Effect of Different Fermentation Methods on the Physicochemical, Bioactive and Volatile Characteristics of Wolfberry Vinegar
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Strains and Chemicals
2.2. Sample Preparation
2.3. Physicochemical Analysis
2.4. Determination of Total Phenols and Total Flavonoids
2.5. Determination of Antioxidant Activity In Vitro
2.6. Determination of Phenolic Compounds
2.7. Determination of Organic Acids
2.8. Determination of Amino Acids
2.9. Determination of Volatile Organic Compounds
2.10. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Property of Wolfberry Vinegar
3.2. TPC, TFC and Antioxidant Capacities of Wolfberry Vinegar
3.3. Phenolic Compounds of Wolfberry Vinegar
3.4. The Relationship Between Phenolic Compounds and Antioxidant Activities of MFV
3.5. Organic Acids of Wolfberry Vinegar
3.6. Comparison of Amino Acid Between MFV and SFV
3.7. Volatile Organic Compounds of Wolfberry Vinegar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.J.; Liang, X.J.; Guo, S.J.; Li, Y.K.; Zhang, B.; Yin, Y.; Wei, A.; Cao, Y.L.; Zhao, J.H. Evaluation of nutrients and related environmental factors for wolfberry (Lycium barbarum) fruits grown in the different areas of China. Biochem. Syst. Ecol. 2019, 86, 103916. [Google Scholar]
- Feng, L.; Tang, N.C.; Liu, R.J.; Nie, R.; Guo, Y.W.; Liu, R.R.; Chang, M. Effects of different processing methods on bioactive substances and antioxidation properties of Lycium barbarum (goji berry) from China. Food Biosci. 2021, 42, 101048. [Google Scholar]
- Wang, Y.; Han, Q.Q.; Bai, F.; Luo, Q.; Wu, M.L.; Song, G.; Zhang, H.M.; Wang, Y.Q. The assembly and antitumor activity of Lycium barbarum polysaccharide-platinum-based conjugates. J. Inorg. Biochem. 2020, 205, 111001. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Liang, T.S.; Liu, Y.L.; Ding, G.T.; Zhang, F.M.; Ma, Z.R. Extraction, structural characterization, and biological functions of Lycium barbarum polysaccharides: A review. Biomolecules 2019, 9, 389. [Google Scholar] [CrossRef]
- Sun, W.L.; Shahrajabian, M.H.; Qi, C. Health benefits of wolfberry (Gou Qi Zi, Fructus barbarum L.) on the basis of ancient Chinese herbalism and Western modern medicine. Avicenna J. Phytomed. 2021, 11, 109–119. [Google Scholar]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a potential natural antioxidant medicine: An insight into their molecular mechanisms of action. Oxid. Med. Cell. Longev. 2019, 2019, 2437397. [Google Scholar]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar]
- Qiang, X.; Xia, T.; Geng, B.B.; Zhao, M.; Li, X.; Zheng, Y.; Wang, M. Bioactive components of Lycium barbarum and deep-processing fermentation products. Molecules 2023, 28, 8044. [Google Scholar] [CrossRef]
- Luzón-Quintana, L.; Castro, R.; Durán-Guerrero, E. Biotechnological processes in fruit vinegar production. Foods 2021, 10, 945. [Google Scholar] [CrossRef]
- Valdes, D.S.; So, D.; Gill, P.A.; Kellow, N.J. Effect of dietary acetic acid supplementation on plasma glucose, lipid profiles, and body mass index in human adults: A systematic review and meta-analysis. J. Acad. Nutr. Diet. 2021, 121, 895–914. [Google Scholar] [CrossRef]
- Ousaaid, D.; Laaroussi, H.; Bakour, M.; Ghouizi, A.; Aboulghazi, A.; Lyoussi, B.; Arabi, I. Beneficial effects of apple vinegar on hyperglycemia and hyperlipidemia in hypercaloric-fed rats. J. Diabetes Res. 2020, 2020, 9284987. [Google Scholar] [CrossRef]
- Tian, Y.; Xia, T.; Qiang, X.; Zhao, Y.; Li, S.; Wang, Y.; Zheng, Y.; Yu, J.; Wang, J.; Wang, M. Nutrition, bioactive components, and hepatoprotective activity of fruit vinegar produced from Ningxia wolfberry. Molecules 2022, 27, 4422. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, B.; Duan, W.H.; Zhang, J.; Wang, M. Nutrients and bioactive components from vinegar: A fermented and functional food. J. Funct. Foods 2020, 64, 103681. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Sun, C.; Wang, Q.; Yao, H.; Yang, W.; Zheng, Z.; Jiang, S.; Wu, X. Effects of mixed cultures of Candida tropicalis and aromatizing yeast in alcoholic fermentation on the quality of apple vinegar. 3 Biotech 2019, 9, 128. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Bai, Y.; Fu, C.X.; Zhou, M.Z.; Gao, B.; Wang, C.; Li, D.S.; Hu, Y.; Xu, N. Effects of mixed cultures of Saccharomyces cerevisiae and Lactobacillus plantarum in alcoholic fermentation on the physicochemical and sensory properties of citrus vinegar. LWT-Food Sci. Technol. 2017, 84, 753–763. [Google Scholar] [CrossRef]
- Peyer, L.C.; Zannini, E.; Arendt, E.K. Lactic acid bacteria as sensory biomodulators for fermented cereal-based beverages. Trends Food Sci. Technol. 2017, 61, 16–25. [Google Scholar] [CrossRef]
- Li, Y.; Nguyen, T.T.H.; Jin, J.; Lim, J.; Lee, J.; Piao, M.; Mok, I.K.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.J.B.; Qi, Y.; Liu, C.H.; Chen, Q.J.; Mai, X.Y.; Zhu, Z.J.; Ma, B. Effects of Lactobacillus plantarum cofermentation on the flavor and taste characteristics of mango vinegar. J. Food Meas. Charact. 2024, 18, 3744–3756. [Google Scholar] [CrossRef]
- GB/T 12456-2008; Determination of Total Acid in Foods. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2008.
- GB 5009 7-2016; Determination of Total Sugars in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 2023-2003; Food Additive-Lactic Acid. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2003.
- GB 5009.225-2016National Food Safety Standard for the Determination of Ethanol Concentration in Wine; National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Xia, T.; Qiang, X.; Geng, B.; Zhang, X.; Wang, Y.; Li, S.; Meng, Y.; Zheng, Y.; Wang, M. Changes in the phytochemical and bioactive compounds and the antioxidant properties of wolfberry during vinegar fermentation processes. Int. J. Mol. Sci. 2022, 23, 15839. [Google Scholar] [CrossRef]
- Zhuang, J.; Dai, X.; Zhu, M.; Zhang, S.; Dai, Q.; Jiang, X.; Liu, Y.; Gao, L.; Xia, T. Evaluation of astringent taste of green tea through mass spectrometry-based targeted metabolic profiling of polyphenols. Food Chem. 2020, 305, 125507. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, M.; Zhao, N.; Tu, L.; Xue, D.; Zhang, X.; Zhao, C.; Cheng, Y.; Zheng, Y.; Wang, M. Metabolic profile of main organic acids and its regulatory mechanism in solid-state fermentation of Chinese cereal vinegar. Food Res. Int. 2021, 145, 110400. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, A.; Zhou, Y.; Zhang, W.; Liang, K.; Román-Camacho, J.J.; Zhou, J.; Song, J.; Zheng, Y.; Wang, M. Identification of aroma active compounds in Shanxi aged vinegar and tracing the source in the entire production process. Food Chem. X 2024, 24, 101918. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Wu, S.Q.; Li, Y.R.; Huang, Y.Y.; Yang, X.P. Effects of different acetic acid bacteria strains on the bioactive compounds, volatile compounds and antioxidant activity of black tea vinegar. LWT-Food Sci. Technol. 2022, 171, 114131. [Google Scholar] [CrossRef]
- Yin, R.; Jiang, J.; Ma, X.; Xie, Y.; Cui, M.; Chen, Y.; Li, Y.; Hu, Y.; Cheng, W.; Gao, F. Dynamic changes of physicochemical parameters, antioxidant activity, organic acids, polyphenols, and volatile components in prune vinegar during fermentation. Food Biosci. 2024, 59, 104042. [Google Scholar] [CrossRef]
- Roda, A.; Lucini, L.; Torchio, F.; Dordoni, R.; De Faveri, D.M.; Lambri, M. Metabolite profiling and volatiles of pineapple wine and vinegar obtained from pineapple waste. Food Chem. 2017, 229, 734–742. [Google Scholar] [CrossRef]
- Kim, S.; Abbas, A.; Moon, G. Improved Functions of Fermented Coffee by Lactic Acid Bacteria. Appl. Sci. 2024, 14, 7596. [Google Scholar] [CrossRef]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT-Food Sci. Technol. 2022, 154, 112716. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106–125. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.; Wu, M.; Sackey, A.; Xiao, L.; Tahir, H. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, Y.; Tong, Q.; Liu, Y.; Xu, W. Effects of different lactic acid bacteria on phenolic profiles, antioxidant capacities, and volatile compounds in purple sweet potato juice. J. Food Sci. Technol. 2024, 61, 1800–1810. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Tang, F.X.; Cai, W.C.; Peng, B.; Zhang, P.L.; Shan, C.H. Effect of fermentation by lactic acid bacteria on the phenolic composition, antioxidant activity, and flavor substances of jujube–wolfberry composite juice. LWT-Food Sci. Technol. 2023, 184, 114884. [Google Scholar] [CrossRef]
- Yong, D.O.C.; Saker, S.R.; Chellappan, D.K.; Madheswaran, T.; Panneerselvam, J.; Choudhury, H.; Pandey, M.; Chan, Y.L.; Collet, T.; Gupta, G.; et al. Molecular and immunological mechanisms underlying the various pharmacological properties of the potent bioflavonoid, rutin. Endocr. Metab. Immune 2020, 20, 1590–1596. [Google Scholar] [CrossRef] [PubMed]
- Akash, S.R.; Tabassum, A.; Aditee, L.M.; Rahman, A.; Hossain, M.I.; Hannan, M.A.; Uddin, M.J. Pharmacological insight of rutin as a potential candidate against peptic ulcer. Biomed. Pharmacother. 2024, 177, 116961. [Google Scholar] [CrossRef]
- Liu, H.; Li, N.; Wang, Y.; Cheng, T.; Yang, H.; Peng, Q. Study on fermentation kinetics, antioxidant activity and flavor characteristics of Lactobacillus plantarum CCFM1050 fermented wolfberry pulp. Food Innov. Adv. 2024, 3, 126–134. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Tong, W.; Chen, G.; Yu, H.; Wang, X.; Xue, R.; Yang, Y.; Luo, H.; Huang, D. Dynamic changes and correlation of organic acids, physicochemical properties, and microbial communities during fermentation of Sichuan bran vinegar. J. Food Compos. Anal. 2024, 132, 106355. [Google Scholar] [CrossRef]
- Li, Y.; Wang, A.; Dang, B.; Yang, X.; Nie, M.; Chen, Z.; Lin, R.; Wang, L.; Wang, F.; Tong, L.T. Deeply analyzing dynamic fermentation of highland barley vinegar: Main physicochemical factors, key flavors, and dominate microorganisms. Food Res. Int. 2024, 177, 113919. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Li, J.; Zhang, P.; Tang, F.; Shan, C. Influence of lactic acid bacteria on physicochemical indexes, sensory and flavor characteristics of fermented sea buckthorn juice. Food Biosci. 2021, 46, 101519. [Google Scholar]
- Bergentall, M.K.; Malafronte, L.; As, D.; Calmet, E.; Melin, P. Reduction of malicacid in bilberry juice by Lactiplantibacillus plantarum-mediated malolactic fermentation. Eur. Food Res. Technol. 2024, 250, 811–820. [Google Scholar] [CrossRef]
- Cui, W.; Wang, X.; Han, S.; Guo, W.; Meng, N.; Li, J.; Sun, B.; Zhang, X. Research progress of tartaric acid stabilization on wine characteristics. Food Chem. X 2024, 23, 101728. [Google Scholar] [CrossRef]
- Ji, G.; Liu, G.; Li, B.; Tan, H.; Zheng, R.; Sun, X.; He, F. Influence on the aroma substances and functional ingredients of apple juice by lactic acid bacteria fermentation. Food Biosci. 2022, 51, 102337. [Google Scholar] [CrossRef]
- Chinnici, F.; Durán-Guerrero, E.; Riponi, C. Discrimination of some European vinegars with protected denomination of origin as a function of their amino acid and biogenic amine content. J. Sci. Food Agric. 2016, 96, 3762–3771. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, F.; Wang, Y.; Han, J.; Gao, F.; Tian, J.; Zhang, K.; Jin, Y. The changes occurring in proteins during processing and storage of fermented meat products and their regulation by lactic acid bacteria. Foods 2022, 11, 2427. [Google Scholar] [CrossRef] [PubMed]
- Urbina, Á.; Calderón, F.; Benito, S. The combined use of Lachancea thermotolerans and Lactiplantibacillus plantarum (former Lactobacillus plantarum) in wine technology. Foods 2021, 10, 1356. [Google Scholar] [CrossRef]
- Wang, C.; Sun, S.; Zhou, H.; Cheng, Z. The influence of Lactiplantibacillus plantarum and Oenococcus oeni starters on the volatile and sensory properties of black raspberry wine. Foods 2023, 12, 4212. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Özdemir, N.; Zannou, O.; Koca, I. Antioxidant capacity, phytochemical compounds, and volatile compounds related to aromatic property of vinegar produced from black rosehip (Rosa pimpinellifolia L.) juice. Food Biosci. 2021, 44, 101318. [Google Scholar] [CrossRef]
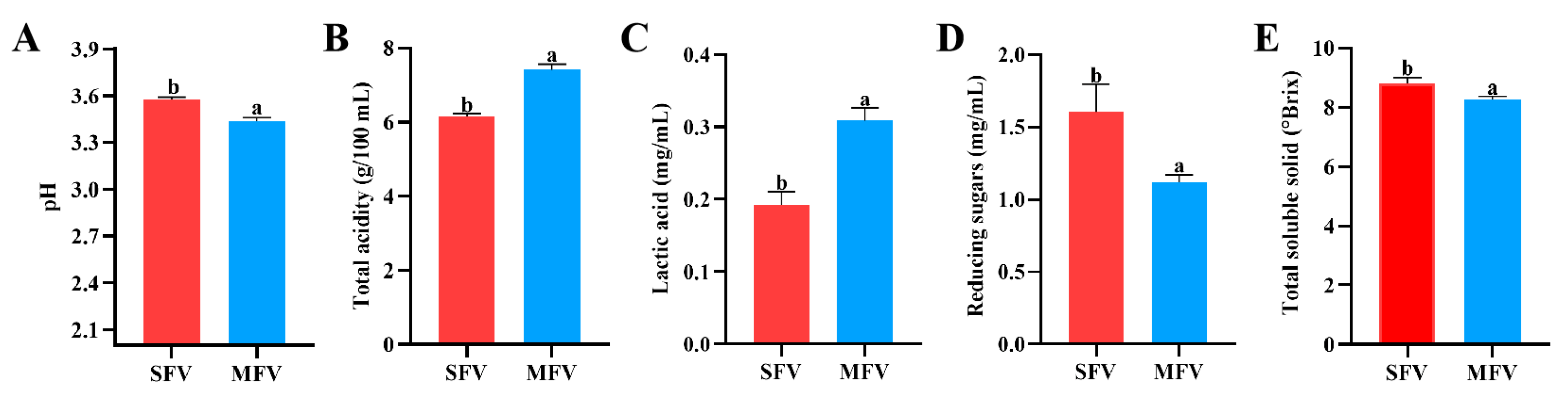
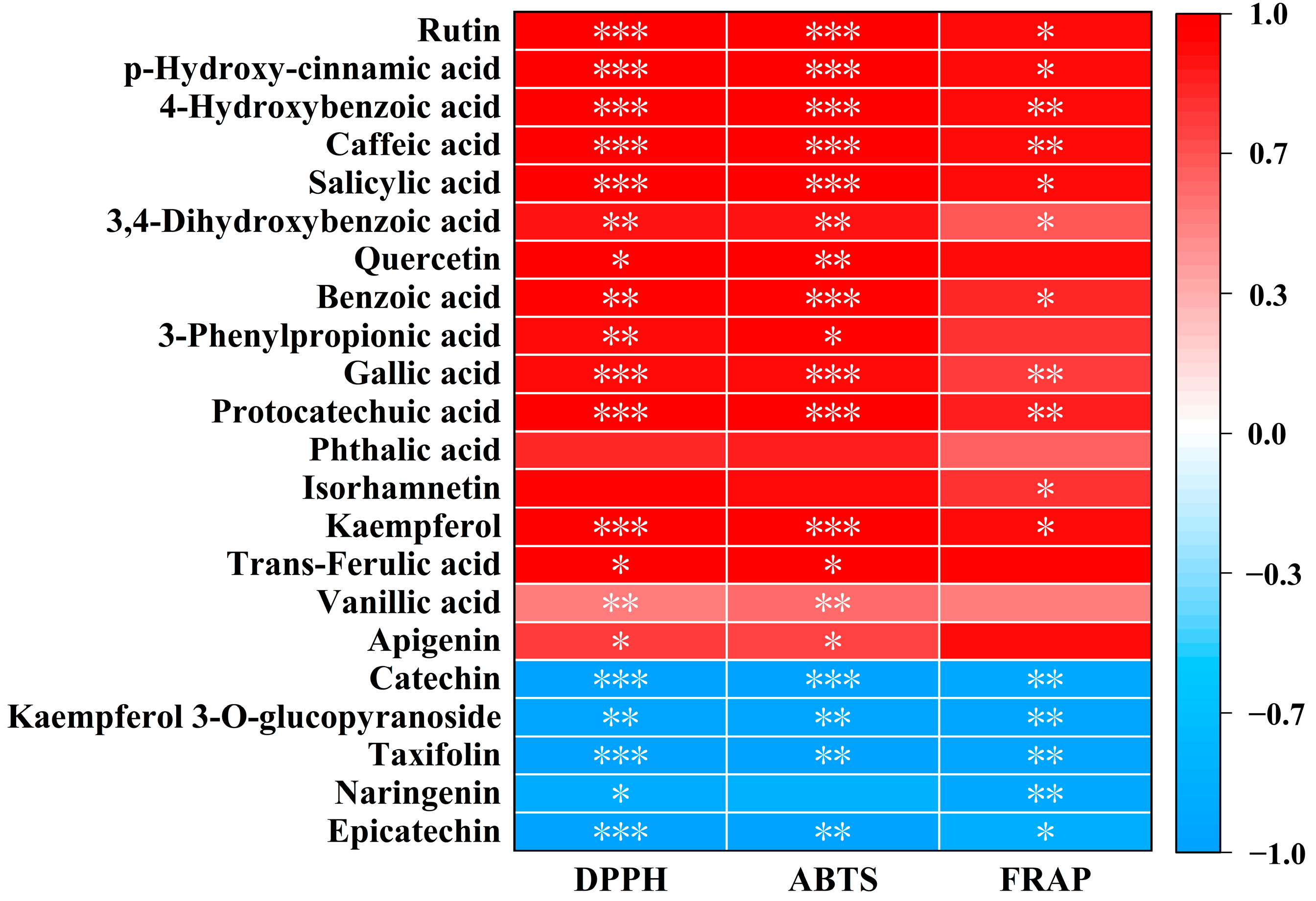
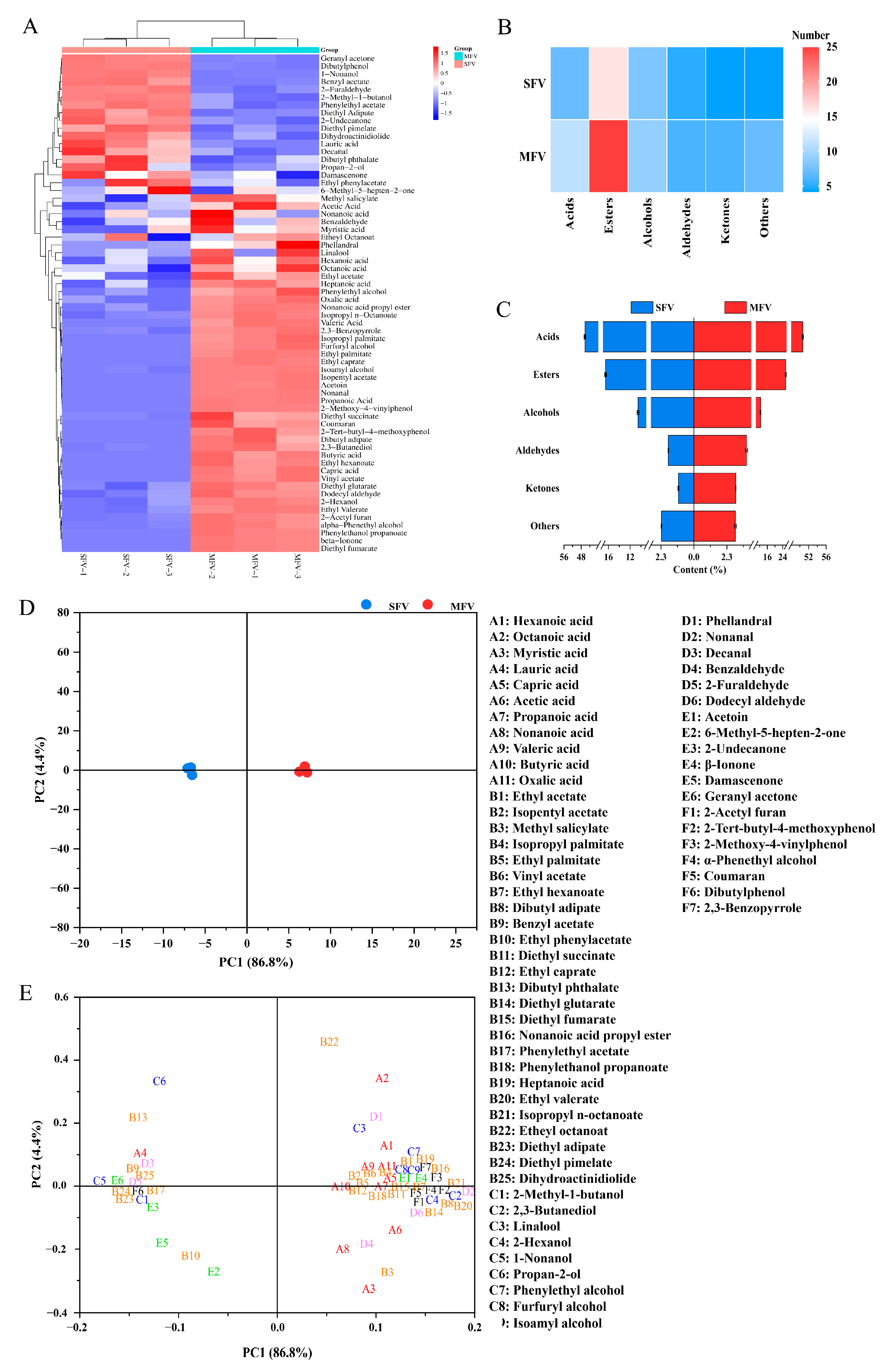
| Indexes | Content | ||
|---|---|---|---|
| SFV | MFV | ||
| Active ingredients | TPC (mg GAE/mL) | 2.64 ± 0.04 b | 2.99 ± 0.05 a |
| TFC (mg RE/mL) | 1.21 ± 0.06 b | 1.81 ± 0.01 a | |
| Antioxidant activities | DPPH (mM Trolox/L) | 17.61 ± 0.11 b | 20.84 ± 0.13 a |
| ABTS (mM Trolox/L) | 16.21 ± 0.43 b | 18.67 ± 0.07 a | |
| FRAP (mM Trolox/L) | 4.40 ± 0.23 b | 5.79 ± 0.26 a | |
| Phenolics | Retention Time (min) | SFV | MFV |
|---|---|---|---|
| Protocatechuic acid | 3.24 | 23.57 ± 2.48 b | 36.07 ± 1.20 a |
| Catechin | 3.97 | 68.41 ± 2.55 a | 14.61 ± 0.25 b |
| Vanillic acid | 4.20 | 2.08 ± 0.65 a | 2.46 ± 0.33 a |
| Salicylic acid | 5.72 | 116.86 ± 7.36 b | 420.92 ± 8.66 a |
| Gallic acid | 5.99 | 39.19 ±4.43 b | 49.84 ±3.04 a |
| Benzoic acid | 6.04 | 57.91 ± 7.23 b | 80.59 ± 2.19 a |
| Kaempferol 3-Oglucopyranoside | 6.79 | 7.53 ± 0.22 a | 6.22 ± 0.49 b |
| Quercetin | 7.09 | 67.50 ± 1.80 b | 113.77 ± 0.89 a |
| Apigenin | 7.59 | 0.11 ± 0.01 b | 0.20 ± 0.09 a |
| Isorhamnetin | 7.74 | 2.36 ± 0.33 b | 11.42 ± 0.17 a |
| Trans-Ferulic acid | 9.69 | 3.51 ± 0.02 b | 5.31 ± 0.21 a |
| 3-Phenylpropionic acid | 11.90 | 3.91 ± 0.57 b | 56.04 ± 1.92 a |
| 4-Hydroxybenzoic acid | 13.43 | 579.90 ± 24.37 b | 724.86 ± 56.12 a |
| Epicatechin | 14.82 | 1.07 ± 0.01 a | 0.55 ± 0.09 b |
| p-Hydroxy-cinnamic acid | 17.15 | 1706.41 ± 82.69 b | 1954.46 ± 59.74 a |
| Kaempferol | 17.67 | 0.82 ± 0.12 b | 8.06 ± 0.09 a |
| Naringenin | 19.59 | 0.86 ± 0.11 a | 0.69 ± 0.05 b |
| Taxifolin | 21.69 | 2.45 ± 0.10 a | 1.07 ± 0.09 b |
| Phthalic acid | 23.31 | 19.44 ± 3.72 b | 25.74 ± 2.96 a |
| Caffeic acid | 24.35 | 72.38 ± 0.77 b | 525.01 ± 1.06 a |
| Protocatechuic acid | 26.04 | 111.90 ± 8.30 b | 202.46 ± 0.21 a |
| Rutin | 35.61 | 4501.29 ± 384.81 b | 5457.97 ± 450.30 a |
| Total contents | 7389.46 ± 483.44 b | 9698.31 ± 571.20 a |
| Organic Acids | Retention Time (min) | SFV | MFV |
|---|---|---|---|
| Tartaric acid | 6.03 | 1.34 ± 0.03 b | 1.62 ± 0.02 a |
| Oxalic acid | 6.74 | 0.95 ± 0.02 a | 1.01 ± 0.02 a |
| Citric acid | 7.76 | 5.16 ± 0.03 a | 5.05 ± 0.03 a |
| Ascorbic acid | 8.20 | 0.36 ± 0.02 a | 0.44 ± 0.04 a |
| Malic acid | 9.36 | 5.14 ± 0.05 a | 4.98 ± 0.06 a |
| Acetic Acid | 9.89 | 64.85 ± 0.20 b | 70.92 ± 0.12 a |
| Lactic acid | 11.16 | 1.38 ± 0.07 a | 1.47 ± 0.02 a |
| Succinic acid | 12.72 | 1.25 ± 0.06 a | 1.30 ± 0.06 a |
| Fumaric acid | 15.72 | 1.29 ± 0.03 a | 1.34 ± 0.04 a |
| Total organic acids | 81.72 ± 0.12 b | 88.13 ± 0.13 a |
| Amino Acids | Taste Attributes | Concentration (mg/mL) | |
|---|---|---|---|
| SFV | MFV | ||
| Proline | sweet | 1.595 ± 0.386 b | 1.936 ± 0.173 a |
| Alanine | sweet | 0.716 ± 0.523 b | 1.138 ± 0.108 a |
| Serine | sweet | 0.397 ± 0.232 b | 0.743 ± 0.022 a |
| Threonine | sweet | 0.204 ± 0.069 b | 0.349 ± 0.015 a |
| Glycine | sweet | 0.088 ± 0.018 a | 0.117 ± 0.011 a |
| Histidine | sweet | 0.028 ± 0.010 a | 0.043 ± 0.017 a |
| Lysine | sweet and bitter | 0.123 ± 0.025 b | 0.890 ± 0.042 a |
| Arginine | bitter | 0.464 ± 0.165 a | 0.048 ± 0.007 b |
| Methionine | bitter | 0.071 ± 0.063 a | 0.107 ± 0.015 a |
| Isoleucine | bitter | 0.094 ± 0.069 b | 0.148 ± 0.024 a |
| Leucine | bitter | 0.066 ± 0.023 b | 0.121 ± 0.027 a |
| Valine | bitter | 0.054 ± 0.026 a | 0.082 ± 0.013 a |
| Phenylalanine | bitter | 0.098 ± 0.030 a | 0.140 ± 0.057 a |
| Tyrosine | bitter | 0.087 ± 0.036 a | 0.105 ± 0.047 a |
| Aspartic acid | umami | 0.015 ± 0.003 a | 0.051 ± 0.029 a |
| Glutamic acid | umami | 0.216 ± 0.083 b | 0.309 ± 0.018 a |
| Cysteine | tasteless | 0.117 ± 0.095 b | 0.178 ± 0.006 a |
| Total essential free amino acids | 0.708 ± 0.217 b | 1.835 ± 0.186 a | |
| Content of total free amino acids | 4.43 ± 0.12 b | 6.50 ± 0.17 a | |
| Class | Compounds | Relative Content (%) | |
|---|---|---|---|
| SFV | MFV | ||
| Acids | Hexanoic acid | 0.596 ± 0.127 b | 0.682 ± 0.133 a |
| Octanoic acid | 1.593 ± 0.220 b | 2.17 ± 0.994 a | |
| Myristic acid | 1.035 ± 0.381 a | 1.263 ± 0.373 a | |
| Lauric acid | 0.489 ± 0.097 a | 0.159 ± 0.112 b | |
| Nonanoic acid | 0.593 ± 0.182 b | 0.703 ± 0.347 a | |
| Capric acid | - | 0.822 ± 0.427 a | |
| Acetic Acid | 41.673 ± 0.623 b | 43.13 ± 0.513 a | |
| Propanoic Acid | - | 0.892 ± 0.276 a | |
| Valeric Acid | - | 0.13 ± 0.082 a | |
| Butyric acid | - | 0.235 ± 0.109 a | |
| Oxalic acid | 0.294 ± 0.120 b | 0.477 ± 0.095 a | |
| Total | 46.273 ± 0.330 b | 50.664 ± 0.132 a | |
| Esters | Ethyl acetate | 0.536 ± 0.068 b | 0.650 ± 0.131 a |
| Isopentyl acetate | - | 0.834 ± 0.279 a | |
| Methyl salicylate | 0.356 ± 0.069 b | 0.469 ± 0.159 a | |
| Isopropyl palmitate | - | 0.268 ± 0.093 a | |
| Ethyl palmitate | - | 0.353 ± 0.199 a | |
| Dibutyl adipate | - | 0.157 ± 0.096 a | |
| Benzyl acetate | 0.417 ± 0.106 a | 0.137 ± 0.102 b | |
| Ethyl phenylacetate | 0.270 ± 0.114 a | 0.239 ± 0.163 a | |
| Dihydroactinidiolide | 0.657 ± 0.067 a | 0.167 ± 0.076 b | |
| Ethyl hexanoate | - | 0.340 ± 0.129 a | |
| Vinyl acetate | - | 0.369 ± 0.221 a | |
| Diethyl succinate | 0.572 ± 0.182 b | 1.373 ± 0.474 a | |
| Ethyl caprate | - | 6.300 ± 0.151 a | |
| Dibutyl phthalate | 0.426 ± 0.197 a | 0.350 ± 0.022 b | |
| Diethyl glutarate | 0.353 ± 0.267 b | 0.670 ± 0.465 a | |
| Etheyl Octanoat | 0.238 ± 0.218 a | 0.254 ± 0.263 a | |
| Nonanoic acid propyl ester | 0.337 ± 0.114 b | 0.530 ± 0.091 a | |
| phenylethyl acetate | 9.882 ± 2.045 a | 8.656 ± 2.361 b | |
| Phenylethanol propanoate | - | 0.286 ± 0.198 a | |
| Heptanoic acid | 0.448 ± 0.278 b | 0.580 ± 0.252 a | |
| Ethyl Valerate | 0.212 ± 0.100 b | 0.514 ± 0.086 a | |
| Isopropyl n-Octanoate | 0.442 ± 0.286 b | 0.765 ± 0.420 a | |
| Diethyl Adipate | 0.662 ± 0.075 a | 0.461 ± 0.094 b | |
| Diethyl pimelate | 0.71 ± 0.294 a | 0.461 ± 0.149 b | |
| Diethyl fumarate | - | 0.465 ± 0.268 a | |
| Total | 16.517 ± 0.141 b | 25.646 ± 0.260 a | |
| Alcohols | 2-Hexanol | 0.278 ± 0.218 b | 0.491 ± 0.195 a |
| 2-Methyl-1-butanol | 0.808 ± 0.264 a | 0.559 ± 0.282 b | |
| Linalool | 0.128 ± 0.058 a | 0.160 ± 0.080 a | |
| 1-Nonanol | 0.840 ± 0.435 a | 0.157 ± 0.537 b | |
| Isoamyl alcohol | 1.175 ± 0.124 b | 2.498 ± 0.666 a | |
| 2,3-Butanediol | 0.180 ± 0.006 b | 0.451 ± 0.099 a | |
| Phenylethyl alcohol | 6.873 ± 2.027 b | 7.721 ± 2.021 a | |
| 1-Nonanol | 0.254 ± 0.108 a | 0.206 ± 0.010 a | |
| Furfuryl alcohol | - | 0.266 ± 0.034 a | |
| Total | 10.537 ± 0.170 b | 12.509 ± 0.137 a | |
| Aldehydes | Nonanal | - | 1.864 ± 0.616 a |
| 2-Furaldehyde | 0.492 ± 0.347 a | 0.358 ± 0.181 b | |
| Decanal | 0.368 ± 0.251 a | 0.318 ± 0.180 b | |
| Benzaldehyde | 0.347 ± 0.107 a | 0.363 ± 0.216 a | |
| Phellandral | 0.208 ± 0.100 b | 0.301 ± 0.110 a | |
| Dodecyl aldehyde | 0.373 ± 0.181 b | 0.468 ± 0.269 a | |
| Total | 1.789 ± 1.589 b | 3.672 ± 0.470 a | |
| Ketones | 6-Methyl-5-hepten-2-one | 0.168 ± 0.062 a | 0.155 ± 0.089 a |
| 2-Undecanone | 0.184 ± 0.077 a | 0.105 ± 0.051 b | |
| Damascenone | 0.168 ± 0.080 a | 0.125 ± 0.094 a | |
| Geranyl acetone | 0.567 ± 0.334 a | 0.112 ± 0.017 b | |
| β-Ionone | - | 0.346 ± 0.265 a | |
| Acetoin | - | 2.070 ± 0.651 a | |
| Total | 1.087 ± 1.005 b | 2.914 ± 1.130 a | |
| Others | Dibutylphenol | 1.224 ± 0.054 a | 0.344 ± 0.054 b |
| α-Phenethyl alcohol | 0.535 ± 0.005 b | 0.768 ± 0.054 a | |
| 2-Methoxy-4-vinylphenol | - | 0.516 ± 0.054 a | |
| 2-Acetyl furan | 0.135 ± 0.010 b | 0.353 ± 0.054 a | |
| 2,3-Benzopyrrole | 0.375 ± 0.054 b | 0.603 ± 0.054 a | |
| 2-Tert-butyl-4-methoxyphenol | - | 0.149 ± 0.054 a | |
| Coumaran | - | 0.157 ± 0.054 a | |
| Total | 2.269 ± 0.054 b | 2.892 ± 0.054 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, X.; Zhao, M.; Xia, T.; Wang, Q.; Yu, J.; Qiao, C.; Zhang, H.; Lv, S.; Liu, Y.; Wang, M. Effect of Different Fermentation Methods on the Physicochemical, Bioactive and Volatile Characteristics of Wolfberry Vinegar. Foods 2025, 14, 1078. https://doi.org/10.3390/foods14061078
Qiang X, Zhao M, Xia T, Wang Q, Yu J, Qiao C, Zhang H, Lv S, Liu Y, Wang M. Effect of Different Fermentation Methods on the Physicochemical, Bioactive and Volatile Characteristics of Wolfberry Vinegar. Foods. 2025; 14(6):1078. https://doi.org/10.3390/foods14061078
Chicago/Turabian StyleQiang, Xiao, Man Zhao, Ting Xia, Qi Wang, Junwei Yu, Changsheng Qiao, Huimin Zhang, Shiyang Lv, Yanhua Liu, and Min Wang. 2025. "Effect of Different Fermentation Methods on the Physicochemical, Bioactive and Volatile Characteristics of Wolfberry Vinegar" Foods 14, no. 6: 1078. https://doi.org/10.3390/foods14061078
APA StyleQiang, X., Zhao, M., Xia, T., Wang, Q., Yu, J., Qiao, C., Zhang, H., Lv, S., Liu, Y., & Wang, M. (2025). Effect of Different Fermentation Methods on the Physicochemical, Bioactive and Volatile Characteristics of Wolfberry Vinegar. Foods, 14(6), 1078. https://doi.org/10.3390/foods14061078







