Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Settings
2.2. Spectral Data Acquisition
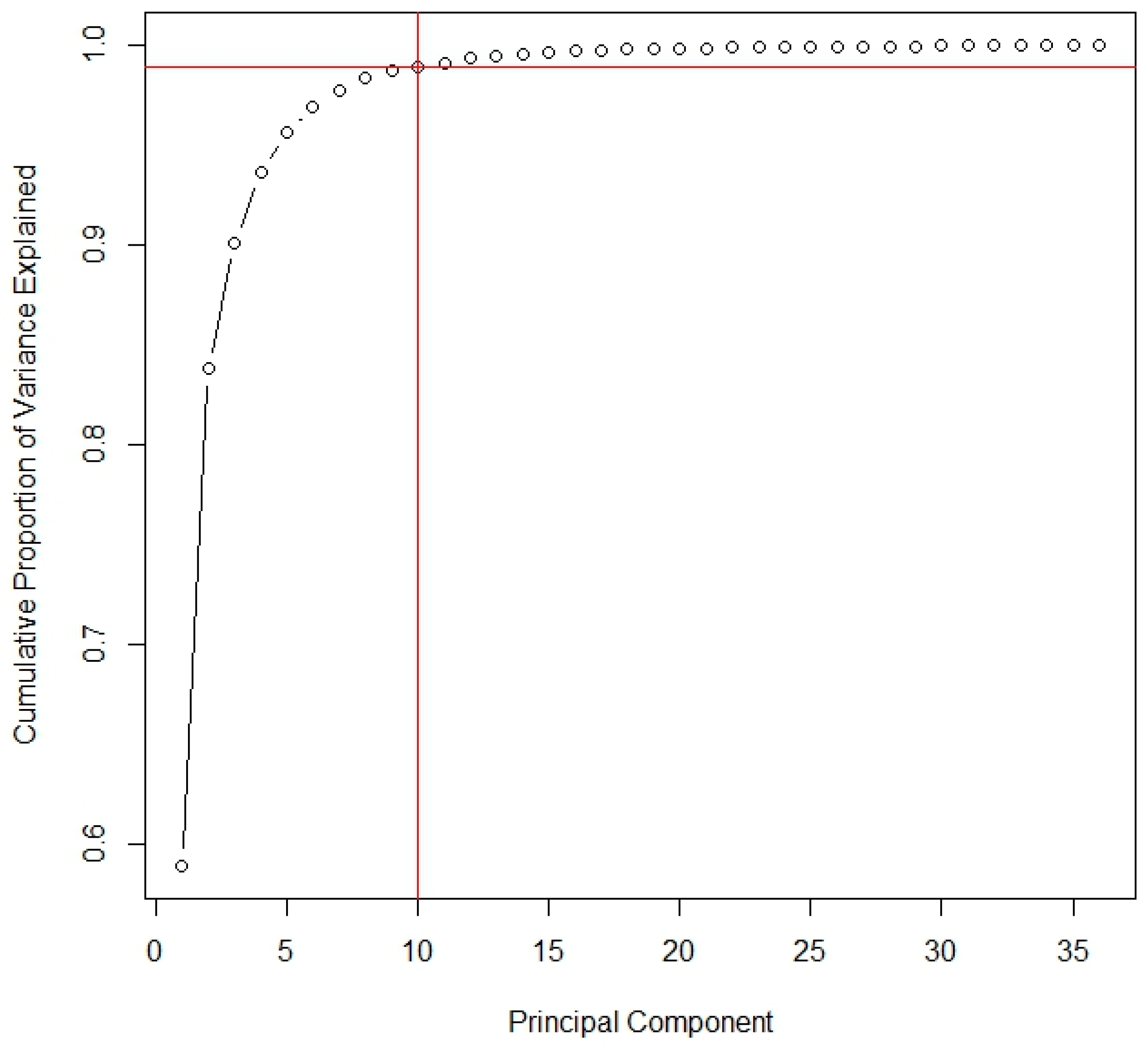
2.3. Data Analysis
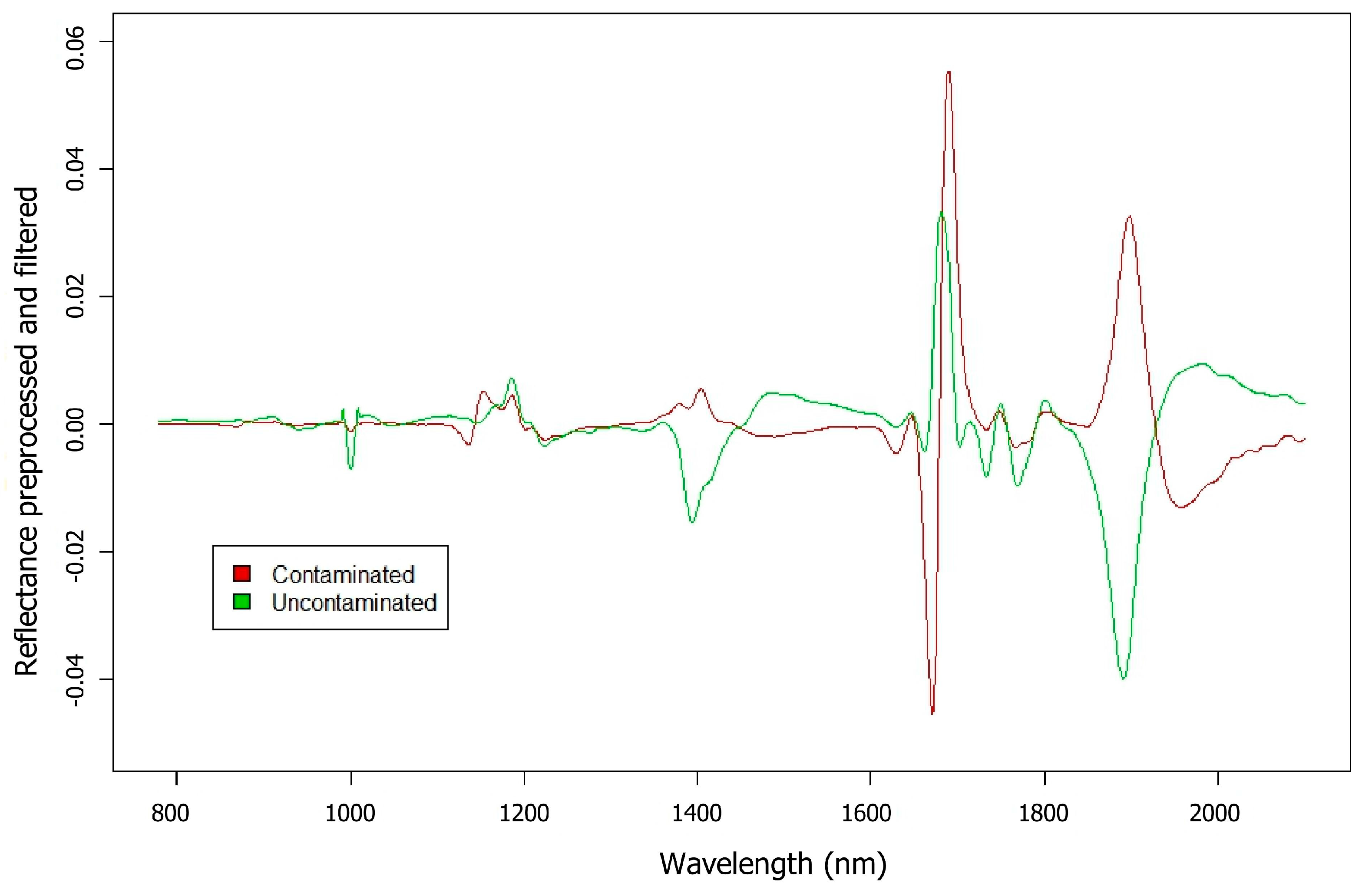
3. Results and Discussion
3.1. Biofilms Formed by Different Bacterial Strains
3.2. Biofilms Formed by S. aureus on Glass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, J.; Tarar, S.M.; Gul, I.; Nawaz, U.; Arshad, M. Challenges of Antibiotic Resistance Biofilms and Potential Combating Strategies: A Review. 3 Biotech 2021, 11, 169. [Google Scholar] [CrossRef]
- Highmore, C.J.; Melaugh, G.; Morris, R.J.; Parker, J.; Direito, S.O.L.; Romero, M.; Soukarieh, F.; Robertson, S.N.; Bamford, N.C. Translational Challenges and Opportunities in Biofilm Science: A BRIEF for the Future. NPJ Biofilms Microbiomes 2022, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus Aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Chatre, C.; Lavigne, J.P.; Pantel, A.; Reynes, J.; Dunyach-Remy, C. Effect of Antibiotic Exposure on Staphylococcus Epidermidis Responsible for Catheter-Related Bacteremia. Int. J. Mol. Sci. 2023, 24, 1547. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, J.M.O.; Martín-Gutiérrez, G.; Casimiro-Soriguer, C.S.; Gimeno-Gascón, M.A.; Cisneros, J.M.; de Alarcón, A.; Lepe, J.A. C-Terminal Deletion of RelA Protein Is Suggested as a Possible Cause of Infective Endocarditis Recurrence with Enterococcus Faecium. Antimicrob. Agents Chemother. 2024, 68, e0108323. [Google Scholar] [CrossRef]
- Carvalho, F.M.; Teixeira-Santos, R.; Mergulhão, F.J.M.; Gomes, L.C. Effect of Lactobacillus Plantarum Biofilms on the Adhesion of Escherichia Coli to Urinary Tract Devices. Antibiotics 2021, 10, 966. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S. Do Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry—A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef]
- Funari, R.; Shen, A.Q. Detection and Characterization of Bacterial Biofilms and Biofilm-Based Sensors. ACS Sens. 2022, 7, 347–357. [Google Scholar] [CrossRef]
- Lorenzo, F.; Sanz-Puig, M.; Bertó, R.; Orihuel, E. Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination. Appl. Sci. 2020, 10, 744. [Google Scholar] [CrossRef]
- Fratamico, P.M.; Annous, B.A.; Guenther, N.W. Biofilms in the Food and Beverage Industries; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier Science: Amsterdam, The Netherlands, 2009; ISBN 9781845694777. [Google Scholar]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)Control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Weinstein, R.A. Controlling Antimicrobial Resistance in Hospitals: Infection and Use of Antibiotics. Emerg. Infect. Dis. 2001, 7, 188–192. [Google Scholar] [CrossRef]
- Gieroba, B.; Krysa, M.; Wojtowicz, K.; Wiater, A.; Pleszczyńska, M.; Tomczyk, M.; Sroka-Bartnicka, A. The FT-IR and Raman Spectroscopies as Tools for Biofilm Characterization Created by Cariogenic Streptococci. Int. J. Mol. Sci. 2020, 21, 3811. [Google Scholar] [CrossRef] [PubMed]
- Kamnev, A.A.; Dyatlova, Y.A.; Kenzhegulov, O.A.; Fedonenko, Y.P.; Evstigneeva, S.S.; Tugarova, A.V. Fourier Transform Infrared (FTIR) Spectroscopic Study of Biofilms Formed by the Rhizobacterium Azospirillum Baldaniorum Sp245: Aspects of Methodology and Matrix Composition. Molecules 2023, 28, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zimmerleiter, R.; Leiss-Holzinger, E.; Wagner, E.M.; Kober-Rychli, K.; Wagner, M.; Brandstetter, M. Inline Biofilm Monitoring Based on Near-Infrared Spectroscopy with Ultracompact Spectrometer Technology. NIR News 2020, 31, 9–13. [Google Scholar] [CrossRef]
- Fernández, L.; Allende-Prieto, C.; Peón, J.; Recondo, C.; Rodríguez-Gonzálvez, P.; Gutiérrez, D.; Martínez, B.; García, P.; Rodríguez, A. Preliminary Assessment of Visible, near-Infrared, and Short-Wavelength–Infrared Spectroscopy with a Portable Instrument for the Detection of Staphylococcus Aureus Biofilms on Surfaces. J. Food Prot. 2019, 82, 1314–1319. [Google Scholar] [CrossRef]
- And, A.; Microioilogy, E. Use of Lactobacillus Plantarum LPCO10, a Bacteriocin Producer, as a Starter Culture in Spanish-Style Green Olive Fermentations. Appl. Environ. Microbiol. 1994, 60, 2059–2064. [Google Scholar]
- Valle, J.; Toledo-Arana, A.; Berasain, C.; Ghigo, J.M.; Amorena, B.; Penadés, J.R.; Lasa, I. SarA and Not ΣB Is Essential for Biofilm Development by Staphylococcus Aureus. Mol. Microbiol. 2003, 48, 1075–1087. [Google Scholar] [CrossRef]
- Delgado, S.; Arroyo, R.; Jiménez, E.; Marín, M.L.; Del Campo, R.; Fernández, L.; Rodríguez, J.M. Staphylococcus Epidermidis Strains Isolated from Breast Milk of Women Suffering Infectious Mastitis: Potential Virulence Traits and Resistance to Antibiotics. BMC Microbiol. 2009, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Rehaiem, A.; Martínez, B.; Manai, M.; Rodríguez, A. Production of Enterocin A by Enterococcus Faecium MMRA Isolated from ‘Rayeb’, a Traditional Tunisian Dairy Beverage. J. Appl. Microbiol. 2010, 108, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Analizador LabSpec 4 Standard-Res–Bonsai Advanced. Available online: https://www.bonsaiadvanced.com/producto/analisis-espectral/analizadores-nir/labspec/labspec-4-standard-res-lab-analyzer-copiar/ (accessed on 27 May 2024).
- ProLab Systems. ASD Instrument Spectroscopy Probes and Accessories, 2020. Available online: https://prolabsystems.com/wp/2020/03/15/asd-instrument-spectroscopy-probes-and-accessories/ (accessed on 9 January 2025).
- Kastanek, M. Calibrating with a White Reference for a Baseline, 2020. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/insights/calibrating-with-a-white-reference-for-a-baseline (accessed on 9 January 2025).
- Liu, Y. Principal Component Analysis in the Development of Optical and Imaging Spectroscopic Inspections for Agricultural/Food Safety and Quality; IntechOpen: London, UK, 2012; ISBN 978-953-51-0182-6. [Google Scholar]
- Ronningen, I.G.; Peterson, D.G. Identification of Aging-Associated Food Quality Changes in Citrus Products Using Untargeted Chemical Profiling. J. Agric. Food Chem. 2018, 66, 682–688. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2024. [Google Scholar]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Veerasakulwat, S.; Sitorus, A.; Udompetaikul, V. Rapid Classification of Sugarcane Nodes and Internodes Using Near-Infrared Spectroscopy and Machine Learning Techniques. Sensors 2024, 24, 7102. [Google Scholar] [CrossRef]
- Lanjewar, M.G.; Asolkar, S.; Parab, J.S.; Morajkar, P.P. Detecting Starch-Adulterated Turmeric Using Vis-NIR Spectroscopy and Multispectral Imaging with Machine Learning. J. Food Compos. Anal. 2024, 136, 106700. [Google Scholar] [CrossRef]
- Sui, M.; Li, Y.; Jiang, Y.; Zhang, Y.; Wang, L.; Zhang, W.; Wang, X. Light Exposure Interferes with Electroactive Biofilm Enrichment and Reduces Extracellular Electron Transfer Efficiency. Water Res. 2021, 188, 116512. [Google Scholar] [CrossRef]
- Elahi, Y.; Baker, M.A.B. Light Control in Microbial Systems. Int. J. Mol. Sci. 2024, 25, 4001. [Google Scholar] [CrossRef]
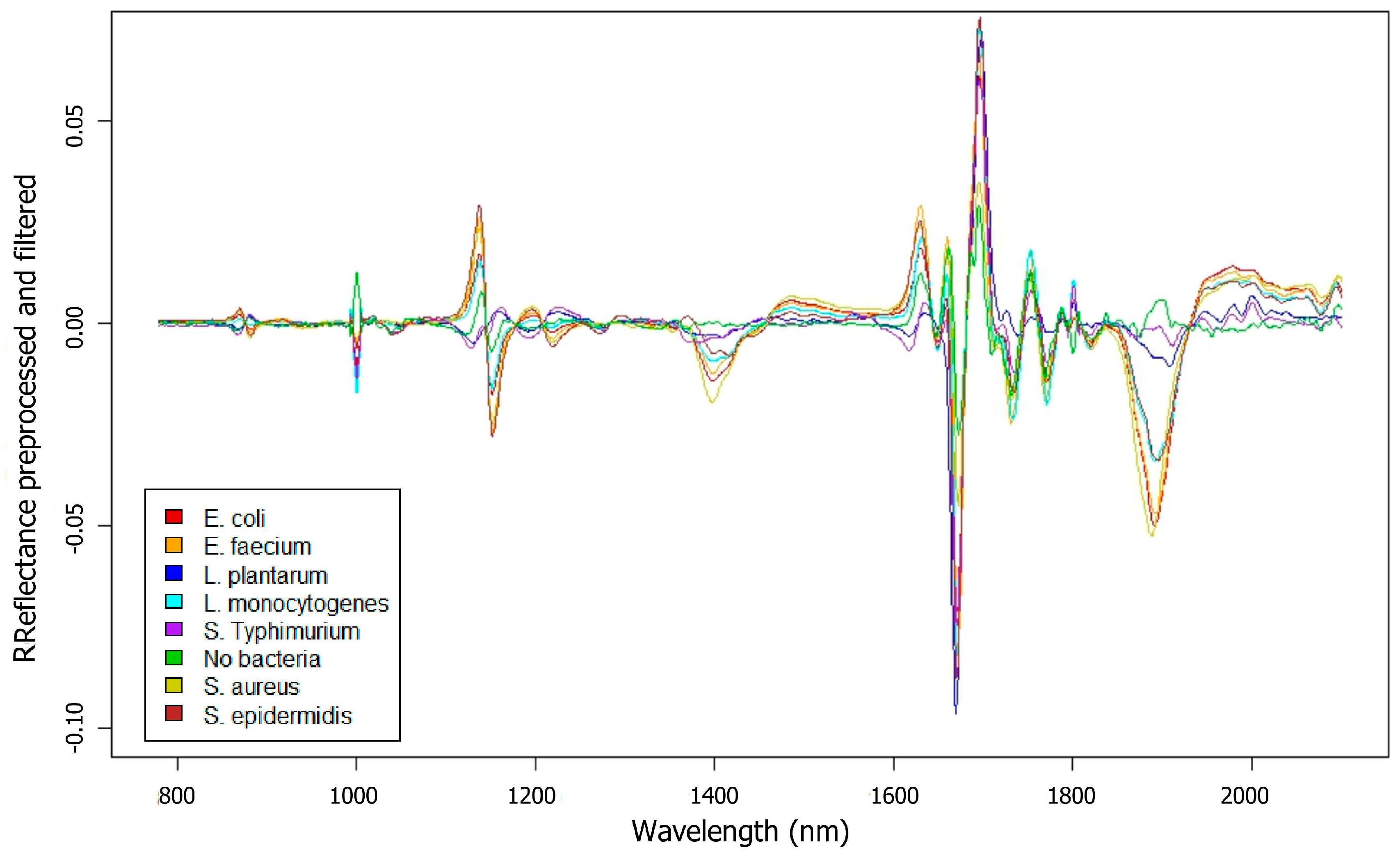
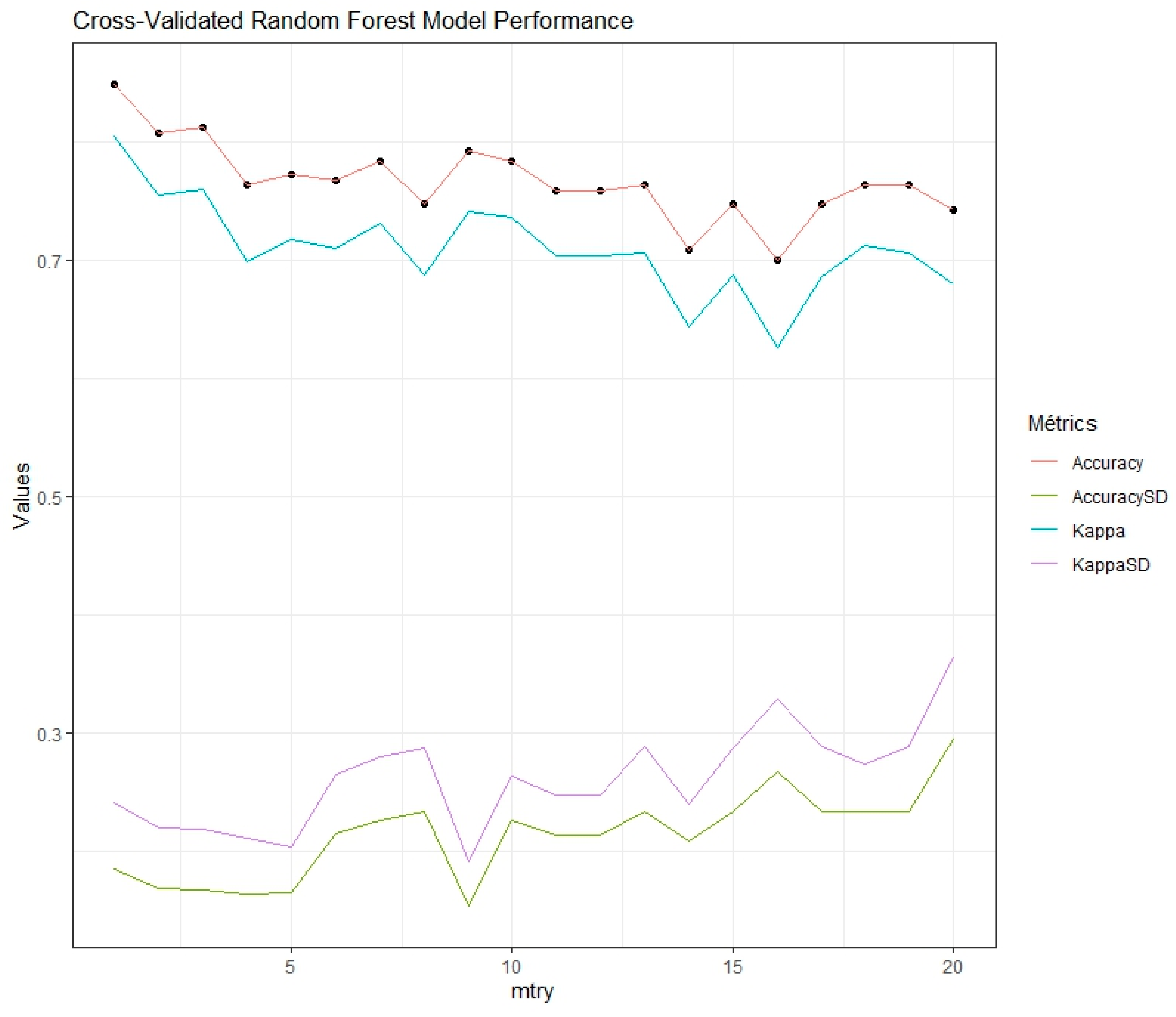
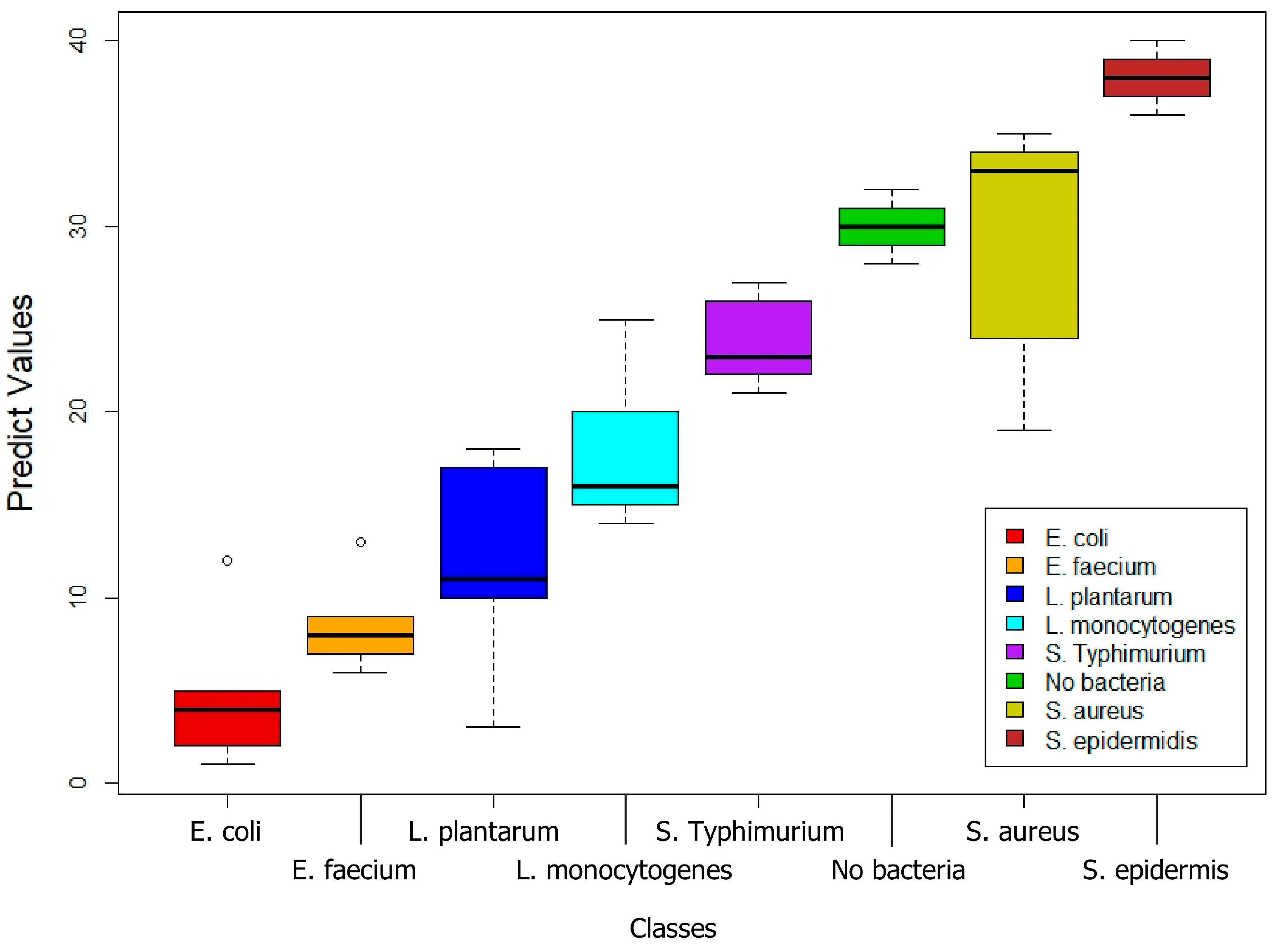
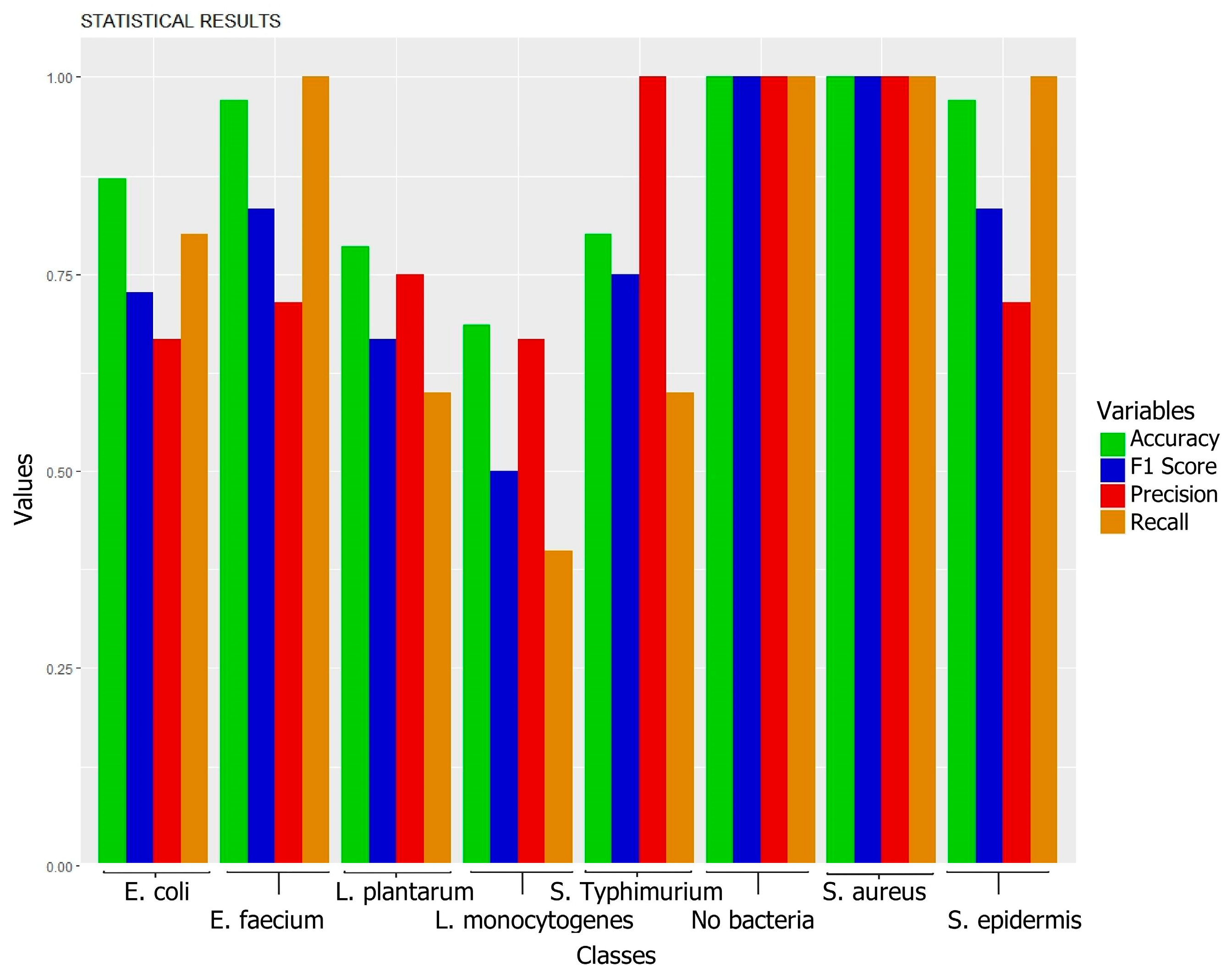
| E. coli | E. faecium | L. plantarum | L. monocytogenes | S. typhimurium | Control | S. aureus | S. epidermidis | |
| E. coli | 80.00% | 0.00% | 40.00% | 20.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| E. faecium | 0.00% | 100.00% | 0.00% | 40.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| L. plantarum | 20.00% | 0.00% | 60.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| L. monocytogenes | 0.00% | 0.00% | 0.00% | 40.00% | 20.00% | 0.00% | 0.00% | 0.00% |
| S. typhimurium | 0.00% | 0.00% | 0.00% | 0.00% | 60.00% | 0.00% | 0.00% | 0.00% |
| Control | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 100.00% | 0.00% | 0.00% |
| S. aureus | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% | 100.00% | 0.00% |
| S. epidermidis | 0.00% | 0.00% | 0.00% | 0.00% | 20.00% | 0.00% | 0.00% | 100.00% |
| Contaminated | Uncontaminated | |
| Contaminated | 100.00% | 9.09% |
| Uncontaminated | 0.00% | 90.91% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allende-Prieto, C.; Fernández, L.; Rodríguez-Gonzálvez, P.; García, P.; Rodríguez, A.; Recondo, C.; Martínez, B. Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy. Foods 2025, 14, 913. https://doi.org/10.3390/foods14060913
Allende-Prieto C, Fernández L, Rodríguez-Gonzálvez P, García P, Rodríguez A, Recondo C, Martínez B. Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy. Foods. 2025; 14(6):913. https://doi.org/10.3390/foods14060913
Chicago/Turabian StyleAllende-Prieto, Cristina, Lucía Fernández, Pablo Rodríguez-Gonzálvez, Pilar García, Ana Rodríguez, Carmen Recondo, and Beatriz Martínez. 2025. "Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy" Foods 14, no. 6: 913. https://doi.org/10.3390/foods14060913
APA StyleAllende-Prieto, C., Fernández, L., Rodríguez-Gonzálvez, P., García, P., Rodríguez, A., Recondo, C., & Martínez, B. (2025). Advances in the Detection and Identification of Bacterial Biofilms Through NIR Spectroscopy. Foods, 14(6), 913. https://doi.org/10.3390/foods14060913









