Carotenoids in Paprika Fruits and Ajvar: Chemical Characterization and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Products from Analyzed Paprika Genotypes
- 2 kg of roasted paprika
- 100 mL of oil
- 20 g of salt
- 20 mL of 9% vinegar
2.3. Extraction of Carotenoids
2.4. HPTLC Analysis
2.5. Determination of the Total Carotenoids Content (TCC)
2.6. Raman Spectroscopy
2.7. Determination of the Ability to Scavenge DPPH Radicals
Purple color → Yellow color
2.8. Fe3+ Reduction Ability—FRP
2.9. Cupric Ion Reducing Antioxidant Capacity—CUPRAC
2.10. In Vitro Digestion
2.11. Statistical Analysis
3. Results
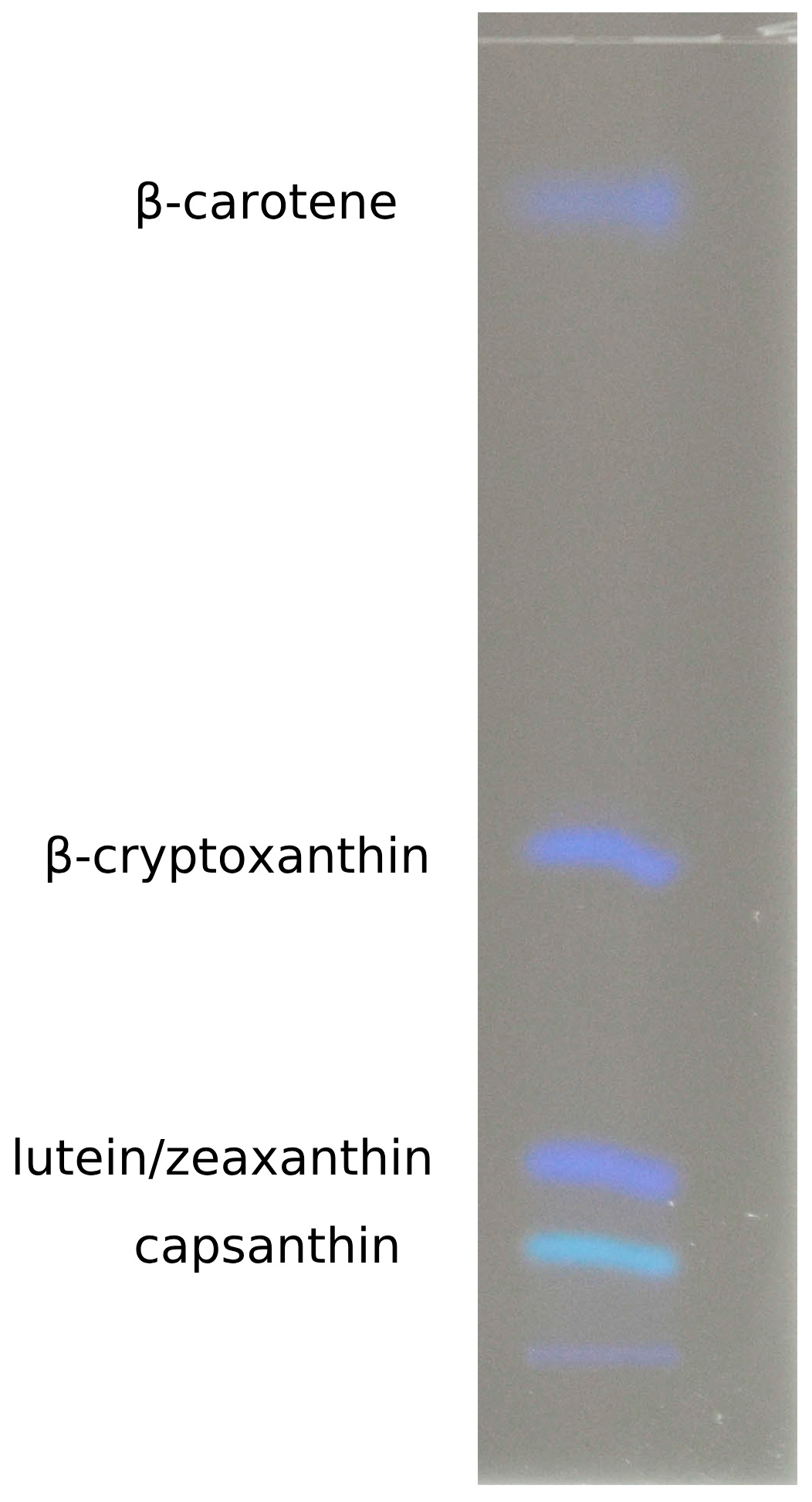
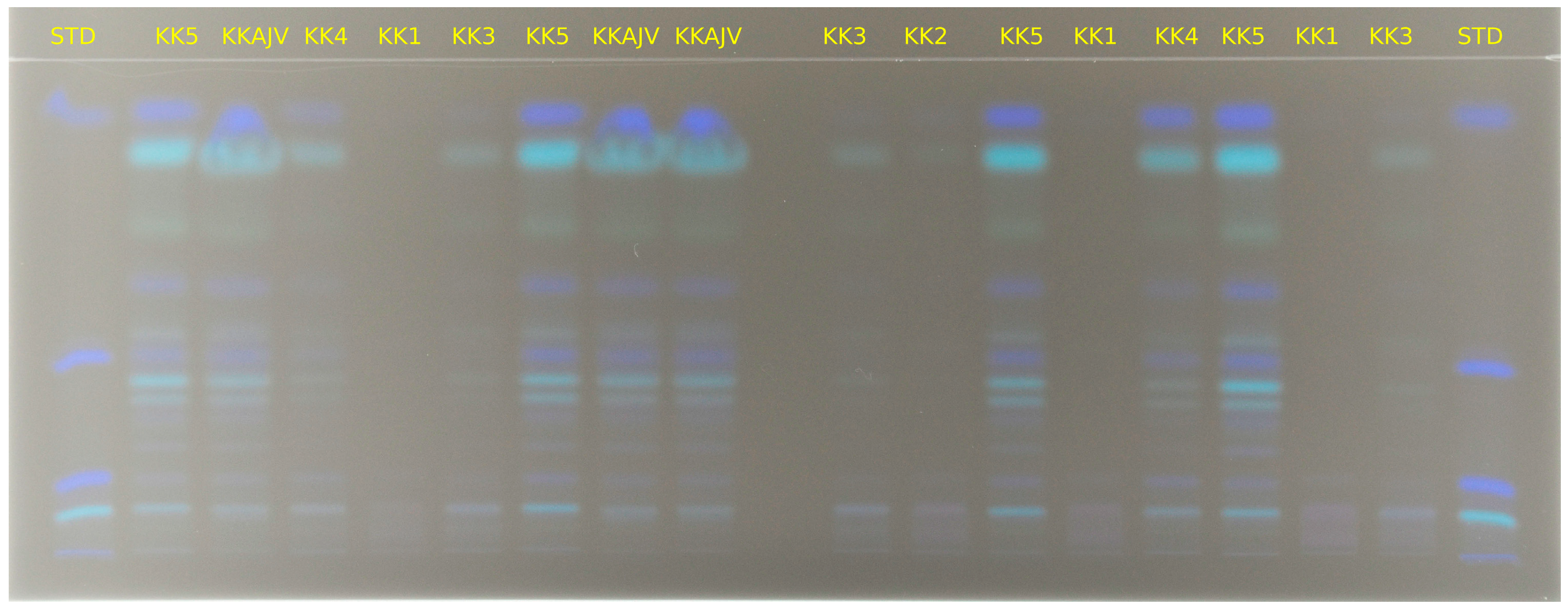
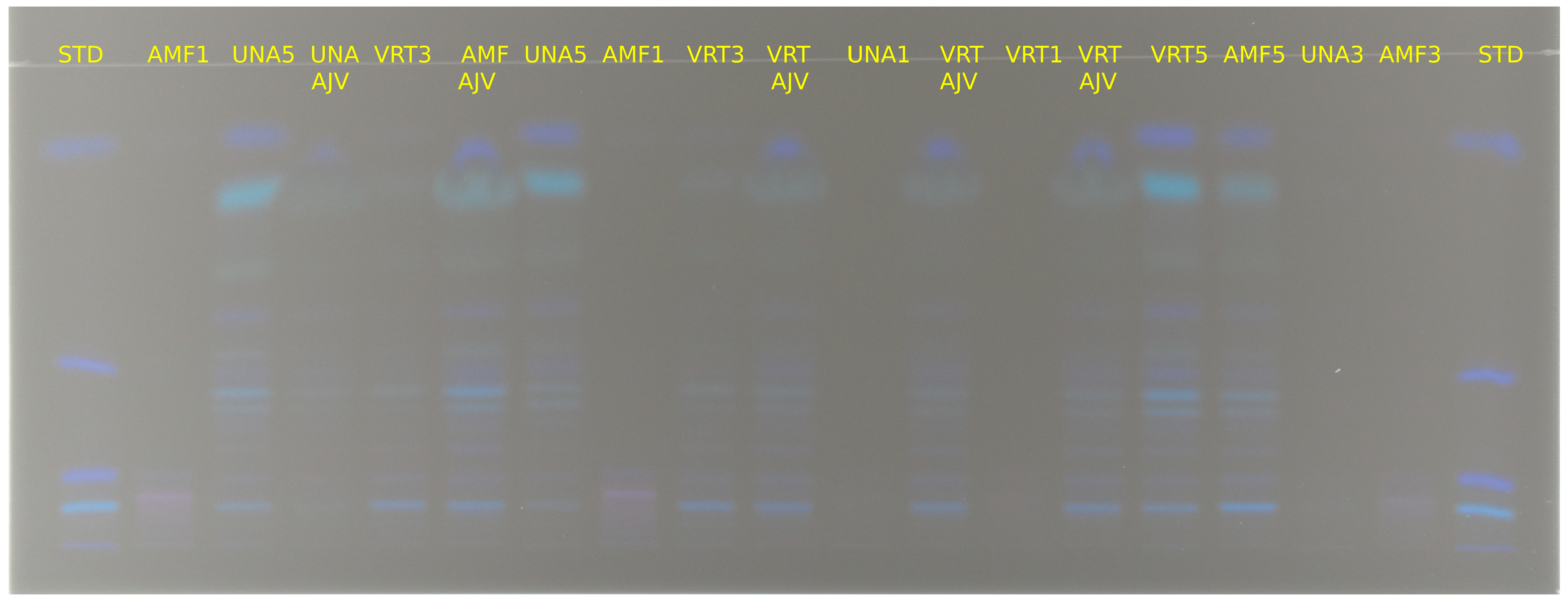
3.1. Qualitative and Quantitative Analysis of Carotenoids from Paprika and Ajvar Extracts Using HPTLC Analytical Method
3.2. Total Carotenoids Analysis
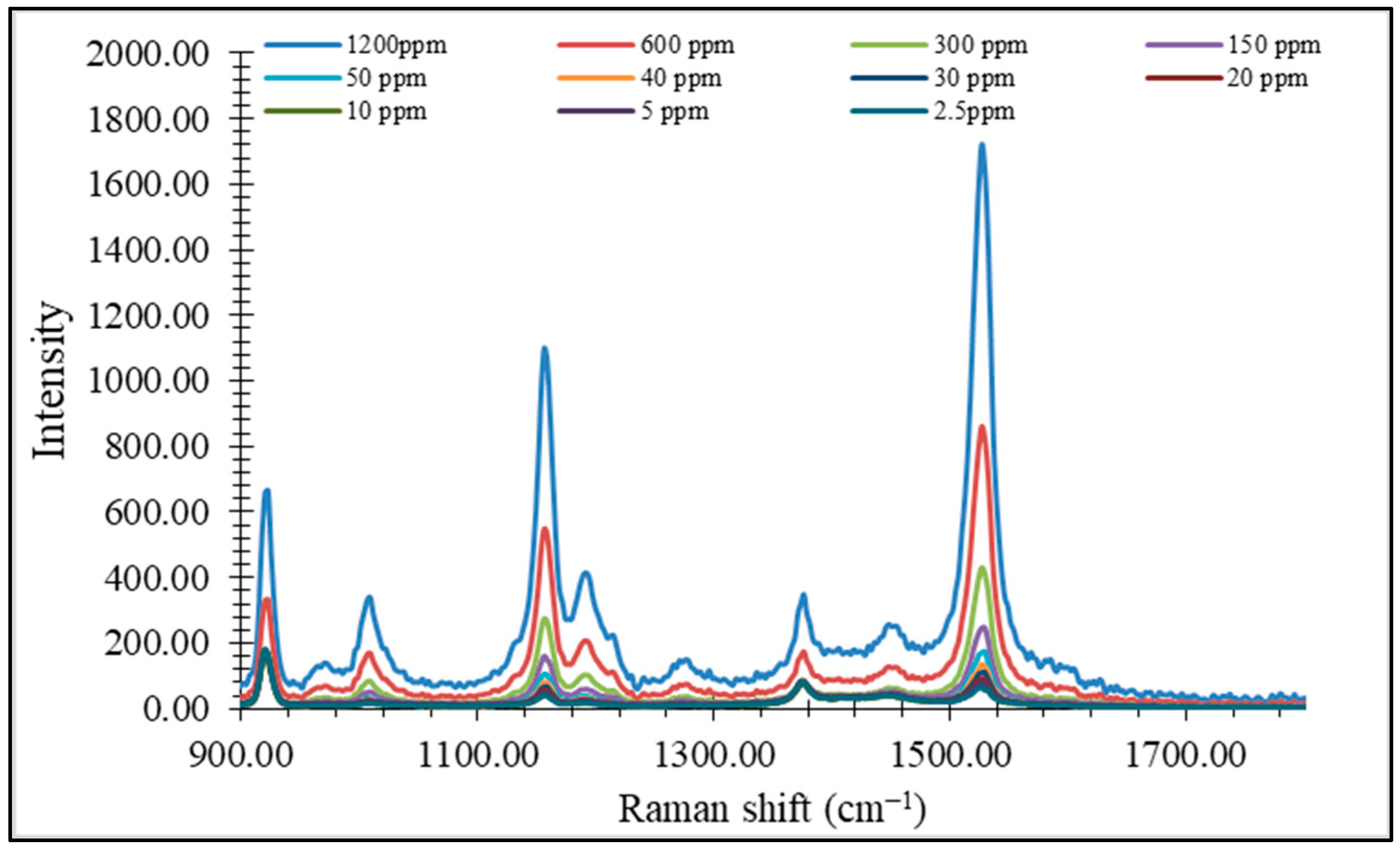
3.3. Quantitative Analysis of Total Carotenoids in Paprika Fruit Using Raman Spectroscopy
3.4. Results of DPPH Test
3.5. Results of CUPRAC Test
3.6. Results of the FRP Test
3.7. Bioavailability of Carotenoids from Paprika Fruit and Ajvar of Genotype Una
4. Discussion
4.1. Influence of Genotype on Carotenoid Composition and Stability During Thermal Processing
4.2. Quantification of Carotenoids Using Raman Spectroscopy
4.3. Influence of Genotype, Ripening, and Thermal Processing on Antioxidant Capacity
4.4. Bioavailability of Total Carotenoids in Paprika Samples at Various Ripening Stages and in Ajvar
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A







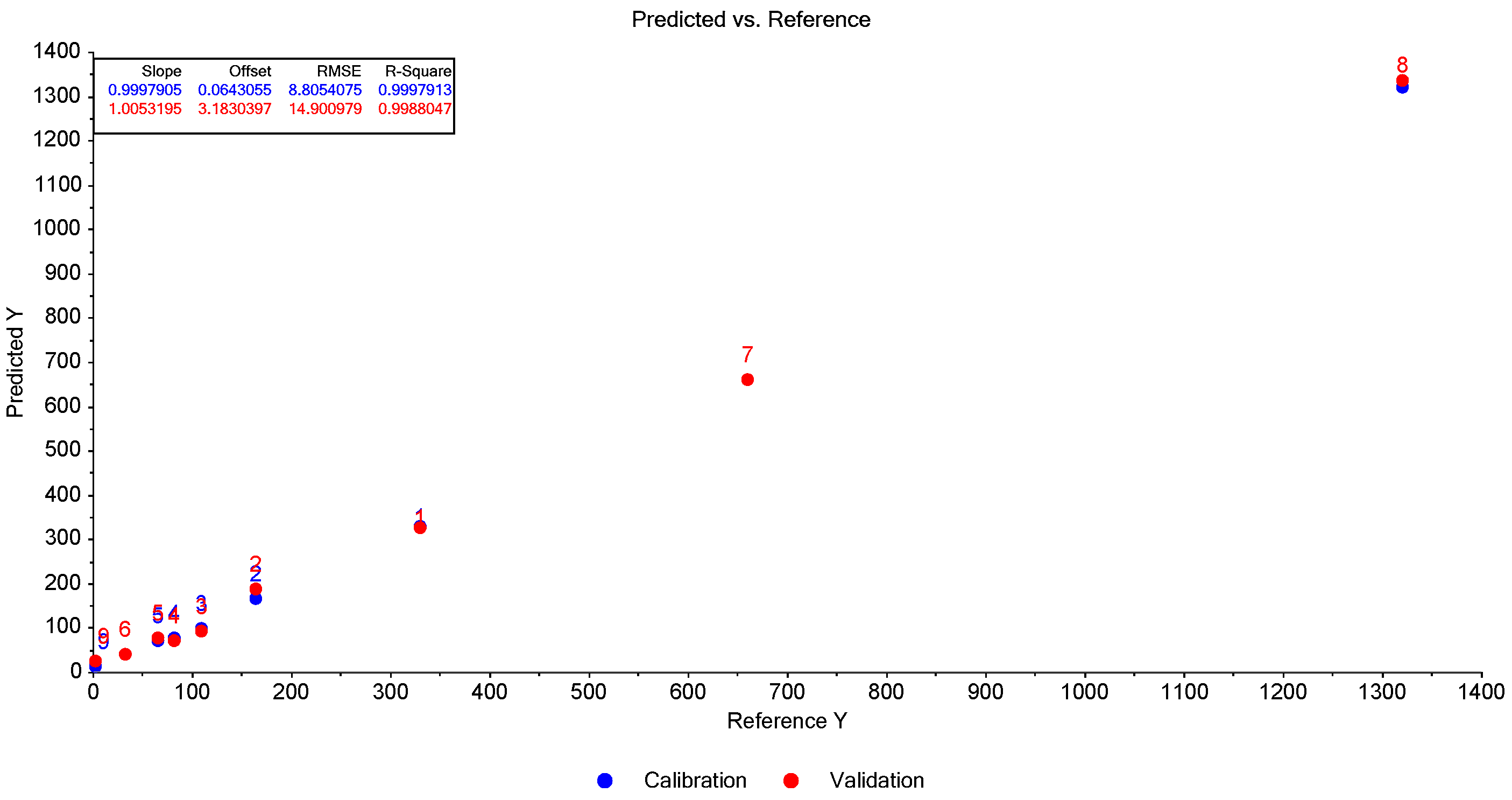
| Correlations Examined | Sorta | Amfora | Kurtovska Kapija | Una | Vrtka |
|---|---|---|---|---|---|
| Correlations between DPPH analysis results and total carotenoids measured spectrophotometrically | Pearson correlation coefficient | 0.965 * | 0.907 * | 0.589 | 0.992 ** |
| p-value | 0.035 | 0.013 | 0.411 | 0.008 | |
| Correlations between the results of the DPPH analysis and total carotenoids obtained by summing individual carotenoids measured by the HPTLC method | Pearson correlation coefficient | 0.963 * | 0.879 * | 0.682 | 0.994 ** |
| p-value | 0.037 | 0.021 | 0.318 | 0.006 |
References
- Hernández-Pérez, T.; Gómez-García, M.R.; Valverde, M.E.; Peredes-López, O. Capsicum annuum (hot pepper): An ancient Latin-American crop with outstanding bioactive compounds and nutraceutical potential. A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; An, C.G.; Park, J.S.; Lim, Y.P.; Kim, S. Carotenoid profiling from 27 types of paprika (Capsicum annuum L.) with different colours, shapes, and cultivation methods. Food Chem. 2016, 201, 64–71. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814616300371 (accessed on 11 February 2025). [CrossRef] [PubMed]
- Kolašinac, S.; Dajić Stevanović, Z.; Kilibarda, S.; Kostić, A. Carotenoids: New Applications of “Old” Pigments. Phyton 2021, 90, 1041–1062. Available online: https://www.techscience.com/phyton/v90n4/42400 (accessed on 1 February 2025). [CrossRef]
- Bufka, J.; Vaňková, L.; Sýkora, J.; Křížková, V. Exploring carotenoids: Metabolism, antioxidants, and impacts on human health. J. Funct. Foods 2024, 118, 106284. Available online: https://linkinghub.elsevier.com/retrieve/pii/S175646462400286X (accessed on 4 February 2025). [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Stevanović, Z.D. Raman spectroscopy coupled with chemometric modeling approaches for authentication of different paprika varieties at physiological maturity. LWT 2022, 162, 113402. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643822003371 (accessed on 1 February 2025). [CrossRef]
- Richins, R.D.; Hernandez, L.; Dungan, B.; Hambly, S.; Holguin, F.O.; O’Connell, M.A. A “Green” Extraction Protocol to Recover Red Pigments from Hot Capsicum Fruit. HortScience 2010, 45, 1084–1087. Available online: https://journals.ashs.org/view/journals/hortsci/45/7/article-p1084.xml (accessed on 12 February 2025). [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Aćić, S.; Stevanović, Z.D. Raman spectroscopic-based chemometric modeling in assessment of red pepper ripening phases and carotenoids accumulation. J. Raman Spectrosc. 2021, 52, 1598–1605. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.6197 (accessed on 2 February 2025). [CrossRef]
- Bogdanović, S.; Ranđelović, D.; Zlatanović, Z. Control analysis of energy values and mycological quality of homemade—Ajvar. J. ASEAN Fed. Endocr. Soc. 2022, 76, 67–69. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. Res. J. Chem. Sci. 2014, 4, 2231–2606. [Google Scholar]
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical studies on antioxidant mechanisms and free radical scavenging properties of lignans. Org. Biomol. Chem. 2005, 3, 3336. Available online: https://xlink.rsc.org/?DOI=b506739a (accessed on 4 February 2025). [CrossRef]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of Chemical Composition and Antifungal and Antioxidant Activities of the Essential Oils from Three Turkish Artemisia Species. J. Agric. Food Chem. 2005, 53, 1408–1416. Available online: https://pubs.acs.org/doi/10.1021/jf048429n (accessed on 4 February 2025). [CrossRef] [PubMed]
- Rodriguez-Amaya, D. Food Carotenoids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Gawron-Gzella, A.; Królikowska, A.; Pietrzak, M. Antioxidant activity of teas obtained from leaves of Camellia sinensis (L.) Kuntze in course of various production processes available on Polish market. Herba Pol. 2018, 64, 60–67. [Google Scholar]
- Nibir, Y.M.; Sumit, A.F.; Akhand, A.A.; Ahsan, N.; Hossain, M.S. Comparative assessment of total polyphenols, antioxidant and antimicrobial activity of different tea varieties of Bangladesh. Asian Pac. J. Trop. Biomed. 2017, 7, 352–357. Available online: http://linkinghub.elsevier.com/retrieve/pii/S2221169116308309 (accessed on 4 February 2025). [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla species (P. speciosa L. and P. reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 23, 8. Available online: http://journal.frontiersin.org/article/10.3389/fphar.2017.00290/full (accessed on 4 February 2025). [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. Available online: http://xlink.rsc.org/?DOI=C3FO60702J (accessed on 14 February 2025). [CrossRef]
- Baranska, M.; Roman, M.; Dobrowolski, J.C.; Schulz, H.; Baranski, R. Recent Advances in Raman Analysis of Plants: Alkaloids, Carotenoids, and Polyacetylenes. Curr. Anal. Chem. 2013, 9, 108–127. Available online: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1573-4110&volume=9&issue=1&spage=108 (accessed on 5 February 2025). [CrossRef]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; de Diego, A.; Madariaga, J.M. Portable Raman spectroscopy for an in-situ monitoring the ripening of tomato (Solanum lycopersicum) fruits. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 138–143. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1386142517301920 (accessed on 10 January 2025). [CrossRef]
- Oliveira, V.E.; Castro, H.V.; Edwards, H.G.; de Oliveira LF, C. Carotenes and carotenoids in natural biological samples: A Raman spectroscopic analysis. J. Raman Spectrosc. 2010, 41, 642–650. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. Available online: https://onlinelibrary.wiley.com/doi/10.1002/bip.20215 (accessed on 4 February 2025). [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. Available online: https://pubs.acs.org/doi/10.1021/ac061220j (accessed on 4 February 2025). [CrossRef]
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT Food Sci. Technol. 2007, 40, 121–129. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643805002264 (accessed on 14 February 2025). [CrossRef]
- Ro, N.; Oh, H.; Ko, H.C.; Yi, J.; Na, Y.W.; Haile, M. Genome-Wide Analysis of Fruit Color and Carotenoid Content in Capsicum Core Collection. Plants 2024, 13, 2562. [Google Scholar] [CrossRef] [PubMed]
- Topuz, A.; Ozdemir, F. Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0889157507000567 (accessed on 4 February 2025). [CrossRef]
- Rodríguez-Rodríguez, E.; Sánchez-Prieto, M.; Olmedilla-Alonso, B. Assessment of carotenoid concentrations in red peppers (Capsicum annuum) under domestic refrigeration for three weeks as determined by HPLC-DAD. Food Chem. X 2020, 6, 100092. Available online: https://linkinghub.elsevier.com/retrieve/pii/S259015752030016X (accessed on 4 February 2025). [CrossRef]
- Deli, J.; Molnár, P.; Matus, Z.; Tóth, G. Carotenoid Composition in the Fruits of Red Paprika (Capsicum annuum var. lycopersiciforme rubrum) during Ripening; Biosynthesis of Carotenoids in Red Paprika. J. Agric. Food Chem. 2001, 49, 1517–1523. Available online: https://pubs.acs.org/doi/10.1021/jf000958d (accessed on 4 February 2025).
- Flores, P.; Sánchez, E.; Fenoll, J.; Hellín, P. Genotypic variability of carotenoids in traditional tomato cultivars. Food Res. Int. 2017, 100, 510–516. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0963996916302903 (accessed on 4 February 2025). [CrossRef]
- Hornero-Méndez, D.; Gómez-Ladrón de Guevara, R.; Mínguez-Mosquera, M.I. Carotenoid Biosynthesis Changes in Five Red Pepper (Capsicum annuum L.) Cultivars during Ripening. Cultivar Selection for Breeding. J. Agric. Food Chem. 2000, 48, 3857–3864. Available online: https://pubs.acs.org/doi/10.1021/jf991020r (accessed on 22 February 2025). [CrossRef]
- Morais, H.; Rodrigues, P.; Ramos, C.; Almeida, V.; Forgács, E.; Cserháti, T.; Oliveira, J.S. Note. Effect of Blanching and Frozen Storage on the Stability of β-Carotene and Capsanthin in Red Pepper (Capsicum annuum) Fruit. Food Sci. Technol. Int. 2002, 8, 55–59. Available online: http://journals.sagepub.com/doi/10.1106/1082013022914 (accessed on 12 February 2025). [CrossRef]
- Deli, J.; Matus, Z.; Molnár, P.; Tóth, G. Separation and identification of carotenoids from different coloured paprika (Capsicum annuum) by reversed-phase high-performance liquid chromatography. Eur. Food Res. Technol. 2001, 213, 301–305. Available online: http://link.springer.com/10.1007/s002170100377 (accessed on 14 February 2025). [CrossRef]
- Sevindik Baç, H.; Yemiş, O.; Özkan, M. Thermal stabilities of lycopene and β-carotene in tomato pulp and pink grapefruit juice. J. Food Eng. 2023, 337, 111217. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0260877422002710 (accessed on 4 February 2025). [CrossRef]
- Liu, W.; Wang, J.; McClements, D.J.; Zou, L. Encapsulation of β-carotene-loaded oil droplets in caseinate/alginate microparticles: Enhancement of carotenoid stability and bioaccessibility. J. Funct. Foods 2018, 40, 527–535. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1756464617307181 (accessed on 4 February 2025). [CrossRef]
- Liu, Y.; Hou, Z.; Yang, J.; Gao, Y. Effects of antioxidants on the stability of β-Carotene in O/W emulsions stabilized by Gum Arabic. J. Food Sci. Technol. 2014, 52, 3300–3311. Available online: http://link.springer.com/10.1007/s13197-014-1380-0 (accessed on 4 February 2025). [CrossRef] [PubMed][Green Version]
- Cornacchia, L.; Roos, Y.H. Stability of β-Carotene in Protein-Stabilized Oil-in-Water Delivery Systems. J. Agric. Food Chem. 2011, 59, 7013–7020. Available online: https://pubs.acs.org/doi/10.1021/jf200841k (accessed on 4 February 2025). [CrossRef] [PubMed]
- Gheonea, I.; Aprodu, I.; Enachi, E.; Horincar, G.; Bolea, C.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Investigations on thermostability of carotenoids from tomato peels in oils using a kinetic approach. J. Food Process. Preserv. 2020, 44, e14303. Available online: https://onlinelibrary.wiley.com/doi/10.1111/jfpp.14303 (accessed on 4 February 2025).
- Qiu, D.; Shao Sx Zhao, B.; Wu, Y.C.; Shi, L.F.; Zhou, J.C.; Chen, Z.R. Stability of β-carotene in thermal oils. J. Food Biochem. 2012, 36, 198–206. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1745-4514.2010.00526.x (accessed on 4 February 2025). [CrossRef]
- Sebben, J.A.; da Silveira Espindola, J.; Ranzan, L.; Fernandes de Moura, N.; Trierweiler, L.F.; Trierweiler, J.O. Development of a quantitative approach using Raman spectroscopy for carotenoids determination in processed sweet potato. Food Chem. 2018, 245, 1224–1231. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814617319052 (accessed on 4 February 2025). [CrossRef]
- Hara, R.; Ishigaki, M.; Kitahama, Y.; Ozaki, Y.; Genkawa, T. Excitation wavelength selection for quantitative analysis of carotenoids in tomatoes using Raman spectroscopy. Food Chem. 2018, 258, 308–313. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814618305223 (accessed on 4 February 2025). [CrossRef]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Akpolat, M.Z.; Francis, D.M.; Chen, Y.J.; Rodriguez-Saona, L.E. High-Throughput Phenotyping Approach for Screening Major Carotenoids of Tomato by Handheld Raman Spectroscopy Using Chemometric Methods. Sensors 2020, 20, 3723. Available online: https://www.mdpi.com/1424-8220/20/13/3723 (accessed on 4 February 2025). [CrossRef]
- Killeen, D.P.; Sansom, C.E.; Lill, R.E.; Eason, J.R.; Gordon, K.C.; Perry, N.B. Quantitative Raman Spectroscopy for the Analysis of Carrot Bioactives. J. Agric. Food Chem. 2013, 61, 2701–2708. Available online: https://pubs.acs.org/doi/10.1021/jf3053669 (accessed on 4 February 2025). [CrossRef]
- Lawaetz, A.J.; Christensen, S.M.U.; Clausen, S.K.; Jørnsgaard, B.; Rasmussen, S.K.; Andersen, S.B.; Rinnan, Å. Fast, cross cultivar determination of total carotenoids in intact carrot tissue by Raman spectroscopy and Partial Least Squares calibration. Food Chem. 2016, 204, 7–13. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814616302771 (accessed on 4 February 2025). [CrossRef]
- El-Ghorab, A.H.; Javed, Q.; Anjum, F.M.; Hamed, S.F.; Shaaban, H.A. Pakistani Bell Pepper (Capsicum annum L.): Chemical Compositions and its Antioxidant Activity. Int. J. Food Prop. 2013, 16, 18–32. Available online: http://www.tandfonline.com/doi/abs/10.1080/10942912.2010.513616 (accessed on 4 February 2025). [CrossRef]
- Elkholy, N.S.; Hariri, M.L.M.; Mohammed, H.S.; Shafaa, M.W. Lutein and β-Carotene Characterization in Free and Nanodispersion Forms in Terms of Antioxidant Activity and Cytotoxicity. J. Pharm. Innov. 2023, 18, 1727–1744. Available online: https://link.springer.com/10.1007/s12247-023-09745-2 (accessed on 4 February 2025). [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1756464611000077 (accessed on 4 February 2025). [CrossRef]
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant Activities of Different Coloured Sweet Bell Peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1750-3841.2006.00245.x (accessed on 13 February 2025). [CrossRef]
- Fitriansyah, S.N.; Aulifa, D.L.; Febriani, Y.; Sapitri, E. Correlation of Total Phenolic, Flavonoid and Carotenoid Content of Phyllanthus emblica Extract from Bandung with DPPH Scavenging Activities. Pharmacogn. J. 2018, 10, 447–452. Available online: http://www.phcogj.com/article/578 (accessed on 10 January 2025). [CrossRef]
- Al-Rimawi, F.; Abu-Lafi, S.; Abbadi, J.; Alamarneh, A.A.A.; Sawahreh, R.A.; Odeh, I. analysis of phenolic and flavonoids of wild ephedra alata plant extracts by LC/PDA and LC /MS and their antioxidant activity. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 130–141. Available online: http://journals.athmsi.org/index.php/ajtcam/article/view/4579 (accessed on 4 February 2025). [CrossRef]
- Kovács, Z.; Bedő, J.; Pápai, B.; Tóth-Lencsés, A.K.; Csilléry, G.; Szőke, A.; Bányai-Stefanovits, E.; Kiss, E.; Veres, A. Ripening-Induced Changes in the Nutraceutical Compounds of Differently Coloured Pepper (Capsicum annuum L.) Breeding Lines. Antioxidants 2022, 11, 637. Available online: https://www.mdpi.com/2076-3921/11/4/637 (accessed on 4 February 2025). [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends Food Sci. Technol. 2020, 96, 114–126. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0924224419304959 (accessed on 10 January 2025). [CrossRef]
- Tan, Y.; Zhou, H.; McClements, D.J. Application of static in vitro digestion models for assessing the bioaccessibility of hydrophobic bioactives: A review. Trends Food Sci. Technol. 2022, 122, 314–327. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0924224422000826 (accessed on 4 February 2025). [CrossRef]
- Xiao, S.; Ahn, D.U. Enhanced lutein stability under UV-Light and high temperature by loading it into alginate-chitosan complex. LWT 2022, 164, 113663. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643822005989 (accessed on 4 February 2025). [CrossRef]
- Davidov-Pardo, G.; Gumus, C.E.; McClements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of pH and temperature on physical and chemical stability. Food Chem. 2016, 196, 821–827. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814615300182 (accessed on 4 February 2025). [CrossRef] [PubMed]
- Shao, P.; Qiu, Q.; Xiao, J.; Zhu, Y.; Sun, P. Chemical Stability and in vitro release properties of β-carotene in emulsions stabilized by Ulva fasciata polysaccharide. Int. J. Biol. Macromol. 2017, 102, 225–231. Available online: https://linkinghub.elsevier.com/retrieve/pii/S014181301730003X (accessed on 4 February 2025). [CrossRef] [PubMed]
- Sheng, B.; Li, L.; Zhang, X.; Jiao, W.; Zhao, D.; Wang, X.; Wan, L.; Li, B.; Rong, H. Physicochemical Properties and Chemical Stability of β-Carotene Bilayer Emulsion Coated with Bovine Serum Albumin and Arabic Gum Compared to Monolayer Emulsions. Molecules 2018, 23, 495. Available online: https://www.mdpi.com/1420-3049/23/2/495 (accessed on 4 February 2025). [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Bandino, E.; Hormaza, J.I.; Cano, M.P. Characterization and the impact of in vitro simulated digestion on the stability and bioaccessibility of carotenoids and their esters in two Pouteria lucuma varieties. Food Chem. 2020, 316, 126369. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814620302272 (accessed on 4 February 2025). [CrossRef]
- Milinčić, D.D.; Salević-Jelić, A.S.; Kostić, A.Ž.; Stanojević, S.P.; Nedović, V.; Pešić, M.B. Food nanoemulsions: How simulated gastrointestinal digestion models, nanoemulsion, and food matrix properties affect bioaccessibility of encapsulated bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 64, 8091–8113. Available online: https://www.tandfonline.com/doi/full/10.1080/10408398.2023.2195519 (accessed on 9 January 2025). [CrossRef]
- Hedrén, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. Available online: https://www.nature.com/articles/1601329 (accessed on 4 February 2025). [CrossRef]
- Hornero-Méndez, D.; Mínguez-Mosquera, M.I. Bioaccessibility of carotenes from carrots: Effect of cooking and addition of oil. Innov. Food Sci. Emerg. Technol. 2007, 8, 407–412. Available online: https://linkinghub.elsevier.com/retrieve/pii/S146685640700046X (accessed on 9 January 2025). [CrossRef]
- Kirkhus, B.; Afseth, N.K.; Borge, G.I.A.; Grimsby, S.; Steppeler, C.; Krona, A.; Langton, M. Increased release of carotenoids and delayed in vitro lipid digestion of high pressure homogenized tomato and pepper emulsions. Food Chem. 2019, 285, 282–289. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814619301992 (accessed on 9 January 2025). [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. Available online: https://pubs.acs.org/doi/10.1021/acs.jafc.8b03174 (accessed on 9 January 2025). [CrossRef]




| Chemicals | SSF | SGF | SIF |
|---|---|---|---|
| pH 7.0 | pH 3.0 | pH 7.0 | |
| mmol∙L−1 | mmol∙L−1 | mmol∙L−1 | |
| KCl | 15.1 | 6.9 | 6.8 |
| KH2PO4 | 3.7 | 0.9 | 0.8 |
| NaHCO3 | 13.6 | 25 | 85 |
| NaCl | - | 47.2 | 38.4 |
| MgCl2·6H2O | 0.15 | 0.1 | 0.33 |
| (NH4)2CO3 | 0.06 | 0.5 | - |
| NaOH | - | - | 8.4 |
| HCl | 1.1 | 15.6 | - |
| CaCl2·2H2O | 0.75 | 0.075 | 0.3 |
| Genotype | Ripening Stage | Investigated Carotenoids | TICC (Capsanthin + Lutein/Zeaxanthin + β-Cryptoxanthin + β-Carotene (g/100 g % DM) | Change in the TICC Through Ripening Stages and in the Product Compared to the Initial Stage—Stage 1 (%) | Change in the TICC Through Ripening Stages and in the Product Compared to the Previous Stage (%) | |||
|---|---|---|---|---|---|---|---|---|
| Capsanthin (g/100 g DM ± SD) | Lutein/Zeaksantin (g/100 g DM ± SD) | β-Cryptoxanthin (g/100 g DM ± SD) | β-Carotene (g/100 g DM ± SD) | |||||
| Kurtovska kapija | 1 | 0.13 ± 0.06 a | 0.03 ± 0.01 a,B | n.d. | 0.09 ± 0.01 a,A | 0.25 | 0.00 | 0.00 |
| 2 | 0.13 ± 0.003 a | 0.05 ± 0.003 b | n.d. | 0.13 ± 0.01 bc | 0.31 | 22.26 | 22.26 | |
| 3 | 0.13 ± 0.04 a,A | 0.04 ± 0.02 a,C | n.d. | 0.12 ± 0.01 b,B | 0.28 | 10.37 | −9.73 | |
| 4 | 0.14 ± 0.03 a | 0.11 ± 0.03 c | n.d. | 0.47 ± 0.11 d | 0.72 | 183.94 | 157.27 | |
| 5 | 0.36 ± 0.08 c,B | 0.15 ± 0.04 e,B | 0.05 ± 0.01 b,A | 0.99 ± 0.14 e,D | 1.55 | 509.62 | 114.70 | |
| Ajvar | 0.30 ± 0.08 b,A | 0.14 ± 0.03 d,B | 0.04 ± 0.01 a,B | 0.98 ± 0.12 e,C | 1.52 | 498.04 | −1.90 | |
| Amfora | 1 | n.d. | 0.07 ± 0.009 b,C | n.d. | 0.10 ± 0.01 b,A | 0.17 | 0.00 | 0.00 |
| 3 | 0.07 ± 0.0 08 a,B | 0.02 ± 0.006 a,B | n.d. | 0.09 ± 0.008 a,A | 0.18 | 6.49 | 6.49 | |
| 5 | 0.50 ± 0.09 c,C | 0.15 ± 0.04 d,B | 0.07 ± 0.03 b,B | 0.54 ± 0.14 c,B | 1.28 | 652.42 | 606.59 | |
| Ajvar | 0.35 ± 0.13 b,A | 0.12 ± 0.04 c,B | 0.03 ± 0.01 a,A | 0.53 ± 0.04 c,B | 1.03 | 505.48 | −19.53 | |
| Una | 1 | n.d. | 0.01 ± 0.001 a,A | n.d. | n.d. | 0.01 | 0.00 | 0.00 |
| 3 | n.d. | 0.02 ± 0.005 b,A | n.d. | n.d. | 0.01 | 6.58 | 6.58 | |
| 5 | 0.11 ± 0.07 A | 0.08 ± 0.05 d,A | 0.01 ± 0.007 | 0.17 ± 0.02 b,A | 0.38 | 2394.74 | 2240.74 | |
| Ajvar | n.d. | 0.033 ± 0.09 c,A | n.d. | 0.09 ± 0.008 a,A | 0.12 | 723.68 | −66.98 | |
| Vrtka | 1 | n.d. | 0.03 ± 0.03 a,A | n.d. | n.d. | 0.03 | 0.00 | 0.00 |
| 3 | 0.26 ± 0.04 a,C | 0.09 ± 0.02 b,D | n.d. | 0.12 ± 0.01 a,B | 0.47 | 1580.50 | 1580.50 | |
| 5 | 0.51 ± 0.04 c,C | 0.16 ± 0.02 c,C | 0.08 ± 0.04 b,C | 0.72 ± 0.07 c,C | 1.47 | 5131.21 | 211.29 | |
| Ajvar | 0.456 ± 0.18 b,B | 0.16 ± 0.05 c,C | 0.071 ± 0.03 a,C | 0.55 ± 0.05 b,B | 1.23 | 4279.79 | −16.28 | |
| Name of the Sample | Total Carotenoids mg/100 g | Change in TCC Through Ripening Stages and in the Product Compared to the Initial Stage—Stage 1 (%) | Change in TCC Through Ripening Stages and in the Product Compared to the Previous Stage (%) |
|---|---|---|---|
| Amfora stage 1 | 190.60 ± 3.23 a | 0.00 | 0.00 |
| Amfora stage 3 | 200.21 ± 10.55 a | 5.04 | 5.04 |
| Amfora stage 5 | 1311.01 ± 15.84 b | 587.83 | 554.82 |
| Amfora Ajvar | 1099.35 ± 15.62 c | 476.78 | −16.14 |
| Kurtovska kapija stage 1 | 301.98 ± 13.98 a | 0.00 | 0.00 |
| Kurtovska kapija stage 2 | 401.56 ± 10.23 b | 32.98 | 32.98 |
| Kurtovska kapija stage 3 | 419.31 ± 15.96 b | 38.85 | 4.42 |
| Kurtovska kapija stage 4 | 805.45 ± 32.23 c | 166.72 | 92.09 |
| Kurtovska kapija stage 5 | 1498.21 ± 60.89 d | 396.13 | 86.01 |
| Kurtovska kapija Ajvar | 1401.11 ± 59.01 e | 363.97 | −6.48 |
| Una stage 1 | 20.22 ± 1.63 a | 0.00 | 0.00 |
| Una stage 3 | 22.01 ± 1.21 a | 8.85 | 8.85 |
| Una stage 5 | 289.57 ± 14.58 b | 1332.10 | 1215.63 |
| Una Ajvar | 51.08 ± 2.66 c | 152.62 | −82.36 |
| Vrtka stage 1 | 31.21 ± 1.28 a | 0.00 | 0.00 |
| Vrtka stage 3 | 515.05 ± 20.21 b | 1550.27 | 1550.27 |
| Vrtka stage 5 | 1502.21 ± 70.74 d | 4713.23 | 191.66 |
| Vrtka Ajvar | 1299.09 ± 60.21 c | 4062.42 | −13.52 |
| Selected Samples | TCC by RS Coupled with MLR Method (g/100 g DM) | TICC by HPTLC (g/100 g DM) | TCC Spectrophotometrically (g/100 g DM) |
|---|---|---|---|
| Kurtovska kapija stage 5 | 1.952 | 1.5527 | 1.4982 |
| Kurtovska kapija Ajvar | 1.899 | 1.5232 | 1.4011 |
| Amfora stage 5 | 1.726 | 1.2791 | 1.3110 |
| Amfora Ajvar | 1.614 | 1.0269 | 1.0993 |
| Name of the Sample | DPPH• | Change in the Inhibition of DPPH Radicals Through the Ripening Stages and in the Product in Relation to the Initial Stage—Stage 1 (%) | Change in DPPH Radical Inhibition Through the Ripening Stages and in the Product Compared to the Previous Stage (%) |
|---|---|---|---|
| μmol/g TEAC | |||
| Amfora stage 1 | 0.23 ± 0.03 a | 0.00 | 0.00 |
| Amfora stage 3 | 0.61 ± 0.02 b | 165.22 | 165.22 |
| Amfora stage 5 | 1.46 ± 0.19 d | 534.78 | 139.34 |
| Amfora Ajvar | 1.31 ± 0.15 c | 469.57 | −10.27 |
| Kurtovska kapija stage 1 | 0.21 ± 0.06 a | 0.00 | 0.00 |
| Kurtovska kapija stage 2 | 0.40 ± 0.04 b | 90.48 | 90.48 |
| Kurtovska kapija stage 3 | 0.91 ± 0.07 c | 333.33 | 127.50 |
| Kurtovska kapija stage 4 | 1.04 ± 0.09 d | 395.24 | 14.29 |
| Kurtovska kapija stage 5 | 1.50 ± 0.11 f | 614.29 | 44.23 |
| Kurtovska kapija Ajvar | 1.34 ± 0.15 e | 538.10 | −10.67 |
| Una stage 1 | 0.30 ± 0.04 a | 0.00 | 0.00 |
| Una stage 3 | 0.89 ± 0.04 b | 196.67 | 196.67 |
| Una stage 5 | 1.18 ± 0.02 c | 293.33 | 32.58 |
| Una Ajvar | 1.10 ± 0.02 d | 266.67 | −6.78 |
| Vrtka stage 1 | 0.21 ± 0.01 a | 0.00 | 0.00 |
| Vrtka stage 3 | 0.46 ± 0.04 b | 223.91 | 223.91 |
| Vrtka stage 5 | 1.49 ± 0.09 d | 184.78 | −12.08 |
| Vrtka Ajvar | 1.31 ± 0.07 c | 139.13 | −16.03 |
| Name of the Sample | mg AsA/g DM | Change in CUPRAC Activity Through the Maturation Stages and in the Product in Relation to the Initial Stage—Stage 1 (%) | Change in CUPRAC Activity Through the Maturation Stages and in the Pro-Extract Compared to the Previous Stage (%) |
|---|---|---|---|
| Amfora stage 1 | 0.194 ± 0.03 a | 0.00 | 0.00 |
| Amfora stage 3 | 0.205 ± 0.02 a | 5.67 | 5.67 |
| Amfora stage 5 | 0.319 ± 0.02 c | 64.43 | 55.61 |
| Amfora phase | 0.254 ± 0.02 b | 30.93 | −20.38 |
| Kurtovska kapija stage 1 | 0.205 ± 0.02 a | 0.00 | 0.00 |
| Kurtovska kapija stage 2 | 0.209 ± 0.02 a | 1.95 | 1.95 |
| Kurtovska kapija stage 3 | 0.200 ± 0.02 a | −2.44 | −4.31 |
| Kurtovska kapija stage 4 | 0.256 ± 0.02 b | 24.88 | 28.00 |
| Kurtovska kapija stage 5 | 0.297 ± 0.02 c | 44.88 | 16.02 |
| Kurtovska kapija Ajvar | 0.291 ± 0.02 c | 41.95 | −2.02 |
| Una stage 1 | 0.185 ± 0.02 a | 0.00 | 0.00 |
| Una stage 3 | 0.185 ± 0.02 a | 0.00 | 0.00 |
| Una stage 5 | 0.249 ± 0.03 c | 34.59 | 34.59 |
| Una Ajvar | 0.198 ± 0.02 b | 7.03 | −20.48 |
| Vrtka faza 1 | 0.178 ± 0.02 a | 0.00 | 0.00 |
| Vrtka faza 3 | 0.199 ± 0.02 b | 11.80 | 11.80 |
| Vrtka faza 5 | 0.225 ± 0.02 c | 26.40 | 13.07 |
| Vrtka Ajvar | 0.205 ± 0.02 b | 15.17 | −8.89 |
| Name of the Sample | mg AsA (GA)/g DM | Change in FRP Activity Through the Ripening Stages and in the Product in Relation to the Initial Stage—Stage 1 (%) | Change in FRP Activity Through the Ripening Stages and in the Product in Relation to the Previous Stage (%) |
|---|---|---|---|
| Amfora stage 1 | 12.36 ± 0.59 a | 0.00 | 0.00 |
| Amfora stage 3 | 16.54 ± 0.73 b | 33.82 | 33.82 |
| Amfora stage 5 | 24.23 ± 1.10 c | 96.04 | 46.49 |
| Amfora Ajvar | 23.08 ± 1.15 c | 86.73 | −4.75 |
| Kurtovska kapija stage 1 | 10.37 ± 0.52 a | 0.00 | 0.00 |
| Kurtovska kapija stage 2 | 10.23 ± 0.51 a | −1.35 | −1.35 |
| Kurtovska kapija stage 3 | 13.74 ± 0.72 b | 32.50 | 34.31 |
| Kurtovska kapija stage 4 | 16.36 ± 0.75 c | 57.76 | 319.07 |
| Kurtocska kapija stage 5 | 25.66 ± 1.30 d | 147.44 | 56.85 |
| Kurtovska kapija Ajvar | 25.12 ± 1.26 d | 142.24 | −2.10 |
| Una stage 1 | 9.33 ± 0.47 a | 0.00 | 0.00 |
| Una stage 3 | 10.11 ± 0.51 a | 8.36 | 8.36 |
| Una stage 5 | 15.81 ± 0.79 b | 69.45 | 56.38 |
| Una Ajvar | 16.13 ± 0.75 b | 72.88 | 2.02 |
| Vrtka stage 1 | 10.11 ± 0.61 a | 0.00 | 0.00 |
| Vrtka faza 3 | 13.16 ± 0.66 b | 11.80 | 11.80 |
| Vrtka stage 5 | 15.22 ± 0.77 d | 26.40 | 13.07 |
| Vrtka Ajvar | 14.89 ± 0.80 c | 15.17 | −8.89 |
| Sample | Before In Vitro Digestion mg/100 g | After In Vitro Digestion mg/100 g | Bioavailability Relative to Initial Extract (%) |
|---|---|---|---|
| Una stage 1 | 20.22 | 7.52 | 37.19 |
| Una stage 3 | 22.01 | 7.62 | 34.60 |
| Una stage 5 | 289.57 | 16.19 | 5.59 |
| Una Ajvar | 51.08 | 66.82 | 130.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolašinac, S.; Pećinar, I.; Cvetković, M.; Gođevac, D.; Stanisavljević, N.; Veljović, M.; Šoštarić, I.; Aćić, S.; Rančić, D.; Mačukanović-Jocić, M.; et al. Carotenoids in Paprika Fruits and Ajvar: Chemical Characterization and Biological Activity. Foods 2025, 14, 914. https://doi.org/10.3390/foods14060914
Kolašinac S, Pećinar I, Cvetković M, Gođevac D, Stanisavljević N, Veljović M, Šoštarić I, Aćić S, Rančić D, Mačukanović-Jocić M, et al. Carotenoids in Paprika Fruits and Ajvar: Chemical Characterization and Biological Activity. Foods. 2025; 14(6):914. https://doi.org/10.3390/foods14060914
Chicago/Turabian StyleKolašinac, Stefan, Ilinka Pećinar, Mirjana Cvetković, Dejan Gođevac, Nemanja Stanisavljević, Mile Veljović, Ivan Šoštarić, Svetlana Aćić, Dragana Rančić, Marina Mačukanović-Jocić, and et al. 2025. "Carotenoids in Paprika Fruits and Ajvar: Chemical Characterization and Biological Activity" Foods 14, no. 6: 914. https://doi.org/10.3390/foods14060914
APA StyleKolašinac, S., Pećinar, I., Cvetković, M., Gođevac, D., Stanisavljević, N., Veljović, M., Šoštarić, I., Aćić, S., Rančić, D., Mačukanović-Jocić, M., Kolašinac, J., & Dajić Stevanović, Z. (2025). Carotenoids in Paprika Fruits and Ajvar: Chemical Characterization and Biological Activity. Foods, 14(6), 914. https://doi.org/10.3390/foods14060914










