Influence of Thermal Processing on In Vitro Starch Digestibility in Cereal-Based Infant Foods †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation and Autoclaving of Cereal Samples
2.2.2. Polarized and Light Microscopy
2.2.3. Rapid Visco-Analysis (RVA)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Infant Food Preparation
2.2.6. Phytic Acid Content in Infant Food
2.2.7. In Vitro Digestion of Infant Food
2.2.8. Statistical Analysis
3. Results and Discussion
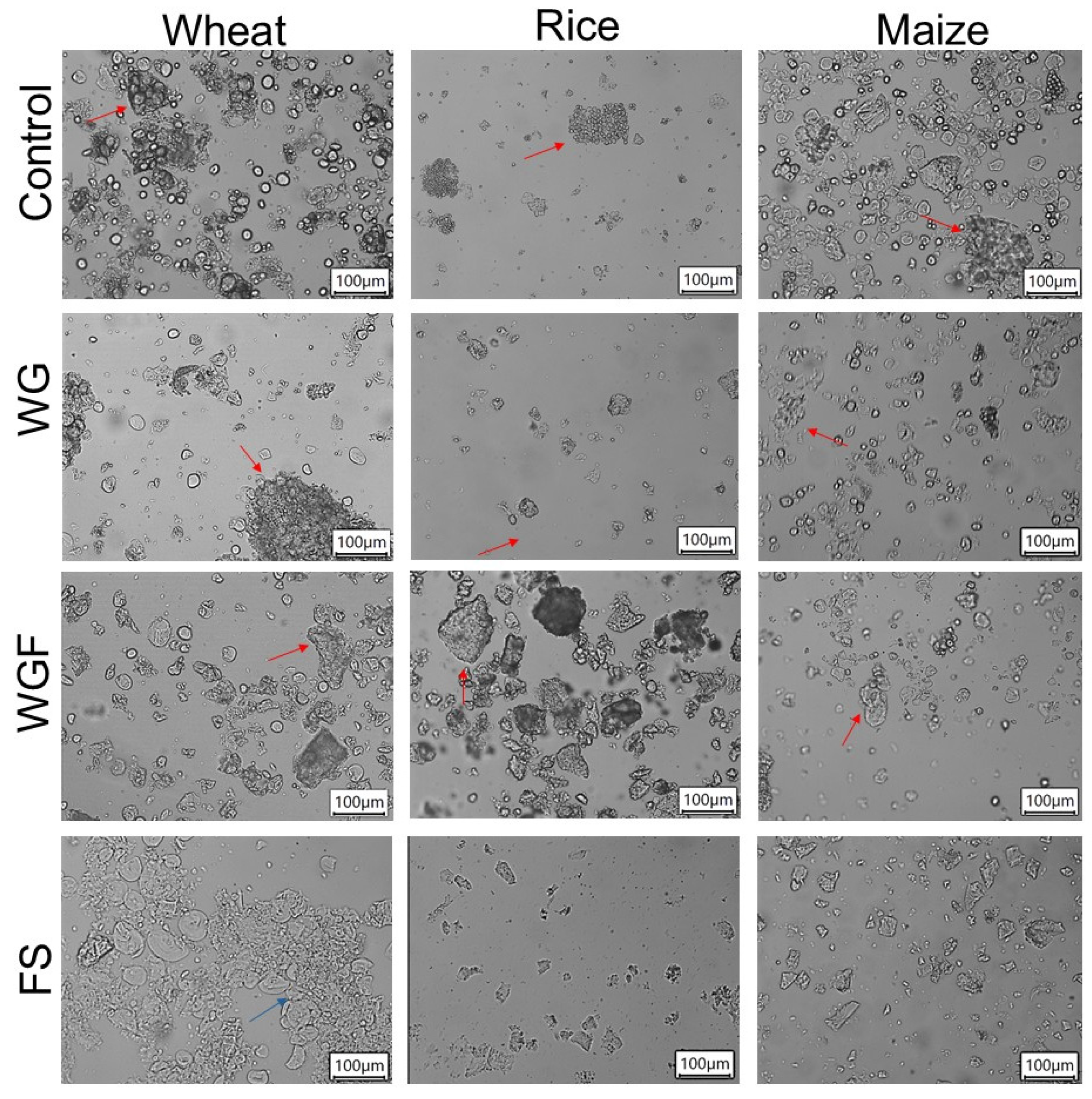
3.1. Optical Microscopy
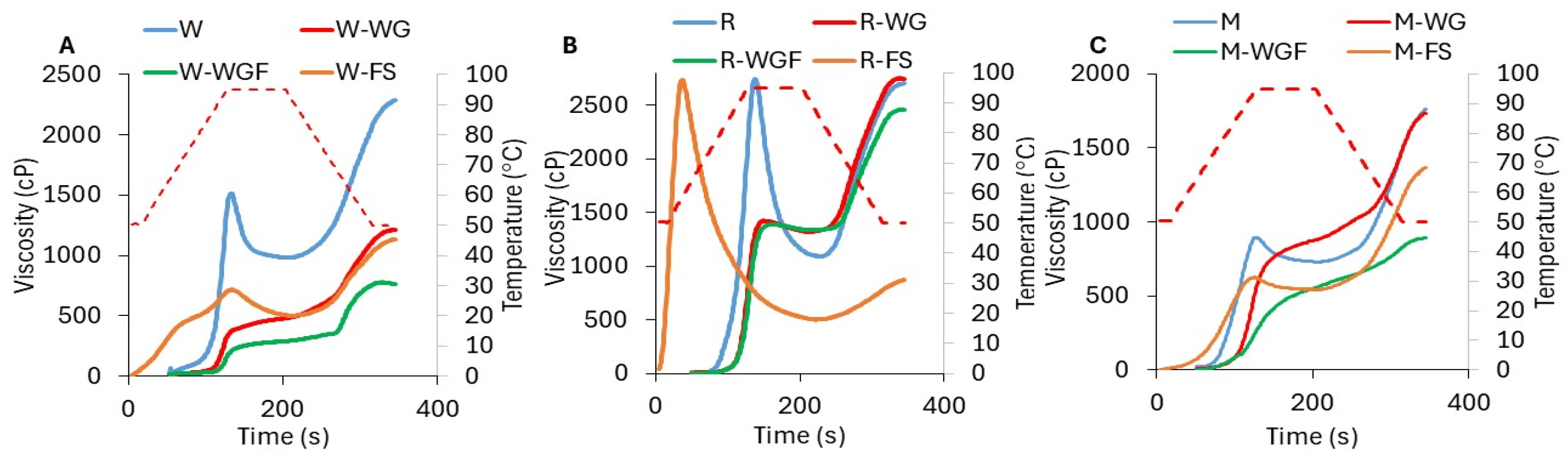
3.2. Pasting Properties of Samples
3.3. Thermal Properties of Samples
3.4. Phytic Acid Content in Infant Purees
3.5. In Vitro Digestion of Infant Purees
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WG | whole grains |
| WGF | Whole grain flours |
| FS | Flour suspension |
| THS | Total hydrolyzed starch |
| W | Wheat |
| R | Rice |
| M | Maize |
| RVA | Rapid Visco-Analysis |
| PV | Peak viscosity |
| BD | Breakdown |
| FV | Final viscosity |
| SB | Setback |
| tPV | Peak viscosity time |
| db. | Dry basis |
| DSC | Differential scanning calorimetry |
| Tp | Peak temperature |
| ΔH | Endotherm enthalpy |
| DNS | 3,5-dinitrosalicylic acid |
| RS | Resistant starch |
| ANOVA | Analysis of variance |
| PAP | Available phosphorus phytate |
References
- Rai, D.; Larson, B. Driving Research in Infant and Children’s Nutrition: A Perspective on Industry. Am. J. Clin. Nutr. 2009, 89, 1530S–1532S. [Google Scholar] [CrossRef] [PubMed]
- Were, F.N.; Lifschitz, C. Complementary Feeding: Beyond Nutrition. Ann. Nutr. Metab. 2018, 73, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.; Alles, M.; De Graaf, C.; Fleith, M.; Hadjilucas, E.; Isaacs, E.; Maffeis, C.; Zeinstra, G.; Matthys, C.; Gil, A. The Role and Requirements of Digestible Dietary Carbohydrates in Infants and Toddlers. Eur. J. Clin. Nutr. 2012, 66, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Gibson, R.S. Traditional Food-Processing and Preparation Practices to Enhance the Bioavailability of Micronutrients in Plant-Based Diets1. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef]
- Schafranski, K.; Cristina, V.; Gustavo, L. Impacts and Potential Applications: A Review of the Modification of Starches by Heat-Moisture Treatment (HMT). Food Hydrocoll. 2021, 117, 106690. [Google Scholar] [CrossRef]
- Niba, L.L. Processing Effects on Susceptibility of Starch to Digestion in Some Dietary Starch Sources. Int. J. Food Sci. Nutr. 2003, 54, 97–109. [Google Scholar] [CrossRef]
- Klerks, M.; Bernal, M.J.; Roman, S.; Bodenstab, S.; Gil, A.; Sanchez-Siles, L.M. Infant Cereals: Current Status, Challenges, and Future Opportunities for Whole Grains. Nutrients 2019, 11, 473. [Google Scholar] [CrossRef]
- Särkkä-Tirkkonen, M.; Väisänen, H.-M.; Beck, A. Overview on Different Sterilization Techniques for Baby Food. 2010. ISSN 1796-0630. Available online: https://orgprints.dk/id/eprint/17236/1/Reports60.pdf (accessed on 30 March 2025).
- Sevenich, R.; Kleinstueck, E.; Crews, C.; Anderson, W.; Pye, C.; Riddellova, K.; Hradecky, J.; Moravcova, E.; Reineke, K.; Knorr, D. High-Pressure Thermal Sterilization Food Safety and Food Quality of Baby Food. J. Food Sci. 2014, 79, 230–237. [Google Scholar] [CrossRef]
- Passannanti, F.; Nigro, F.; Gallo, M.; Tornatore, F.; Frasso, A.; Saccone, G.; Budelli, A.; Barone, M.V.; Nigro, R. In Vitro Dynamic Model Simulating the Digestive Tract of 6-Month-Old Infants. PLoS ONE 2017, 12, 0189807. [Google Scholar] [CrossRef]
- Özkaya, B.; Turksoy, S.; Özkaya, H.; Duman, B. Dephytinization of Wheat and Rice Brans by Hydrothermal Autoclaving Process and the Evaluation of Consequences for Dietary Fiber Content, Antioxidant Activity and Phenolics. Innov. Food Sci. Emerg. Technol. 2017, 39, 209–215. [Google Scholar] [CrossRef]
- Arns, B.; Bartz, J.; Radunz, M.; do Evangelho, J.A.; Pinto, V.Z.; da Rosa Zavareze, E.; Dias, A.R.G. Impact of Heat-Moisture Treatment on Rice Starch, Applied Directly in Grain Paddy Rice or in Isolated Starch. LWT 2015, 60, 708–713. [Google Scholar] [CrossRef]
- Mathobo, V.M.; Silungwe, H.; Ramashia, S.E.; Anyasi, T.A. Effects of Heat-Moisture Treatment on the Thermal, Functional Properties and Composition of Cereal, Legume and Tuber Starches—A Review. J. Food Sci. Technol. 2021, 58, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.D.; León, A.E.; Bustos, M.C. Starch Digestion in Infants: An Update of Available In Vitro Methods—A Mini Review. Plant Foods Hum. Nutr. 2022, 77, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G. The Challenge of Meeting Nutrient Needs of Infants and Young Children during the Period of Complementary Feeding: An Evolutionary. J. Nutr. 2013, 143, 2050–2054. [Google Scholar] [CrossRef]
- Auria, E.D.; Borsani, B.; Pendezza, E.; Bosetti, A.; Paradiso, L.; Zuccotti, G.V.; Verduci, E. Complementary Feeding: Pitfalls for Health Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 7931. [Google Scholar] [CrossRef]
- Miniello, V.L.; Verga, M.C.; Miniello, A.; Di Mauro, C.; Diaferio, L.; Francavilla, R. Complementary Feeding and Iron Status: “The Unbearable Lightness of Being” Infants. Nutrients 2021, 13, 4201. [Google Scholar] [CrossRef]
- Li, P.; Dhital, S.; Fu, X.; Huang, Q.; Liu, R.; Zhang, B.; He, X. Starch Digestion in Intact Pulse Cotyledon Cells Depends on the Extent of Thermal Treatment. Food Chem. 2020, 315, 126268. [Google Scholar] [CrossRef]
- Selma-Gracia, R.; Laparra, J.M.; Haros, C.M. Potential Beneficial Effect of Hydrothermal Treatment of Starches from Various Sources on in Vitro Digestion. Food Hydrocoll. 2020, 103, 105687. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Penci, M.C.; Calderón-Domínguez, G.; Ribotta, P.D. Chemical Composition and Physical Properties of Sorghum Flour Prepared from Different Sorghum Hybrids Grown in Argentina. Starch-Stärke 2016, 68, 1055–1064. [Google Scholar] [CrossRef]
- Rodriguez, M.D.; León, A.E.; Bustos, M.C. Co-Ingestion of Cereals and Legumes during Infant Complementary Feeding: Starch and Protein in Vitro Digestion. Plant Foods Hum. Nutr. 2024, 79, 489–496. [Google Scholar] [CrossRef]
- Navarro, J.L.; Richard, P.L.; Moiraghi, M.; Bustos, M.; León, A.E.; Steffolani, M.E. Effect of Different Wheat Sprouting Conditions on the Characteristics of Whole-Wheat Flour. Food Technol. Biotechnol. 2024, 62, 8435. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.S.; Parimalavalli, R. Effect of Autoclaving on Functional, Chemical, Pasting and Morphological Properties of Sweet Potato Starch. J. Root Crops 2013, 39, 78–83. [Google Scholar]
- Dundar, A.N.; Gocmen, D. Effects of Autoclaving Temperature and Storing Time on Resistant Starch Formation and Its Functional and Physicochemical Properties. Carbohydr. Polym. 2013, 97, 764–771. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Physical Modification of Starch. In Starch in Food: Structure, Function and Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 223–253. ISBN 9780081008966. [Google Scholar]
- Puncha-arnon, S.; Uttapap, D. Rice Starch vs. Rice Flour: Differences in Their Properties When Modified by Heat—Moisture Treatment. Carbohydr. Polym. 2013, 91, 85–91. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. A Review of the Hydrothermal Treatments Impact on Starch Based Systems Properties. Crit. Rev. Food Sci. Nutr. 2020, 60, 3890–3915. [Google Scholar] [CrossRef]
- Rodriguez, M.D.; León, A.E.; Bustos, M.C. Cereal-based infant purees: Effects of thermal treatments on the properties and in vitro starch digestion (Papi-llas infantiles a base de cereales: Efectos de tratamientos térmicos sobre las propiedades y la digestión in vitro del almidón). In Proceedings of the IX International Conference of Food Science and Technology (IX Congreso Internacional de Ciencia y Tecnología de los Alimentos), Córdoba, Argentina, 16–18 October 2024. [Google Scholar]
- Chung, H.; Hoover, R.; Liu, Q. The Impact of Single and Dual Hydrothermal Modifications on the Molecular Structure and Physicochemical Properties of Normal Corn Starch. Int. J. Biol. Macromol. 2009, 44, 203–210. [Google Scholar] [CrossRef]
- Ozturk, S.; Koksel, H.; Ng, P.K.W. Production of Resistant Starch from Acid-Modified Amylotype Starches with Enhanced Functional Properties. J. Food Eng. 2011, 103, 156–164. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Y.; Wen, L.; Yang, B.; Farag, M.A.; Jiang, Y. The Occurrence, Role, and Management Strategies for Phytic Acid in Foods. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13416. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant Food Anti-Nutritional Factors and Their Reduction Strategies: An Overview. Food Prod. Process. Nutr. 2020, 5, 6. [Google Scholar] [CrossRef]
- Liu, H.; Lv, M.; Wang, L.; Li, Y.; Fan, H.; Wang, M. Comparative Study: How Annealing and Heat-Moisture Treatment Affect the Digestibility, Textural, and Physicochemical Properties of Maize Starch. Starch/Staerke 2016, 68, 1158–1168. [Google Scholar] [CrossRef]
- Li, H.T.; Li, Z.; Fox, G.P.; Gidley, M.J.; Dhital, S. Protein-Starch Matrix Plays a Key Role in Enzymic Digestion of High-Amylose Wheat Noodle. Food Chem. 2021, 336, 127719. [Google Scholar] [CrossRef] [PubMed]
- De La Rosa-Millán, J. Physicochemical, Molecular, and Digestion Characteristics of Annealed and Heat-Moisture Treated Starches under Acidic, Neutral, or Alkaline PH. Cereal Chem. 2017, 94, 770–779. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Changes of Starch during Thermal Processing of Foods: Current Status and Future Directions. Trends Food Sci. Technol. 2022, 119, 320–337. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, B.; Zhang, W.; Shu, Z.; Wang, P.; Zeng, X. Structural and Functional Characteristics of Japonica Rice Starches with Different Amylose Contents. CYTA-J. Food 2021, 19, 532–540. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Kumar, A.; Sahu, C.; Panda, P.A.; Biswal, M.; Sah, R.P.; Lal, M.K.; Baig, M.J.; Swain, P.; Behera, L.; Chattopadhyay, K.; et al. Phytic Acid Content May Affect Starch Digestibility and Glycemic Index Value of Rice (Oryza sativa L.). J. Sci. Food Agric. 2020, 100, 1598–1607. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Hoover, R. Impact of Annealing and Heat-Moisture Treatment on Rapidly Digestible, Slowly Digestible and Resistant Starch Levels in Native and Gelatinized Corn, Pea and Lentil Starches. Carbohydr. Polym. 2009, 75, 436–447. [Google Scholar] [CrossRef]
- Englyst, K.; Goux, A.; Meynier, A.; Quigley, M.; Englyst, H.; Brack, O.; Vinoy, S. Inter-Laboratory Validation of the Starch Digestibility Method for Determination of Rapidly Digestible and Slowly Digestible Starch. Food Chem. 2018, 245, 1183–1189. [Google Scholar] [CrossRef]
- McKeen, S.; Young, W.; Mullaney, J.; Fraser, K.; McNabb, W.C.; Roy, N.C. Infant Complementary Feeding of Prebiotics for the Microbiome and Immunity. Nutrients 2019, 11, 364. [Google Scholar] [CrossRef]
- Neufeld, M.; Prentice, A.M.; Michaelsen, K.F.; Neufeld, L.M.; Andrew, G.; Prentice, M. Environmental and Physiological Barriers to Child Growth and Development. In Global Landscape of Nutrition Challenges in Infants and Children: 93rd Nestlé Nutrition Institute Workshop; S. Karger Publishing: Basel, Switzerland, 2019; p. 125. [Google Scholar]



| Samples | Tp (°C) | Temperature Range (°C) | ΔH (J/g) |
|---|---|---|---|
| W | 65.2 ± 0.4 b | 11.0 ± 0.2 a | 8.6 ± 0.4 c |
| W-WG | 64.8 ± 0.1 b | 13.1 ± 0.0 a | 7.2 ± 0.5 b |
| W-WGF | 67.9 ± 0.2 c | 11.1 ± 0.0 a | 10.4 ± 1.3 d |
| W-FS | 51.8 ± 0.3 a | 12.1 ± 1.8 b | 3.1 ± 0.4 a |
| R | 77.5 ± 0.1 e | 22.9 ± 2.3 b | 8.7 ± 0.1 c |
| R-WG | 78.5 ± 0.0 e | 21.3 ± 4.5 b | 10.1 ± 0.1 d |
| R-WGF | 78.5 ± 0.4 e | 21.8 ± 3.9 b | 13.2 ± 0.8 e |
| R-FS | 51.9 ± 0.1 a | 14.1 ± 0.9 a | 6.7 ± 0.0 b |
| M | 71.0 ± 2.6 d | 21.4 ± 2.1 b | 7.2 ± 0.7 b |
| M-WG | 72.1 ± 0.0 d | 17.3 ± 0.9 a | 7.2 ± 0.4 b |
| M-WGF | 71.6 ± 0.7 d | 16.3 ± 1.9 a | 10.1 ± 0.8 d |
| M-FS | 52.3 ± 0.6 a | 14.1 ± 1.2 a | 5.6 ± 0.0 b |
| Samples Used to Made Infant Food | Total Starch Hydrolyzed (g/100 g Starch) | RS (g/100 g Starch) |
|---|---|---|
| W | 33.3 ± 1.6 c | 66.7 ± 1.6 e |
| W-WG | 27.8 ± 0.7 c | 72.2 ± 0.7 e |
| W-WGF | 43.1 ± 3.1 e | 56.9 ± 3.1 c |
| W-FS | 57.4 ± 2.1 g | 42.6 ± 2.1 a |
| R | 30.3 ± 1.8 c | 69.8 ± 1.8 e |
| R-WG | 38.1 ± 3.0 d | 61.9 ± 3.0 d |
| R-WGF | 51.6 ± 1.9 f | 48.4 ± 1.9 b |
| R-FS | 39.3 ± 2.9 d | 60.7 ± 2.9 d |
| M | 24.1± 0.2 b | 75.9 ± 0.2 f |
| M-WG | 11.3 ± 0.2 a | 88.7 ± 0.2 g |
| M-WGF | 46.2 ± 0.6 e | 53.8 ± 0.6 c |
| M-FS | 45.4 ± 0.3 e | 54.6 ± 0.3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, M.D.; Bongianino, N.F.; León, A.E.; Bustos, M.C. Influence of Thermal Processing on In Vitro Starch Digestibility in Cereal-Based Infant Foods. Foods 2025, 14, 1367. https://doi.org/10.3390/foods14081367
Rodríguez MD, Bongianino NF, León AE, Bustos MC. Influence of Thermal Processing on In Vitro Starch Digestibility in Cereal-Based Infant Foods. Foods. 2025; 14(8):1367. https://doi.org/10.3390/foods14081367
Chicago/Turabian StyleRodríguez, Marianela D., Nicolás F. Bongianino, Alberto E. León, and Mariela C. Bustos. 2025. "Influence of Thermal Processing on In Vitro Starch Digestibility in Cereal-Based Infant Foods" Foods 14, no. 8: 1367. https://doi.org/10.3390/foods14081367
APA StyleRodríguez, M. D., Bongianino, N. F., León, A. E., & Bustos, M. C. (2025). Influence of Thermal Processing on In Vitro Starch Digestibility in Cereal-Based Infant Foods. Foods, 14(8), 1367. https://doi.org/10.3390/foods14081367






