Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetable Oil
2.2. Microorganisms
Effect of Oil on Starter Culture Strains
2.3. Effectiveness in Fermented Sausage
2.3.1. Fermented Sausage Preparation
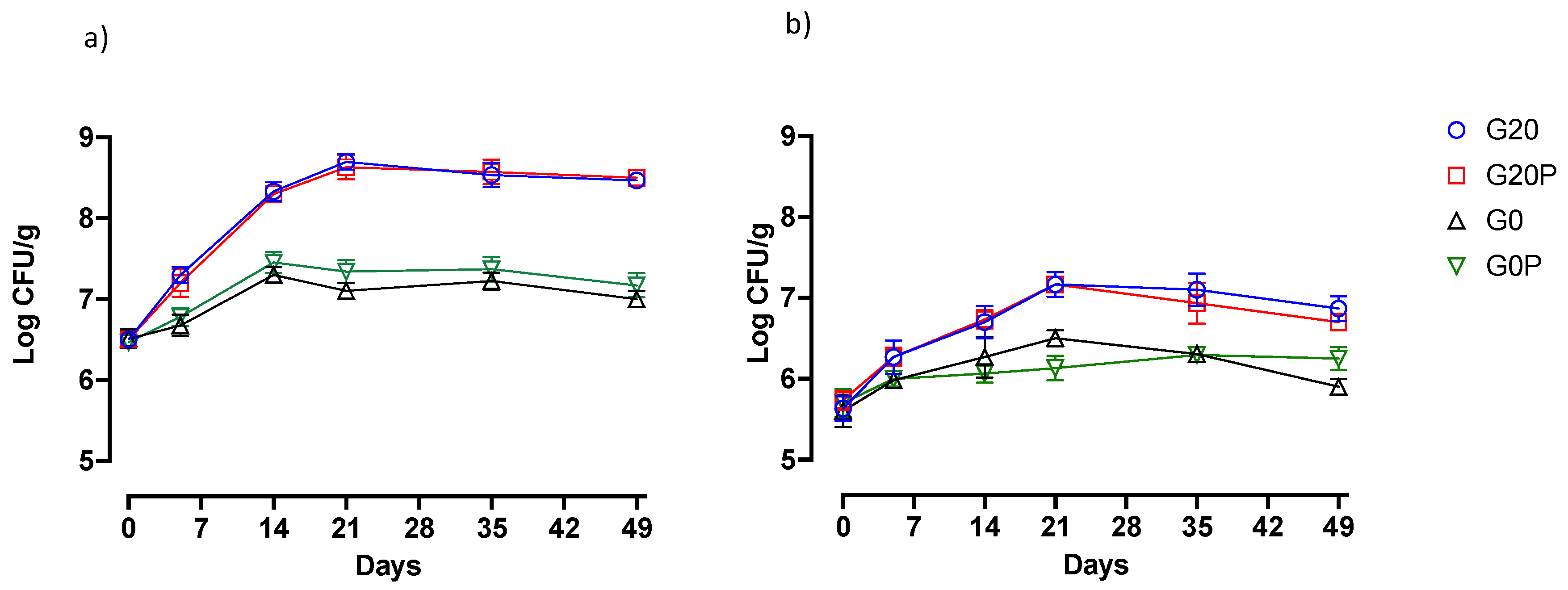
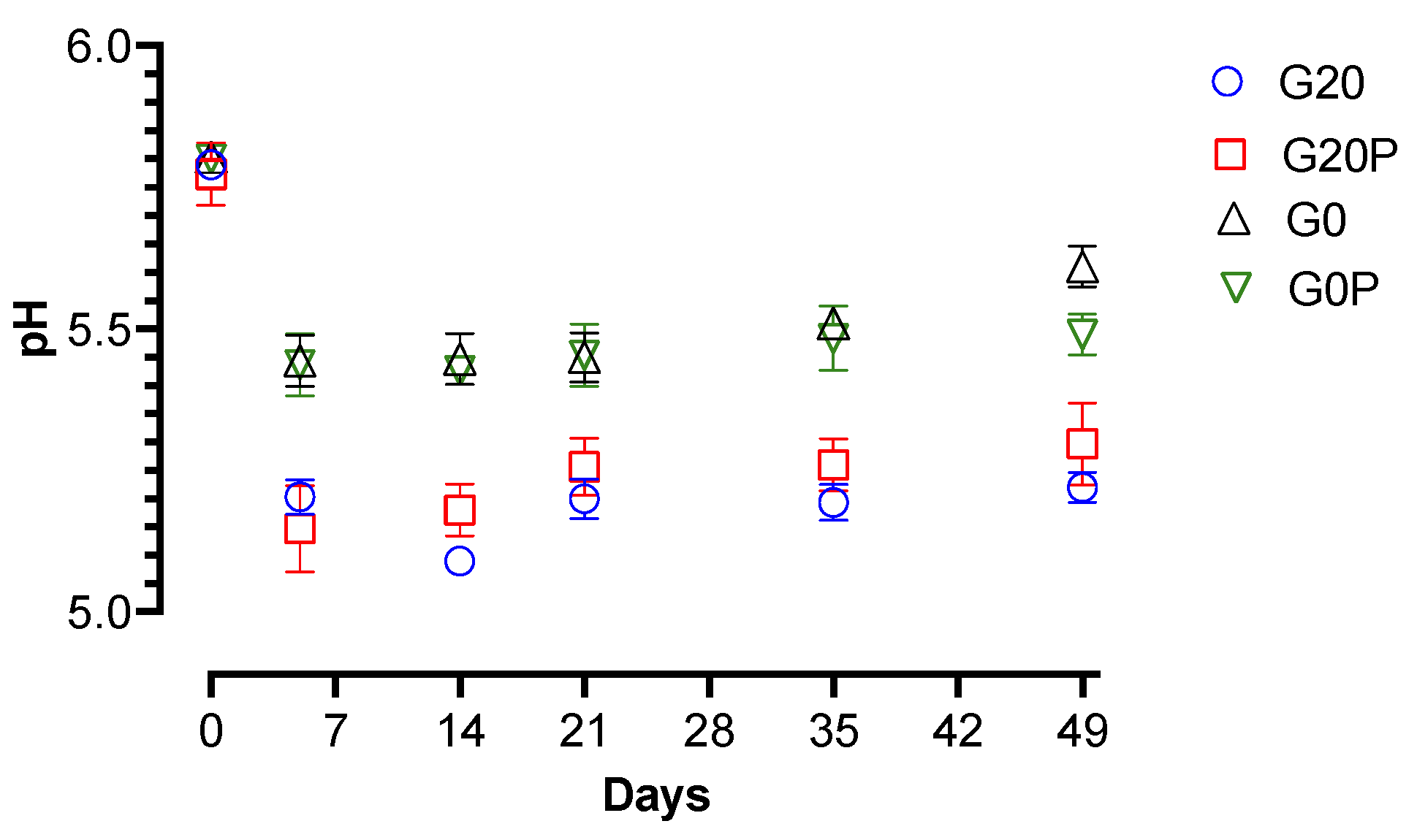
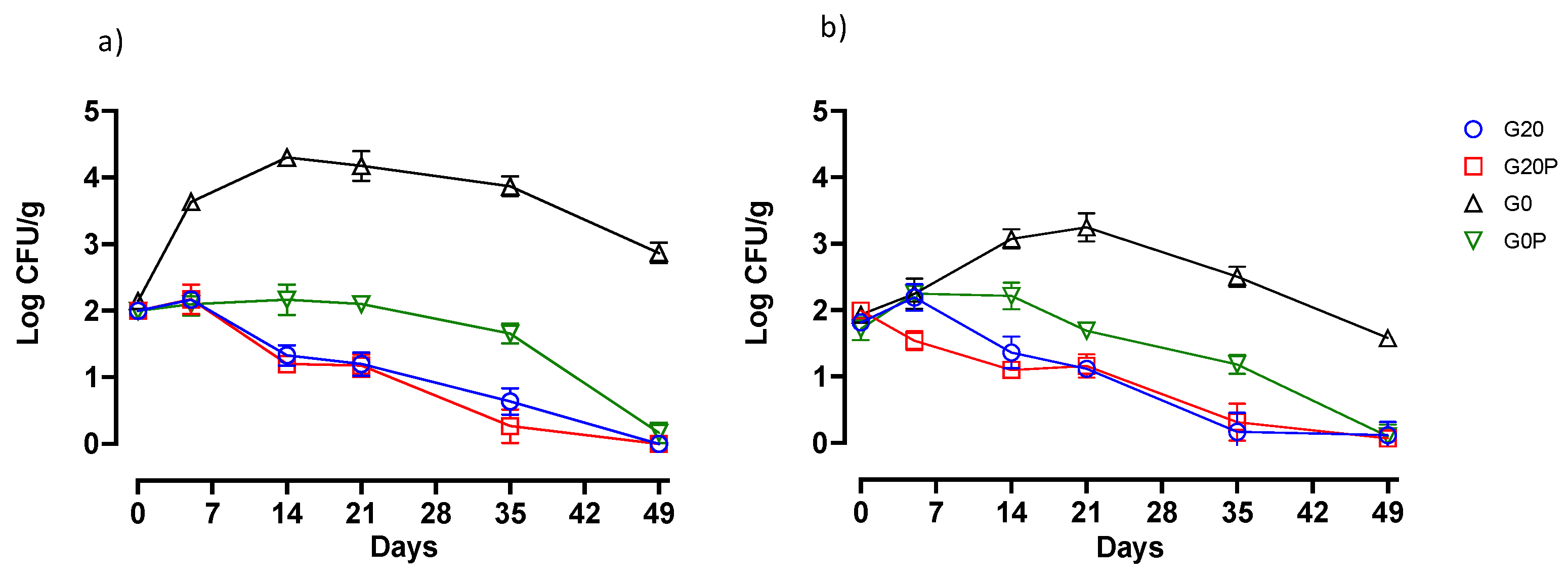
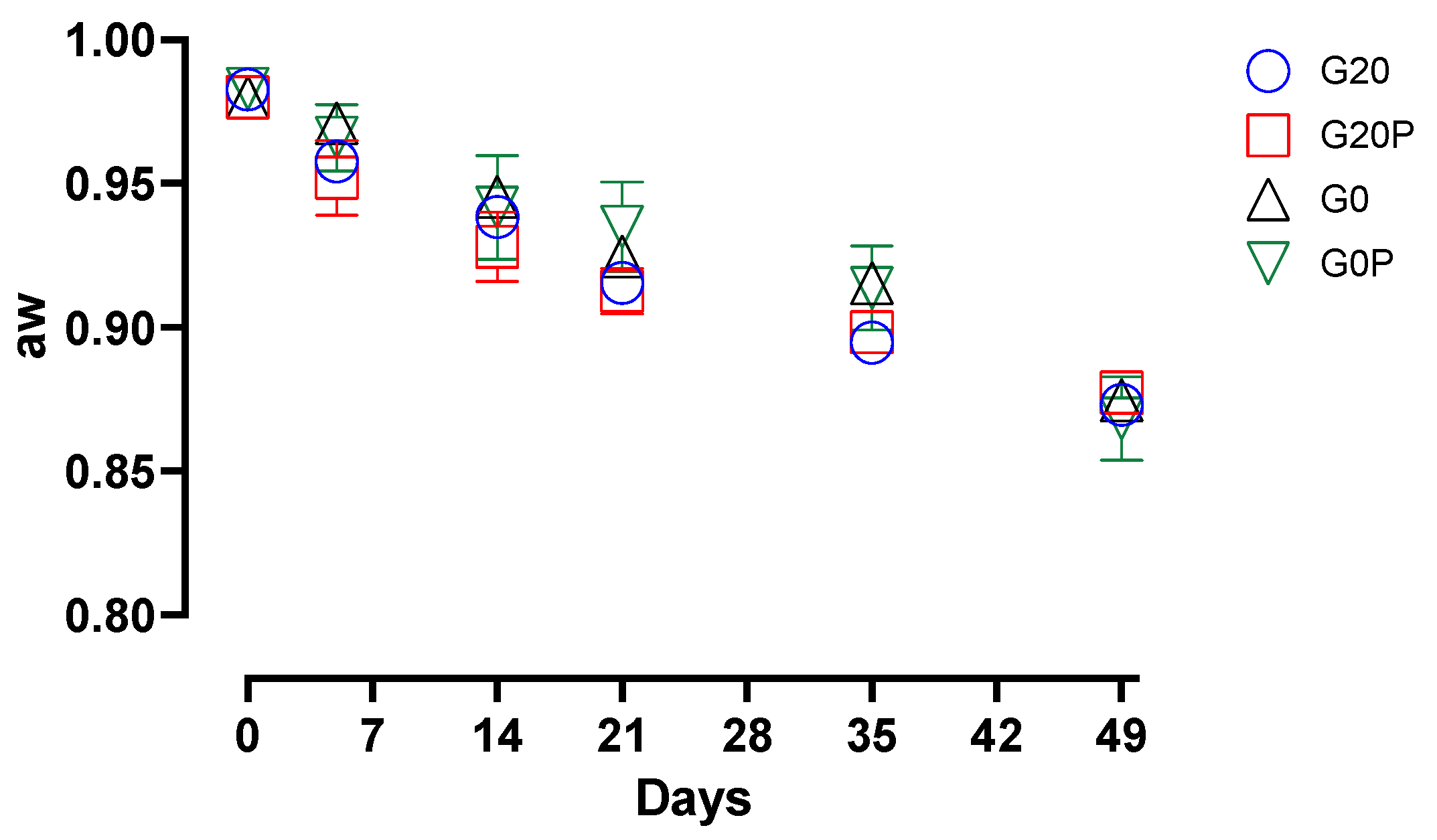
- G20, glucose (0.2%) and pork lard (3%) were added to the mixture, and the batch was used as a control.
- G20P, produced with the addition of glucose (0.2%), pork lard (2.5%), and pumpkin oil (0.5%) sprayed during the second mixing.
- G0, produced without glucose addition, and pork lard (3%) was added to the mixture.
- G0P, produced without glucose addition, pork lard (2.5%) and pumpkin oil (0.5%) sprayed during the second mixing.
2.3.2. Chemical Composition
2.3.3. Lipid Peroxidation
2.3.4. Physicochemical and Microbiological Analyses
2.3.5. Sensorial Characterization
2.4. Statistical Analyses
3. Results and Discussion
3.1. Vegetal Oil as Alternative Ingredient
Culture Starters—Pumpkin Oil Compatibility
3.2. Effect of Pumpkin Oil on Sugar-Free Buffalo Salami Quality
3.2.1. Effect on Chemical Composition and Fatty Acids
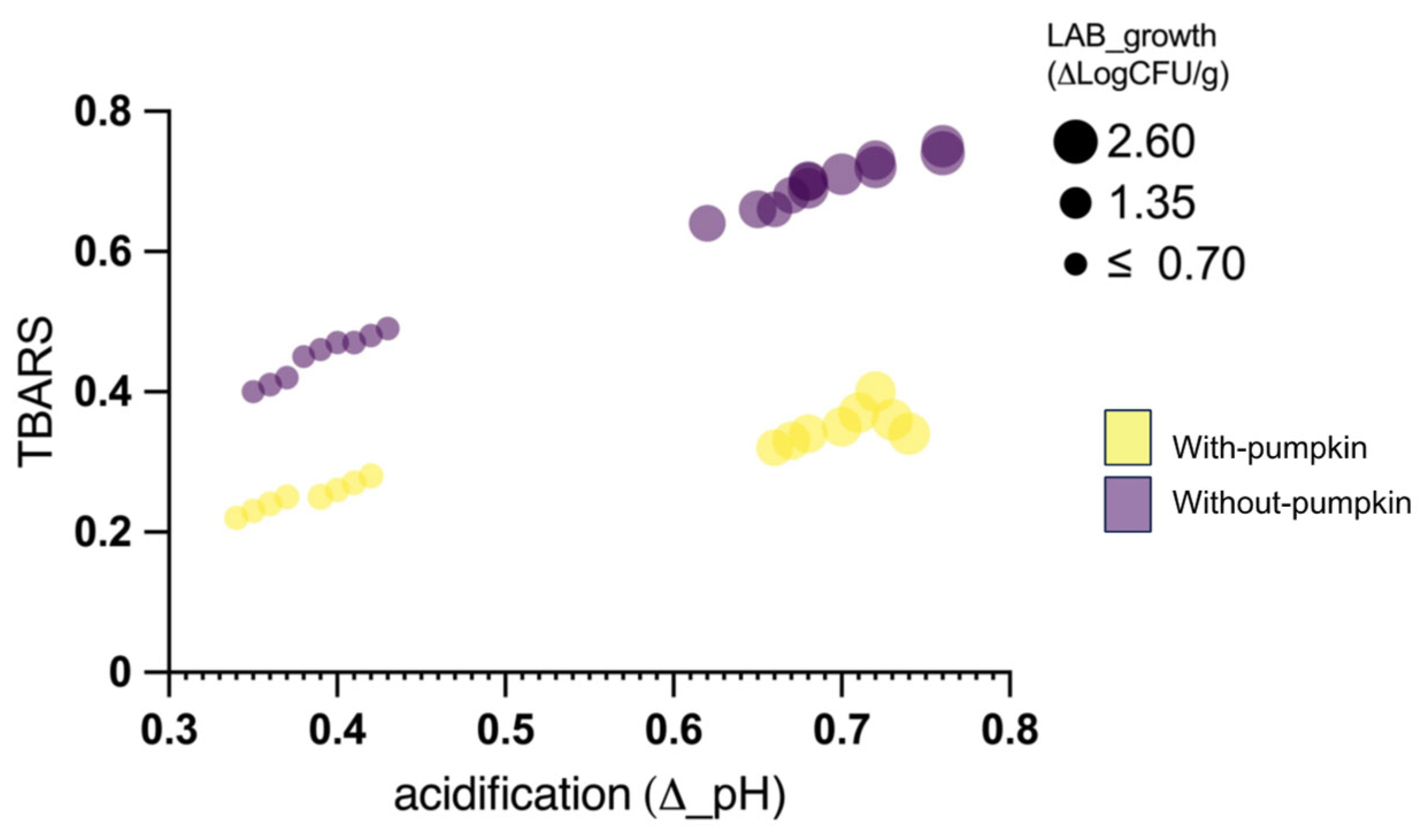
3.2.2. Effect on Microbial Populations and Physicochemical Parameters
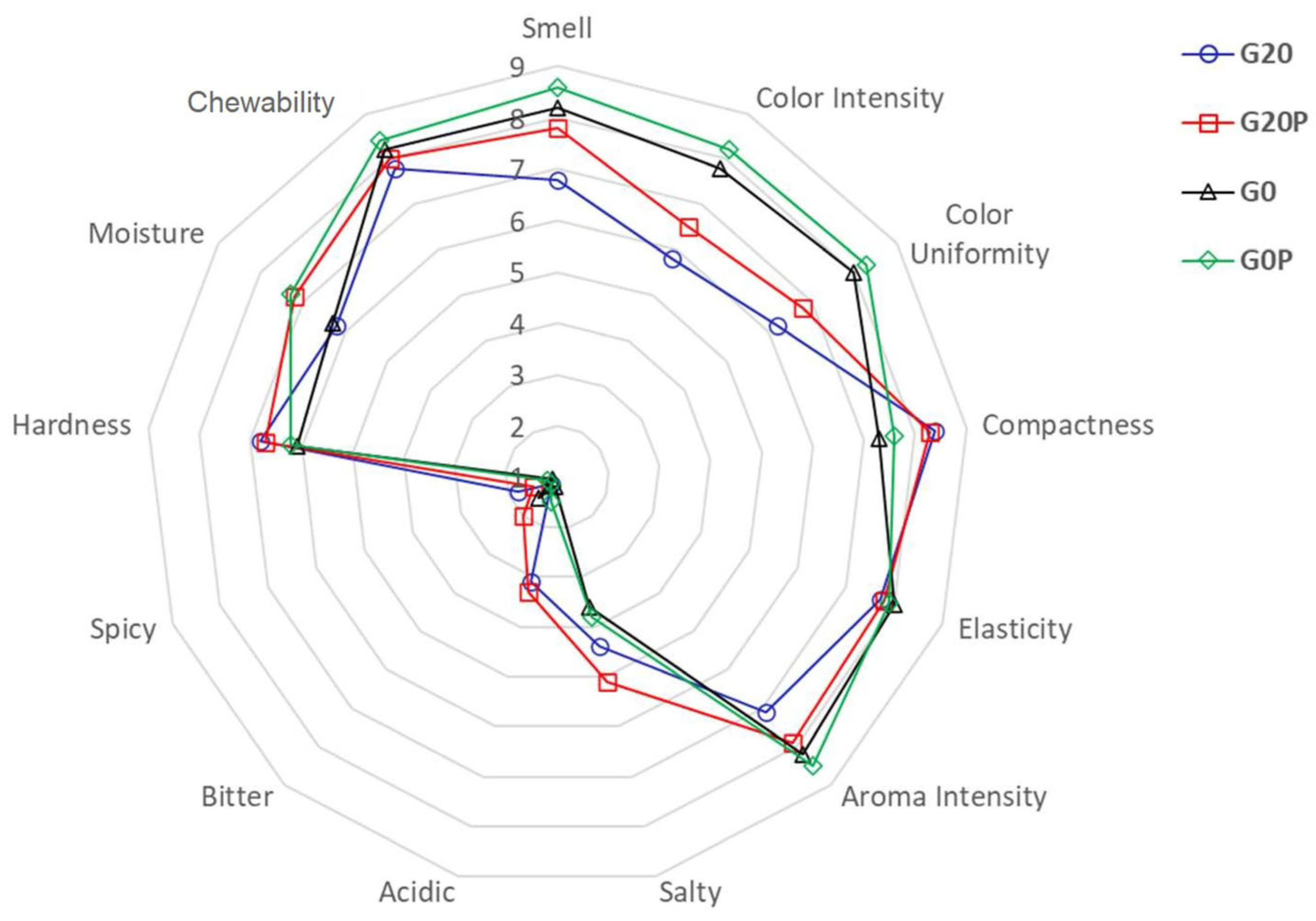
3.2.3. Effect on Lipid Peroxidation and Sensorial Features
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salter, A. The Effects of Meat Consumption on Global Health. Rev. Sci. Tech. 2018, 37, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, M.; Siatka, T.; Krčmová, L.K.; Matoušová, K.; Mladěnka, P. Biological Properties of Vitamin B12. Nutr. Res. Rev. 2024, 1–33. [Google Scholar] [CrossRef]
- Contò, M.; Miarelli, M.; Di Giovanni, S.; Failla, S. Variability of Sialic Acids in Beef Breeds and Nutritional Implications in Red Meat. Molecules 2025, 30, 710. [Google Scholar] [CrossRef]
- Gaeini, Z.; Alvirdizadeh, S.; Hosseinpour-Niazi, S.; Mirmiran, P.; Azizi, F. Dietary Fat Quality Indices and Risk of Pre-Diabetes and Type 2 Diabetes Mellitus: Tehran Lipid and Glucose Study. Public Health Nutr. 2025, 28, e8. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Carrascal, J.R.; Bügel, S.; Astrup, A. Meat and Human Health—Current Knowledge and Research Gaps. Foods 2021, 10, 1556. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; Redondo, N.; Cofrades, S.; Bastida, S.; Nova, E.; Benedí, J.; Sánchez-Muniz, F.J.; Marcos, A.; López-Oliva, M.E. Carob Fruit Extract-Enriched Meat, as Preventive and Curative Treatments, Improves Gut Microbiota and Colonic Barrier Integrity in a Late-Stage T2DM Model. Food Res. Int. 2021, 141, 110124. [Google Scholar] [CrossRef]
- Lombardi, S.J.; Pannella, G.; Coppola, F.; Vergalito, F.; Maiuro, L.; Succi, M.; Sorrentino, E.; Tremonte, P.; Coppola, R. Plant-Based Ingredients Utilized as Fat Replacers and Natural Antimicrobial Agents in Beef Burgers. Foods 2024, 13, 3229. [Google Scholar] [CrossRef]
- Mounir, S.; Mohamed, R.; Sunooj, K.V.; El-Saidy, S.; Farid, E. Assessing the Effects of Partially Substituting Chicken Breast Meat with Oyster Mushroom Stalk Powder on the Quality Attributes of Mushroom-Chicken Burgers. Sci. Rep. 2025, 15, 4361. [Google Scholar] [CrossRef]
- Dasiewicz, K.; Szymańska, I.; Opat, D.; Hąc-Szymańczuk, E. Development and Characterization of Hybrid Burgers Made from Pork and Multi-Ingredient Plant Mixtures and Protected with Lactic Acid Bacteria. Appl. Sci. 2024, 14, 6272. [Google Scholar] [CrossRef]
- Espinales, C.; Baldeón, M.; Bravo, C.; Toledo, H.; Carballo, J.; Romero-Peña, M.; Cáceres, P.J. Strategies for Healthier Meat Foods: An Overview. Prev. Nutr. Food Sci. 2024, 29, 18–30. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, J.; Fan, Y.; Kou, T. Effect of Flavonoids from Lycium Barbarum Leaves on the Oxidation of Myofibrillar Proteins in Minced Mutton during Chilled Storage. J. Food Sci. 2021, 86, 1766–1777. [Google Scholar] [CrossRef]
- Puvača, N.; Milenković, J.; Galonja Coghill, T.; Bursić, V.; Petrović, A.; Tanasković, S.; Pelić, M.; Ljubojević Pelić, D.; Miljković, T. Antimicrobial Activity of Selected Essential Oils against Selected Pathogenic Bacteria: In Vitro Study. Antibiotics 2021, 10, 546. [Google Scholar] [CrossRef] [PubMed]
- Nilofar; Zengin, G.; Uba, A.I.; Abul, N.; Gulcin, I.; Koyuncu, I.; Yuksekdag, O.; Ponnaiya, S.K.M.; Tessappan, S.; Nazzaro, F.; et al. A Multifunctional Natural Treasure Based on a “One Stone, Many Birds” Strategy for Designing Health-Promoting Applications: Tordylium apulum. Food Biosci. 2024, 62, 105088. [Google Scholar] [CrossRef]
- Comi, G.; Colautti, A.; Bernardi, C.E.M.; Stella, S.; Orecchia, E.; Coppola, F.; Iacumin, L. Leuconostoc Gelidum Is the Major Species Responsible for the Spoilage of Cooked Sausage Packaged in a Modified Atmosphere, and Hop Extract Is the Best Inhibitor Tested. Microorganisms 2024, 12, 1175. [Google Scholar] [CrossRef]
- Francolino, R.; Martino, M.; Nazzaro, F.; Sirignano, C.; Fratianni, F.; Coppola, F.; De Martino, L.; Formisano, C.; De Feo, V. Chemical Profile and Bioactivities of Three Species of Mentha Growing in the Campania Region, Southern Italy. Plants 2025, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Coppola, F.; Lombardi, S.J.; Tremonte, P. Edible Insect Meals as Bioactive Ingredients in Sustainable Snack Bars. Foods 2025, 14, 702. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, S. Influence of Vegetable Oils on pH Profile during Processing of Semidry Fermented Buffalo Meat Sausage. J. Food Process. Preserv. 2018, 9, 2. [Google Scholar] [CrossRef]
- Nazir, S.; Azad, Z.R.A.A. Food Nanotechnology: An Emerging Technology in Food Processing and Preservation. In Health and Safety Aspects of Food Processing Technologies; Malik, A., Erginkaya, Z., Erten, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 567–576. [Google Scholar] [CrossRef]
- Da Silva, J.A.R.; Garcia, A.R.; De Almeida, A.M.; Bezerra, A.S.; de Brito Lourenço Junior, J. Water Buffalo Production in the Brazilian Amazon Basin: A Review. Trop. Anim. Health Prod. 2021, 53, 343. [Google Scholar] [CrossRef]
- Tamburrano, A.; Tavazzi, B.; Callà, C.A.M.; Amorini, A.M.; Lazzarino, G.; Vincenti, S.; Zottola, T.; Campagna, M.C.; Moscato, U.; Laurenti, P. Biochemical and Nutritional Characteristics of Buffalo Meat and Potential Implications on Human Health for a Personalized Nutrition. Ital. J. Food Saf. 2019, 8, 8317. [Google Scholar] [CrossRef]
- Guerrero-Legarreta, I.; Napolitano, F.; Cruz-Monterrosa, R.; Mota-Rojas, D.; Mora-Medina, P.; Ramírez-Bribiesca, E.; Bertoni, A.; Berdugo-Gutiérrez, J.; Braghieri, A. River Buffalo Meat Production and Quality: Sustainability, Productivity, Nutritional and Sensory Properties. J. Buffalo Sci. 2020, 9, 159–169. [Google Scholar] [CrossRef]
- Martuscelli, M.; Serio, A.; Capezio, O.; Mastrocola, D. Safety, Quality and Analytical Authentication of Ḥalāl Meat Products, with Particular Emphasis on Salami: A Review. Foods 2020, 9, 1111. [Google Scholar] [CrossRef]
- da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical Composition, Extraction Sources and Action Mechanisms of Essential Oils: Natural Preservative and Limitations of Use in Meat Products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, M.; Purriños, L.; Martínez, S.; Carballo, J. Spices Affect the Biochemical Events Taking Place during the Manufacture of Galician Chorizo Sausage. LWT—Food Sci. Technol. 2025, 216, 117322. [Google Scholar] [CrossRef]
- Di Stasio, L.; Brugiapaglia, A. Current Knowledge on River Buffalo Meat: A Critical Analysis. Animals 2021, 11, 2111. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Swelum, A.A.; Al-Sultan, S.I.; El-Ghareeb, W.R.; Hussein, E.O.S.; Ba-Awadh, H.A.; Akl, B.A.; Nader, M.M. Enhancing Quality and Safety of Raw Buffalo Meat Using the Bioactive Peptides of Pea and Red Kidney Bean under Refrigeration Conditions. Ital. J. Anim. Sci. 2021, 20, 762–776. [Google Scholar] [CrossRef]
- Haque, A.; Ahmad, S.; Adnan, M.; Khan, M.I.; Ashraf, S.A.; Azad, Z.R.A.A. Fortification of Conventional Buffalo Meat Sausage with Ash Gourd Peel Enhances Shelf Life, Nutritional, Functional and Microstructural Characteristics. NFS J. 2024, 35, 100179. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, S. Studies on Physicochemical Properties of Cooked Buffalo Meat Sausage as Influenced by Incorporation of Carrot Powder during Refrigerated Storage. J. Food Process. Technol. 2015, 6, 436. [Google Scholar] [CrossRef]
- Grispoldi, L.; Ianni, F.; Blasi, F.; Pollini, L.; Crotti, S.; Cruciani, D.; Cenci-Goga, B.T.; Cossignani, L. Apple Pomace as Valuable Food Ingredient for Enhancing Nutritional and Antioxidant Properties of Italian Salami. Antioxidants 2022, 11, 1221. [Google Scholar] [CrossRef]
- Younis, K.; Ahmad, S. Waste Utilization of Apple Pomace as a Source of Functional Ingredient in Buffalo Meat Sausage. Cogent Food Agric. 2015, 1, 1119397. [Google Scholar] [CrossRef]
- Petridis, D.; Zotos, A.; Skapetas, B.; Bampidis, V.A. The Effect of Buffalo Meat on Composition, Instrumental and Sensory Characteristics of Traditional Greek Sausages. J. Food Res. 2015, 4, 26. [Google Scholar] [CrossRef]
- Sirini, N.; Munekata, P.E.S.; Lorenzo, J.M.; Stegmayer, M.Á.; Pateiro, M.; Pérez-Álvarez, J.Á.; Sepúlveda, N.; Sosa-Morales, M.E.; Teixeira, A.; Fernández-López, J.; et al. Development of Healthier and Functional Dry Fermented Sausages: Present and Future. Foods 2022, 11, 1128. [Google Scholar] [CrossRef]
- Bassi, D.; Milani, G.; Belloso Daza, M.V.; Barbieri, F.; Montanari, C.; Lorenzini, S.; Šimat, V.; Gardini, F.; Tabanelli, G. Taxonomical Identification and Safety Characterization of Lactobacillaceae from Mediterranean Natural Fermented Sausages. Foods 2022, 11, 2776. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Tabanelli, G.; Barbieri, F.; Montanari, C.; Gardini, F.; Daza, M.V.B.; Castellone, V.; Bozzetti, M.; Cocconcelli, P.S.; Bassi, D. Technological Traits and Mitigation Activity of Autochthonous Lactic Acid Bacteria from Mediterranean Fermented Meat-Products. LWT 2024, 196, 115861. [Google Scholar] [CrossRef]
- Francesca, N.; Sannino, C.; Moschetti, G.; Settanni, L. Microbial Characterisation of Fermented Meat Products from the Sicilian Swine Breed “Suino Nero Dei Nebrodi”. Ann. Microbiol. 2013, 63, 53–62. [Google Scholar] [CrossRef]
- Sgroi, F. Food Traditions and Consumer Preferences for Cured Meats: Role of Information in Geographical Indications. Int. J. Gastron. Food Sci. 2021, 25, 100386. [Google Scholar] [CrossRef]
- Coppola, S.; Mauriello, G.; Aponte, M.; Moschetti, G.; Villani, F. Microbial Succession during Ripening of Naples-Type Salami, a Southern Italian Fermented Sausage. Meat Sci. 2000, 56, 321–329. [Google Scholar] [PubMed]
- Polizzi, G.; Casalino, L.; Di Paolo, M.; Sardo, A.; Vuoso, V.; Franco, C.M.; Marrone, R. Influence of Different Starter Cultures on Physical–Chemical, Microbiological, and Sensory Characteristics of Typical Italian Dry-Cured “Salame Napoli”. Appl. Sci. 2024, 14, 3035. [Google Scholar] [CrossRef]
- Mauriello, G.; Casaburi, G.; Villani, F. Proteolytic Activity of Staphylococcus xylosus Strains on Pork Myofibrillar and Sarcoplasmic Proteins and Use of Selected Strains in the Production of ‘Naples Type’ Salami. J. Appl. Microbiol. 2002, 92, 482–490. [Google Scholar] [CrossRef]
- Fratianni, F.; Amato, G.; De Feo, V.; d’Acierno, A.; Coppola, R.; Nazzaro, F. Potential Therapeutic Benefits of Unconventional Oils: Assessment of the Potential in Vitro Biological Properties of Some Rubiaceae, Cucurbitaceae, and Brassicaceae Seed Oils. Front. Nutr. 2023, 10, 1171766. [Google Scholar] [CrossRef]
- Tremonte, P.; Pannella, G.; Lombardi, S.J.; Iorizzo, M.; Vergalito, F.; Cozzolino, A.; Maiuro, L.; Succi, M.; Sorrentino, E.; Coppola, R. Low-Fat and High-Quality Fermented Sausages. Microorganisms 2020, 8, 1025. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth, and Its Application in the in Vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Ombra, M.N.; Coppola, F.; De Giulio, B.; d’Acierno, A.; Coppola, R.; Fratianni, F. Antibacterial Activity and Prebiotic Properties of Six Types of Lamiaceae Honey. Antibiotics 2024, 13, 868. [Google Scholar] [CrossRef]
- Baranyi, J.; Roberts, T.A. A dynamic approach to predicting bacterial growth in food. Int. J. Food Microbiol. 1994, 23, 277–294. [Google Scholar] [CrossRef]
- Zullo, A.; Diaferia, C.; Genovino, G.; Palazzo, M.; Matassino, D. Production of Napoli salami from some swine autochthonous genetic types. II. Sensory evaluation. Options Méditerranéennes A 2000, 41, 237–240. [Google Scholar]
- AOAC International. Official Methods of Analysis, 22nd ed.; AOAC International: Rockville, MD, USA, 2023. [Google Scholar]
- Tremonte, P.; Gambacorta, G.; Pannella, G.; Trani, A.; Succi, M.; La Gatta, B.; Tipaldi, L.; Grazia, L.; Sorrentino, E.; Coppola, R. NaCl Replacement with KCl Affects Lipolysis, Microbiological and Sensorial Features of Soppressata Molisana. Eur. J. Lipid Sci. Technol. 2018, 120, 1700449. [Google Scholar] [CrossRef]
- Yildirim, Z.; Cibik, R.; Dertli, E.; Sagdic, O. Functional and Technological Characterization of Lactic Acid Bacteria Isolated from Turkish Dry-Fermented Sausage (Sucuk). Braz. J. Microbiol. 2022, 53, 959–968. [Google Scholar]
- Mangia, N.P.; Cottu, M.; Aponte, M.; Murgia, M.A.; Mura, M.E.; Blaiotta, G. Technological and Safety Characterization of Coagulase-Negative Staphylococci Isolated from Sardinian Fermented Sausage Made by Ovine Meat. Foods 2024, 13, 633. [Google Scholar] [CrossRef]
- Chiavari, C.; Coloretti, F.; Ferri, G.; Nanni, M. Proposta di un metodo per l’analisi sensoriale dei salami. Ind. Aliment. 2007, 46, 27–33. [Google Scholar]
- Joujou, F.M.; Darra, N.E.; Rajha, H.N.; Sokhn, E.S.; Alwan, N. Evaluation of Synergistic/Antagonistic Antibacterial Activities of Fatty Oils from Apricot, Date, Grape, and Black Seeds. Sci. Rep. 2024, 14, 6532. [Google Scholar] [CrossRef]
- Papatzimos, G.; Basdagianni, Z.; Kasapidou, E. Substitution of Animal Fat and Sodium Nitrite with Hemp Seed Oil: Effect on the Nutritional Value, Sensory Characteristics, and Shelf Life of Fermented Salami. Foods 2023, 13, 2584. [Google Scholar] [CrossRef]
- Wadeesirisak, K.; Musigamart, N.; Pantoa, D.T.; Saithong, P.; Chitisankul, W.T. Biochemical Composition and Functional Properties of Pumpkin (Cucurbita sp.) Seed Yogurt: A Functional Plant-Based Product. Available online: https://ssrn.com/abstract=5017870 (accessed on 11 February 2025).
- Laranjo, M.; Elias, M.; Fraqueza, M.J. The Use of Starter Cultures in Traditional Meat Products. J. Food Qual. 2019, 2019, 9546026. [Google Scholar] [CrossRef]
- Montanari, C.; Manfreda, G.; Ndagijimana, M.; Quattrucci, S.; Bernbom, N.; Huerta, R.; Giromini, C.; La Torre, A.; Gänzle, M.G.; Cecchini, F.; et al. Lactic Acid Bacteria in Fermented Meats: Selection and Technological Properties. Front. Microbiol. 2022, 13, 845796. [Google Scholar] [CrossRef]
- Chelladurai, P.; Pandey, A.; Swamy, C.T.; Sivadurga, K.; Anbarasi, K.; Reddy, M.P.; Sen, A.; Purewal, S.S.; Dash, S.K.; Kaur, M. Phenolic Compounds: Extraction, Identification, and Health Aspects, Part I. In Colored Cereals: Properties, Processing, Health Benefits, and Industrial Uses; Kaur, M., Ed.; CRC Press: Boca Raton, FL, USA, 2025; pp. 220–250. [Google Scholar] [CrossRef]
- Poveda, J.M. Polyphenol-Based Antimicrobial Coatings for Food Preservation. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2638–2660. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic Metabolism Revisited: Metabolism of Lactic Acid Bacteria in Food Fermentations and Food Spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- He, X.; Cui, Y.; Jia, Q.; Zhuang, Y.; Gu, Y.; Fan, X.; Ding, Y. Response Mechanisms of Lactic Acid Bacteria under Environmental Stress and Their Application in the Food Industry. Food Biosci. 2025, 64, 105938. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Speranza, B.; Gallo, M.; Campaniello, D.; Sinigaglia, M. Selection of Wild Lactic Acid Bacteria for Sausages: Design of a Selection Protocol Combining Statistic Tools, Technological and Functional Properties. LWT-Food Sci. Technol. 2017, 81, 144–152. [Google Scholar] [CrossRef]
- Sionek, B.; Szydłowska, A.; Trząskowska, M.; Kołożyn-Krajewska, D. The Impact of Physicochemical Conditions on Lactic Acid Bacteria Survival in Food Products. Fermentation 2024, 10, 298. [Google Scholar] [CrossRef]
- Bustos, A.Y.; Taranto, M.P.; Gerez, C.L.; Agriopoulou, S.; Smaoui, S.; Varzakas, T.; Enshasy, H.A.E. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics Antimicrob. Proteins 2024, 17, 138–158. [Google Scholar] [CrossRef]
- Rebucci, R.; Sangalli, L.; Fava, M.; Bersani, C.; Cantoni, C.; Baldi, A. Evaluation of Functional Aspects in Lactobacillus Strains Isolated from Dry Fermented Sausages. J. Food Qual. 2007, 30, 187–201. [Google Scholar] [CrossRef]
- Aquilanti, L.; Garofalo, C.; Osimani, A.; Clementi, F. Ecology of Lactic Acid Bacteria and Coagulase Negative Cocci in Fermented Dry Sausages Manufactured in Italy and Other Mediterranean Countries: An Overview. Int. Food Res. J. 2016, 23, 429–445. [Google Scholar]
- Colautti, A.; Camprini, L.; Ginaldi, F.; Comi, G.; Reale, A.; Coppola, F.; Iacumin, L. Safety Traits, Genetic and Technological Characterization of Lacticaseibacillus rhamnosus Strains. LWT 2024, 207, 116578. [Google Scholar] [CrossRef]
- Petrović, T.Ž.; Tomović, V.M.; Marković, K.G.; Semedo-Lemsaddek, T.; Grujović, M.Ž. Probiotics and Honey: Boosting Functional Properties in Dry Fermented Sausages. Microorganisms 2025, 13, 349. [Google Scholar] [CrossRef]
- Selani, M.M.; Herrero, A.M.; Ruiz-Capillas, C. Plant Antioxidants in Dry Fermented Meat Products with a Healthier Lipid Profile. Foods 2022, 11, 3558. [Google Scholar] [CrossRef]
- Nawaz, A.; Walayat, N.; Khalifa, I.; Harlina, P.W.; Irshad, S.; Qin, Z.; Luo, X. Emerging Challenges and Efficacy of Polyphenols–Proteins Interaction in Maintaining the Meat Safety during Thermal Processing. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13313. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.; Fakhry, S.; Satour, R. Revolutionizing Food Quality with Machine Vision and Machine Learning Techniques. In Food in the Metaverse and Web 3.0 Era: Intersecting Food, Technology, and Culture; IGI Global Scientific Publishing: New York, NY, USA, 2025; pp. 71–124. [Google Scholar] [CrossRef]
- Mahmud, N.; Ferdaus, M.J.; Silva, R.C.D. Exploring the Feasibility of Direct-Dispersion Oleogels in Healthier Sausage Formulations. Gels 2024, 10, 819. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Qian, M.; Wang, Y.; Zhang, K.; Tian, J.; Wang, X. Research Progress on the Gel Properties of Fermented Sausage. Food Mater. Res. 2024, 4, e007. [Google Scholar] [CrossRef]
- de Carvalho, F.A.L.; Munekata, P.E.; Pateiro, M.; Campagnol, P.C.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Effect of Replacing Backfat with Vegetable Oils during the Shelf-Life of Cooked Lamb Sausages. LWT 2020, 122, 109052. [Google Scholar] [CrossRef]
- Monteiro, G.M.; Souza, X.R.; Costa, D.P.B.; Faria, P.B.; Vicente, J. Partial Substitution of Pork Fat with Canola Oil in Toscana Sausage. Innov. Food Sci. Emerg. Technol. 2017, 44, 2–8. [Google Scholar] [CrossRef]
- Heck, R.T.; Dos Santos, B.A.; Lorenzo, J.M.; Ruiz-Capillas, C.; Cichoski, A.J.; de Menezes, C.R.; Campagnol, P.C.B. Replacement of Saturated Fat by Healthy Oils to Improve Nutritional Quality of Meat Products. In Food Lipids: Sources, Health Implications, and Future Trends; Elsevier: Amsterdam, The Netherlands, 2022; pp. 461–487. [Google Scholar] [CrossRef]
- Bilska, A.; Krzywdzińska-Bartkowiak, M. The Influence of Vegetable Oil Addition Levels on the Fatty Acid Profile and Oxidative Transformation Dynamics in Liver Sausage-Type Processed Meats. Foods 2025, 14, 380. [Google Scholar] [CrossRef]
- Toupchi, F.M.; Pirsa, S.; Mohammadi, S. Hydrogel (Pectin), Oleogel (Oil/Triglyceride) and Bigel (Alginate-Oil/Triglyceride) and Their Applications in Meat Products Formulation. Carbohydr. Polym. Technol. Appl. 2025, 9, 100715. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Kralik, Z.; Kralik, G.; Košević, M. Effects of Vegetable Oils Supplemented into Broiler Diet on the Fatty Acid Profile and Lipid Indices in Broiler Meat. Agriculture 2025, 15, 441. [Google Scholar] [CrossRef]
- Hajji, H.; Smeti, S.; Mekki, I.; Atti, N. Effect of Dietary Protein Level and Lamb Breed on Meat Physicochemical Traits, Fatty Acid Profile and Nutritional Indices. Arch. Anim. Breed. 2025, 68, 57–66. [Google Scholar] [CrossRef]
- Cence, K.; Vendruscolo, M.J.D.; da Silva, L.M.; Colet, R.; Junges, A.; Steffens, C.; Zeni, J.; Valduga, E. Impact of Microencapsulated Starter Culture on the Quality Characteristics of Italian-Type Salami during Storage. J. Food Meas. Charact. 2025, 19, 1913–1928. [Google Scholar]
- Fadda, S.; Castellano, P.; Terán, L.; Raya, R.; Vignolo, G. Role of Autochthonous Starter Cultures on Quality, Safety, and Health. Lactic Acid Bacteria: Microbiological and Functional Aspects, 6th ed.; Vinderola, G., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 228–246. [Google Scholar] [CrossRef]
- Li, X.; Zhao, G.; Zheng, Y.; Wang, Y.; Bai, X.; Li, F.; Gu, Y.; Zhu, C. Effects of Single Fermentation of Lactobacillus sakei and Compound Fermentation with Staphylococcus carnosus on the Metabolomics of Beef Sausages. Food Chem. 2025, 464, 141728. [Google Scholar] [CrossRef]
- Montanari, C.; Barbieri, F.; Gardini, G.; Magnani, R.; Gottardi, D.; Gardini, F.; Tabanelli, G. Effects of Starter Cultures and Type of Casings on the Microbial Features and Volatile Profile of Fermented Sausages. Fermentation 2022, 8, 683. [Google Scholar] [CrossRef]
- Sánchez-Giraldo, M.; Vioque-Amor, M.; Gómez-Díaz, R.; Clemente-López, I.; Amaro-López, M.Á.; Avilés-Ramírez, C. Effect of Carbohydrate Formulas on Instrumental and Sensory Parameters in Dry-Fermented Iberian Pork Sausages. Foods 2025, 14, 248. [Google Scholar] [CrossRef]
- Tabanelli, G.; Barbieri, F.; Soglia, F.; Magnani, R.; Gardini, F.; Petracci, M.; Montanari, C. Safety and Technological Issues of Dry Fermented Sausages Produced Without Nitrate and Nitrite. Food Res. Int. 2022, 160, 111685. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. Sausages, Types of Dry and Semi-Dry. In Encyclopedia of Meat Sciences, 3rd ed.; Dikeman, M., Ed.; Elsevier: Oxford, UK, 2024; pp. 413–424. [Google Scholar] [CrossRef]
- Tremonte, P.; Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Ibanez, E.; Coppola, R. Antimicrobial Effect of Malpighia punicifolia and Extension of Water Buffalo Steak Shelf-Life. J. Food Sci. 2016, 81, M97–M105. [Google Scholar] [CrossRef]
- Santchurn, S.J.; Arnaud, E.; Zakhia-Rozis, N.; Collignan, A. Drying: Principles and Applications. In Handbook of Meat and Meat Processing, 2nd ed.; Hui, Y.H., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2012; pp. 505–523. [Google Scholar]
- Dasiewicz, K.; Szymańska, I.; Słowiński, M.; Górska, A.; Dasiewicz, B. Effect of Fermentation Technology and Storage Time on the Quality of Salami-Type Sausages. Appl. Sci. 2024, 14, 8510. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Dhanavel, N.; Nandakrishnan, M.H. A Review of Animal Fat: A Great Source for Industrial Applications. J. Chem. Rev. 2024, 6, 115–137. [Google Scholar] [CrossRef]
- Rai, S.; Rai, S.K. Anti-Bacterial Effect of Zanthoxylum armatum Oleoresin Against Some Bacterial Isolates from Pork and Chicken Meat Sold in Dharan, Nepal. Himalayan J. Sci. Technol. 2017, 1, 35–39. [Google Scholar] [CrossRef]
- Rodrigues, L.S.; da Silva, J.A.R.; da Silva, W.C.; da Silva, É.B.R.; Belo, T.S.; Sousa, C.E.L.; de Carvalho Rodrigues, T.C.G.; e Silva, A.G.M.; Prates, J.A.M.; de Brito Lourenço-Júnior, J. A Review of the Nutritional Aspects and Composition of the Meat, Liver and Fat of Buffaloes in the Amazon. Animals 2024, 14, 1618. [Google Scholar] [CrossRef]
- Škrlep, M.; Ozmec, M.; Čandek-Potokar, M. Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages. Processes 2022, 10, 821. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current Research in Meat Color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Chmiel, M.; Adamczak, L.; Pietrzak, D.; Florowski, T.; Florowska, A. Effect of Differentiated Relative Humidity of Air on the Quality of Traditional Speciality Guaranteed “Krakowska Sucha Staropolska” Sausage. Foods 2022, 11, 811. [Google Scholar] [CrossRef]
- Bernardi, E.; Capri, E.; Pulina, G. Carni e Salumi: Le Nuove Frontiere Della Sostenibilità: Ambiente, Salute, Sicurezza, Cultura, Economia ed Etica Nelle Filiere Nazionali; FrancoAngeli: Milan, Italy, 2023. [Google Scholar]
| Control Media | Media with Pumpkin Oil | |
|---|---|---|
| Lt. sakei | ||
| Initial value | 4.05 ± 0.06 a | 4.05 ± 0.06 a |
| Lag | 3.85 ± 0.28 a | 3.71 ± 0.29 a |
| Maximum Rate | 0.54 ± 0.02 a | 0.53 ± 0.02 a |
| Final Value | 9.36 ± 0.06 a | 9.27 ± 0.06 a |
| S. xylosus | ||
| Initial value | 4.14 ± 0.08 a | 4.06 ± 0.08 a |
| Lag | 4.17 ± 0.5 a | 4.11 ± 0.55 a |
| Maximum Rate | 0.35 ± 0.02 a | 0.34 ± 0.02 a |
| Final Value | 9.09 ± 0.14 a | 9.18 ± 0.14 a |
| G20 | G20P | G0 | G0P | |
|---|---|---|---|---|
| Moisture (%) | 29.91 a | 29.98 a | 30.01 a | 30.12 a |
| Protein (% d.m.) | 58.50 a | 58.58 a | 58.63 a | 58.97 a |
| NPN (% d.m.) | 10.53 a | 10.41 a | 10.66 a | 10.80 a |
| Ash (% d.m.) | 8.85 a | 8.82 a | 8.76 a | 8.22 a |
| Carbohydrates (% d.m.) | 0.11 a | 0.13 a | 0.01 b | 0.01 b |
| Lipid (% d.m.) | 22.01 a | 22.06 a | 21.94 a | 22.00 a |
| SFA (% d.m.) | 9.94 a | 8.01 b | 9.81 a | 8.05 b |
| MUFA (% d.m.) | 8.66 a | 8.44 a | 8.49 a | 8.52 a |
| PUFA (% d.m.) | 3.41 a | 5.61 b | 3.64 a | 5.43 b |
| PUFA/SFA | 0.34 a | 0.70 b | 0.37 a | 0.67 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Nazzaro, F.; Fratianni, F.; Lombardi, S.J.; Grazia, L.; Coppola, R.; Tremonte, P. Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat. Foods 2025, 14, 1077. https://doi.org/10.3390/foods14061077
Coppola F, Nazzaro F, Fratianni F, Lombardi SJ, Grazia L, Coppola R, Tremonte P. Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat. Foods. 2025; 14(6):1077. https://doi.org/10.3390/foods14061077
Chicago/Turabian StyleCoppola, Francesca, Filomena Nazzaro, Florinda Fratianni, Silvia Jane Lombardi, Luigi Grazia, Raffaele Coppola, and Patrizio Tremonte. 2025. "Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat" Foods 14, no. 6: 1077. https://doi.org/10.3390/foods14061077
APA StyleCoppola, F., Nazzaro, F., Fratianni, F., Lombardi, S. J., Grazia, L., Coppola, R., & Tremonte, P. (2025). Pumpkin Oil and Its Effect on the Quality of Naples-Style Salami Produced from Buffalo Meat. Foods, 14(6), 1077. https://doi.org/10.3390/foods14061077










