 | 0.66 * | −0.21 | 0.46 | 0.37 | −0.66 * | 0.85 ** | 1.00 | ||
| BD | −0.27 | −0.16 | −0.46 | 0.12 | 0.50 | −0.25 | 0.34 | 0.37 | −0.57 | 0.98 ** | 0.74 ** | 1.00 | |
| Fin. vis | −0.29 | −0.29 | −0.60 * | −0.20 | 0.69 * | −0.27 | 0.48 | 0.40 | −0.72 ** | 0.90 ** | 0.98 ** | 0.81 ** | 1.00 |
| SB | 0.28 | 0.16 | 0.41 | −0.21 | −0.42 | 0.22 | −0.29 | −0.34 | 0.50 | −0.96 ** | −0.66 * | −0.99 ** | −0.74 ** |
| Pt | 0.19 | −0.17 | −0.06 | 0.76 ** | −0.28 | 0.24 | −0.23 | 0.29 | −0.01 | 0.03 | −0.09 | 0.07 | −0.14 |
| Cons | −0.18 | −0.13 | −0.59 * | −0.19 | 0.69 * | −0.33 | 0.47 | 0.42 | −0.75 ** | 0.92 ** | 0.89 ** | 0.87 ** | 0.96 ** |
| Set/cons | 0.28 | 0.12 | 0.32 | −0.22 | −0.34 | 0.26 | −0.22 | −0.33 | 0.48 | −0.94 ** | −0.63* | −0.98 ** | −0.70 * |
| Max/Min | −0.11 | 0.09 | −0.19 | 0.30 | 0.20 | −0.29 | 0.11 | 0.27 | −0.39 | 0.80 ** | 0.37 | 0.89 ** | 0.49 |
| Max/Fin | −0.26 | −0.09 | −0.29 | 0.24 | 0.32 | −0.28 | 0.20 | 0.32 | −0.49 | 0.93 ** | 0.62 * | 0.97 ** | 0.69 * |
| AAC | −0.31 | −0.36 | −0.26 | 0.10 | −0.15 | 0.56 | −0.15 | −0.06 | 0.24 | −0.45 | −0.32 | −0.46 | −0.40 |
| λmax | −0.11 | −0.14 | −0.60 * | 0.01 | 0.17 | 0.30 | −0.06 | 0.47 | −0.42 | −0.13 | 0.00 | −0.17 | 0.02 |
| Aλmax | −0.32 | −0.36 | −0.17 | 0.10 | −0.19 | 0.56 | −0.15 | −0.15 | 0.34 | −0.46 | −0.35 | −0.46 | −0.44 |
| λmax/Aλmax | 0.33 | 0.37 | 0.13 | −0.10 | 0.21 | −0.55 | 0.15 | 0.19 | −0.38 | 0.45 | 0.35 | 0.45 | 0.44 |
| Fb3 | −0.32 | −0.36 | −0.17 | 0.10 | −0.19 | 0.56 | −0.15 | −0.15 | 0.34 | −0.46 | −0.35 | −0.46 | −0.44 |
| SB | Pt | Cons | Set/Cons | Max/Min | Max/Fin | AAC | λmax | Aλmax | λmax/Aλmax | Fb3 | |||
| SB | 1.00 | ||||||||||||
| Pt | −0.15 | 1.00 | |||||||||||
| Cons | −0.80 ** | −0.21 | 1.00 | ||||||||||
| Set/cons | 0.99 ** | −0.16 | −0.76 ** | 1.00 | |||||||||
| Max/Min | −0.93 ** | 0.13 | 0.63 * | −0.94 ** | 1.00 | ||||||||
| Max/Fin | −0.99 ** | 0.16 | 0.75 ** | −1.00** | 0.95 ** | 1.00 | |||||||
| AAC | 0.42 | 0.17 | −0.49 | 0.46 | −0.47 | −0.47 | 1.00 | ||||||
| λmax | 0.23 | 0.06 | 0.06 | 0.29 | −0.28 | −0.30 | 0.53 | 1.00 | |||||
| Aλmax | 0.42 | 0.18 | −0.54 | 0.45 | −0.46 | −0.46 | 0.99 ** | 0.39 | 1.00 | ||||
| λmax/Aλmax | −0.40 | −0.18 | 0.55 | −0.42 | 0.44 | 0.44 | −0.97 ** | −0.32 | −1.00 ** | 1.00 | |||
| Fb3 | 0.42 | 0.18 | −0.54 | 0.45 | −0.46 | −0.46 | 0.99 ** | 0.39 | 1.00 | −1.00 ** | 1.00 |
| Tenderness | Pliability | Toughness | Brittleness | Hardness | |
|---|---|---|---|---|---|
| (N/cm2) | (N/cm2) | (N/cm2) | |||
| Yumechikara (Purified water) | 910.9 ± 142.7 a | 1.02 ± 0.11 a | 357.2 ± 61.4 a | 1.75 ± 0.17 a | 17.33 ± 2.52 a |
| Yumechikara (Contrex) (pH 4.6) | 691.6 ± 108.4 b | 0.99 ± 0.01 a | 268.6 ±49.1 b | 1.73 ± 0.07 a | 16.67 ± 0.58 a |
| Minamikaori (Purified water) | 762.7 ± 48.4 a | 1.12 ± 0.02 a | 304.8 ± 2.8 a | 1.53 ± 0.03 a | 19.33 ± 1.15 a |
| Minamikaori (Contrex) (pH 4.6) | 744.0 ± 63.7 a | 1.06 ± 0.06 a | 266.3 ± 63.4 a | 1.79 ± 0.28 a | 16.33 ± 3.51 a |
| Haruyokoi (Purified water) | 611.4 ± 62.9 a | 1.00 ± 0.04 a | 184.3 ± 18.9 b | 2.05 ± 0.06 a | 13.33 ± 0.58 a |
| Haruyokoi (Contrex) (pH 4.6) | 675.7 ± 58.1 a | 0.97 ± 0.04 a | 268.2 ± 37.6 a | 1.76 ± 0.16 a | 16.67 ± 1.53 a |
| Kitanokaori (Purified water) | 841.1 ± 218.8 a | 0.92 ± 0.06 a | 302.0 ± 117.9 a | 2.09 ± 0.41 a | 14.00 ± 3.61 a |
| >Kitanokaori (Contrex) (pH 4.6) | 714.6 ± 91.8 a | 0.91 ± 0.02 a | 229.7 ± 26.8 a | 2.15 ± 0.19 a | 13.00 ± 2.00 a |
| Kitahonami (Purified water) | 1106.4 ± 457.0 a | 1.04 ± 0.03 a | 289.5 ± 149.6 a | 2.42 ± 0.67 a | 11.33 ± 3.51 a |
| Kitahonami (Contrex) (pH 4.6) | 727.8 ± 447.8 b | 1.02 ± 0.06 a | 212.9 ± 146.9 b | 5.35 ± 4.70 a | 10.67 ± 7.51 a |
| Sanukinoyume (Purified water) | 1530.8 ± 329.3 a | 0.96 ± 0.03 a | 539.1 ± 181.2 a | 1.94 ± 0.31 a | 14.00 ± 3.00 a |
| Sanukinoyume (Contrex) (pH 4.6) | 1250.5 ± 638.0 a | 1.10 ± 0.10 a | 392.2 ± 226.1 b | 2.37 ± 1.05 a | 13.33 ± 6.11 a |
| WB | ΔE (ab) | a* | b* | |
|---|---|---|---|---|
| Yumechikara (Purified water) | 19.6 ± 2.0 b | 42.0 ± 2.0 a | −1.1 ± 0.1 a | 13.2 ± 0.7 a |
| Yumechikara (Contrex) (pH 4.6) | 24.5 ± 3.2 a | 37.2 ± 2.9 b | −1.4 ± 0.3 a | 12.8 ± 0.8 b |
| Minamikaori (Purified water) | 17.3 ± 1.1 b | 44.3 ± 1.2 a | −1.2 ± 0.0 a | 14.5 ± 0.3 a |
| Minamikaori (Contrex) (pH 4.6) | 21.3 ± 0.9 a | 40.1 ± 1.0 b | −1.2 ± 0.0 a | 13.8 ± 0.2 b |
| Haruyokoi (Purified water) | 21.4 ± 1.2 a | 40.4 ± 0.1 a | −1.3 ± 0.0 a | 12.1 ± 0.2 a |
| Haruyokoi (Contrex) (pH 4.6) | 22.3 ± 1.0 a | 39.6 ± 0.1 a | −1.1 ± 0.0 a | 11.6 ± 0.1 b |
| Kitanokaori(Purified water) | 22.2 ± 0.0 a | 39.1 ± 0.0 a | −1.3 ± 0.1 a | 14.1 ± 0.3 a |
| Kitanokaori (Contrex) (pH 4.6) | 22.3 ± 0.1 a | 38.0 ± 0.1 b | −1.4 ± 0.1 a | 14.7 ± 0.8 a |
| Kitahonami (Purified water) | 21.5 ± 1.5 b | 40.8 ± 2.1 a | −2.1 ± 0.1 a | 10.6 ± 1.8 a |
| Kitahonami (Contrex) (pH 4.6) | 26.2 ± 1.0 a | 36.1 ± 0.7 b | −2.0 ± 0.1 a | 10.8 ± 0.8 a |
| Sanukinoyume (Purified water) | 24.2 ± 1.7 b | 34.7 ± 1.4 a | −2.3 ± 0.1 a | 13.6 ± 0.3 a |
| Sanukinoyume (Contrex) (pH 4.6) | 29.8 ± 1.6 a | 33.0 ± 1.2 b | −2.5 ± 0.0 a | 11.3 ± 0.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Ohtsubo, K. Characteristics of Wheat Noodle “Kitanokaori” Using Weakly Acidic Hard Water in Terms of Functional Qualities, Such as Inhibiting Postprandial Abrupt Increase in Blood Glucose. Foods 2025, 14, 1044. https://doi.org/10.3390/foods14061044
Nakamura S, Ohtsubo K. Characteristics of Wheat Noodle “Kitanokaori” Using Weakly Acidic Hard Water in Terms of Functional Qualities, Such as Inhibiting Postprandial Abrupt Increase in Blood Glucose. Foods. 2025; 14(6):1044. https://doi.org/10.3390/foods14061044
Chicago/Turabian StyleNakamura, Sumiko, and Ken’ichi Ohtsubo. 2025. "Characteristics of Wheat Noodle “Kitanokaori” Using Weakly Acidic Hard Water in Terms of Functional Qualities, Such as Inhibiting Postprandial Abrupt Increase in Blood Glucose" Foods 14, no. 6: 1044. https://doi.org/10.3390/foods14061044
APA StyleNakamura, S., & Ohtsubo, K. (2025). Characteristics of Wheat Noodle “Kitanokaori” Using Weakly Acidic Hard Water in Terms of Functional Qualities, Such as Inhibiting Postprandial Abrupt Increase in Blood Glucose. Foods, 14(6), 1044. https://doi.org/10.3390/foods14061044







