Raman Spectroscopy in the Characterization of Food Carotenoids: Challenges and Prospects
Abstract
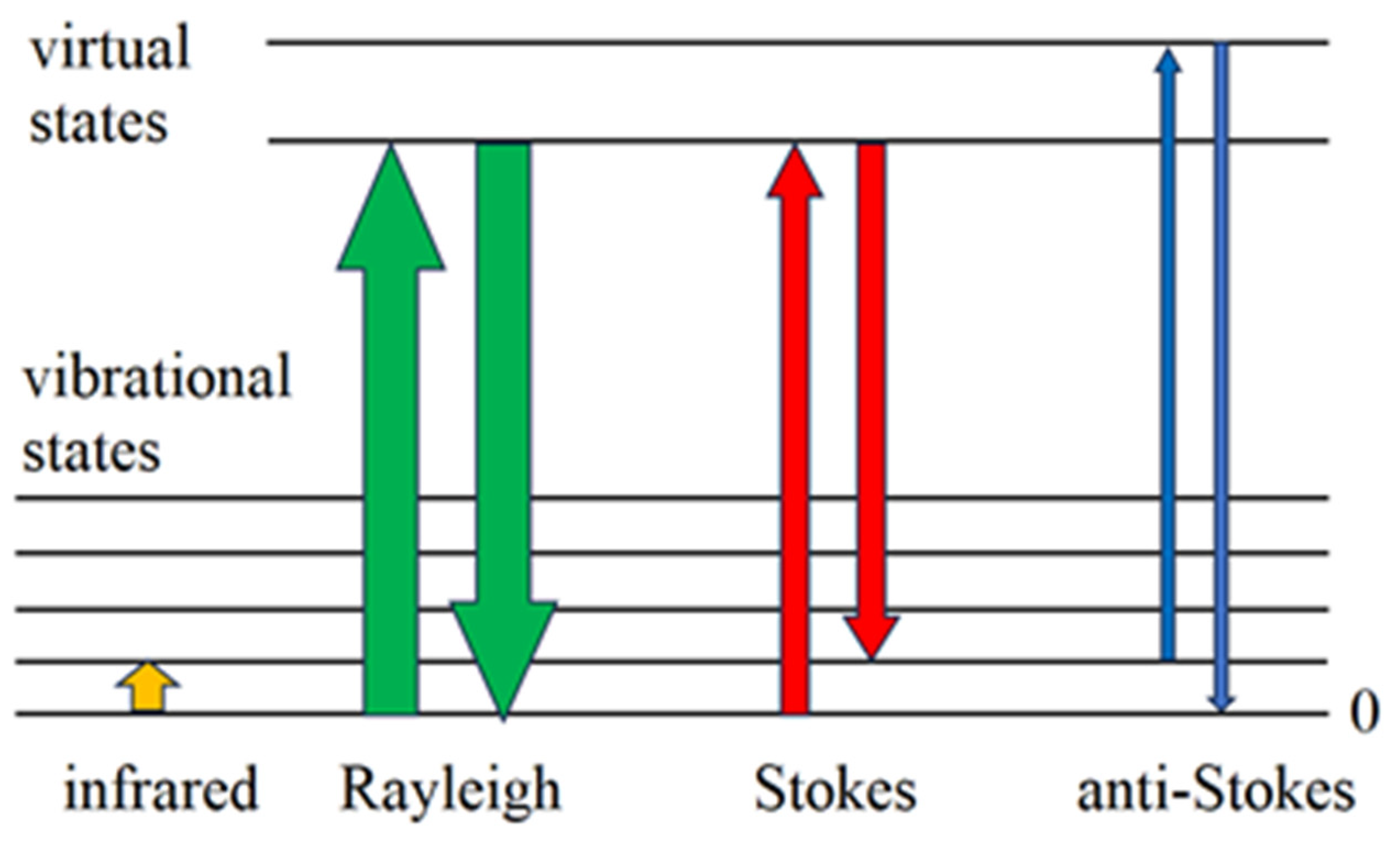
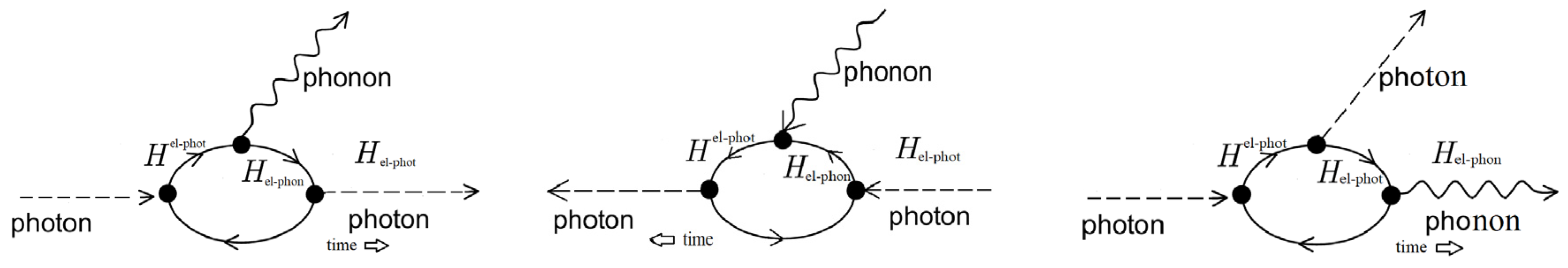
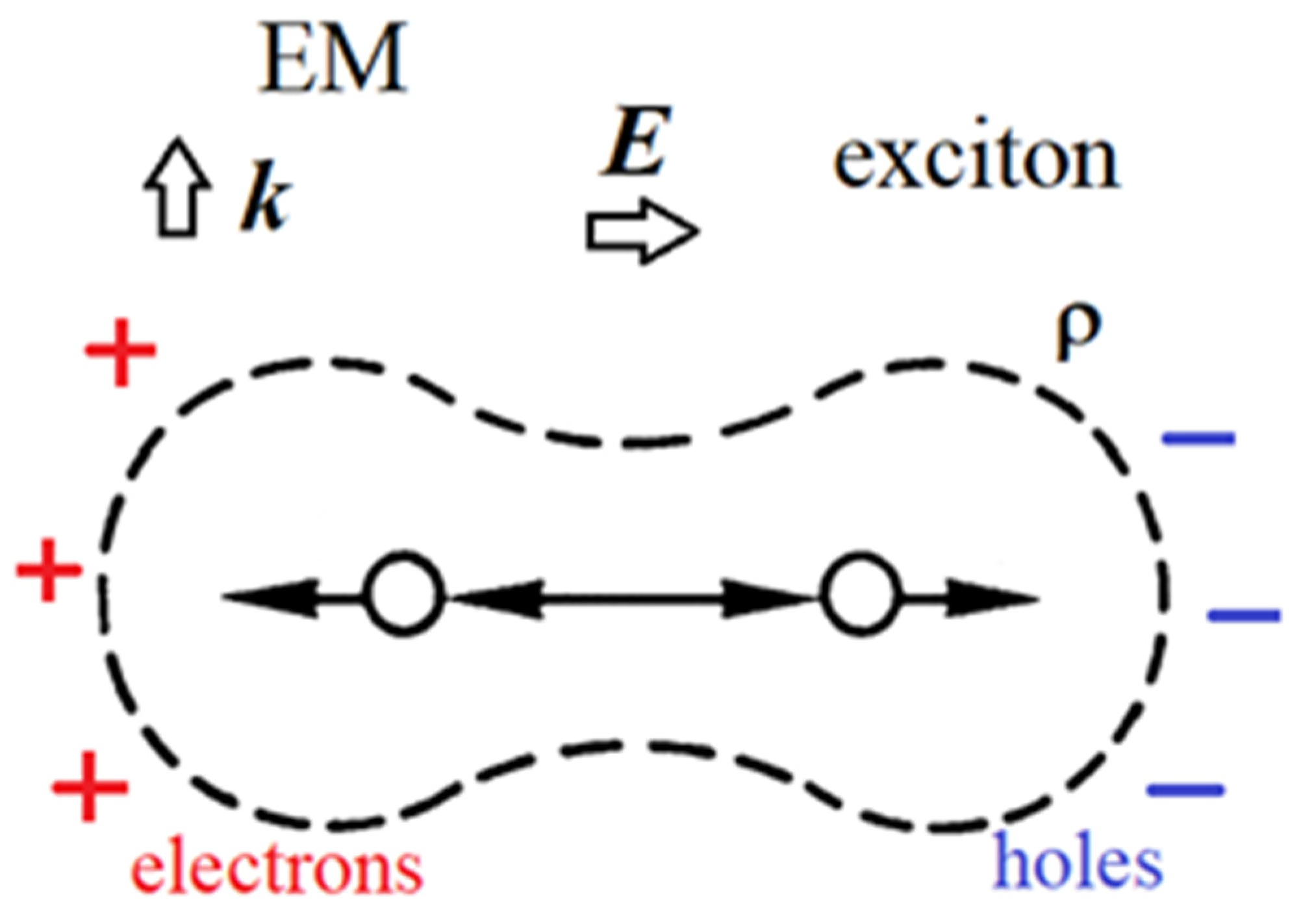
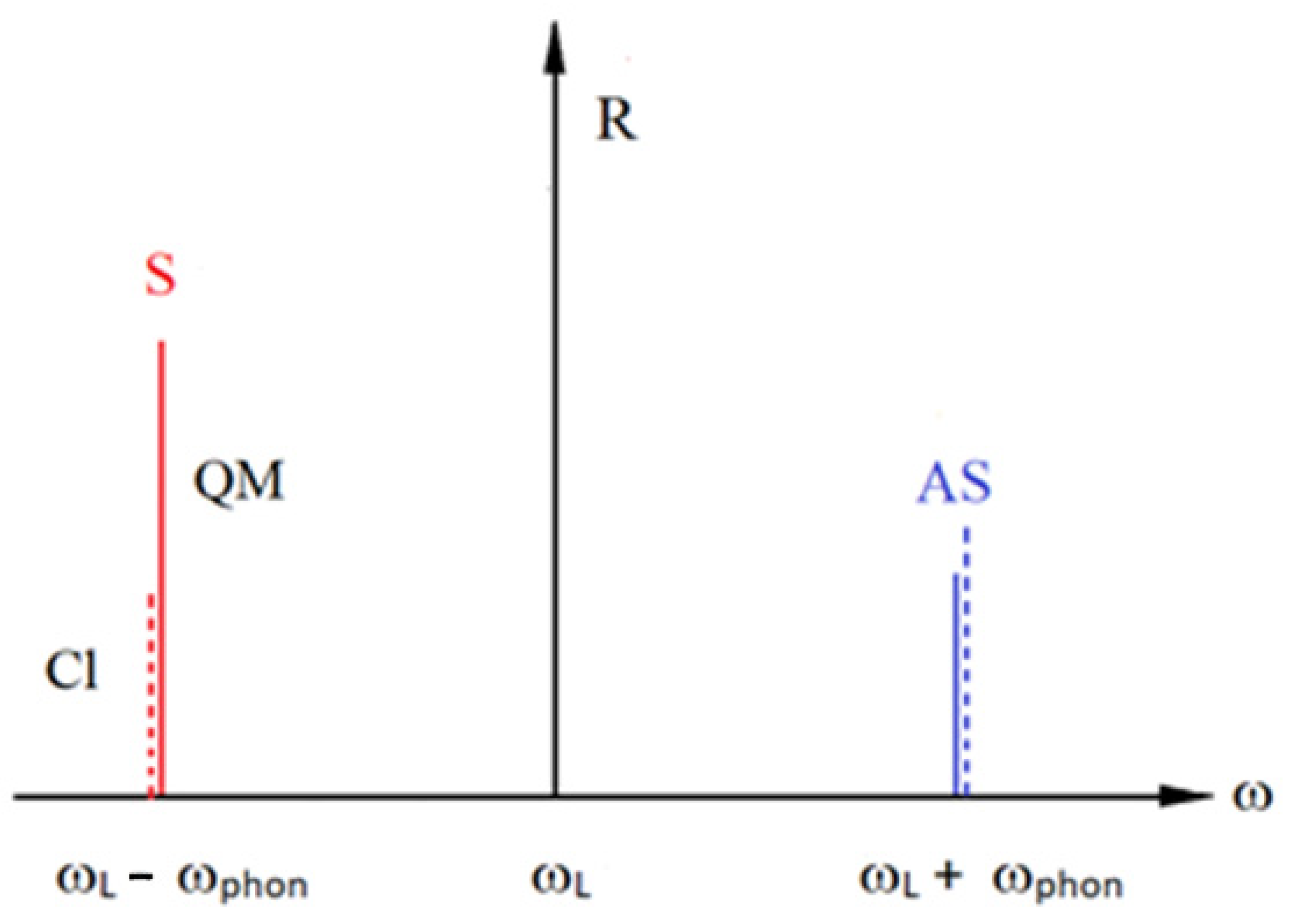
1. Microscopic Theory
- First vertex: The incident photon interacts with an electron in the molecule creating an intermediate (virtual) state of an electron–hole pair (or exciton).
- Second vertex 2: This electron–hole pair is scattered into another virtual state by emitting a phonon via the electron–phonon interaction.
- Third vertex 3: Due to the electron–phonon interaction, the electron–hole pair recombines with the emission of the scattered photon.
2. Overview and Importance of Plant Carotenoids
3. In Situ Raman Spectroscopy Determination and Quantification of Foods Carotenoids
4. Statistical Processing of Raman Spectra for Qualitative and Quantitative Characterization of Carotenoids
- Baseline correction is a pre-processing algorithm used to separate true spectroscopic signals from background effects [63];
- Normalization is a very important part of a pre-processing procedure, especially when samples are measured under different experimental conditions, allowing unbiased analysis [64];
- Smoothing is one of the appropriate mathematical techniques used to reduce the noise in the spectra while preserving all the important spectral information of the analyte. There are a number of methods that can be used to smooth the spectra, even if this involves a distortion of some spectral features [65];
- Spectral calibration is a method that can be used to correct for the occurrence of wavelength shifts. Accurate calibration is essential for comparing and combining spectra obtained from different measurements;
- Outlier detection is the procedure of identifying and handling outliers in the spectra that could significantly impact the validity of further statistical analyses. Outliers may arise from instrument noise, sample artefacts, or from measurement errors;
- Feature selection helps identify relevant spectral characteristics for the analysis, considering the specific information needed for the study. The selection of appropriate variables is required to avoid over-fitting, as well as for improvements in the model’s interpretability.
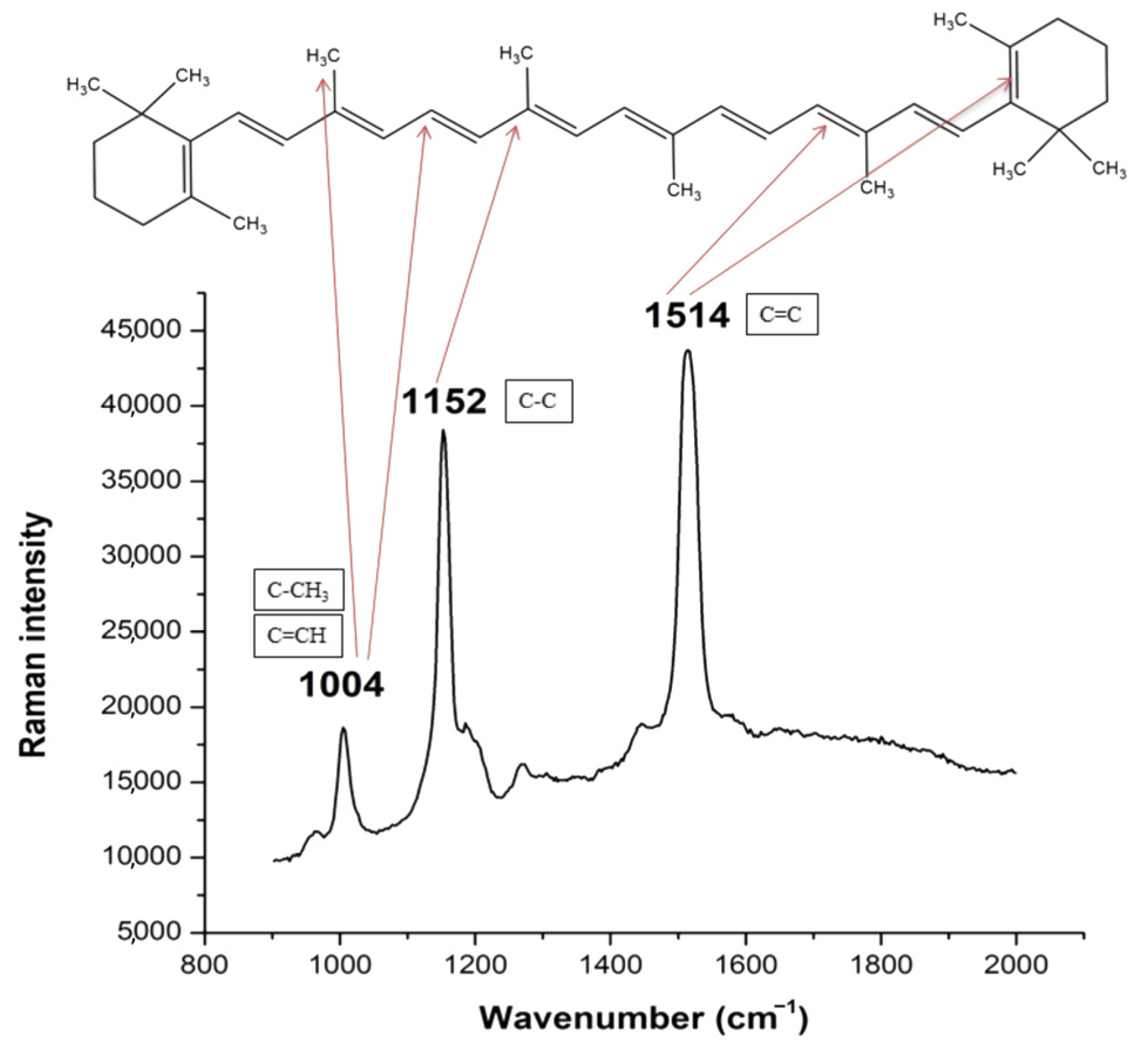
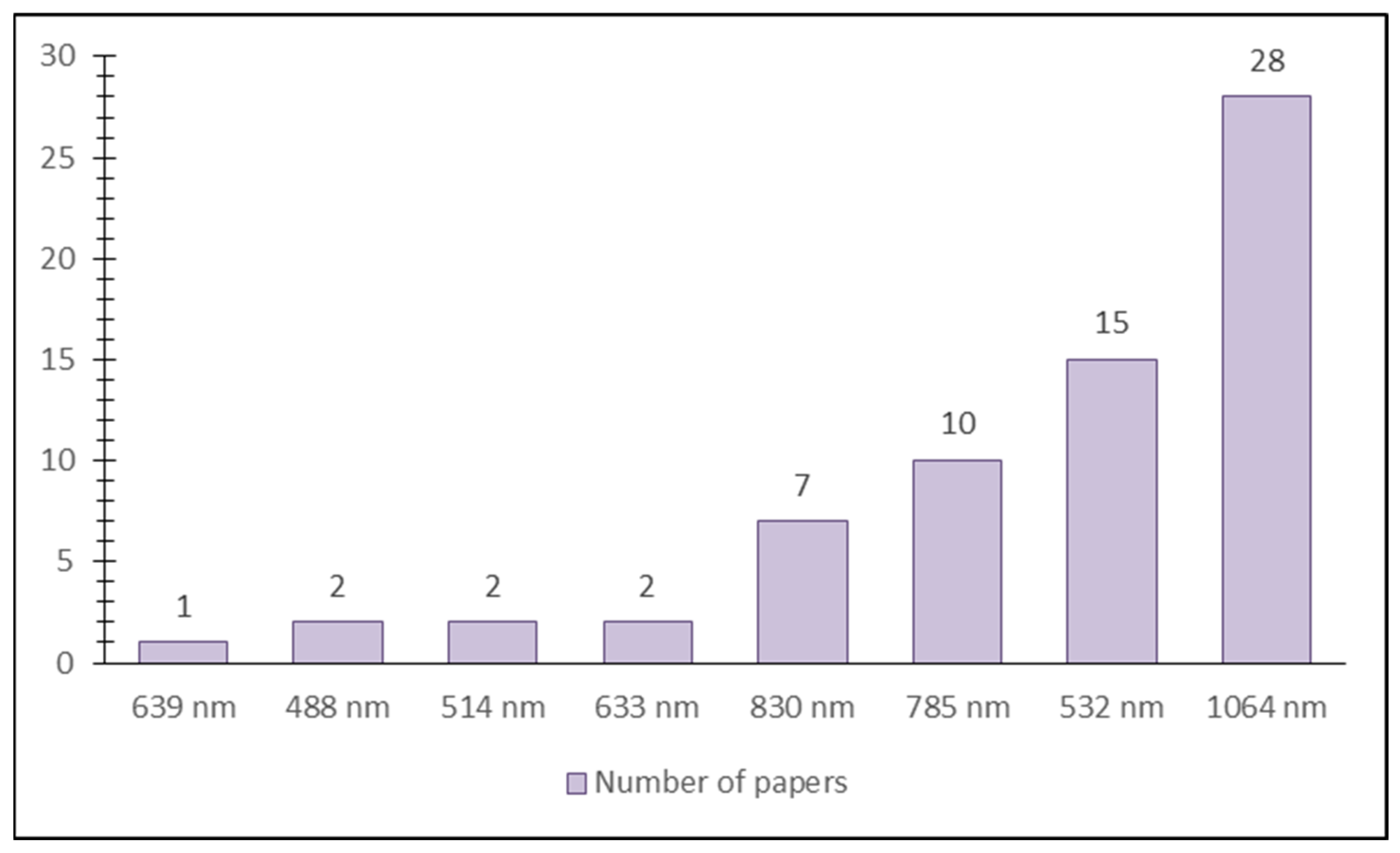
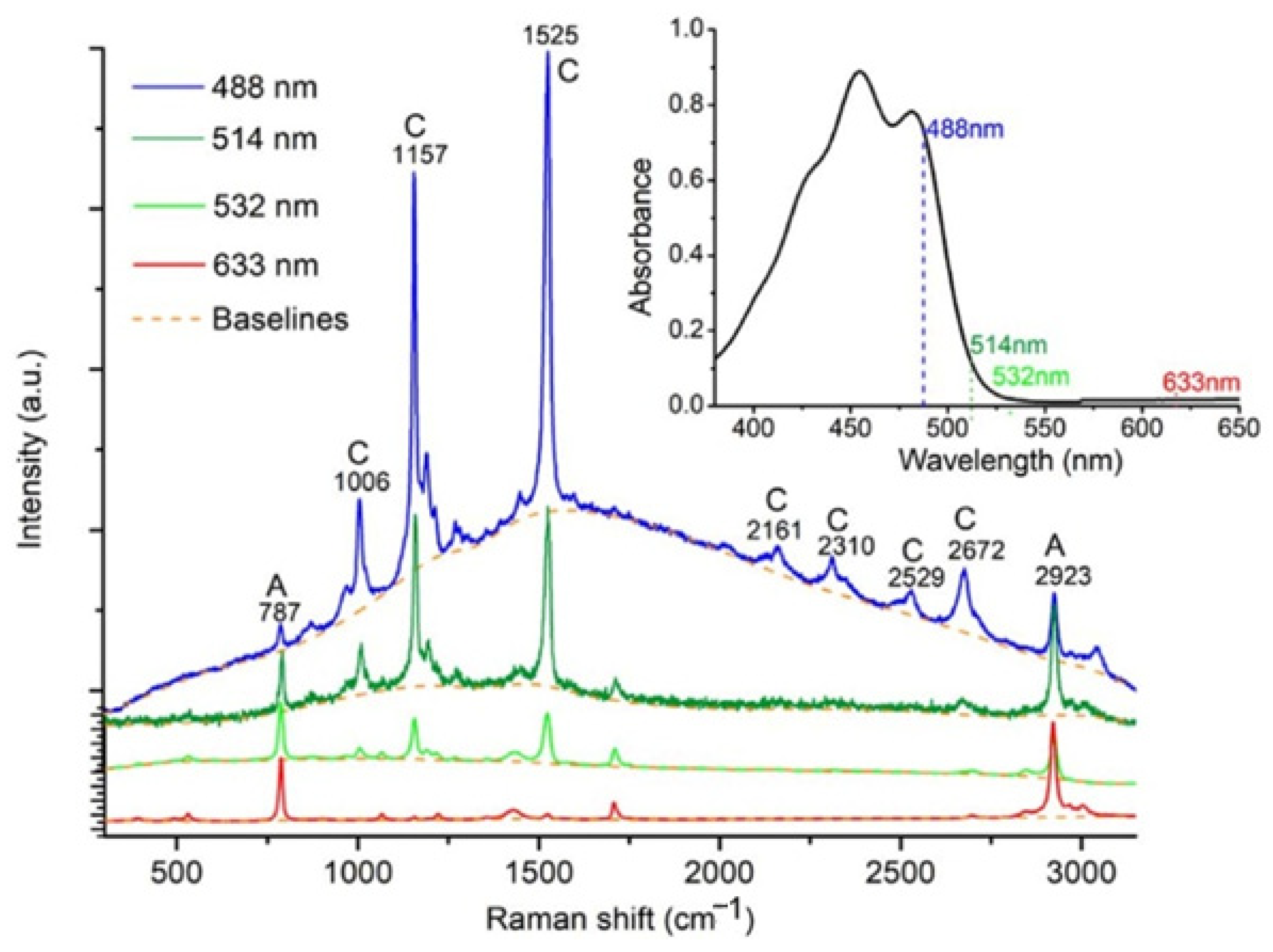
5. Raman Spectroscopy in Analysis of Carotenoids
- n is the required sample size for each group of studied samples/specimens;
- and are the variances within each group;
- is the quantil of the standard normal distribution at the probability level , for a given significance level ;
- is the quantil of the standard normal distribution at the probability level for achieving a power of 1 − β;
- is the minimum effect size one wants to detect.
6. Carotenoids Quantification Modeling: From Starting Trials to Current Approaches
- Laser power and environmental (i.e., external) conditions should be stable and very similar during continuous RS measurements. As recommended, dividing the spectra into training and test datasets for the calibration and validation of the model is preferable;
- Recording the same samples in different terms (days, time-scale) for external validation is preferable so as to minimize the sensitivity of the Raman instrumentation. Upon obtaining results, it will be obvious whether the performed model is adequate or not. Testing different Raman spectra traits/variables (i.e., the height of the observed band, the area under the band, height/area band ratio, etc.) will provide the best matching with analytical data regarding the concentration of the analyte;
- Raman spectra are often shifted due to spectral variations that mostly originate from the sample, but could be also caused by external factors, which should be minimized during RS measurements.
| Species | Type of Analysis | Aim of Investigation | Training and Test Sets | Applied Pre-Processing | Applied Models | External Validation (Yes/No) | RMSE (Yes/No) | Conclusion | References |
|---|---|---|---|---|---|---|---|---|---|
| Allium (Allium sp.) | QLT | Qualitative and classification analysis of different Allium species. | No | Baseline correction, normalization, PCA | PCA | No | NA | The analysis of the Raman spectra, combined with PCA, provided clear differences in the chemical profiles of the different Allium species samples. | [76] |
| Apple (Malus domestica L.) | QLT | Potential in monitoring fruit juice production. | NA | Baseline correction | NA | NA | NA | Raman spectroscopy can be used for direct online monitoring of carotenoids in fruit juice samples | [77] |
| Apricot (Prunus armeniaca L.) | QLT | Analyzing carotenoids in situ. | NA | No | NA | NA | NA | It has been found that FT-Raman spectroscopy can be successfully applied for the identification of carotenoids directly in the plant tissue without any preliminary sample preparation. | [48] |
| Apricot (Prunus armeniaca L.) | QLT | Potential in monitoring fruit juice production. | NA | Baseline correction | NA | NA | NA | Raman spectroscopy can be used for direct online monitoring of carotenoids in fruit juice samples. | [77] |
| Cabbage (Brassica oleraceavar. capitata) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of cabbage where β-carotene is detected. | [78] |
| Canabis/hemp (Cannabis sativa L.) | QLT and DIS | Discrimination between cannabis and hemp. | No | Baseline correction, SNV, first derivative. | OPLS-DA | No | NA | Raman spectroscopy can be used in discrimination between hemp and cannabis with 100% accuracy. | [79] |
| Carrot (Daucus carota L.) | QNT | Quantification of carotenoids in carrots using Raman spectroscopy. | No | Baseline correction and PCA | PCR, PLSR and LS-SVM | No | Yes | Models showed better prediction when characteristic bands were used (at 1006, 1154 I 1518 cm−1) compared to when the whole spectra were used. PLS showed the best results (R2 = 0.927–0.959 RMSE 4.66 mg/kg). | [21] |
| Carrot (Daucus carota L.) | QLT | Three complementary instrumental methods (Raman imaging, AFM and SNOM) were applied to reveal differences in carotenoid crystals accumulating in carrot cells. | NA | No | NA | NA | NA | Raman imaging using two excitation lines revealed different compositions of crystals and have provided evidence that crystals of planar structure, i.e., needle-like and rhomboidal, do not differ in their constitution, and they are composed of β-carotene and α-carotene, implying the crystals in the carrot root are the same. | [80] |
| Carrot (Daucus carota L.) | QLT | Monitoring biosynthesis and accumulation of carotenoids during development of carrot. Raman spectroscopy was employed to investigate the evolutions of carotenoids’ development and the influence of light in different stages of growth. | No | Baseline correction | NA | NA | NA | Raman spectroscopy can be used in the monitoring of the biosynthesis, transformation and accumulation of carotenoids during the growth of carrot without any pretreatment of the sample. | [81] |
| Carrot (Daucus carota L.) | QLT, QNT and DIS | Quantification of carotenoids in carrot as well as discrimination between different carrot genotypes. | Cross-validation | Second derivative for quantification | PLSR PCA | No | Yes | FT-Raman spectroscopy can be used for the quantification of carotenoids in carrot with regression coefficients over 0.96. PCA showed potential use in the discrimination of different carrot varieties. | [61] |
| Carrot (Daucus carota L.) 31 carrot lines | QNT | Quantification of carotenoids in carrot samples. | Yes | Smoothing and baseline correction, SNV, MSC, PCA | MLR, PLSR | No | Yes | Raman spectroscopy can be used for carotenoid quantification and discrimination of selected carrot samples. The regression model applied showed a high regression coefficient (R2 = 0.88 and 0.85 for calibration and validation, respectively). | [59] |
| Carrot red, yellow (Daucus carrota L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of red and yellow carrot where β-carotene and lutein were detected. | [78] |
| Carrot red, yellow (Daucus carrota L.) | QNT | Qunatification of total carotenoid content in carrot tissues. | No | Baseline correction and normalization | PLS-R | No | Yes | Results showed that Raman spectroscopy in combination with PLS-R can be used in total carotenoids quantification in carrot (R2 = 0.86). | [82] |
| Chinese chives (Allium odorum L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of Chinese chives where β-carotene was detected. | [78] |
| Chive (Allium schoenoprasum L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of chive where β-carotene was detected. | [78] |
| Citrus sp. | QLT | To investigate the relationship between freshness and obtained Raman spectra. | NA | Normalization | NA | NA | NA | Raman spectroscopy demonstrated that the intensity of the carotenoid (pre)-resonance Raman signal is an excellent indicator of the intact citrus freshness, thus introducing objective criteria of appreciation and quality control. | [83] |
| Corn (Zea mays L.) | QLT | Discrimination of different maize varieties. | No | No | PLS-DA | No | NA | Raman spectroscopy in combination with PLS-DA can be used for the discrimination of different maize varieties (precision 88–99%). | [84] |
| Corn (Zea mays L.) | QLT | Application of hand-held Raman spectrometer in combination with chemometrics to discriminate healthy and diseased maize kernels. | No | Baseline correction, normalization, PCA | OPLS-DA | No | NA | Raman spectroscopy coupled with OPL-DA discrimination model showed 100% precision. | [16] |
| Different vegetables | QNT | Determination of carotenoids in vegetables before and during boiling. | No | No | No | No | No | Raman spectroscopy can be used for the monitoring of changing of carotenoids contents during boiling of carotenoid-rich vegetables. | [85] |
| Dog rose (Rosa canina L.) | QLT and DIS | To discriminate different rosehip samples using Raman spectroscopy and chemometrics. | No | Baseline correction and normalization | PCA | No | NA | The results confirm the potential use of Raman spectroscopy as a fast and sophisticated method for obtaining detailed information concerning the spatial distribution of plant metabolites in the studied rosehips. | [86] |
| Dog rose (Rosa canina L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of dog rose where carotenoids were detected. | [78] |
| Extravirgin olive oil—EVOO | QNT | Quantification of carotenoids in EVOO. | No | Nonlinear curve fit based on Levenberg–Marquardt minimization algorithm | Simple linear regression | NE | No | Raman spectroscopy can be used to quantify the lutein/β carotene ratio in EVOOs from a single drop of oil. | [58] |
| Falso guarana (Bunchosia glandulifera (Jacq.) Kunth | QNT | To evaluate the carotenoids content of processed Bunchosia glandulifera. | No | PCA | PLSR | No | Yes | Raman spectroscopy can be used for the determination of carotenoids without sample preparation. There is a correlation between the area of characteristic bands and carotenoids content in the samples. | [60] |
| Garlic (Allium sativum L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of garlic where β-carotene and lutein were detected. | [78] |
| Grape (Vitis vinifera L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of grape where zeaxhantine, β-carotene and lutein were detected. | [78] |
| Lettuce butter (Lactuca sativa var. capitata) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of butter lettuce where β-carotene was detected. | [78] |
| Mandarine orange (Citrus reticulata Blanco) | QLT | Application of Raman spectroscopy in combination with pattern recognition approach to classification of citrus fruit. | No | Baseline correction and normalization | PCA, HCA | No | NA | It is concluded that Raman spectroscopy in combination with PCA and HCA can be used as a tool for quality assessment. According to PCA, two groups were obtained, while HCA showed slightly different results. | [87] |
| Mango (Mangífera indica L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in the carotenoids analysis of mango, where β-carotene was detected. | [78] |
| Nectarine (Prunus perica L. var. nucipersica) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of nectarine where β-Cryptoxanthin was detected. | [78] |
| Orange (Citrus × aurantium L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of orange where β-carotene and β-Cryptoxanthin were detected. | [78] |
| Palm oil | QLT | Determination of ripeness level of oil palm fruitlets. | Yes—fivefold validation scheme | Baseline correction, smoothing | CRT, SVM, KNN | No | NA | Raman spectroscopy can be used to assess the ripeness level of oil palm fruitlets using carotenoids information and classification models. The best model was KNN (100% accuracy). | [88] |
| Papaya (Carica papaya L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of papaya pulp where lycopene, β-Cryptoxanthin, and β-carotene were detected. | [78] |
| Corn (Zea mays L.), Pumpkin (Cucurbita pepo L.), Paprika (Capsicum annuum L.), Watermelon (Citrullus lanatus L.), Apricot Prunus armeniaca L.), achiote tree (Bixa orellana L.), Nectarine(Prunus perica L. var. nucipersica), French bean (Phaseolus vulgaris L.), Saffron (Crocus sativus L.), Broccoli (Brassica oleracea var. italica) | QLT | To demonstrate potential of FT-Raman for in situ analysis of carotenoids-rich samples. | NA | No | NA | NA | NA | The results show that Raman spectroscopy can be used in the investigation of the cis-trans isomerization of carotenoids ruing processing. Besides this, 2-D Raman imaging presents the possibility of evaluating the distribution of carotenoids in plant tissue. | [48] |
| Peanut (Arachis hypogaea L.) | QLT and DIS | Discrimination of different peanut genotypes. | Cross-validation | Baseline correction | OPLS-DA | No | NA | Raman spectroscopy can be used for accurate identification of peanuts based on spectroscopic signatures of their leaves and seeds. OPLS-DA results show that peanut seeds can be identified with, on average, 95% accuracy, whereas the accuracy of Raman-based identification of leaves is, on average, 80%. | [89] |
| Pepper (Capsicum annuum L.) | QLT, QNT and DIS | Application of Raman spectroscopy to discriminate adulterated samples of paprika powder from the original one. Besides this, the quantification of ASTA color and Sundan I (adulterant). | Yes, 66 traiining, 22 test sets | Polynomial fitting | PLSR, PCA za klasifikaciju | NE | Yes | Raman spectroscopy showed two groups in the PCA score plot. Quantification displayed high regression coefficient for both ASTA and Sudan I concentrations (0.945 and 0.982, respectively). | [82] |
| Pepper (Capsicum annuum L.) | QLT, DIS | Classification of different paprika varieties at their final maturity stages. | Yes | Baseline correction, normalization, smoothing, PCA | PCA-LDA, PLS-DA, QDA | No | NA | Raman spectroscopy in combination with chemometrics can be used as a tool for the discrimination of different paprika varieties. The best classification results were acquired using QDA (100%). | [90] |
| Pepper (Capsicum annuum L.) | QLT and QNT | Qualitative and quantitative changes of carotenoids in paprika samples during plant development or under stress condition. Spatial distribution of carotenoids (Raman mapping). | No | Baseline correction | No | No | No | Raman spectroscopy can be used in qualitative and quantitative carotenoid analyses in paprika samples. Besides this, changes in these parameters during development can be monitored using Raman imaging where the distributions and relative amounts of carotenoids are presented. | [47] |
| Pepper (Capsicum annuum L.) | QLT, DIS | Assessment of red pepper ripening phases and carotenoids Accumulation. | Yes | Baseline correction, normalization, PCA | PCA-LDA, PLS-DA, SIMCA | No | No | Raman spectroscopy coupled with chemometrics can be used in determination of ripening phase. SIMCA showed the best classification accuracy (100%). | [56] |
| Pepper (Capsicum annuum L.) | QLT and DIS | Application of Raman spectroscopy in monitoring ripeness of hot paprika. PCA was applied to discriminate paprika fruits in different maturity stages. | No | SNV | PCA | No | NA | Raman spectroscopy can be used in the monitoring of ripeness of hot paprika as well as to discriminate different maturity stages. | [41] |
| Potato (Solanum tuberosum L.) | QLT | Identification of nine different potato varieties as well as determination of geographic origin of potato. | No | Third-order derivative | PLS-DA | No | NA | Raman spectroscopy can be used to identify nine different potato varieties, as well as to determine the origin of their cultivation. | [91] |
| Pumpkin (Cucurbita pepo L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of pumpkin where β-carotene was detected. | [78] |
| Purple onion (Allium cepa L.) | QLT | Application of Raman spectroscopy to evaluate carotenoid composition in different green and red fruits, vegetables and spices. | NA | NA | NA | NA | NA | Raman spectroscopy can be used in carotenoids analysis of purple onion where β-carotene was detected. | [78] |
| Rice (Oryza sativa L.) | QLT and DIS | Application of Raman spectroscopy to evaluate abiotic stresses. | No | Baseline correction, smoothing | PLS-DA | No | NA | Raman spectroscopy in combination with PLS-DA can be used in the discrimination of different stage of stresses. The accuracy of the applied model depends on the stage of stress (lower accuracy was obtained in samples with low stress and vice versa). | [79] |
| Sweet potato (Ipomoea batatas (L.) Lam.) | QNT | Quantitative analysis of carotenoids in processed sweet potato. | No | SNV, PCA | PLSR | No | Yes | Raman spectroscopy coupled with chemometrics can be used to quantify carotenoids in real time. | [59] |
| Tea leaves (Camellia sinensis L.) | QNT | In situ quantitative visualization of chlorophyll and carotenoid with calibration transfer model. | No | MSC, WT, SNV, RCF and airPLS. | PLSR | Yes, to second instrument | Yes | It can be concluded that Raman spectroscopy was applicable for in situ, non-destructive and rapid quantitative detection and imaging of photosynthetic pigment concentration in tea leaves, and the spectral detection model established based on the laboratory Raman spectrometer can be applied to a portable field spectrometer for quantitatively imaging the foliar pigments. | [92] |
| Tomato (Lycopersicon esculentum L.) | QNT | Quantification of carotenoids. | Cross-validation | Baseline correction | PLSR | No | No | The 785 nm laser has the most suitable excitation wavelength to perform a quantitative analysis of carotenoid concentrations in tomatoes. | [20] |
| Tomato (Lycopersicon esculentum L.) | QLT, QNT and DIS | To develop models for the quantification of carotenoids and discrimination of analyzed samples. | Cross-validation | Mean centering and smoothing, PCA | SIMCA, ANN, PLSR | No | Yes | SIMCA classification accuracy 93%, ANN 100%. PLSR and ANN for quantification regression coefficients over 0.9. | [55] |
| Tomato (Lycopersicon esculentum L.) | QLT | Monitoring carotenoids’ biosynthesis in situ during fruit development. | NA | Baseline correction | NA | NA | NA | Raman spectroscopy equipped with 1064 and 785 nm lasers and DFT calculations can be used for monitoring the main carotenoids during the ripening process. | [40] |
| Tomato (Lycopersicon esculentum L.) | QLT, QNT and DIS | It was found that Raman spectroscopy with a short exposure time can be used in the quantitative and discriminative analysis of carotenoids in intact tomato. | Yes, 40 for training and 20 for test set | Baseline correction | PLSR and PLS-DA | No | Yes | The results show that longer exposure times provide better R2 values. Accordingly, when the exposure time was 10 s and 0.7 s, R2 was 0.87 and 0.69, respectively. When it comes to discrimination, there were no significant differences depending on exposure time. | [20] |
| Tomato (Lycopersicon esculentum L.) | QLT | To evaluate the fruit quality of three traditional tomato genotypes with different colors from the Balkans. | NA | Normalization | PCA | No | NA | Carotenoid information obtained by Raman spectroscopy can be used in discrimination of different tomato varieties. | [93] |
| Tomato (Lycopersicon esculentum L.) | QNT | Quantification of lycopene and β-carotene in tomato fruits and related products. | Cross-validation | Baseline correction and normalization | PLSR | No | Yes | Results show that Raman spectroscopy can be used in quantification of lycopene and β-carotene. Accordingly, R2 was 0.91 and 0.89 for lycopene and β-carotene, respectively. | [46] |
| Tomato (Lycopersicon esculentum L.) | QLT, DIS and QNT | Does exposure time affect the precision of quantitative and discrimination analysis? | Yes, 40 for training and 20 for validation. | Mean centering | PLSR and PLS-DA | No | Yes | When it comes to quantification analysis, the best results were obtained when 10 s integration time was applied, and the worst when 0.7 s was applied. On the other hand, in the PLS-DA result, integration time did not affect precision. | [21] |
| Watermelon (Citrullus lanatus L.) | QLT | Determination of maturity stage of watermelon. | NA | Baseline correction | PLS-DA | No | Yes | Raman spectroscopy can be used to determinate the stage of maturity of watermelon. | [39] |
| Wheat (Triticum aestivum L.) | QLT and DIS | Discrimination of healthy and infected wheat. | No | Baseline correction | PLS-DA | No | NA | Raman spectroscopy can be used to differentiate between healthy wheat and wheat infected with viruses. | [57] |
| Species | Raman Scattering Technique | Laser Wavelength | Laser Power | Exposure Time | Accumulation Time | Grating | Number of Recorded Spectra | Objective | Spectral Resolution | Recorded Spectral Range Used in Analysis | Carotenoid Band Position | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allium sp. | point scan | 532 nm | No | 10 s | 15 | 600 g/mm | No | - | 3 cm−1 | No | 1527, 1153, 1002 cm−1 | [76] |
| Apple (Malus domestica L.) | mapping | 633 nm | 17 mW | 60–300 s | No | 1800 g/mm | No | 50x | 4 cm−1 | 0–2000 cm−1 | 1008, 1159 and 1520 cm−1 | [77] |
| Apricot (Prunus armeniaca L.) | point-scan | 1064 nm | 300 mW | No | No | No | 512 | No | 4 cm−1 | 100–4000 cm−1 | 1524, 1156, 1003 cm−1 | [48] |
| Apricot (Prunus armeniaca L.) | mapping | 633 nm | 17 mW | 60–300 s | No | 1800 g/mm | No | 50× | 4 cm−1 | 0–2000 cm−1 | 1520, 1159, 1008 cm−1 | [77] |
| Cabbage (Brassica oleracea var. capitata) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1517, 1158, 1008 cm−1 | [78] |
| Canabis/hemp (Cannabis sativa L.) | point scan | 831 nm | 495 mW | 10 s | No | 20–23 | No | 15 cm−1 | 701–1700 cm−1 | 1527, 1155, 1000 cm−1 | [79] | |
| Carrot (Daucus carota L.) | Point-scan | 785 nm | 300 mW | 10 s | 2 | No | No | No | 6 cm−1 | 100–3200 cm−1 | 1006, 1154, 1518 cm−1 | [21] |
| Carrot (Daucus carota L.) | imaging | 532 nm | 20 mW | 0.1 s | No | No | No | No | 5–6 steps/1 μm | No | 1007–1010; 1159–1160; 1519–1523 cm−1 | [80] |
| Carrot (Daucus carota L.) | imaging | 488 nm | 10 mW | 0.3 s | No | No | No | No | 2 steps/1 μm | No | 1007–1010; 1159–1160; 1519–1523 cm−2 | [80] |
| Carrot (Daucus carota L.) | point scan | 532 nm | 0.4 mW | 10 s | No | No | No | 50x | 0.1 cm−1 | 400–1800 cm−1 | 1519, 1156, 1008 cm−1 | [81] |
| Carrot (Daucus carota L.) | point scan | 1064 nm | 500 mW | No | 64 | No | 5 | No | 4 cm−1 | total carotenoids—4000–3619 cm−1; 2481–1339 cm−1; 961–199 cm−1 | 1527, 1157, 1108 cm−1 | [61] |
| Carrot (Daucus carota L.) | point scan | 1064 nm | 500 mW | No | 64 | No | 5 | No | 4 cm−1 | α-carotene—3621–3239 cm−1; 1721–959 cm−1 | 1527, 1157, 1108 cm−2 | [61] |
| Carrot (Daucus carota L.) | point scan | 1064 nm | 500 mW | No | 64 | No | 5 | No | 4 cm−1 | β-carotene—4000–3619 cm−1; 1721–199 cm−1 | 1527, 1157, 1108 cm−3 | [61] |
| Carrot (Daucus carota L.) | point scan | 1064 nm | 500 mW | No | 64 | No | 5 | No | 4 cm−1 | lutein—4000–3619 cm−1; 1721–959 cm−1; 581–199 cm−1 | 1527, 1157, 1108 cm−4 | [61] |
| Carrot (Daucus carota L.) | point scan | 785 nm | 200 mW | 2 s | No | No | No | No | No | 100–1900 cm−1 | 1520, 1156, 1007 cm−1 | [82] |
| Carrot (Daucus carota L.), 31 carrot lines | point scan | 1064 nm | 120 mW | 3 min | 100 | No | 101 | No | 4 cm−1 | 0–3500 cm−1; | 1520, 1156, 1006 cm−0 | [69] |
| Carrot (Daucus carota L.), 31 carrot lines | point scan | 830 nm | 271 mW | 4 min | 16 | No | 101 | No | No | 200–2300 cm−1; | 1520, 1156, 1006 cm−1 | [69] |
| Carrot (Daucus carota L.), 31 carrot lines | point scan | 785 nm | No | 4 min | 16 | No | 101 | No | No | 200–2300 cm−1 | 1520, 1156, 1006 cm−2 | [69] |
| Carrot red, yellow (Daucus carrota L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1527, 1157, 1108 cm−1 | [78] |
| Carrot red, yellow (Daucus carrota L.) | point scan | 785 nm | 400 mW | 0.1 s | 150 | No | 88 | - | No | 300–1800 cm−1 | 1521, 1157, 1107 cm−1 | [82] |
| Carrot red, yellow (Daucus carrota L.) | point scan | 785 nm | 100 mW | No | No | No | 64 | No | 13 cm−1 | 400–2300 cm−1 | 1001, 1156, 1515 cm−0 | [41] |
| Carrot red, yellow (Daucus carrota L.) | point scan | 639 nm | 35 mW | No | No | No | 64 | No | 7–10 cm−1 | 150–3150 cm−1 | 1001, 1156, 1515 cm−1 | [41] |
| Carrot red, yellow (Daucus carrota L.) | point scan | 1064 nm | 900 mW | No | No | No | 64 | No | 6 cm−1 | 100–3800 cm−1 | 1001, 1156, 1515 cm−2 | [41] |
| Carrot red, yellow (Daucus carrota L.) | image | 1064 nm | 300 mW | 3000 s | No | No | No | No | 4 cm−1 | 100–4000 cm−1 | 1524, 1510 cm−1 | [47] |
| Chinese chives (Allium odorum L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1527, 1153, 1002 cm−1 | [78] |
| Chive (Allium schoenoprasum L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1527, 1158, 1006 cm−1 | [78] |
| Citrus sp. | point scan | 532 nm | Clementine-200 mW, mandarins and tangarins 20 mW | 1 s | 1 | No | No | 20× | 0.5 cm−1 | 100–1800 cm−1 | 1520–1523, 1153–1155, 1003 cm−1 | [83] |
| Corn (Zea mays L.) | point scan | 830 nm | 432 mW | 1 s | No | No | Around 100 per variety | - | 15 cm−1 | 400–1800 cm−1 | 1527, 1153 cm−1 | [84] |
| Corn (Zea mays L.) | point scan | 1064 nm | 200 mW | 10 s | No | No | - | No | No | 400–1700 cm−1 | 1523, 1155, 1003 cm−1 | [16] |
| Corn (Zea mays L.), Pumpkin (Cucurbita pepo L.), Pepper (Capsicum annuum L.), Watermelon (Citrullus lanatus L.), Apricot Prunus armeniaca L.), achiote tree (Bixa orellana L.), Nectarine (Prunus perica L. var. nucipersica), French bean (Phaseolus vulgaris L.), Saffron (Crocus sativus L.), Broccoli (Brassica oleracea var. italica) | point scan | 1064 nm | Samples—300 | No | No | No | No | No | No | 800–1800 cm−1 | Apricot-1526, 1156, 1000 cm−1 French bean—1522, 1157, 1005 cm−1; Corn-1522, 1157, 1005; Nectaraine-1527, 1157, 1005 cm−1; Pumpkin-1550–1600, 1450, 1360–1390, 1100, 950–980, 520–530; Tomato-1510, 1156, 1005 cm−1; Watermelon-1510, 1158, 1008 cm−1; Saffron-1536, 1165, 1020 cm−1; Broccoli-1524, 1157, 1005 cm−1; Carrot—1526, 1157, 1004 cm−1; Annatto—1518, 1154, 1011 cm−1 | [48] |
| Different vegetables | point scan | 488 nm | / | No | No | No | No | 6 | No | No | 1525 cm−1 | [85] |
| Dog rose (Rosa canina L.) | point scan | 532 nm | No | 10 | 10 | 1200 g/mm | No | No | 3 cm−1 | 200–1800 cm−1 | 1513, 1153, 1003 cm−1 | [86] |
| Dog rose (Rosa canina L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1513, 1153, 1000 cm−1 | [78] |
| Extravirgin olive oil EVOO | point scan | 532 nm | 5 mW | 10 s | 5 | No | 9 | 10× | No | 750–1800 cm−1 | 1520, 1156 cm−1 | [58] |
| Falso guarana (Bunchosia glandulifera (Jacq.) Kunth | point scan | 532 nm | 50 mW | 10 s | 3 | 1800 g/mm | 6 | No | No | 400–2000 cm−1 | 1524, 1157, 1005 cm−1 | [60] |
| Garlic (Allium sativum L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1511, 1179, 1005 cm−1 | [78] |
| Grape (Vitis vinifera L.) | point scan | 1064 nm | 70–300 mW | No | 3000 scans | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1525, 1155, 1004 cm−1 | [78] |
| Lettuce butter (Lactuca sativa var capitata) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1517, 1158, 1008 cm−1 | [78] |
| Mandarine orange (Citrus reticulata Blanco) | point scan | 514 nm | 20 mW | 10 | No | No | No | No | 1.5 cm−1 | 100–3200 cm−1 | 1520, 1155, 1003 cm−1 | [87] |
| Mango (Mangífera indica L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1513, 1153, 1000 cm−1 | [78] |
| Nectarine (Prunus perica L. var. nucipersica) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1529, 1157, 1004 cm−1 | [78] |
| Orange (Citrus × aurantium L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1531, 1156, 1012 cm−1 | [78] |
| Palm oil | point scan | 532 nm | 2 mW | 3 s | 3 | 900 g/mm | No | No | No | No | 1000, 1150, 1515 cm−1 | [88] |
| Papaya pulp (Carica papaya L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1525, 1155, 1008 cm−1 | [78] |
| Peanut (Arachis hypogaea L.) | point scan | 830 nm | 495 mW | 1 s | No | No | No | No | No | 400–1800 cm−1 | 1526, 1155, 1000 cm−1 | [89] |
| Pepper (Capsicum annuum L.) | point scan | 532 nm | 20–25 mW | 5 s | 10 | 1200 g/mm | 50 | 50× | 3 cm−1 | 900–1800 cm−1 | 1511–1519, ~1149–1151, | [90] |
| Pepper (Capsicum annuum L.) | point scan | 532 nm | 20–25 mW | 5 s | 10 | 1200 g/mm | 50 | 50× | 3 cm−1 | 900–1800 cm−1 | ~998–1006 cm−1 | [90] |
| Pepper (Capsicum annuum L.) | point scan | 532 nm | 20–25 mW | 3 s | 10 | 1200 g/mm | 50 | 50× | 3 cm−1 | 900–1800 cm−1 | 1003, 1151, 1514–1517 cm−1 | [56] |
| Pumpkin (Cucurbita pepo L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1527, 1157, 1108 cm−1 | [78] |
| Purple onion (Allium cepa L.) | point scan | 1064 nm | 70–300 mW | No | 3000 | No | No | No | 1–3 cm−1 | 900–2000 cm−1 | 1515, 1151, 1014 cm−1 | [78] |
| Rice (Oryza sativa L) | point scan | 830 nm | 495 mW | 1 s | No | No | No | No | No | 600–1800 cm−1 | 1000, 1155, 1527 cm−0 | [79] |
| Sweet orange (Citrus × sinensis) | point scan | 514 nm | 20 mW | 10 s | No | No | No | No | 1.5 cm−1 | 100–3200 cm−1 | 1520, 1155, 1003 cm−1 | [87] |
| Sweet potato (Ipomoea batatas (L.) Lam.) | point scan | 532 nm | 50 mW | No | 5 | 1800 g/mm | 30 | 50x | No | 250–2000 cm−1 | 1520, 1157, 1006 cm−1 | [59] |
| Tea leaves (Camellia sinensis L.) | point scan | 532 nm | 50 mW | 1 s | No | No | 315 | No | 0.2 cm−1 | 792–1961 cm−1 | 1008, 1159, 1528 cm−1 | [92] |
| Tomato (Lycopersicon esculentum L.) | point scan | 532 nm | 25 mW | 0.2 s | 1 | No | No | No | 1.45 cm−1 | 800–1800 cm−1 | 1006, 1156, 1518 cm−1 | [20] |
| Tomato (Lycopersicon esculentum L.) | point scan | 785 nm | 176 mW | 10 s | 1 | No | No | No | 8 cm−1 | 800–1800 cm−1 | 1006, 1156, 1518 cm−2 | [20] |
| Tomato (Lycopersicon esculentum L.) | point scan | 1064 nm | 490 mW | 0.5 s | 3 | No | No | No | 11 cm−1 | 800–1800 cm−1 | 1006, 1156, 1518 cm−3 | [20] |
| Tomato (Lycopersicon esculentum L.) | point scan | 1064 nm | / | 10 s | 30 | No | No | No | 8 cm−1 | 200–2500 cm−1 | 1007, 1158, 1520 cm−1 | [55] |
| Tomato (Lycopersicon esculentum L.) | point scan | 785 nm | 176 mW | 0.7 s and 10 s | 1 | No | No | No | 1.4 cm−1 | 975–1615 cm−1 | 1156 cm−1 | [21] |
| Tomato (Lycopersicon esculentum L.) | point scan | 1064 nm | 1064–150 mW | No | 1024 | No | No | No | 4 cm−1 | No | 1519–1528, 1158, 1007 cm−1 | [40] |
| Tomato (Lycopersicon esculentum L.) | point scan | 785 nm | 785–100 mW | 10 s | No | No | No | 50× | No | No | 1519–1528, 1158, 1007 cm−2 | [40] |
| Tomato (Lycopersicon esculentum L.) | point scan | 785 nm | 176 mW | 10 and 0.7 | No | No | 60 | - | 1.45 cm−1 | 800–1800 cm−1 for discrimination model | 1516, 1156, 1006 cm−1 | [20] |
| Tomato (Lycopersicon esculentum L.) | point scan | 785 nm | 176 mW | 10 and 0.7 | No | No | 60 | - | 1.45 cm−1 | 1615–975 cm−1 f or regression model | 1516, 1156, 1006 cm−2 | [20] |
| Tomato (Lycopersicon esculentum L.) | point scan | 532 nm | / | 5 s | 5 | 1200 g/mm | No | 50× | 3 cm−1 | 200–1800 cm−1 | 1505–1515, 1148, 997 cm−1 | [93] |
| Tomato (Lycopersicon esculentum L.) | point scan | 1064 nm | 150 mW | No | 512 | No | No | No | 4 cm−1 | 100–4000 cm−1 | 1370, 965–960 cm−1 | [46] |
| Watermelon (Citrullus lanatus L.) | point scan | 830 nm | 495 mW | 1 s | No | No | 20–43 | No | No | 400–2000 cm−1 | 1002, 1,156, 1,186, 1,217, and 1,525 cm−1 | [39] |
| Wheat (Triticum aestivum L.) | point scan | 830 nm | 320 mW | 1 s | No | No | 5–10 per leaf | - | 15 cm−1 | 400–1800 cm−1 | 1525, 1156, 1000 cm−1 | [57] |
7. New Raman Approaches in Food Carotenoids Analysis
7.1. Spatially Offset Raman Spectroscopy
7.2. Coherent Anti-Stokes Raman Spectroscopy
7.3. Stimulated Raman Spectroscopy
8. Application of Artificial Intelligence (AI) in Carotenoid Analysis Using Raman Spectroscopy
9. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, D.A. The Raman Effect; Wiley: Chichester, UK, 2002; ISBN 9780471490289. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/0470845767 (accessed on 29 January 2025).
- Yu, P.Y.; Cardona, M. Fundamentals of Semiconductors; Graduate Texts in Physics; Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-642-00709-5. Available online: https://link.springer.com/10.1007/978-3-642-00710-1 (accessed on 21 January 2025).
- Brüesch, P. Phonons: Theory and Experiments II; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 1986; Volume 65, ISBN 978-3-642-52265-9. Available online: http://link.springer.com/10.1007/978-3-642-52263-5 (accessed on 25 January 2025).
- Feynman. The Feynman Lectures on Physics; The New Mi; Feynman, R.P., Leighton, R.B., Sands, M., Eds.; Addison Wesley: Chichester, UK, 2010; Volume 3, ISBN 9788578110796. [Google Scholar]
- Simkin, A.J. Carotenoids and Apocarotenoids in Planta: Their Role in Plant Development, Contribution to the Flavour and Aroma of Fruits and Flowers, and Their Nutraceutical Benefits. Plants 2021, 10, 2321. Available online: https://www.mdpi.com/2223-7747/10/11/2321 (accessed on 21 January 2025). [CrossRef] [PubMed]
- Sun, T.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0168945219315043 (accessed on 25 January 2025). [CrossRef] [PubMed]
- Roy, S.; Deshmukh, R.K.; Tripathi, S.; Gaikwad, K.K.; Das, S.S.; Sharma, D. Recent Advances in the Carotenoids Added to Food Packaging Films: A Review. Foods 2023, 12, 4011. Available online: https://www.mdpi.com/2304-8158/12/21/4011 (accessed on 7 January 2025). [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2214662821000098 (accessed on 7 January 2025). [CrossRef]
- Zulfiquar, S.; Sharif, S.; Saeed, M.; Tahir, A. Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Available online: https://link.springer.com/10.1007/978-3-030-46459-2 (accessed on 21 January 2025)ISBN 978-3-030-46458-5.
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. Available online: https://molhort.biomedcentral.com/articles/10.1186/s43897-022-00023-2 (accessed on 21 January 2025). [CrossRef]
- Harrison, P.J.; Bugg, T.D.H. Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch. Biochem. Biophys. 2014, 544, 105–111. Available online: https://linkinghub.elsevier.com/retrieve/pii/S000398611300307X (accessed on 5 January 2025). [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Esquivel, P.; Meléndez-Martínez, A.J. Comprehensive Update on Carotenoid Colorants from Plants and Microalgae: Challenges and Advances from Research Laboratories to Industry. Foods 2023, 12, 4080. Available online: https://www.mdpi.com/2304-8158/12/22/4080 (accessed on 20 January 2025). [CrossRef]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid Metabolism in Plants: The Role of Plastids. Mol. Plant 2018, 11, 58–74. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1674205217302733 (accessed on 10 January 2025). [CrossRef]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. Available online: https://www.mdpi.com/2223-7747/12/2/313 (accessed on 20 January 2025). [CrossRef]
- Fuenmayor, C.A.; Baron-Cangrejo, O.G.; Salgado-Rivera, P.A. Encapsulation of Carotenoids as Food Colorants via Formation of Cyclodextrin Inclusion Complexes: A Review. Polysaccharides 2021, 2, 454–476. Available online: https://www.mdpi.com/2673-4176/2/2/28 (accessed on 21 January 2025). [CrossRef]
- Farber, C.; Kurouski, D. Detection and Identification of Plant Pathogens on Maize Kernels with a Hand-Held Raman Spectrometer. Anal. Chem. 2018, 90, 3009–3012. Available online: https://pubs.acs.org/doi/10.1021/acs.analchem.8b00222 (accessed on 15 January 2025). [CrossRef] [PubMed]
- Perry, A.; Rasmussen, H.; Johnson, E.J. Xanthophyll (lutein, zeaxanthin) content in fruits, vegetables and corn and egg products. J. Food Compos. Anal. 2009, 22, 9–15. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0889157508001336 (accessed on 11 January 2025). [CrossRef]
- Ponder, A.; Kulik, K.; Hallmann, E. Occurrence and Determination of Carotenoids and Polyphenols in Different Paprika Powders from Organic and Conventional Production. Molecules 2021, 26, 2980. Available online: https://www.mdpi.com/1420-3049/26/10/2980 (accessed on 5 December 2024). [CrossRef] [PubMed]
- Xu, J.; Lin, J.; Peng, S.; Zhao, H.; Wang, Y.; Rao, L.; Liao, X.; Zhao, L. Development of an HPLC-PDA Method for the Determination of Capsanthin, Zeaxanthin, Lutein, β-Cryptoxanthin and β-Carotene Simultaneously in Chili Peppers and Products. Molecules 2023, 28, 2362. Available online: https://www.mdpi.com/1420-3049/28/5/2362 (accessed on 11 January 2025). [CrossRef] [PubMed]
- Hara, R.; Ishigaki, M.; Ozaki, Y.; Ahamed, T.; Noguchi, R.; Miyamoto, A.; Genkawa, T. Effect of Raman exposure time on the quantitative and discriminant analyses of carotenoid concentrations in intact tomatoes. Food Chem. 2021, 360, 129896. Available online: https://linkinghub.elsevier.com/retrieve/pii/S030881462100902X (accessed on 15 December 2024). [CrossRef]
- Wang, X.; Zhang, X.; Hong, H.; Guan, C.; Zhao, C. Non-destructive quantitative analysis of carotene content in carrots using Raman spectroscopy. Eur. Food Res. Technol. 2021, 247, 2299–2307. Available online: https://link.springer.com/10.1007/s00217-021-03788-w (accessed on 5 December 2024). [CrossRef]
- Takemura, M.; Sahara, T.; Misawa, N. Violaxanthin: Natural function and occurrence, biosynthesis, and heterologous production. Appl. Microbiol. Biotechnol. 2021, 105, 6133–6142. Available online: https://link.springer.com/10.1007/s00253-021-11452-2 (accessed on 20 January 2025). [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. Available online: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.13625 (accessed on 5 December 2024). [CrossRef]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0271531714001171 (accessed on 29 January 2025). [CrossRef]
- Monago-Maraña, O.; Eskildsen, C.E.; Afseth, N.K.; Galeano-Díaz, T.; Muñoz de la Peña, A.; Wold, J.P. Non-destructive Raman spectroscopy as a tool for measuring ASTA color values and Sudan I content in paprika powder. Food Chem. 2019, 274, 187–193. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814618315437 (accessed on 15 December 2024). [CrossRef]
- Stra, A.; Almarwaey, L.O.; Alagoz, Y.; Moreno, J.C.; Al-Babili, S. Carotenoid metabolism: New insights and synthetic approaches. Front. Plant Sci. 2023, 13, 1072061. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2022.1072061/full (accessed on 20 January 2025). [CrossRef] [PubMed]
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0001868619302738 (accessed on 20 January 2025). [CrossRef] [PubMed]
- Alqarni, M.H.; Alam, P.; Alam, A.; Ali, A.; Foudah, A.I.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. A Greener HPTLC Approach for the Determination of β-Carotene in Traditional and Ultrasound-Based Extracts of Different Fractions of Daucus carota (L.), Ipomea batatas (L.), and Commercial Formulation. Agronomy 2021, 11, 2443. Available online: https://www.mdpi.com/2073-4395/11/12/2443 (accessed on 22 December 2024). [CrossRef]
- LaFountain, A.M.; Pacheco, C.; Prum, R.O.; Frank, H.A. Nuclear magnetic resonance analysis of carotenoids from the burgundy plumage of the Pompadour Cotinga (Xipholena punicea). Arch. Biochem. Biophys. 2013, 539, 133–141. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0003986113002555 (accessed on 20 January 2025). [CrossRef] [PubMed]
- López, G.-D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. Available online: https://www.tandfonline.com/doi/full/10.1080/10408347.2021.2016366 (accessed on 5 December 2024). [CrossRef]
- Maia, L.F.; Fernandes, R.F.; Lobo-Hajdu, G.; de Oliveira, L.F.C. Conjugated polyenes as chemical probes of life signature: Use of Raman spectroscopy to differentiate polyenic pigments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20140200. Available online: https://royalsocietypublishing.org/doi/10.1098/rsta.2014.0200 (accessed on 20 January 2025). [CrossRef] [PubMed][Green Version]
- Rodriguez-Amaya, D. Food Carotenoids; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Pirutin, S.K.; Jia, S.; Yusipovich, A.I.; Shank, M.A.; Parshina, E.Y.; Rubin, A.B. Vibrational Spectroscopy as a Tool for Bioanalytical and Biomonitoring Studies. Int. J. Mol. Sci. 2023, 24, 6947. Available online: https://www.mdpi.com/1422-0067/24/8/6947 (accessed on 20 January 2025). [CrossRef] [PubMed]
- Jones, R.R.; Hooper, D.C.; Zhang, L.; Wolverson, D.; Valev, V.K. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res. Lett. 2019, 14, 231. Available online: https://link.springer.com/10.1186/s11671-019-3039-2 (accessed on 15 January 2025). [CrossRef]
- Jin, H.; Lu, Q.; Chen, X.; Ding, H.; Gao, H.; Jin, S. The use of Raman spectroscopy in food processes: A review. Appl. Spectrosc. Rev. 2016, 51, 12–22. Available online: http://www.tandfonline.com/doi/full/10.1080/05704928.2015.1087404 (accessed on 20 January 2025). [CrossRef]
- Li, N.; Hussain, N.; Ding, Z.; Qu, C.; Li, Y.; Chu, L.; Liu, H. Guidelines for Raman spectroscopy and imaging techniques in food safety analysis. Food Saf. Heal. 2024, 2, 221–237. Available online: https://onlinelibrary.wiley.com/doi/10.1002/fsh3.12040 (accessed on 5 December 2024). [CrossRef]
- Park, M.; Somborn, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Raman spectroscopy in crop quality assessment: Focusing on sensing secondary metabolites: A review. Hortic. Res. 2023, 10, uhad074. Available online: https://academic.oup.com/hr/article/doi/10.1093/hr/uhad074/7128290 (accessed on 20 January 2025). [CrossRef] [PubMed]
- Rimai, L.; Heyde, M.E.; Gill, D. Vibrational spectra of some carotenoids and related linear polyenes. Raman spectroscopic study. J. Am. Chem. Soc. 1973, 95, 4493–4501. Available online: https://pubs.acs.org/doi/abs/10.1021/ja00795a005 (accessed on 20 January 2025). [CrossRef] [PubMed]
- Dhanani, T.; Dou, T.; Biradar, K.; Jifon, J.; Kurouski, D.; Patil, B.S. Raman Spectroscopy Detects Changes in Carotenoids on the Surface of Watermelon Fruits During Maturation. Front. Plant Sci. 2022, 13, 832522. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2022.832522/full (accessed on 5 December 2024). [CrossRef]
- Campos, M.T.C.; Maia, L.F.; Dos Santos, H.F.; Edwards, H.G.M.; de Oliveira, L.F.C. Revealing the chemical synergism in coloring tomatoes by Raman spectroscopy. J. Raman Spectrosc. 2023, 54, 1314–1326. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.6471 (accessed on 10 January 2025). [CrossRef]
- Legner, R.; Voigt, M.; Servatius, C.; Klein, J.; Hambitzer, A.; Jaeger, M. A Four-Level Maturity Index for Hot Peppers (Capsicum annum) Using Non-Invasive Automated Mobile Raman Spectroscopy for On-Site Testing. Appl. Sci. 2021, 11, 1614. Available online: https://www.mdpi.com/2076-3417/11/4/1614 (accessed on 12 December 2024). [CrossRef]
- Guo, S.; Ryabchykov, O.; Ali, N.; Houhou, R.; Bocklitz, T. Comprehensive Chemometrics. In Comprehensive Chemometrics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–359. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780124095472146001 (accessed on 10 January 2025).
- Ryabchykov, O.; Schie, I.; Popp, J.; Bocklitz, T. Errors and Mistakes to Avoid when Analyzing Raman Spectra. Spectroscopy 2022, 37, 48–50. Available online: https://www.spectroscopyonline.com/view/errors-and-mistakes-to-avoid-when-analyzing-raman-spectra (accessed on 5 December 2024). [CrossRef]
- Gill, D.; Kilponen, R.G.; Rimai, L. Resonance Raman Scattering of Laser Radiation by Vibrational Modes of Carotenoid Pigment Molecules in Intact Plant Tissues. Nature 1970, 227, 743–744. Available online: https://www.nature.com/articles/227743a0 (accessed on 20 January 2025). [CrossRef]
- Rimai, L.; Kilponen, R.G.; Gill, D. Excitation profiles of laser Raman spectra in the resonance region of two carotenoid pigments in solution. J. Am. Chem. Soc. 1970, 92, 3824–3825. Available online: https://pubs.acs.org/doi/abs/10.1021/ja00715a066 (accessed on 12 January 2025). [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of Lycopene and β-Carotene Content in Tomato Fruits and Related Products: Comparison of FT-Raman, ATR-IR, and NIR Spectroscopy. Anal. Chem. 2006, 78, 8456–8461. Available online: https://pubs.acs.org/doi/10.1021/ac061220j (accessed on 20 January 2025). [CrossRef]
- Baranski, R.; Baranska, M.; Schulz, H. Changes in carotenoid content and distribution in living plant tissue can be observed and mapped in situ using NIR-FT-Raman spectroscopy. Planta 2005, 222, 448–457. Available online: http://link.springer.com/10.1007/s00425-005-1566-9 (accessed on 20 January 2025). [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. Available online: https://onlinelibrary.wiley.com/doi/10.1002/bip.20215 (accessed on 13 December 2024). [CrossRef] [PubMed]
- Novikov, V.S.; Kuzmin, V.V.; Darvin, M.E.; Lademann, J.; Sagitova, E.A.; Prokhorov, K.A.; Ustynyuk, L.Y.; Nikolaeva, G.Y. Relations between the Raman spectra and molecular structure of selected carotenoids: DFT study of α-carotene, β-carotene, γ-carotene and lycopene. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 270, 120755. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1386142521013329 (accessed on 15 December 2024). [CrossRef]
- Vítek, P.; Veselá, B.; Klem, K. Spatial and Temporal Variability of Plant Leaf Responses Cascade after PSII Inhibition: Raman, Chlorophyll Fluorescence and Infrared Thermal Imaging. Sensors 2020, 20, 1015. Available online: https://www.mdpi.com/1424-8220/20/4/1015 (accessed on 5 December 2024). [CrossRef] [PubMed]
- Liedtke, I.; Diehn, S.; Heiner, Z.; Seifert, S.; Obenaus, S.; Büttner, C.; Kneipp, J. Multivariate Raman mapping for phenotypic characterization in plant tissue sections. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 251, 119418. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1386142520313974 (accessed on 10 January 2025). [CrossRef] [PubMed]
- Weng, S.; Hu, X.; Wang, J.; Tang, L.; Li, P.; Zheng, S.; Zheng, L.; Huang, L.; Xin, Z. Advanced Application of Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy in Plant Disease Diagnostics: A Review. J. Agric. Food Chem. 2021, 69, 2950–2964. Available online: https://pubs.acs.org/doi/10.1021/acs.jafc.0c07205 (accessed on 15 January 2025). [CrossRef] [PubMed]
- Lahr, R.H.; Vikesland, P.J. Surface-Enhanced Raman Spectroscopy (SERS) Cellular Imaging of Intracellulary Biosynthesized Gold Nanoparticles. ACS Sustain. Chem. Eng. 2014, 2, 1599–1608. Available online: https://pubs.acs.org/doi/10.1021/sc500105n (accessed on 5 December 2024). [CrossRef]
- Zhou, H.; Kneipp, J. Potential Regulation for Surface-Enhanced Raman Scattering Detection and Identification of Carotenoids. Anal. Chem. 2023, 95, 3363–3370. Available online: https://pubs.acs.org/doi/10.1021/acs.analchem.2c04658 (accessed on 12 January 2025). [CrossRef] [PubMed]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Akpolat, M.Z.; Francis, D.M.; Chen, Y.-J.; Rodriguez-Saona, L.E. High-Throughput Phenotyping Approach for Screening Major Carotenoids of Tomato by Handheld Raman Spectroscopy Using Chemometric Methods. Sensors 2020, 20, 3723. Available online: https://www.mdpi.com/1424-8220/20/13/3723 (accessed on 15 January 2025). [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Aćić, S.; Stevanović, Z.D. Raman spectroscopic-based chemometric modeling in assessment of red pepper ripening phases and carotenoids accumulation. J. Raman Spectrosc. 2021, 52, 1598–1605. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.6197 (accessed on 5 January 2025). [CrossRef]
- Farber, C.; Bryan, R.; Paetzold, L.; Rush, C.; Kurouski, D. Non-Invasive Characterization of Single-, Double- and Triple-Viral Diseases of Wheat with a Hand-Held Raman Spectrometer. Front. Plant Sci. 2020, 11, 01300. Available online: https://www.frontiersin.org/article/10.3389/fpls.2020.01300/full (accessed on 15 December 2024). [CrossRef]
- Portarena, S.; Anselmi, C.; Leonardi, L.; Proietti, S.; Bizzarri, A.R.; Brugnoli, E.; Baldacchini, C. Lutein/β-carotene ratio in extra virgin olive oil: An easy and rapid quantification method by Raman spectroscopy. Food Chem. 2023, 404, 134748. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814622027108 (accessed on 12 December 2024). [CrossRef] [PubMed]
- Sebben, J.A.; da Silveira Espindola, J.; Ranzan, L.; Fernandes de Moura, N.; Trierweiler, L.F.; Trierweiler, J.O. Development of a quantitative approach using Raman spectroscopy for carotenoids determination in processed sweet potato. Food Chem. 2018, 245, 1224–1231. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814617319052 (accessed on 15 January 2025). [CrossRef] [PubMed]
- Carvalho, D.G.; Sebben, J.A.; de Moura, N.F.; Trierweiler, J.O.; da Silveira Espindola, J. Raman spectroscopy for monitoring carotenoids in processed Bunchosia glandulifera pulps. Food Chem. 2019, 294, 565–571. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814619307927 (accessed on 12 December 2024). [CrossRef] [PubMed]
- Krähmer, A.; Böttcher, C.; Rode, A.; Nothnagel, T.; Schulz, H. Quantifying biochemical quality parameters in carrots (Daucus carota L.)—FT-Raman spectroscopy as efficient tool for rapid metabolite profiling. Food Chem. 2016, 212, 495–502. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814616308676 (accessed on 11 January 2025). [CrossRef] [PubMed]
- Bocklitz, T.; Walter, A.; Hartmann, K.; Rösch, P.; Popp, J. How to pre-process Raman spectra for reliable and stable models? Anal. Chim. Acta 2011, 704, 47–56. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0003267011008749 (accessed on 5 December 2024). [CrossRef]
- Liland, K.H.; Rukke, E.-O.; Olsen, E.F.; Isaksson, T. Customized baseline correction. Chemom. Intell. Lab. Syst. 2011, 109, 51–56. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0169743911001535 (accessed on 14 December 2024). [CrossRef]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 8. Available online: http://www.epjtechniquesandinstrumentation.com/content/2/1/8 (accessed on 5 December 2024). [CrossRef]
- Schulze, H.G.; Rangan, S.; Blades, M.W.; Piret, J.M.; Turner, R.F.B. Smoothing Raman Spectra with Contiguous Single-Channel Fitting of Voigt Distributions: An Automated, High-Quality Procedure. Appl. Spectrosc. 2019, 73, 47–58. [Google Scholar] [CrossRef]
- Xu, Y.; Goodacre, R. On Splitting Training and Validation Set: A Comparative Study of Cross-Validation, Bootstrap and Systematic Sampling for Estimating the Generalization Performance of Supervised Learning. J. Anal. Test. 2018, 2, 249–262. Available online: http://link.springer.com/10.1007/s41664-018-0068-2 (accessed on 5 December 2024). [CrossRef]
- Forrest, G.; Vilcins, G. Determination of tobacco carotenoids by resonance Raman spectroscopy. J. Agric. Food Chem. 1979, 27, 609–612. Available online: https://pubs.acs.org/doi/abs/10.1021/jf60223a046 (accessed on 5 December 2024). [CrossRef]
- Bhosale, P.; Ermakov, I.V.; Ermakova, M.R.; Gellermann, W.; Bernstein, P.S. Resonance Raman Quantification of Nutritionally Important Carotenoids in Fruits, Vegetables, and Their Juices in Comparison to High-Pressure Liquid Chromatography Analysis. J. Agric. Food Chem. 2004, 52, 3281–3285. Available online: https://pubs.acs.org/doi/10.1021/jf035345q (accessed on 5 December 2024). [CrossRef] [PubMed]
- Killeen, D.P.; Sansom, C.E.; Lill, R.E.; Eason, J.R.; Gordon, K.C.; Perry, N.B. Quantitative Raman Spectroscopy for the Analysis of Carrot Bioactives. J. Agric. Food Chem. 2013, 61, 2701–2708. Available online: https://pubs.acs.org/doi/10.1021/jf3053669 (accessed on 15 January 2025). [CrossRef] [PubMed]
- Creasey, D.; Akkus, O. 1064-nm Raman: The Right Choice for Biological Samples? Spectroscopy 2017, 32, 46–54. Available online: https://www.spectroscopyonline.com/view/1064-nm-raman-right-choice-biological-samples (accessed on 15 December 2024).
- Lu, L.; Shi, L.; Secor, J.; Alfano, R. Resonance Raman scattering of β-carotene solution excited by visible laser beams into second singlet state. J. Photochem. Photobiol. B Biol. 2018, 179, 18–22. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1011134417309818 (accessed on 10 January 2025). [CrossRef] [PubMed]
- Instruments, E. How to Choose Your Lasers for Raman Spectroscopy. Available online: https://www.edinst.com/blog/lasers-for-raman-spectroscopy (accessed on 11 January 2025).
- Wei, D.; Chen, S.; Liu, Q. Review of Fluorescence Suppression Techniques in Raman Spectroscopy. Appl. Spectrosc. Rev. 2015, 50, 387–406. Available online: http://www.tandfonline.com/doi/abs/10.1080/05704928.2014.999936 (accessed on 29 January 2025). [CrossRef]
- Adar, F. Considerations of Grating Selection in Optimizing a Raman Specrograph. Spectroscopy 2013, 28, 1–17. Available online: https://www.spectroscopyonline.com/view/considerations-grating-selection-optimizing-raman-spectrograph (accessed on 11 January 2025).
- Dubessy, J.; Caumon, M.-C.; Rull, F.; Sharma, S. Instrumentation in Raman spectroscopy. In Raman Spectroscopy Applied to EARTH Sciences and Cultural Heritage; European Mineralogical Union: Caen, France, 2012; pp. 83–172. Available online: https://pubs.geoscienceworld.org/books/book/948/chapter/106822777/ (accessed on 11 January 2025).
- Vuković, S.; Moravčević, Đ.; Gvozdanović-Varga, J.; Kostić, A.Ž.; Vujošević, A.; Kilibarda, S.; Pećinar, I. Raman Spectroscopy as a Useful Tool for Tentative Identification of Nutritional Ingredients and Distinction of Allium Species. Biol. Life Sci. Forum 2022, 16, 21. Available online: https://www.mdpi.com/2673-9976/16/1/21 (accessed on 15 January 2025). [CrossRef]
- Camerlingo, C.; Zenone, F.; Delfino, I.; Diano, N.; Mita, D.; Lepore, M. Investigation on Clarified Fruit Juice Composition by Using Visible Light Micro-Raman Spectroscopy. Sensors 2007, 7, 2049–2061. Available online: https://www.mdpi.com/1424-8220/7/10/2049 (accessed on 25 January 2025). [CrossRef]
- de Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveira, L.F.C. Carotenes and carotenoids in natural biological samples: A Raman spectroscopic analysis. J. Raman Spectrosc. 2010, 41, 642–650. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.2493 (accessed on 15 January 2025). [CrossRef]
- Sanchez, L.; Ermolenkov, A.; Biswas, S.; Septiningsih, E.M.; Kurouski, D. Raman Spectroscopy Enables Non-invasive and Confirmatory Diagnostics of Salinity Stresses, Nitrogen, Phosphorus, and Potassium Deficiencies in Rice. Front. Plant Sci. 2020, 11, 573321. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2020.573321/full (accessed on 29 January 2025). [CrossRef]
- Rygula, A.; Oleszkiewicz, T.; Grzebelus, E.; Pacia, M.Z.; Baranska, M.; Baranski, R. Raman, AFM and SNOM high resolution imaging of carotene crystals in a model carrot cell system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 47–55. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1386142518300787 (accessed on 15 January 2025). [CrossRef] [PubMed]
- Badgujar, P.M.; Wang, Y.; Cheng, C. A light-mediated study of carotenoids in carrots (Daucus carota) using resonance Raman spectroscopy. J. Raman Spectrosc. 2021, 52, 2609–2620. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.6176 (accessed on 15 January 2025). [CrossRef]
- Lawaetz, A.J.; Christensen, S.M.U.; Clausen, S.K.; Jørnsgaard, B.; Rasmussen, S.K.; Andersen, S.B.; Rinnan, Å. Fast, cross cultivar determination of total carotenoids in intact carrot tissue by Raman spectroscopy and Partial Least Squares calibration. Food Chem. 2016, 204, 7–13. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814616302771 (accessed on 29 January 2025). [CrossRef] [PubMed]
- Nekvapil, F.; Brezestean, I.; Barchewitz, D.; Glamuzina, B.; Chiş, V.; Cintă Pinzaru, S. Citrus fruits freshness assessment using Raman spectroscopy. Food Chem. 2018, 242, 560–567. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814617315820 (accessed on 15 December 2024). [CrossRef] [PubMed]
- Krimmer, M.; Farber, C.; Kurouski, D. Rapid and Noninvasive Typing and Assessment of Nutrient Content of Maize Kernels Using a Handheld Raman Spectrometer. ACS Omega 2019, 4, 16330–16335. Available online: https://pubs.acs.org/doi/10.1021/acsomega.9b01661 (accessed on 11 January 2025). [CrossRef]
- Gehse, S.; Knorr, F.; Patzelt, A.; Zastrow, L.; Meinke, M.C.; Lademann, J.; Darvin, M.E. Determination of the effect of boiling on the bioavailability of carotenoids in vegetables using resonance Raman spectroscopy. Laser Phys. 2018, 28, 105602. Available online: https://iopscience.iop.org/article/10.1088/1555-6611/aad1b4 (accessed on 15 December 2024). [CrossRef]
- Pećinar, I.; Krstić, D.; Caruso, G.; Popović-Djordjević, J.B. Rapid characterization of hypanthium and seed in wild and cultivated rosehip: Application of Raman microscopy combined with multivariate analysis. R. Soc. Open Sci. 2021, 8, rsos.202064. Available online: https://royalsocietypublishing.org/doi/10.1098/rsos.202064 (accessed on 13 December 2024). [CrossRef]
- Feng, X.; Zhang, Q.; Zhu, Z. Rapid Classification of Citrus Fruits Based on Raman Spectroscopy and Pattern Recognition Techniques. Food Sci. Technol. Res. 2013, 19, 1077–1084. Available online: https://www.jstage.jst.go.jp/article/fstr/19/6/19_1077/_article (accessed on 11 January 2025). [CrossRef]
- Raj, T.; Hashim, F.H.; Huddin, A.B.; Hussain, A.; Ibrahim, M.F.; Abdul, P.M. Classification of oil palm fresh fruit maturity based on carotene content from Raman spectra. Sci. Rep. 2021, 11, 18315. Available online: https://www.nature.com/articles/s41598-021-97857-5 (accessed on 15 January 2025). [CrossRef]
- Farber, C.; Sanchez, L.; Rizevsky, S.; Ermolenkov, A.; McCutchen, B.; Cason, J.; Simpson, C.; Burow, M.; Kurouski, D. Raman Spectroscopy Enables Non-Invasive Identification of Peanut Genotypes and Value-Added Traits. Sci. Rep. 2020, 10, 7730. Available online: https://www.nature.com/articles/s41598-020-64730-w (accessed on 12 December 2024). [CrossRef]
- Kolašinac, S.; Pećinar, I.; Danojević, D.; Stevanović, Z.D. Raman spectroscopy coupled with chemometric modeling approaches for authentication of different paprika varieties at physiological maturity. LWT 2022, 162, 113402. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643822003371 (accessed on 5 December 2024). [CrossRef]
- Morey, R.; Ermolenkov, A.; Payne, W.Z.; Scheuring, D.C.; Koym, J.W.; Vales, M.I.; Kurouski, D. Non-invasive identification of potato varieties and prediction of the origin of tuber cultivation using spatially offset Raman spectroscopy. Anal. Bioanal. Chem. 2020, 412, 4585–4594. Available online: https://link.springer.com/10.1007/s00216-020-02706-5 (accessed on 12 December 2024). [CrossRef]
- Zeng, J.; Ping, W.; Sanaeifar, A.; Xu, X.; Luo, W.; Sha, J.; Huang, Z.; Huang, Y.; Liu, X.; Zhan, B.; et al. Quantitative visualization of photosynthetic pigments in tea leaves based on Raman spectroscopy and calibration model transfer. Plant Methods 2021, 17, 4. Available online: https://plantmethods.biomedcentral.com/articles/10.1186/s13007-020-00704-3 (accessed on 22 December 2024). [CrossRef] [PubMed]
- Petrović, I.; Marjanović, M.; Pećinar, I.; Savić, S.; Jovanović, Z.; Stikić, R. Chemical Characterization of Different Colored Tomatoes: Application of Biochemical and Spectroscopic Tools. Biol. Life Sci. Forum 2022, 16, 32. Available online: https://www.mdpi.com/2673-9976/16/1/32 (accessed on 29 January 2025). [CrossRef]
- Al-Attili, M.; Ferreira, C.; Price, C.; Faulds, K.; Chen, Y.-C. Development of a spatially offset Raman spectroscopy probe for monitoring pharmaceutical drying. Chem. Eng. Res. Des. 2023, 192, 510–520. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0263876223001296 (accessed on 5 December 2024). [CrossRef]
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568. Available online: https://xlink.rsc.org/?DOI=D0CS00855A (accessed on 11 January 2025). [CrossRef]
- Arroyo-Cerezo, A.; Jimenez-Carvelo, A.M.; González-Casado, A.; Koidis, A.; Cuadros-Rodríguez, L. Deep (offset) non-invasive Raman spectroscopy for the evaluation of food and beverages—A review. Lebensm. Wiss. Technol. 2021, 149, 111822. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643821009750 (accessed on 15 December 2024). [CrossRef]
- Hargreaves, M. Handheld Raman, SERS, and SORS. In Portable Spectroscopy and Spectrometry; Wiley: Chichester, UK, 2021; pp. 347–376. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119636489.ch38 (accessed on 15 December 2024).
- Mosca, S.; Conti, C.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy. Nat. Rev. Methods Prim. 2021, 1, 21. Available online: https://www.nature.com/articles/s43586-021-00019-0 (accessed on 15 January 2025). [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S. Investigation of Raman chemical imaging for detection of lycopene changes in tomatoes during postharvest ripening. J. Food Eng. 2011, 107, 277–288. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0260877411003979 (accessed on 15 December 2024). [CrossRef]
- Trebolazabala, J.; Maguregui, M.; Morillas, H.; de Diego, A.; Madariaga, J.M. Portable Raman spectroscopy for an in-situ monitoring the ripening of tomato (Solanum lycopersicum) fruits. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 138–143. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1386142517301920 (accessed on 11 January 2025). [CrossRef]
- Cicerone, M. Molecular imaging with CARS micro-spectroscopy. Curr. Opin. Chem. Biol. 2016, 33, 179–185. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1367593116300503 (accessed on 15 December 2024). [CrossRef] [PubMed]
- Brackmann, C.; Bengtsson, A.; Alminger, M.L.; Svanberg, U.; Enejder, A. Visualization of β-carotene and starch granules in plant cells using CARS and SHG microscopy. J. Raman Spectrosc. 2011, 42, 586–592. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.2778 (accessed on 29 January 2025). [CrossRef]
- Dementjev, A.; Kostkevičiene, J. Applying the method of Coherent Anti-stokes Raman microscopy for imaging of carotenoids in microalgae and cyanobacteria. J. Raman Spectrosc. 2013, 44, 973–979. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jrs.4321 (accessed on 15 January 2025). [CrossRef]
- Chen, K.; Wu, T.; Chen, T.; Wei, H.; Yang, H.; Zhou, T.; Li, Y. Spectral focusing dual-comb coherent anti-Stokes Raman spectroscopic imaging. Opt. Lett. 2017, 42, 3634. Available online: https://opg.optica.org/abstract.cfm?URI=ol-42-18-3634 (accessed on 29 January 2025). [CrossRef] [PubMed]
- CARS and SRS Raman Microscopy. Available online: https://www.newport.com/n/cars-srs?srsltid=AfmBOorwPxyeDgS0evHsc7Ok--AWGn5ArT_Y6xeKq8HINWmkrQ1eaX6R (accessed on 29 January 2025).
- Cheng, J.-X.; Xie, X.S. Vibrational spectroscopic imaging of living systems: An emerging platform for biology and medicine. Science 2015, 350, aaa8870. Available online: https://www.science.org/doi/10.1126/science.aaa8870 (accessed on 29 January 2025). [CrossRef]
- Mansfield, J.C.; Littlejohn, G.R.; Seymour, M.P.; Lind, R.J.; Perfect, S.; Moger, J. Label-free Chemically Specific Imaging in Planta with Stimulated Raman Scattering Microscopy. Anal. Chem. 2013, 85, 5055–5063. Available online: https://pubs.acs.org/doi/10.1021/ac400266a (accessed on 15 January 2025). [CrossRef] [PubMed]
- Takaya, T.; Iwata, K. Relaxation Mechanism of β-Carotene from S2 (1Bu+) State to S1 (2Ag−) State: Femtosecond Time-Resolved Near-IR Absorption and Stimulated Resonance Raman Studies in 900–1550 nm Region. J. Phys. Chem. A 2014, 118, 4071–4078. Available online: https://pubs.acs.org/doi/10.1021/jp504272h (accessed on 11 January 2025). [CrossRef]
- Dobrowolski, J.C. Vibrational Spectroscopy as a Tool to Investigate Carotenoids. In Carotenoids; Wiley: Chichester, UK, 2016; pp. 75–102. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118622223.ch6 (accessed on 15 January 2025).
- Antonio, K.A.; Schultz, Z.D. Advances in Biomedical Raman Microscopy. Anal. Chem. 2014, 86, 30–46. Available online: https://pubs.acs.org/doi/10.1021/ac403640f (accessed on 15 January 2025). [CrossRef]
- Pan, L.; Pipitsunthonsan, P.; Zhang, P.; Daengngam, C.; Booranawong, A.; Chongcheawchamnan, M. Noise Reduction Technique for Raman Spectrum using Deep Learning Network. In Proceedings of the 2020 13th International Symposium on Computational Intelligence and Design (ISCID), Hangzhou, China, 12–13 December 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 159–163. Available online: https://ieeexplore.ieee.org/document/9325766/ (accessed on 5 December 2024).
- Cooman, T.; Trejos, T.; Romero, A.H.; Arroyo, L.E. Implementing machine learning for the identification and classification of compound and mixtures in portable Raman instruments. Chem. Phys. Lett. 2022, 787, 139283. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0009261421009660 (accessed on 5 December 2024). [CrossRef]
- Pan, L.; Pipitsunthonsan, P.; Daengngam, C.; Channumsin, S.; Sreesawet, S.; Chongcheawchamnan, M. Identification of Complex Mixtures for Raman Spectroscopy Using a Novel Scheme Based on a New Multi-Label Deep Neural Network. IEEE Sens. J. 2021, 21, 10834–10843. Available online: https://ieeexplore.ieee.org/document/9355178/ (accessed on 11 January 2025). [CrossRef]
- Liu, J.; Osadchy, M.; Ashton, L.; Foster, M.; Solomon, C.J.; Gibson, S.J. Deep convolutional neural networks for Raman spectrum recognition: A unified solution. Analyst 2017, 142, 4067–4074. Available online: https://xlink.rsc.org/?DOI=C7AN01371J (accessed on 15 December 2024). [CrossRef] [PubMed]
- Xu, Y.; Deng, S.; Li, X.; He, Y. A sparse unmixing model based on NMF and its application in Raman image. Neurocomputing 2016, 207, 120–130. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0925231216302922 (accessed on 25 December 2024). [CrossRef]
- Luce, R.; Hildebrandt, P.; Kuhlmann, U.; Liesen, J. Using Separable Nonnegative Matrix Factorization Techniques for the Analysis of Time-Resolved Raman Spectra. Appl. Spectrosc. 2016, 70, 1464–1475. Available online: https://journals.sagepub.com/doi/10.1177/0003702816662600 (accessed on 15 December 2024). [CrossRef] [PubMed]
- Szymańska-Chargot, M.; Pieczywek, P.M.; Chylińska, M.; Zdunek, A. Hyperspectral image analysis of Raman maps of plant cell walls for blind spectra characterization by nonnegative matrix factorization algorithm. Chemom. Intell. Lab. Syst. 2016, 151, 136–145. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0169743915003305 (accessed on 13 January 2025). [CrossRef]
- Yaghoobi, M.; Wu, D.; Clewes, R.J.; Davies, M.E. Fast sparse Raman spectral unmixing for chemical fingerprinting and quantification. Opt. Photonics Counterterrorism Crime Fight. Def. XII 2016, 9995, 99–109. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/9995/2241834/Fast-sparse-Raman-spectral-unmixing-for-chemical-fingerprinting-and-quantification/10.1117/12.2241834.full (accessed on 11 January 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolašinac, S.M.; Pećinar, I.; Gajić, R.; Mutavdžić, D.; Dajić Stevanović, Z.P. Raman Spectroscopy in the Characterization of Food Carotenoids: Challenges and Prospects. Foods 2025, 14, 953. https://doi.org/10.3390/foods14060953
Kolašinac SM, Pećinar I, Gajić R, Mutavdžić D, Dajić Stevanović ZP. Raman Spectroscopy in the Characterization of Food Carotenoids: Challenges and Prospects. Foods. 2025; 14(6):953. https://doi.org/10.3390/foods14060953
Chicago/Turabian StyleKolašinac, Stefan M., Ilinka Pećinar, Radoš Gajić, Dragosav Mutavdžić, and Zora P. Dajić Stevanović. 2025. "Raman Spectroscopy in the Characterization of Food Carotenoids: Challenges and Prospects" Foods 14, no. 6: 953. https://doi.org/10.3390/foods14060953
APA StyleKolašinac, S. M., Pećinar, I., Gajić, R., Mutavdžić, D., & Dajić Stevanović, Z. P. (2025). Raman Spectroscopy in the Characterization of Food Carotenoids: Challenges and Prospects. Foods, 14(6), 953. https://doi.org/10.3390/foods14060953







