Celiac Disease—Narrative Review on Progress in Celiac Disease
Abstract
:1. Introduction
1.1. Classification
1.2. Epidemiology
1.3. Etiopathogenesis
1.4. Diagnosis
1.5. New Diagnostic Techniques for Celiac Disease
1.6. Treatment
2. New Potential Treatment Strategies
2.1. The Use of Bacteria in the Treatment of Celiac Disease
2.2. Oral Supplementation of Endopeptidases
2.3. Modification of Immune Response
2.4. Zonulin Inhibitors
2.5. Tissue Transglutaminase 2 Inhibitors
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Garampazzi, A.; Rapa, A.; Mura, S.; Capelli, A.; Valori, A.; Boldorini, R.; Oderda, G. Clinical pattern of celiac disease is still changing. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Dinler, G.; Atalay, E.; Kalayci, A.G. Celiac disease in 87 children with typical and atypical symptoms in Black Sea region of Turkey. World J. Pediatr. 2009, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, G.; Failla, P.; Rotolo, N.; Sanfilippo, G.; Azzaro, F.; Spina, M.; Patane, R. Changes in coeliac disease behaviour over the years. Acta Paediatr. 1993, 82, 566–568. [Google Scholar] [CrossRef]
- Faulkner-Hogg, K.B.; Selby, W.S.; Loblay, R.H. Dietary analysis in symptomatic patients with coeliac disease on a gluten-free diet: The role of trace amounts of gluten and non-gluten food intolerances. Scand. J. Gastroenterol. 1999, 34, 784–789. [Google Scholar] [CrossRef]
- Marek, K.; Kowalski, A.G. Czy choroba trzewna predysponuje do rozwoju chorób nowotworowych? Onkol. Prakt. Klin. 2015, 2, 140–148. [Google Scholar]
- Schosler, L.; Christensen, L.A.; Hvas, C.L. Symptoms and findings in adult-onset celiac disease in a historical Danish patient cohort. Scand. J. Gastroenterol. 2016, 51, 288–294. [Google Scholar] [CrossRef]
- Zingone, F.; Bai, J.C.; Cellier, C.; Ludvigsson, J.F. Celiac Disease-Related Conditions: Who to Test? Gastroenterology 2024, 167, 64–78. [Google Scholar] [CrossRef]
- Hansen, S.; Osler, M.; Thysen, S.M.; Rumessen, J.J.; Linneberg, A.; Kårhus, L.L. Celiac disease and risk of neuropsychiatric disorders: A nationwide cohort study. Acta Psychiatr. Scand. 2023, 148, 60–70. [Google Scholar] [CrossRef]
- Marsh, M.N. Grains of truth: Evolutionary changes in small intestinal mucosa in response to environmental antigen challenge. Gut 1990, 31, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology 1980, 79, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Caspary, W.F.; Kirchner, T.; Borchard, F.; Stolte, M. Study Group of Gastroenterological Pathology of the German Society of Pathology. Recommendations for celiac disease/sprue diagnosis. Z. Gastroenterol. 2001, 39, 157–166. [Google Scholar] [CrossRef]
- Oberhuber, G. Histopathology of celiac disease. Biomed. Pharmacother. 2000, 54, 368–372. [Google Scholar] [CrossRef]
- Bingley, P.J.; Williams, A.J.; Norcross, A.J.; Unsworth, D.J.; Lock, R.J.; Ness, A.R.; Jones, R.W. Undiagnosed coeliac disease at age seven: Population based prospective birth cohort study. BMJ 2004, 328, 322–323. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Montón Rodríguez, C.; Sánchez Serrano, J.; Poyatos García, P.; Abril García, C.; Gómez Medina, C.; Capilla-Lozano, M.; Lluch Garcia, P.; Pascual Moreno, I. Liver disorders and celiac disease. Rev. Esp. Enferm. Dig. 2024, 116, 41–42. [Google Scholar] [CrossRef]
- Kalayci, A.G.; Kansu, A.; Girgin, N.; Kucuk, O.; Aras, G. Bone mineral density and importance of a gluten-free diet in patients with celiac disease in childhood. Pediatrics 2001, 108, E89. [Google Scholar] [CrossRef]
- Mautalen, C.; Gonzalez, D.; Mazure, R.; Vazquez, H.; Lorenzetti, M.P.; Maurino, E.; Niveloni, S.; Pedreira, S.; Smecuol, E.; Boerr, L.A.; et al. Effect of treatment on bone mass, mineral metabolism, and body composition in untreated celiac disease patients. Am. J. Gastroenterol. 1997, 92, 313–318. [Google Scholar]
- Bai, J.C.; Gonzalez, D.; Mautalen, C.; Mazure, R.; Pedreira, S.; Vazquez, H.; Smecuol, E.; Siccardi, A.; Cataldi, M.; Niveloni, S.; et al. Long-term effect of gluten restriction on bone mineral density of patients with coeliac disease. Aliment. Pharmacol. Ther. 1997, 11, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Maurelli, L.; Klain, M.; Savino, G.; Salvatore, M.; Mazzacca, G.; Cirillo, M. Effects of dietary treatment on bone mineral density in adults with celiac disease: Factors predicting response. Am. J. Gastroenterol. 1997, 92, 992–996. [Google Scholar] [PubMed]
- Zanchetta, M.B.; Longobardi, V.; Costa, F.; Longarini, G.; Mazure, R.M.; Moreno, M.L.; Vazquez, H.; Silveira, F.; Niveloni, S.; Smecuol, E.; et al. Impaired Bone Microarchitecture Improves After One Year On Gluten-Free Diet: A Prospective Longitudinal HRpQCT Study in Women with Celiac Disease. J. Bone Miner. Res. 2016, 32, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Santonicola, A.; Wieser, H.; Gizzi, C.; Soldaini, C.; Ciacci, C. Associations between Celiac Disease, Extra-Gastrointestinal Manifestations, and Gluten-Free Diet: A Narrative Overview. Nutrients 2024, 16, 1814. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Tian, D.; Zhou, J.; Dong, S. Relationship between vitamin D levels and pediatric celiac disease: A systematic review and meta-analysis. BMC Pediatr. 2024, 24, 185. [Google Scholar] [CrossRef]
- Bolland, M.J.; Hofman, P.; Grey, A. Prevalence of Vitamin D Deficiency With Biochemical Abnormalities in Children Undergoing Vitamin D Testing. Clin. Endocrinol. 2024, 102, 255–263. [Google Scholar] [CrossRef]
- Asseri, A.A. Serum Vitamin D Profiles of Children with Asthma in Southwest Saudi: A Comparative Cross-Sectional Study. Int. J. Gen. Med. 2024, 17, 6323–6333. [Google Scholar] [CrossRef]
- Zanchetta, M.B.; Costa, A.F.; Longobardi, V.; Mazure, R.; Silveira, F.; Temprano, M.P.; Vázquez, H.; Bogado, C.; Niveloni, S.I.; Smecuol, E.; et al. Improved Bone Microarchitecture in Patients With Celiac Disease After 3 Years on a Gluten-Free Diet. Clin. Gastroenterol. Hepatol. 2018, 16, 774–775. [Google Scholar] [CrossRef]
- Galli, G.; Carabotti, M.; Conti, L.; Scalamonti, S.; Annibale, B.; Lahner, E. Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up. Nutrients 2023, 15, 2151. [Google Scholar] [CrossRef]
- Ganji, R.; Moghbeli, M.; Sadeghi, R.; Bayat, G.; Ganji, A. Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: A systematic review. Nutr. J. 2019, 18, 9. [Google Scholar] [CrossRef]
- Ganji, A.; Moghbeli, M.; Moradi, Y.; Babaei, N.; Baniasad, A. Bone Loss Correlated with Parathyroid Hormone Levels in Adult Celiac Patients. Middle East J. Dig. Dis. 2022, 14, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.D.; Williams, J.; Lewis, S.K.; Bai, J.C.; Lebwohl, B.; Green, P.H.R. Measurement of Forearm Bone Density by Dual Energy X-Ray Absorptiometry Increases the Prevalence of Osteoporosis in Men With Celiac Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sayar, S.; Aykut, H.; Kaya, Ö.; Kürbüz, K.; Ak, Ç.; Gökçen, P.; Mutlu Bilgiç, N.; Adalı, G.; Kahraman, R.; Doganay, L.; et al. Bone Mineral Density Screening and the Frequency of Osteopenia/Osteoporosis in Turkish Adult Patients With Celiac Disease. Turk. J. Gastroenterol. 2021, 32, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Kinga, S.; Marciniak, M.D.; Michalak, M.; Zawada, A.; Ratajczak-Pawłowska, A.E.; Dobrowolska, A.; Krela-Kaźmierczak, I. The other side of celiac disease—Assessment of bone mineral density and body composition in patients with celiac disease. Gastroenterol. Rev. Przegląd Gastroenterol. 2024, 19, 434–438. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Walters, A.S.; Mullin, G.E.; Duntley, S.P. Celiac disease is associated with restless legs syndrome. Dig. Dis. Sci. 2010, 55, 1667–1673. [Google Scholar] [CrossRef]
- Manchanda, S.; Davies, C.R.; Picchietti, D. Celiac disease as a possible cause for low serum ferritin in patients with restless legs syndrome. Sleep. Med. 2009, 10, 763–765. [Google Scholar] [CrossRef]
- Rodrigo, L.; Hernandez-Lahoz, C.; Lauret, E.; Rodriguez-Pelaez, M.; Soucek, M.; Ciccocioppo, R.; Kruzliak, P. Gluten ataxia is better classified as non-celiac gluten sensitivity than as celiac disease: A comparative clinical study. Immunol. Res. 2016, 64, 558–564. [Google Scholar] [CrossRef]
- Isikay, S.; Hizli, S.; Coskun, S.; Yilmaz, K. Increased tissue transglutaminase levels are associated with increased epileptiform activity in electroencephalography among patients with celiac disease. Arq. Gastroenterol. 2015, 52, 272–277. [Google Scholar] [CrossRef]
- Smith, D.F.; Gerdes, L.U. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr. Scand. 2012, 125, 189–193. [Google Scholar] [CrossRef]
- Chen, S.J.; Chao, Y.L.; Chen, C.Y.; Chang, C.M.; Wu, E.C.; Wu, C.S.; Yeh, H.H.; Chen, C.H.; Tsai, H.J. Prevalence of autoimmune diseases in in-patients with schizophrenia: Nationwide population-based study. Br. J. Psychiatry 2012, 200, 374–380. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Osby, U.; Ekbom, A.; Montgomery, S.M. Coeliac disease and risk of schizophrenia and other psychosis: A general population cohort study. Scand. J. Gastroenterol. 2007, 42, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Garud, S.; Leffler, D.; Dennis, M.; Edwards-George, J.; Saryan, D.; Sheth, S.; Schuppan, D.; Jamma, S.; Kelly, C.P. Interaction between psychiatric and autoimmune disorders in coeliac disease patients in the Northeastern United States. Aliment. Pharmacol. Ther. 2009, 29, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, B.; Verdino, V.; Bossini, L.; Terzuoli, L.; Fagiolini, A. Celiac and non-celiac gluten sensitivity: A review on the association with schizophrenia and mood disorders. Auto. Immun. Highlights 2014, 5, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, M.; Sbravati, F.; Allegri, D.; Labriola, F.; Lombardo, V.; Spisni, E.; Zarbo, C.; Alvisi, P. Is the clinical pattern of pediatric celiac disease changing? A thirty-years real-life experience of an Italian center. Ital. J. Pediatr. 2021, 47, 235. [Google Scholar] [CrossRef]
- Popp, A.; Mäki, M. Changing Pattern of Childhood Celiac Disease Epidemiology: Contributing Factors. Front. Pediatr. 2019, 7, 357. [Google Scholar] [CrossRef]
- Bianchi, P.I.; Lenti, M.V.; Petrucci, C.; Gambini, G.; Aronico, N.; Varallo, M.; Rossi, C.M.; Pozzi, E.; Groppali, E.; Siccardo, F.; et al. Diagnostic Delay of Celiac Disease in Childhood. JAMA Netw. Open 2024, 7, e245671. [Google Scholar] [CrossRef]
- Smith, L.B.; Lynch, K.F.; Kurppa, K.; Koletzko, S.; Krischer, J.; Liu, E.; Johnson, S.B.; Agardh, D. Psychological Manifestations of Celiac Disease Autoimmunity in Young Children. Pediatrics 2017, 139, e20162848. [Google Scholar] [CrossRef]
- Iwańczak, F.; Iwańczak, B. New guidelines for diagnosis and treatment of coeliac disease in children and adolescents. Gastroenterol. Rev. Przegląd Gastroenterol. 2012, 7, 185–191. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Villanacci, V.; Ciacci, C.; Salviato, T.; Leoncini, G.; Bonetti, L.R.; Ragazzini, T.; Limarzi, F.; Saragoni, L. Histopathology of Celiac Disease. Position Statements of the Italian Group of Gastrointestinal Pathologists (GIPAD-SIAPEC). Transl. Med. UniSa 2020, 23, 28–36. [Google Scholar] [CrossRef]
- Green, P.H.R.; Paski, S.; Ko, C.W.; Rubio-Tapia, A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology 2022, 163, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Ondrejka, S.; Jagadeesh, D. Enteropathy-Associated T-Cell Lymphoma. Curr. Hematol. Malig. Rep. 2016, 11, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Olaussen, R.W.; Løvik, A.; Tollefsen, S.; Andresen, P.A.; Vatn, M.H.; De Lange, T.; Bratlie, J.; Brandtzaeg, P.; Farstad, I.N.; Lundin, K.E. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin. Gastroenterol. Hepatol. 2005, 3, 875–885. [Google Scholar] [CrossRef]
- Demiroren, K. Possible relationship between refractory celiac disease and malignancies. World J. Clin. Oncol. 2022, 13, 200–208. [Google Scholar] [CrossRef]
- Daum, S.; Cellier, C.; Mulder, C.J. Refractory coeliac disease. Best. Pract. Res. Clin. Gastroenterol. 2005, 19, 413–424. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.; Bouma, G.; Bontkes, H.J.; Neefjes-Borst, A.; van Grieken, N.C.; von Blomberg, B.M.; Mulder, C.J. Outcome of Referrals for Non-Responsive Celiac Disease in a Tertiary Center: Low Incidence of Refractory Celiac Disease in the Netherlands. Clin. Transl. Gastroenterol. 2017, 8, e218. [Google Scholar] [CrossRef]
- Bai, J.C.; Ciacci, C. World Gastroenterology Organisation Global Guidelines: Celiac Disease February 2017. J. Clin. Gastroenterol. 2017, 51, 755–768. [Google Scholar] [CrossRef]
- Parfenov, A.I.; Bykova, S.V.; Sabel’nikova, E.A.; Maev, I.V.; Baranov, A.A.; Bakulin, I.G.; Krums, L.M.; Bel’mer, S.V.; Borovik, T.E.; Zakharova, I.N.; et al. All-Russian Consensus on Diagnosis and Treatment of Celiac Disease in Children and Adults. Ter. Arkh. 2017, 89, 94–107. [Google Scholar] [CrossRef]
- Downey, L.; Houten, R.; Murch, S.; Longson, D. Recognition, assessment, and management of coeliac disease: Summary of updated NICE guidance. BMJ 2015, 351, h4513. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Bai, J.C.; Biagi, F.; Card, T.R.; Ciacci, C.; Ciclitira, P.J.; Green, P.H.; Hadjivassiliou, M.; Holdoway, A.; van Heel, D.A.; et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014, 63, 1210–1228. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676, quiz 677. [Google Scholar] [CrossRef] [PubMed]
- Remes-Troche, J.M.; Uscanga-Domínguez, L.F.; Aceves-Tavares, R.G.; Calderón de la Barca, A.M.; Carmona-Sánchez, R.I.; Cerda-Contreras, E.; Coss-Adame, E.; Icaza-Chávez, M.E.; Lopéz-Colombo, A.; Milke-García, M.P.; et al. Clinical guidelines on the diagnosis and treatment of celiac disease in Mexico. Rev. Gastroenterol. México 2018, 83, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022, 28, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Maki, M.; Mustalahti, K.; Kokkonen, J.; Kulmala, P.; Haapalahti, M.; Karttunen, T.; Ilonen, J.; Laurila, K.; Dahlbom, I.; Hansson, T.; et al. Prevalence of Celiac disease among children in Finland. N. Engl. J. Med. 2003, 348, 2517–2524. [Google Scholar] [CrossRef]
- Laass, M.W.; Schmitz, R.; Uhlig, H.H.; Zimmer, K.P.; Thamm, M.; Koletzko, S. The prevalence of celiac disease in children and adolescents in Germany. Dtsch. Arztebl. Int. 2015, 112, 553–560. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Singh, A.; Strand, T.A.; Makharia, G.K. Prevalence of Celiac disease in Asia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2015, 31, 1095–1101. [Google Scholar] [CrossRef]
- Choung, R.S.; Ditah, I.C.; Nadeau, A.M.; Rubio-Tapia, A.; Marietta, E.V.; Brantner, T.L.; Camilleri, M.J.; Rajkumar, S.V.; Landgren, O.; Everhart, J.E.; et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: Findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am. J. Gastroenterol. 2015, 110, 455–461. [Google Scholar] [CrossRef]
- Fasano, A.; Berti, I.; Gerarduzzi, T.; Not, T.; Colletti, R.B.; Drago, S.; Elitsur, Y.; Green, P.H.; Guandalini, S.; Hill, I.D.; et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: A large multicenter study. Arch. Intern. Med. 2003, 163, 286–292. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Lal, S.; Strand, T.A.; Makharia, G.K. Risk of Celiac Disease in the First- and Second-Degree Relatives of Patients with Celiac Disease: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 1539–1548. [Google Scholar] [CrossRef]
- Sahin, Y.; Mermer, S. Frequency of celiac disease and distribution of HLA-DQ2/DQ8 haplotypes among siblings of children with celiac disease. World J. Clin. Pediatr. 2022, 11, 351–359. [Google Scholar] [CrossRef]
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: Results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, E.; Gatti, S.; Pulvirenti, A.; Catassi, C. Celiac disease from a global perspective. Best. Pract. Res. Clin. Gastroenterol. 2015, 29, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836.e2. [Google Scholar] [CrossRef] [PubMed]
- El-Metwally, A.; Toivola, P.; AlAhmary, K.; Bahkali, S.; AlKhathaami, A.; AlSaqabi, M.K.; Al Ammar, S.A.; Jawed, M.; Alosaimi, S.M. The Epidemiology of Celiac Disease in the General Population and High-Risk Groups in Arab Countries: A Systematic Review. Biomed. Res. Int. 2020, 2020, 6865917. [Google Scholar] [CrossRef]
- Tamai, T.; Ihara, K. Celiac Disease Genetics, Pathogenesis, and Standard Therapy for Japanese Patients. Int. J. Mol. Sci. 2023, 24, 2075. [Google Scholar] [CrossRef]
- Poddighe, D.; Abdukhakimova, D. Celiac Disease in Asia beyond the Middle East and Indian subcontinent: Epidemiological burden and diagnostic barriers. World J. Gastroenterol. 2021, 27, 2251–2256. [Google Scholar] [CrossRef]
- Ashtari, S.; Najafimehr, H.; Pourhoseingholi, M.A.; Rostami, K.; Asadzadeh-Aghdaei, H.; Rostami-Nejad, M.; Tavirani, M.R.; Olfatifar, M.; Makharia, G.K.; Zali, M.R. Prevalence of celiac disease in low and high risk population in Asia-Pacific region: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 2383. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Card, T.R.; Kaukinen, K.; Bai, J.; Zingone, F.; Sanders, D.S.; Murray, J.A. Screening for celiac disease in the general population and in high-risk groups. United Eur. Gastroenterol. J. 2015, 3, 106–120. [Google Scholar] [CrossRef]
- Parra-Medina, R.; Molano-Gonzalez, N.; Rojas-Villarraga, A.; Agmon-Levin, N.; Arango, M.T.; Shoenfeld, Y.; Anaya, J.M. Prevalence of celiac disease in latin america: A systematic review and meta-regression. PLoS ONE 2015, 10, e0124040. [Google Scholar] [CrossRef]
- Savvateeva, L.V.; Erdes, S.I.; Antishin, A.S.; Zamyatnin, A.A., Jr. Overview of Celiac Disease in Russia: Regional Data and Estimated Prevalence. J. Immunol. Res. 2017, 2017, 2314813. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Liu, X.Y.; Wang, M.M.; Liang, L.P.; Liu, L.; Zheng, K.; Silvester, J.A.; Ma, W.J.; Wu, W.; Ji, G.Y.; et al. Prevalence of celiac disease in China: Meta-analysis and serological survey in high-risk populations. J. Dig. Dis. 2021, 22, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Makharia, G.K.; Verma, A.K.; Amarchand, R.; Bhatnagar, S.; Das, P.; Goswami, A.; Bhatia, V.; Ahuja, V.; Datta Gupta, S.; Anand, K. Prevalence of celiac disease in the northern part of India: A community based study. J. Gastroenterol. Hepatol. 2011, 26, 894–900. [Google Scholar] [CrossRef]
- Chin, M.W.; Mallon, D.F.; Cullen, D.J.; Olynyk, J.K.; Mollison, L.C.; Pearce, C.B. Screening for coeliac disease using anti-tissue transglutaminase antibody assays, and prevalence of the disease in an Australian community. Med. J. Aust. 2009, 190, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.M.; Haghighat, M.; Mobayen, A.; Rezaianzadeh, A.; Geramizadeh, B. Prevalence of celiac disease in healthy Iranian school children. Ann. Saudi Med. 2013, 33, 159–161. [Google Scholar] [CrossRef]
- Al-Ajlan, A.S. Screening of coeliac disease in undetected adults and patients diagnosed with irritable bowel syndrome in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, 462–466. [Google Scholar] [CrossRef]
- Lechtman, N.; Shamir, R.; Cohen, S.; Chodick, G.; Kariv, R.; Supino-Rosin, L.; Weintraub, Y.; Yerushalmy-Feler, A.; Ben Tov, A. Increased incidence of coeliac disease autoimmunity rate in Israel: A 9-year analysis of population-based data. Aliment. Pharmacol. Ther. 2021, 53, 696–703. [Google Scholar] [CrossRef]
- Mankaï, A.; Landolsi, H.; Chahed, A.; Gueddah, L.; Limem, M.; Ben Abdessalem, M.; Yacoub-Jemni, S.; Ghannem, H.; Jeddi, M.; Ghedira, I. Celiac disease in Tunisia: Serological screening in healthy blood donors. Pathol. Biol. 2006, 54, 10–13. [Google Scholar] [CrossRef]
- Abu-Zekry, M.; Kryszak, D.; Diab, M.; Catassi, C.; Fasano, A. Prevalence of celiac disease in Egyptian children disputes the east-west agriculture-dependent spread of the disease. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 136–140. [Google Scholar] [CrossRef]
- Teresi, S.; Crapisi, M.; Vallejo, M.D.; Castellaneta, S.P.; Francavilla, R.; Iacono, G.; Ravelli, A.; Menegazzi, P.; Louali, M.; Catassi, C. Celiac disease seropositivity in Saharawi children: A follow-up and family study. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 506–509. [Google Scholar] [CrossRef]
- Aboulaghras, S.; Piancatelli, D.; Taghzouti, K.; Balahbib, A.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Oumhani, K. Meta-Analysis and Systematic Review of HLA DQ2/DQ8 in Adults with Celiac Disease. Int. J. Mol. Sci. 2023, 24, 1188. [Google Scholar] [CrossRef]
- Comino, I.; Moreno Mde, L.; Sousa, C. Role of oats in celiac disease. World J. Gastroenterol. 2015, 21, 11825–11831. [Google Scholar] [CrossRef] [PubMed]
- Pulido, O.M.; Gillespie, Z.; Zarkadas, M.; Dubois, S.; Vavasour, E.; Rashid, M.; Switzer, C.; Godefroy, S.B. Introduction of oats in the diet of individuals with celiac disease: A systematic review. Adv. Food Nutr. Res. 2009, 57, 235–285. [Google Scholar] [CrossRef] [PubMed]
- La Vieille, S.; Pulido, O.M.; Abbott, M.; Koerner, T.B.; Godefroy, S. Celiac Disease and Gluten-Free Oats: A Canadian Position Based on a Literature Review. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1870305. [Google Scholar] [CrossRef] [PubMed]
- Aboulaghras, S.; Piancatelli, D.; Taghzouti, K.; Balahbib, A.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A.; Oumhani, K. Safety of Adding Oats to a Gluten-Free Diet for Patients with Celiac Disease: Systematic Review and Meta-analysis of Clinical and Observational Studies. Gastroenterology 2017, 153, 395–409. [Google Scholar] [CrossRef]
- Lionetti, E.; Gatti, S.; Galeazzi, T.; Caporelli, N.; Francavilla, R.; Cucchiara, S.; Roggero, P.; Malamisura, B.; Iacono, G.; Tomarchio, S.; et al. Safety of Oats in Children with Celiac Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Pediatr. 2018, 194, 116–122.e2. [Google Scholar] [CrossRef]
- Tanner, G.; Juhász, A.; Florides, C.G.; Nye-Wood, M.; Békés, F.; Colgrave, M.L.; Russell, A.K.; Hardy, M.Y.; Tye-Din, J.A. Preparation and Characterization of Avenin-Enriched Oat Protein by Chill Precipitation for Feeding Trials in Celiac Disease. Front. Nutr. 2019, 6, 162. [Google Scholar] [CrossRef]
- Palova-Jelinkova, L.; Danova, K.; Drasarova, H.; Dvorak, M.; Funda, D.P.; Fundova, P.; Kotrbova-Kozak, A.; Cerna, M.; Kamanova, J.; Martin, S.F.; et al. Pepsin digest of wheat gliadin fraction increases production of IL-1beta via TLR4/MyD88/TRIF/MAPK/NF-kappaB signaling pathway and an NLRP3 inflammasome activation. PLoS ONE 2013, 8, e62426. [Google Scholar] [CrossRef]
- Garcia-Horsman, J.A.; Venalainen, J.I.; Lohi, O.; Auriola, I.S.; Korponay-Szabo, I.R.; Kaukinen, K.; Maki, M.; Mannisto, P.T. Deficient activity of mammalian prolyl oligopeptidase on the immunoactive peptide digestion in coeliac disease. Scand. J. Gastroenterol. 2007, 42, 562–571. [Google Scholar] [CrossRef]
- Matysiak-Budnik, T.; Candalh, C.; Cellier, C.; Dugave, C.; Namane, A.; Vidal-Martinez, T.; Cerf-Bensussan, N.; Heyman, M. Limited efficiency of prolyl-endopeptidase in the detoxification of gliadin peptides in celiac disease. Gastroenterology 2005, 129, 786–796. [Google Scholar] [CrossRef]
- Wei, G.; Helmerhorst, E.J.; Darwish, G.; Blumenkranz, G.; Schuppan, D. Gluten Degrading Enzymes for Treatment of Celiac Disease. Nutrients 2020, 12, 2095. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Belozersky, M.A.; Filippova, I.Y.; Oppert, B.; Elpidina, E.N. Effective Degradation of Gluten and Its Fragments by Gluten-Specific Peptidases: A Review on Application for the Treatment of Patients with Gluten Sensitivity. Pharmaceutics 2021, 13, 1603. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Caminero, A.; Vivas, S.; Ruiz de Morales, J.M.; Saenz de Miera, L.E.; Rodriguez-Aparicio, L.B.; Casqueiro, J. Differences in faecal bacteria populations and faecal bacteria metabolism in healthy adults and celiac disease patients. Biochimie 2012, 94, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult humans. Gut Microbes 2010, 1, 135–137. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Rizzello, C.G.; Gagliardi, F.; Ricciuti, P.; Ndagijimana, M.; Francavilla, R.; Guerzoni, M.E.; Crecchio, C.; Gobbetti, M.; De Angelis, M. Different fecal microbiotas and volatile organic compounds in treated and untreated children with celiac disease. Appl. Environ. Microbiol. 2009, 75, 3963–3971. [Google Scholar] [CrossRef]
- Bonder, M.J.; Tigchelaar, E.F.; Cai, X.; Trynka, G.; Cenit, M.C.; Hrdlickova, B.; Zhong, H.; Vatanen, T.; Gevers, D.; Wijmenga, C.; et al. The influence of a short-term gluten-free diet on the human gut microbiome. Genome Med. 2016, 8, 45. [Google Scholar] [CrossRef]
- Szczuciński, W.; Salamon, D.; Sopel, A.; Gosiewski, T. Celiac disease and human gut microbiota—How can we study the composition of microorganisms? Gastroenterol. Rev. Przegląd Gastroenterol. 2024, 19, 139574. [Google Scholar] [CrossRef]
- Caminero, A.; Nistal, E.; Herran, A.R.; Perez-Andres, J.; Ferrero, M.A.; Vaquero Ayala, L.; Vivas, S.; Ruiz de Morales, J.M.; Albillos, S.M.; Casqueiro, F.J. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br. J. Nutr. 2015, 114, 1157–1167. [Google Scholar] [CrossRef]
- Zamakhchari, M.; Wei, G.; Dewhirst, F.; Lee, J.; Schuppan, D.; Oppenheim, F.G.; Helmerhorst, E.J. Identification of Rothia bacteria as gluten-degrading natural colonizers of the upper gastro-intestinal tract. PLoS ONE 2011, 6, e24455. [Google Scholar] [CrossRef]
- Helmerhorst, E.J.; Zamakhchari, M.; Schuppan, D.; Oppenheim, F.G. Discovery of a novel and rich source of gluten-degrading microbial enzymes in the oral cavity. PLoS ONE 2010, 5, e13264. [Google Scholar] [CrossRef]
- Stepniak, D.; Spaenij-Dekking, L.; Mitea, C.; Moester, M.; de Ru, A.; Baak-Pablo, R.; van Veelen, P.; Edens, L.; Koning, F. Highly efficient gluten degradation with a newly identified prolyl endoprotease: Implications for celiac disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G621–G629. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Marti, T.; Sollid, L.M.; Gray, G.M.; Khosla, C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: Implications for coeliac sprue. Biochem. J. 2004, 383 Pt 2, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Walter, R.; Tsuru, D. Proline-specific endopeptidase from Flavobacterium. Purification and properties. J. Biol. Chem. 1980, 255, 4786–4792. [Google Scholar] [CrossRef] [PubMed]
- Meresse, B.; Chen, Z.; Ciszewski, C.; Tretiakova, M.; Bhagat, G.; Krausz, T.N.; Raulet, D.H.; Lanier, L.L.; Groh, V.; Spies, T.; et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity 2004, 21, 357–366. [Google Scholar] [CrossRef]
- Londei, M.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Quaratino, S.; Maiuri, L. Gliadin as a stimulator of innate responses in celiac disease. Mol. Immunol. 2005, 42, 913–918. [Google Scholar] [CrossRef]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Picard, J.; Osman, M.; Quaratino, S.; Londei, M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003, 362, 30–37. [Google Scholar] [CrossRef]
- Andre, P.; Castriconi, R.; Espeli, M.; Anfossi, N.; Juarez, T.; Hue, S.; Conway, H.; Romagne, F.; Dondero, A.; Nanni, M.; et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur. J. Immunol. 2004, 34, 961–971. [Google Scholar] [CrossRef]
- Hue, S.; Mention, J.J.; Monteiro, R.C.; Zhang, S.; Cellier, C.; Schmitz, J.; Verkarre, V.; Fodil, N.; Bahram, S.; Cerf-Bensussan, N.; et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity 2004, 21, 367–377. [Google Scholar] [CrossRef]
- Vilasi, S.; Sirangelo, I.; Irace, G.; Caputo, I.; Barone, M.V.; Esposito, C.; Ragone, R. Interaction of ‘toxic’ and ‘immunogenic’ A-gliadin peptides with a membrane-mimetic environment. J. Mol. Recognit. 2010, 23, 322–328. [Google Scholar] [CrossRef]
- Nanayakkara, M.; Kosova, R.; Lania, G.; Sarno, M.; Gaito, A.; Galatola, M.; Greco, L.; Cuomo, M.; Troncone, R.; Auricchio, S.; et al. A celiac cellular phenotype, with altered LPP sub-cellular distribution, is inducible in controls by the toxic gliadin peptide P31-43. PLoS ONE 2013, 8, e79763. [Google Scholar] [CrossRef]
- Barone, M.V.; Gimigliano, A.; Castoria, G.; Paolella, G.; Maurano, F.; Paparo, F.; Maglio, M.; Mineo, A.; Miele, E.; Nanayakkara, M.; et al. Growth factor-like activity of gliadin, an alimentary protein: Implications for coeliac disease. Gut 2007, 56, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, C.; Matarrese, P.; Scazzocchio, B.; Vari, R.; D’Archivio, M.; Straface, E.; Masella, R.; Malorni, W.; De Vincenzi, M. Wheat gliadin induces apoptosis of intestinal cells via an autocrine mechanism involving Fas-Fas ligand pathway. FEBS Lett. 2003, 540, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Lebreton, C.; Schumann, M.; Matysiak-Budnik, T.; Dugave, C.; Bouhnik, Y.; Malamut, G.; Cellier, C.; Allez, M.; Crenn, P.; et al. Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am. J. Pathol. 2012, 180, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lammers, K.M.; Lu, R.; Brownley, J.; Lu, B.; Gerard, C.; Thomas, K.; Rallabhandi, P.; Shea-Donohue, T.; Tamiz, A.; Alkan, S.; et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008, 135, 194–204.e3. [Google Scholar] [CrossRef]
- Clemente, M.G.; De Virgiliis, S.; Kang, J.S.; Macatagney, R.; Musu, M.P.; Di Pierro, M.R.; Drago, S.; Congia, M.; Fasano, A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003, 52, 218–223. [Google Scholar] [CrossRef]
- Drago, S.; El Asmar, R.; Di Pierro, M.; Grazia Clemente, M.; Tripathi, A.; Sapone, A.; Thakar, M.; Iacono, G.; Carroccio, A.; D’Agate, C.; et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand. J. Gastroenterol. 2006, 41, 408–419. [Google Scholar] [CrossRef]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc. Natl. Acad. Sci. USA 2009, 106, 16799–16804. [Google Scholar] [CrossRef]
- Sapone, A.; de Magistris, L.; Pietzak, M.; Clemente, M.G.; Tripathi, A.; Cucca, F.; Lampis, R.; Kryszak, D.; Carteni, M.; Generoso, M.; et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006, 55, 1443–1449. [Google Scholar] [CrossRef]
- El Asmar, R.; Panigrahi, P.; Bamford, P.; Berti, I.; Not, T.; Coppa, G.V.; Catassi, C.; Fasano, A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002, 123, 1607–1615. [Google Scholar] [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. J. Cell Sci. 2000, 113 Pt 24, 4435–4440. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, K.; Blomqvist, T.; Juuti-Uusitalo, K.; Stenman, S.; Venalainen, J.; Maki, M.; Kaukinen, K. Live probiotic Bifidobacterium lactis bacteria inhibit the toxic effects induced by wheat gliadin in epithelial cell culture. Clin. Exp. Immunol. 2008, 152, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Arentz-Hansen, H.; Korner, R.; Molberg, O.; Quarsten, H.; Vader, W.; Kooy, Y.M.; Lundin, K.E.; Koning, F.; Roepstorff, P.; Sollid, L.M.; et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. J. Exp. Med. 2000, 191, 603–612. [Google Scholar] [CrossRef]
- Molberg, O.; McAdam, S.; Lundin, K.E.; Kristiansen, C.; Arentz-Hansen, H.; Kett, K.; Sollid, L.M. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur. J. Immunol. 2001, 31, 1317–1323. [Google Scholar] [CrossRef]
- Quarsten, H.; Molberg, O.; Fugger, L.; McAdam, S.N.; Sollid, L.M. HLA binding and T cell recognition of a tissue transglutaminase-modified gliadin epitope. Eur. J. Immunol. 1999, 29, 2506–2514. [Google Scholar] [CrossRef]
- Camarca, A.; Anderson, R.P.; Mamone, G.; Fierro, O.; Facchiano, A.; Costantini, S.; Zanzi, D.; Sidney, J.; Auricchio, S.; Sette, A.; et al. Intestinal T cell responses to gluten peptides are largely heterogeneous: Implications for a peptide-based therapy in celiac disease. J. Immunol. 2009, 182, 4158–4166. [Google Scholar] [CrossRef]
- Anderson, R.P.; van Heel, D.A.; Tye-Din, J.A.; Barnardo, M.; Salio, M.; Jewell, D.P.; Hill, A.V. T cells in peripheral blood after gluten challenge in coeliac disease. Gut 2005, 54, 1217–1223. [Google Scholar] [CrossRef]
- van de Wal, Y.; Kooy, Y.; van Veelen, P.; Pena, S.; Mearin, L.; Papadopoulos, G.; Koning, F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J. Immunol. 1998, 161, 1585–1588. [Google Scholar] [CrossRef]
- Dorum, S.; Qiao, S.W.; Sollid, L.M.; Fleckenstein, B. A quantitative analysis of transglutaminase 2-mediated deamidation of gluten peptides: Implications for the T-cell response in celiac disease. J. Proteome Res. 2009, 8, 1748–1755. [Google Scholar] [CrossRef]
- Qiao, S.W.; Bergseng, E.; Molberg, O.; Jung, G.; Fleckenstein, B.; Sollid, L.M. Refining the rules of gliadin T cell epitope binding to the disease-associated DQ2 molecule in celiac disease: Importance of proline spacing and glutamine deamidation. J. Immunol. 2005, 175, 254–261. [Google Scholar] [CrossRef]
- Tollefsen, S.; Arentz-Hansen, H.; Fleckenstein, B.; Molberg, O.; Raki, M.; Kwok, W.W.; Jung, G.; Lundin, K.E.; Sollid, L.M. HLA-DQ2 and -DQ8 signatures of gluten T cell epitopes in celiac disease. J. Clin. Investig. 2006, 116, 2226–2236. [Google Scholar] [CrossRef]
- Vader, W.; Stepniak, D.; Kooy, Y.; Mearin, L.; Thompson, A.; van Rood, J.J.; Spaenij, L.; Koning, F. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proc. Natl. Acad. Sci. USA 2003, 100, 12390–12395. [Google Scholar] [CrossRef] [PubMed]
- Henderson, K.N.; Reid, H.H.; Borg, N.A.; Broughton, S.E.; Huyton, T.; Anderson, R.P.; McCluskey, J.; Rossjohn, J. The production and crystallization of the human leukocyte antigen class II molecules HLA-DQ2 and HLA-DQ8 complexed with deamidated gliadin peptides implicated in coeliac disease. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2007, 63 Pt 12, 1021–1025. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabo, I.R.; Vecsei, Z.; Kiraly, R.; Dahlbom, I.; Chirdo, F.; Nemes, E.; Fesus, L.; Maki, M. Deamidated gliadin peptides form epitopes that transglutaminase antibodies recognize. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Vitoria, J.C.; Arrieta, A.; Arranz, C.; Ayesta, A.; Sojo, A.; Maruri, N.; Garcia-Masdevall, M.D. Antibodies to gliadin, endomysium, and tissue transglutaminase for the diagnosis of celiac disease. J. Pediatr. Gastroenterol. Nutr. 1999, 29, 571–574. [Google Scholar]
- Aleanzi, M.; Demonte, A.M.; Esper, C.; Garcilazo, S.; Waggener, M. Celiac disease: Antibody recognition against native and selectively deamidated gliadin peptides. Clin. Chem. 2001, 47, 2023–2028. [Google Scholar] [CrossRef]
- Agardh, D.; Dahlbom, I.; Daniels, T.; Lorinc, E.; Ivarsson, S.A.; Lernmark, A.; Hansson, T. Autoantibodies against soluble and immobilized human recombinant tissue transglutaminase in children with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 322–327. [Google Scholar] [CrossRef]
- Sulkanen, S.; Halttunen, T.; Laurila, K.; Kolho, K.L.; Korponay-Szabo, I.R.; Sarnesto, A.; Savilahti, E.; Collin, P.; Maki, M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology 1998, 115, 1322–1328. [Google Scholar] [CrossRef]
- Bodd, M.; Raki, M.; Tollefsen, S.; Fallang, L.E.; Bergseng, E.; Lundin, K.E.; Sollid, L.M. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010, 3, 594–601. [Google Scholar] [CrossRef]
- Harris, K.M.; Fasano, A.; Mann, D.L. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: Implications for celiac disease. Clin. Immunol. 2010, 135, 430–439. [Google Scholar] [CrossRef]
- Salvati, V.M.; MacDonald, T.T.; Bajaj-Elliott, M.; Borrelli, M.; Staiano, A.; Auricchio, S.; Troncone, R.; Monteleone, G. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut 2002, 50, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Fina, D.; Sarra, M.; Caruso, R.; Del Vecchio Blanco, G.; Pallone, F.; MacDonald, T.T.; Monteleone, G. Interleukin 21 contributes to the mucosal T helper cell type 1 response in coeliac disease. Gut 2008, 57, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Pickard, K.M.; Gordon, J.N.; Salvati, V.; Mazzarella, G.; Beattie, R.M.; Vossenkaemper, A.; Rovedatti, L.; Leakey, N.A.; Croft, N.M.; et al. Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease. Gastroenterology 2007, 133, 1175–1187. [Google Scholar] [CrossRef]
- Mora, B.; Bonamico, M.; Ferri, M.; Megiorni, F.; Osborn, J.; Pizzuti, A.; Mazzilli, M.C. Association of the matrix metalloproteinase-3 (MMP-3) promoter polymorphism with celiac disease in male subjects. Hum. Immunol. 2005, 66, 716–720. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Di Sabatino, A.; Bauer, M.; Della Riccia, D.N.; Bizzini, F.; Biagi, F.; Cifone, M.G.; Corazza, G.R.; Schuppan, D. Matrix metalloproteinase pattern in celiac duodenal mucosa. Lab. Investig. 2005, 85, 397–407. [Google Scholar] [CrossRef]
- Bister, V.; Kolho, K.L.; Karikoski, R.; Westerholm-Ormio, M.; Savilahti, E.; Saarialho-Kere, U. Metalloelastase (MMP-12) is upregulated in the gut of pediatric patients with potential celiac disease and in type 1 diabetes. Scand. J. Gastroenterol. 2005, 40, 1413–1422. [Google Scholar] [CrossRef]
- Lammers, K.M.; Khandelwal, S.; Chaudhry, F.; Kryszak, D.; Puppa, E.L.; Casolaro, V.; Fasano, A. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 2011, 132, 432–440. [Google Scholar] [CrossRef]
- Nanayakkara, M.; Lania, G.; Maglio, M.; Discepolo, V.; Sarno, M.; Gaito, A.; Troncone, R.; Auricchio, S.; Auricchio, R.; Barone, M.V. An undigested gliadin peptide activates innate immunity and proliferative signaling in enterocytes: The role in celiac disease. Am. J. Clin. Nutr. 2013, 98, 1123–1135. [Google Scholar] [CrossRef]
- Barone, M.V.; Troncone, R.; Auricchio, S. Gliadin peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef]
- Volta, U.; Granito, A.; Fiorini, E.; Parisi, C.; Piscaglia, M.; Pappas, G.; Muratori, P.; Bianchi, F.B. Usefulness of antibodies to deamidated gliadin peptides in celiac disease diagnosis and follow-up. Dig. Dis. Sci. 2008, 53, 1582–1588. [Google Scholar] [CrossRef]
- Majsiak, E.; Cukrowska, B.; Choina, M.; Bielawski, K.; Cielecka-Kuszyk, J.; Konopka, E.; Wysokiński, M.; Bierła, J.B. Evaluation of the Usefulness of a Serological Test for Diagnosis of Celiac Disease Simultaneously Detecting Specific Antibodies and Total IgA. Nutrients 2022, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Dubé, C.; Cranney, A.; Saloojee, N.; Sy, R.; Garritty, C.; Sampson, M.; Zhang, L.; Yazdi, F.; Mamaladze, V.; et al. The diagnostic accuracy of serologic tests for celiac disease: A systematic review. Gastroenterology 2005, 128 (Suppl. S1), S38–S46. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Konopka, E.; Grzywnowicz, M.; Oralewska, B.; Cielecka-Kuszyk, J.; Trojanowska, I.; Cukrowska, B. Clinical utility of quantitative multi-antibody Polycheck immunoassays in the diagnosis of coeliac disease. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 254–260. [Google Scholar] [CrossRef]
- Agardh, D. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 1276–1281. [Google Scholar] [CrossRef]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabo, I.R. Accuracy of diagnostic antibody tests for coeliac disease in children: Summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef]
- Lytton, S.D.; Antiga, E.; Pfeiffer, S.; Matthias, T.; Szaflarska-Poplawska, A.; Ulaganathan, V.K.; Placek, W.; Fabbri, P.; Hall, R.; Caproni, M. Neo-epitope tissue transglutaminase autoantibodies as a biomarker of the gluten sensitive skin disease—Dermatitis herpetiformis. Clin. Chim. Acta 2013, 415, 346–349. [Google Scholar] [CrossRef]
- Lerner, A.; Jeremias, P.; Neidhofer, S.; Matthias, T. Antibodies against neo-epitope tTg complexed to gliadin are different and more reliable then anti-tTg for the diagnosis of pediatric celiac disease. J. Immunol. Methods 2016, 429, 15–20. [Google Scholar] [CrossRef]
- Bodd, M.; Raki, M.; Bergseng, E.; Jahnsen, J.; Lundin, K.E.; Sollid, L.M. Direct cloning and tetramer staining to measure the frequency of intestinal gluten-reactive T cells in celiac disease. Eur. J. Immunol. 2013, 43, 2605–2612. [Google Scholar] [CrossRef]
- Picascia, S.; Mandile, R.; Auricchio, R.; Troncone, R.; Gianfrani, C. Gliadin-Specific T-Cells Mobilized in the Peripheral Blood of Coeliac Patients by Short Oral Gluten Challenge: Clinical Applications. Nutrients 2015, 7, 10020–10031. [Google Scholar] [CrossRef]
- Raki, M.; Fallang, L.E.; Brottveit, M.; Bergseng, E.; Quarsten, H.; Lundin, K.E.; Sollid, L.M. Tetramer visualization of gut-homing gluten-specific T cells in the peripheral blood of celiac disease patients. Proc. Natl. Acad. Sci. USA 2007, 104, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Brottveit, M.; Raki, M.; Bergseng, E.; Fallang, L.E.; Simonsen, B.; Lovik, A.; Larsen, S.; Loberg, E.M.; Jahnsen, F.L.; Sollid, L.M.; et al. Assessing possible celiac disease by an HLA-DQ2-gliadin Tetramer Test. Am. J. Gastroenterol. 2011, 106, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, A.; Raki, M.; Bergseng, E.; Lundin, K.E.; Jahnsen, J.; Sollid, L.M.; Qiao, S.W. Tetramer-visualized gluten-specific CD4+ T cells in blood as a potential diagnostic marker for coeliac disease without oral gluten challenge. United Eur. Gastroenterol. J. 2014, 2, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, A.; Risnes, L.F.; Bergseng, E.; Lundin, K.E.; Sollid, L.M.; Qiao, S.W. Healthy HLA-DQ2.5+ Subjects Lack Regulatory and Memory T Cells Specific for Immunodominant Gluten Epitopes of Celiac Disease. J. Immunol. 2016, 196, 2819–2826. [Google Scholar] [CrossRef]
- Özgenel, Ş.M.; Temel, T.; Üsküdar Teke, H.; Yıldız, P.; Korkmaz, H.; Özakyol, A. HLA-DQ2/DQ8 frequency in adult patients with celiac disease, their first-degree relatives, and normal population in Turkey. Turk. J. Gastroenterol. 2019, 30, 321–325. [Google Scholar] [CrossRef]
- Cecilio, L.A.; Bonatto, M.W. The prevalence of HLA DQ2 and DQ8 in patients with celiac disease, in family and in general population. Arq. Bras. Cir. Dig. 2015, 28, 183–185. [Google Scholar] [CrossRef]
- Mansouri, M.; Dadfar, M.; Rostami-Nejad, M.; Ekhlasi, G.; Shahbazkhani, A.; Shahbazkhani, B. The frequency of HLA-DQ2/DQ8 haplotypes and celiac disease among the first-degree relatives of patients with celiac disease. Gastroenterol. Hepatol. Bed Bench 2021, 14, 36–43. [Google Scholar]
- Paziewska, A.; Cukrowska, B.; Dabrowska, M.; Goryca, K.; Piatkowska, M.; Kluska, A.; Mikula, M.; Karczmarski, J.; Oralewska, B.; Rybak, A.; et al. Combination Testing Using a Single MSH5 Variant alongside HLA Haplotypes Improves the Sensitivity of Predicting Coeliac Disease Risk in the Polish Population. PLoS ONE 2015, 10, e0139197. [Google Scholar] [CrossRef]
- Romanos, J.; Rosen, A.; Kumar, V.; Trynka, G.; Franke, L.; Szperl, A.; Gutierrez-Achury, J.; van Diemen, C.C.; Kanninga, R.; Jankipersadsing, S.A.; et al. Improving coeliac disease risk prediction by testing non-HLA variants additional to HLA variants. Gut 2014, 63, 415–422. [Google Scholar] [CrossRef]
- Koskinen, L.; Romanos, J.; Kaukinen, K.; Mustalahti, K.; Korponay-Szabo, I.; Barisani, D.; Bardella, M.T.; Ziberna, F.; Vatta, S.; Szeles, G.; et al. Cost-effective HLA typing with tagging SNPs predicts celiac disease risk haplotypes in the Finnish, Hungarian, and Italian populations. Immunogenetics 2009, 61, 247–256. [Google Scholar] [CrossRef]
- Monsuur, A.J.; de Bakker, P.I.; Zhernakova, A.; Pinto, D.; Verduijn, W.; Romanos, J.; Auricchio, R.; Lopez, A.; van Heel, D.A.; Crusius, J.B.; et al. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLoS ONE 2008, 3, e2270. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Zhang, N.; Zhou, Z.X.; Huang, C.C.; Zeng, C.L.; Xiao, D.; Guo, C.C.; Han, Y.J.; Ye, X.H.; Ye, X.G.; et al. Association of LPP and TAGAP Polymorphisms with Celiac Disease Risk: A Meta-Analysis. Int. J. Environ. Res. Public. Health 2017, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.P.; Goel, G.; Hardy, M.Y.; Russell, A.K.; Wang, S.; Szymczak, E.; Zhang, R.; Goldstein, K.E.; Neff, K.; Truitt, K.E.; et al. Whole blood interleukin-2 release test to detect and characterize rare circulating gluten-specific T cell responses in coeliac disease. Clin. Exp. Immunol. 2021, 204, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Tye-Din, J.A.; Qiao, S.W.; Russell, A.K.; Mayassi, T.; Ciszewski, C.; Sarna, V.K.; Wang, S.; Goldstein, K.E.; Dzuris, J.L.; et al. Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease. Sci. Adv. 2019, 5, eaaw7756. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Daveson, A.J.M.; Goldstein, K.E.; Hand, H.L.; Neff, K.M.; Goel, G.; Williams, L.J.; Truitt, K.E.; Anderson, R.P. Patient factors influencing acute gluten reactions and cytokine release in treated coeliac disease. BMC Med. 2020, 18, 362. [Google Scholar] [CrossRef]
- Molberg, O.; Lundin, K.E.; Nilsen, E.M.; Scott, H.; Kett, K.; Brandtzaeg, P.; Thorsby, E.; Sollid, L.M. HLA restriction patterns of gliadin- and astrovirus-specific CD4+ T cells isolated in parallel from the small intestine of celiac disease patients. Tissue Antigens 1998, 52, 407–415. [Google Scholar] [CrossRef]
- Anderson, R.P.; Degano, P.; Godkin, A.J.; Jewell, D.P.; Hill, A.V. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat. Med. 2000, 6, 337–342. [Google Scholar] [CrossRef]
- Gjertsen, H.A.; Sollid, L.M.; Ek, J.; Thorsby, E.; Lundin, K.E. T cells from the peripheral blood of coeliac disease patients recognize gluten antigens when presented by HLA-DR, -DQ, or -DP molecules. Scand. J. Immunol. 1994, 39, 567–574. [Google Scholar] [CrossRef]
- Zühlke, S.; Risnes, L.F.; Dahal-Koirala, S.; Christophersen, A.; Sollid, L.M.; Lundin, K.E. CD38 expression on gluten-specific T cells is a robust marker of gluten re-exposure in coeliac disease. United Eur. Gastroenterol. J. 2019, 7, 1337–1344. [Google Scholar] [CrossRef]
- Fritz, R.D.; Chen, Y. Oat safety for celiac disease patients: Theoretical analysis correlates adverse symptoms in clinical studies to contaminated study oats. Nutr. Res. 2018, 60, 54–67. [Google Scholar] [CrossRef]
- Thompson, T.; Keller, A. Gluten cross contact in oats: Retrospective database analysis 2011 to 2023. Front. Nutr. 2023, 10, 1284636. [Google Scholar] [CrossRef] [PubMed]
- Smulders, M.J.M.; van de Wiel, C.C.M.; van den Broeck, H.C.; van der Meer, I.M.; Israel-Hoevelaken, T.P.M.; Timmer, R.D.; van Dinter, B.J.; Braun, S.; Gilissen, L. Oats in healthy gluten-free and regular diets: A perspective. Food Res. Int. 2018, 110, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lahdeaho, M.L.; Maki, M.; Laurila, K.; Huhtala, H.; Kaukinen, K. Small- bowel mucosal changes and antibody responses after low- and moderate-dose gluten challenge in celiac disease. BMC Gastroenterol. 2011, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fabiani, E.; Iacono, G.; D’Agate, C.; Francavilla, R.; Biagi, F.; Volta, U.; Accomando, S.; Picarelli, A.; De Vitis, I.; et al. A prospective, double-blind, placebo-controlled trial to establish a safe gluten threshold for patients with celiac disease. Am. J. Clin. Nutr. 2007, 85, 160–166. [Google Scholar] [CrossRef]
- Bruins, M.J. The clinical response to gluten challenge: A review of the literature. Nutrients 2013, 5, 4614–4641. [Google Scholar] [CrossRef]
- Akobeng, A.K.; Thomas, A.G. Systematic review: Tolerable amount of gluten for people with coeliac disease. Aliment. Pharmacol. Ther. 2008, 27, 1044–1052. [Google Scholar] [CrossRef]
- Gibert, A.; Espadaler, M.; Angel Canela, M.; Sanchez, A.; Vaque, C.; Rafecas, M. Consumption of gluten-free products: Should the threshold value for trace amounts of gluten be at 20, 100 or 200 p.p.m.? Eur. J. Gastroenterol. Hepatol. 2006, 18, 1187–1195. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food labeling: Gluten-free labeling of foods. Final rule. Fed. Regist. 2013, 78, 47154–47179. [Google Scholar]
- Tuire, I.; Marja-Leena, L.; Teea, S.; Katri, H.; Jukka, P.; Paivi, S.; Heini, H.; Markku, M.; Pekka, C.; Katri, K. Persistent duodenal intraepithelial lymphocytosis despite a long-term strict gluten-free diet in celiac disease. Am. J. Gastroenterol. 2012, 107, 1563–1569. [Google Scholar] [CrossRef]
- Hollon, J.R.; Cureton, P.A.; Martin, M.L.; Puppa, E.L.; Fasano, A. Trace gluten contamination may play a role in mucosal and clinical recovery in a subgroup of diet-adherent non-responsive celiac disease patients. BMC Gastroenterol. 2013, 13, 40. [Google Scholar] [CrossRef]
- Bascunan, K.A.; Vespa, M.C.; Araya, M. Celiac disease: Understanding the gluten-free diet. Eur. J. Nutr. 2016, 56, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Samasca, G.; Sur, G.; Lupan, I.; Deleanu, D. Gluten-free diet and quality of life in celiac disease. Gastroenterol. Hepatol. Bed Bench 2014, 7, 139–143. [Google Scholar] [PubMed]
- Balamtekin, N.; Aksoy, C.; Baysoy, G.; Uslu, N.; Demir, H.; Koksal, G.; Saltik-Temizel, I.N.; Ozen, H.; Gurakan, F.; Yuce, A. Is compliance with gluten-free diet sufficient? Diet composition of celiac patients. Turk. J. Pediatr. 2015, 57, 374–379. [Google Scholar]
- Churruca, I.; Miranda, J.; Lasa, A.; Bustamante, M.A.; Larretxi, I.; Simon, E. Analysis of Body Composition and Food Habits of Spanish Celiac Women. Nutrients 2015, 7, 5515–5531. [Google Scholar] [CrossRef]
- Kautto, E.; Ivarsson, A.; Norstrom, F.; Hogberg, L.; Carlsson, A.; Hornell, A. Nutrient intake in adolescent girls and boys diagnosed with coeliac disease at an early age is mostly comparable to their non-coeliac contemporaries. J. Hum. Nutr. Diet. 2014, 27, 41–53. [Google Scholar] [CrossRef]
- Wu, J.H.; Neal, B.; Trevena, H.; Crino, M.; Stuart-Smith, W.; Faulkner-Hogg, K.; Yu Louie, J.C.; Dunford, E. Are gluten-free foods healthier than non-gluten-free foods? An evaluation of supermarket products in Australia. Br. J. Nutr. 2015, 114, 448–454. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Gibson, P.R. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J. Hum. Nutr. Diet. 2013, 26, 349–358. [Google Scholar] [CrossRef]
- Miranda, J.; Lasa, A.; Bustamante, M.A.; Churruca, I.; Simon, E. Nutritional differences between a gluten-free diet and a diet containing equivalent products with gluten. Plant Foods Hum. Nutr. 2014, 69, 182–187. [Google Scholar] [CrossRef]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- MacCulloch, K.; Rashid, M. Factors affecting adherence to a gluten-free diet in children with celiac disease. Paediatr. Child. Health 2014, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Burden, M.; Mooney, P.D.; Blanshard, R.J.; White, W.L.; Cambray-Deakin, D.R.; Sanders, D.S. Cost and availability of gluten-free food in the UK: In store and online. Postgrad. Med. J. 2015, 91, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Missbach, B.; Schwingshackl, L.; Billmann, A.; Mystek, A.; Hickelsberger, M.; Bauer, G.; Konig, J. Gluten-free food database: The nutritional quality and cost of packaged gluten-free foods. PeerJ 2015, 3, e1337. [Google Scholar] [CrossRef] [PubMed]
- Zanini, B.; Marullo, M.; Villanacci, V.; Salemme, M.; Lanzarotto, F.; Ricci, C.; Lanzini, A. Persistent Intraepithelial Lymphocytosis in Celiac Patients Adhering to Gluten-Free Diet Is Not Abolished Despite a Gluten Contamination Elimination Diet. Nutrients 2016, 8, 525. [Google Scholar] [CrossRef]
- Lanzini, A.; Lanzarotto, F.; Villanacci, V.; Mora, A.; Bertolazzi, S.; Turini, D.; Carella, G.; Malagoli, A.; Ferrante, G.; Cesana, B.M.; et al. Complete recovery of intestinal mucosa occurs very rarely in adult coeliac patients despite adherence to gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 1299–1308. [Google Scholar] [CrossRef]
- Tursi, A.; Brandimarte, G.; Giorgetti, G.M.; Elisei, W.; Inchingolo, C.D.; Monardo, E.; Aiello, F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: A 2-year prospective study. Endoscopy 2006, 38, 702–707. [Google Scholar] [CrossRef]
- Daniewski, W.; Wojtasik, A.; Kunachowicz, H. Gluten content in special dietary use gluten-free products and other food products. Rocz. Panstw. Zakl. Hig. 2010, 61, 51–55. [Google Scholar]
- Cichańska, B.A. Problemy z Rozróżnianiem Żywności Bezglutenowej. Pediatria Współczesna. Gastroenterologia, Hepatologia i Żywienie Dziecka. 2009, pp. 117–122. Available online: https://katalogi.bn.org.pl/discovery/fulldisplay?docid=alma9910307223805606&vid=48OMNIS_NLOP:48OMNIS_NLOP (accessed on 8 March 2025).
- Wojtasik, W.D.A.; Kunachowicz, H. Zawartość glutenu (gliadyny) w wybranych produktach spożywczych. Bromat. Chem. Toksykol. 2010, XLIII, 362–371. [Google Scholar]
- Koerner, T.B.; Cleroux, C.; Poirier, C.; Cantin, I.; La Vieille, S.; Hayward, S.; Dubois, S. Gluten contamination of naturally gluten-free flours and starches used by Canadians with celiac disease. Food Addit. Contam. Part. A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 2017–2021. [Google Scholar] [CrossRef]
- Thompson, T.; Lee, A.R.; Grace, T. Gluten contamination of grains, seeds, and flours in the United States: A pilot study. J. Am. Diet. Assoc. 2010, 110, 937–940. [Google Scholar] [CrossRef]
- Miller, K.; McGough, N.; Urwin, H. Catering Gluten-Free When Simultaneously Using Wheat Flour. J. Food Prot. 2016, 79, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Goswami, H.; Byrne, K.; Blundell, M.; Howitt, C.A.; Tanner, G.J. Proteomic profiling of 16 cereal grains and the application of targeted proteomics to detect wheat contamination. J. Proteome Res. 2015, 14, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Gibert, A.; Kruizinga, A.G.; Neuhold, S.; Houben, G.F.; Canela, M.A.; Fasano, A.; Catassi, C. Might gluten traces in wheat substitutes pose a risk in patients with celiac disease? A population-based probabilistic approach to risk estimation. Am. J. Clin. Nutr. 2013, 97, 109–116. [Google Scholar] [CrossRef] [PubMed]
- La Vieille, S.; Dubois, S.; Hayward, S.; Koerner, T.B. Estimated levels of gluten incidentally present in a Canadian gluten-free diet. Nutrients 2014, 6, 881–896. [Google Scholar] [CrossRef]
- Lee, H.J.; Anderson, Z.; Ryu, D. Gluten contamination in foods labeled as “gluten free” in the United States. J. Food Prot. 2014, 77, 1830–1833. [Google Scholar] [CrossRef]
- Silvester, J.A.; Graff, L.A.; Rigaux, L.; Walker, J.R.; Duerksen, D.R. Symptomatic suspected gluten exposure is common among patients with coeliac disease on a gluten-free diet. Aliment. Pharmacol. Ther. 2016, 44, 612–619. [Google Scholar] [CrossRef]
- Roma, E.; Roubani, A.; Kolia, E.; Panayiotou, J.; Zellos, A.; Syriopoulou, V.P. Dietary compliance and life style of children with coeliac disease. J. Hum. Nutr. Diet. 2010, 23, 176–182. [Google Scholar] [CrossRef]
- Black, J.L.; Orfila, C. Impact of coeliac disease on dietary habits and quality of life. J. Hum. Nutr. Diet. 2011, 24, 582–587. [Google Scholar] [CrossRef]
- Sasaki, M.; Bosman, B.W.; Tan, P.S. A new, broad-substrate-specificity aminopeptidase from the dairy organism Lactobacillus helveticus SBT 2171. Microbiology 1996, 142 Pt 4, 799–808. [Google Scholar] [CrossRef]
- Tan, P.S.; Pos, K.M.; Konings, W.N. Purification and characterization of an endopeptidase from Lactococcus lactis subsp. cremoris Wg2. Appl. Environ. Microbiol. 1991, 57, 3593–3599. [Google Scholar] [CrossRef]
- Laloi, P.; Atlan, D.; Blanc, B.; Gilbert, C.; Portalier, R. Cell-wall-associated proteinase of Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397: Differential extraction, purification and properties of the enzyme. Appl. Microbiol. Biotechnol. 1991, 36, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Lavermicocca, P.; De Vincenzi, M.; Giovannini, C.; Faccia, M.; Gobbetti, M. Proteolysis by sourdough lactic acid bacteria: Effects on wheat flour protein fractions and gliadin peptides involved in human cereal intolerance. Appl. Environ. Microbiol. 2002, 68, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; De Angelis, M.; Auricchio, S.; Greco, L.; Clarke, C.; De Vincenzi, M.; Giovannini, C.; D’Archivio, M.; Landolfo, F.; Parrilli, G.; et al. Sourdough bread made from wheat and nontoxic flours and started with selected lactobacilli is tolerated in celiac sprue patients. Appl. Environ. Microbiol. 2004, 70, 1088–1096. [Google Scholar] [CrossRef]
- di Cagno, R.; de Angelis, M.; Alfonsi, G.; de Vincenzi, M.; Silano, M.; Vincentini, O.; Gobbetti, M. Pasta made from durum wheat semolina fermented with selected lactobacilli as a tool for a potential decrease of the gluten intolerance. J. Agric. Food Chem. 2005, 53, 4393–4402. [Google Scholar] [CrossRef]
- Gerez, C.L.; Font de Valdez, G.; Rollan, G.C. Functionality of lactic acid bacteria peptidase activities in the hydrolysis of gliadin-like fragments. Lett. Appl. Microbiol. 2008, 47, 427–432. [Google Scholar] [CrossRef]
- Rollan, G.; De Angelis, M.; Gobbetti, M.; de Valdez, G.F. Proteolytic activity and reduction of gliadin-like fractions by sourdough lactobacilli. J. Appl. Microbiol. 2005, 99, 1495–1502. [Google Scholar] [CrossRef]
- Montserrat, V.; Bruins, M.J.; Edens, L.; Koning, F. Influence of dietary components on Aspergillus niger prolyl endoprotease mediated gluten degradation. Food Chem. 2015, 174, 440–445. [Google Scholar] [CrossRef]
- Tack, G.J.; van de Water, J.M.; Bruins, M.J.; Kooy-Winkelaar, E.M.; van Bergen, J.; Bonnet, P.; Vreugdenhil, A.C.; Korponay-Szabo, I.; Edens, L.; von Blomberg, B.M.; et al. Consumption of gluten with gluten-degrading enzyme by celiac patients: A pilot-study. World J. Gastroenterol. 2013, 19, 5837–5847. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Anderson, R.P.; Ffrench, R.A.; Brown, G.J.; Hodsman, P.; Siegel, M.; Botwick, W.; Shreeniwas, R. The effects of ALV003 pre-digestion of gluten on immune response and symptoms in celiac disease in vivo. Clin. Immunol. 2010, 134, 289–295. [Google Scholar] [CrossRef]
- Siegel, M.; Garber, M.E.; Spencer, A.G.; Botwick, W.; Kumar, P.; Williams, R.N.; Kozuka, K.; Shreeniwas, R.; Pratha, V.; Adelman, D.C. Safety, tolerability, and activity of ALV003: Results from two phase 1 single, escalating-dose clinical trials. Dig. Dis. Sci. 2012, 57, 440–450. [Google Scholar] [CrossRef]
- Lahdeaho, M.L.; Kaukinen, K.; Laurila, K.; Vuotikka, P.; Koivurova, O.P.; Karja-Lahdensuu, T.; Marcantonio, A.; Adelman, D.C.; Maki, M. Glutenase ALV003 attenuates gluten-induced mucosal injury in patients with celiac disease. Gastroenterology 2014, 146, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Syage, J.A.; Wu, T.-T.; Dickason, M.A.; Ramos, A.G.; Van Dyke, C.; Horwath, I.; Lavin, P.T.; Mäki, M.; Hujoel, I.; et al. Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients with Celiac Disease Exposed to a Gluten Challenge. Gastroenterology 2022, 163, 1510–1521.e6. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Siegel, J.B.; Tinberg, C.; Camarca, A.; Gianfrani, C.; Paski, S.; Guan, R.; Montelione, G.; Baker, D.; Pultz, I.S. Engineering of Kuma030: A Gliadin Peptidase That Rapidly Degrades Immunogenic Gliadin Peptides in Gastric Conditions. J. Am. Chem. Soc. 2015, 137, 13106–13113. [Google Scholar] [CrossRef] [PubMed]
- Pultz, I.S.; Hill, M.; Vitanza, J.M.; Wolf, C.; Saaby, L.; Liu, T.; Winkle, P.; Leffler, D.A. Gluten Degradation, Pharmacokinetics, Safety, and Tolerability of TAK-062, an Engineered Enzyme to Treat Celiac Disease. Gastroenterology 2021, 161, 81–93.e3. [Google Scholar] [CrossRef]
- Klemenak, M.; Dolinsek, J.; Langerholc, T.; Di Gioia, D.; Micetic-Turk, D. Administration of Bifidobacterium breve Decreases the Production of TNF-alpha in Children with Celiac Disease. Dig. Dis. Sci. 2015, 60, 3386–3392. [Google Scholar] [CrossRef]
- Olivares, M.; Laparra, M.; Sanz, Y.; Cinova, J.; De Palma, G.; Stepankova, R.; Kofronova, O.; Kverka, M.; Tuckova, L. Influence of Bifidobacterium longum CECT 7347 and gliadin peptides on intestinal epithelial cell proteome. J. Agric. Food Chem. 2011, 59, 7666–7671. [Google Scholar] [CrossRef]
- Olivares, M.; Castillejo, G.; Varea, V.; Sanz, Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br. J. Nutr. 2014, 112, 30–40. [Google Scholar] [CrossRef]
- Smecuol, E.; Hwang, H.J.; Sugai, E.; Corso, L.; Chernavsky, A.C.; Bellavite, F.P.; Gonzalez, A.; Vodanovich, F.; Moreno, M.L.; Vazquez, H.; et al. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J. Clin. Gastroenterol. 2013, 47, 139–147. [Google Scholar] [CrossRef]
- Lloyd-Still, J.D.; Grand, R.J.; Khaw, K.T.; Shwachman, H. The use of corticosteroids in celiac crisis. J. Pediatr. 1972, 81, 1074–1081. [Google Scholar] [CrossRef]
- Abbas, A.; Shahab, T.; Sherwani, R.K.; Alam, S. Addition of a Short Course of Prednisolone to a Gluten-Free Diet vs. Gluten-Free Diet Alone in Recovery of Celiac Disease: A Pilot Randomized Controlled Trial. Cureus 2018, 10, e2118. [Google Scholar] [CrossRef]
- Ali Ibrahim, A.; Kenyon, V.; Fasano, A.; Leonard, M.M. Budesonide and the Gluten Containing Elimination Diet as Treatments for Non-responsive Celiac Disease in Children. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Murray, J.A. Classification and management of refractory coeliac disease. Gut 2010, 59, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.S.; Sharma, A.; Rubio-Tapia, A.; Wu, T.T.; Jabri, B.; Murray, J.A. Open-Capsule Budesonide for Refractory Celiac Disease. Am. J. Gastroenterol. 2017, 112, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Therrien, A.; Silvester, J.A.; Leonard, M.M.; Leffler, D.A.; Fasano, A.; Kelly, C.P. Enteric-Release Budesonide May Be Useful in the Management of Non-Responsive Celiac Disease. Dig. Dis. Sci. 2021, 66, 1989–1997. [Google Scholar] [CrossRef]
- Mauriño, E.; Niveloni, S.; Cherñavsky, A.; Pedreira, S.; Mazure, R.; Vazquez, H.; Reyes, H.; Fiorini, A.; Smecuol, E.; Cabanne, A.; et al. Azathioprine in refractory sprue: Results from a prospective, open-label study. Am. J. Gastroenterol. 2002, 97, 2595–2602. [Google Scholar] [CrossRef]
- Goerres, M.S.; Meijer, J.W.; Wahab, P.J.; Kerckhaert, J.A.; Groenen, P.J.; Van Krieken, J.H.; Mulder, C.J. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment. Pharmacol. Ther. 2003, 18, 487–494. [Google Scholar] [CrossRef]
- Iqbal, U.; Chaudhary, A.; Karim, M.A.; Anwar, H.; Merrell, N. Refractory Celiac Disease Successfully Treated with Azathioprine. Gastroenterol. Res. 2017, 10, 199–201. [Google Scholar] [CrossRef]
- Rawal, N.; Twaddell, W.; Fasano, A.; Blanchard, S.; Safta, A. Remission of Refractory Celiac Disease With Infliximab in a Pediatric Patient. ACG Case Rep. J. 2015, 2, 121–123. [Google Scholar] [CrossRef]
- Valitutti, F.; Barbato, M.; Aloi, M.; Marcheggiano, A.; Di Nardo, G.; Leoni, S.; Iorfida, D.; Corazza, G.R.; Cucchiara, S. Autoimmune enteropathy in a 13-year-old celiac girl successfully treated with infliximab. J. Clin. Gastroenterol. 2014, 48, 264–266. [Google Scholar] [CrossRef]
- Costantino, G.; della Torre, A.; Lo Presti, M.A.; Caruso, R.; Mazzon, E.; Fries, W. Treatment of life-threatening type I refractory coeliac disease with long-term infliximab. Dig. Liver Dis. 2008, 40, 74–77. [Google Scholar] [CrossRef]
- Senolt, L.; Vencovsky, J.; Pavelka, K.; Ospelt, C.; Gay, S. Prospective new biological therapies for rheumatoid arthritis. Autoimmun. Rev. 2009, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Conlon, K.C.; Stewart, D.M.; Worthy, T.A.; Janik, J.E.; Fleisher, T.A.; Albert, P.S.; Figg, W.D.; Spencer, S.D.; Raffeld, M.; et al. Phase 1 trial of IL-15 trans presentation blockade using humanized Mikbeta1 mAb in patients with T-cell large granular lymphocytic leukemia. Blood 2013, 121, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Janik, J.E.; White, J.D.; Fleisher, T.A.; Brown, M.; Tsudo, M.; Goldman, C.K.; Bryant, B.; Petrus, M.; Top, L.; et al. Preclinical and phase I clinical trial of blockade of IL-15 using Mikbeta1 monoclonal antibody in T cell large granular lymphocyte leukemia. Proc. Natl. Acad. Sci. USA 2006, 103, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Lähdeaho, M.L.; Scheinin, M.; Vuotikka, P.; Taavela, J.; Popp, A.; Laukkarinen, J.; Koffert, J.; Koivurova, O.P.; Pesu, M.; Kivelä, L.; et al. Safety and efficacy of AMG 714 in adults with coeliac disease exposed to gluten challenge: A phase 2a, randomised, double-blind, placebo-controlled study. Lancet Gastroenterol. Hepatol. 2019, 4, 948–959. [Google Scholar] [CrossRef]
- Cellier, C.; Bouma, G.; Van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.; Crowe, S.E.; Tsuji, W.; et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: A phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef]
- Dieckman, T.; Schumann, M.; Beaumont, H.; Bontkes, H.J.; Koning, F.; Bouma, G. Enduring Clinical Remission in Refractory Celiac Disease Type II with Tofacitinib: An Open-Label Clinical Study. Clin. Gastroenterol. Hepatol. 2024, 22, 2334–2336. [Google Scholar] [CrossRef]
- Croese, J.; Giacomin, P.; Navarro, S.; Clouston, A.; McCann, L.; Dougall, A.; Ferreira, I.; Susianto, A.; O’Rourke, P.; Howlett, M.; et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol. 2015, 135, 508–516. [Google Scholar] [CrossRef]
- McSorley, H.J.; Gaze, S.; Daveson, J.; Jones, D.; Anderson, R.P.; Clouston, A.; Ruyssers, N.E.; Speare, R.; McCarthy, J.S.; Engwerda, C.R.; et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS ONE 2011, 6, e24092. [Google Scholar] [CrossRef]
- Daveson, A.J.; Jones, D.M.; Gaze, S.; McSorley, H.; Clouston, A.; Pascoe, A.; Cooke, S.; Speare, R.; Macdonald, G.A.; Anderson, R.; et al. Effect of hookworm infection on wheat challenge in celiac disease--a randomised double-blinded placebo controlled trial. PLoS ONE 2011, 6, e17366. [Google Scholar] [CrossRef]
- Giacomin, P.; Zakrzewski, M.; Croese, J.; Su, X.; Sotillo, J.; McCann, L.; Navarro, S.; Mitreva, M.; Krause, L.; Loukas, A.; et al. Experimental hookworm infection and escalating gluten challenges are associated with increased microbial richness in celiac subjects. Sci. Rep. 2015, 5, 13797. [Google Scholar] [CrossRef]
- Cantacessi, C.; Giacomin, P.; Croese, J.; Zakrzewski, M.; Sotillo, J.; McCann, L.; Nolan, M.J.; Mitreva, M.; Krause, L.; Loukas, A. Impact of experimental hookworm infection on the human gut microbiota. J. Infect. Dis. 2014, 210, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Croese, J.; Miller, G.C.; Marquart, L.; Llewellyn, S.; Gupta, R.; Becker, L.; Clouston, A.D.; Welch, C.; Sidorenko, J.; Wallace, L.; et al. Randomized, Placebo Controlled Trial of Experimental Hookworm Infection for Improving Gluten Tolerance in Celiac Disease. Clin. Transl. Gastroenterol. 2020, 11, e00274. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A.; Wassaf, D.; Dunn, K.; Arora, S.; Winkle, P.; Stacey, H.; Cooper, S.; Goldstein, K.E.; Manchanda, R.; Kontos, S.; et al. Safety and tolerability of KAN-101, a liver-targeted immune tolerance therapy, in patients with coeliac disease (ACeD): A phase 1 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 735–747. [Google Scholar] [CrossRef]
- Kelly, C.P.; Murray, J.A.; Leffler, D.A.; Getts, D.R.; Bledsoe, A.C.; Smithson, G.; First, M.R.; Morris, A.; Boyne, M.; Elhofy, A.; et al. TAK-101 Nanoparticles Induce Gluten-Specific Tolerance in Celiac Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Gastroenterology 2021, 161, 66–80.e8. [Google Scholar] [CrossRef]
- Riedmann, E.M. Human vaccines: News. Hum. Vaccin. Immunother. 2012, 8, 1550–1553. [Google Scholar] [CrossRef]
- Daveson, A.J.M.; Ee, H.C.; Andrews, J.M.; King, T.; Goldstein, K.E.; Dzuris, J.L.; MacDougall, J.A.; Williams, L.J.; Treohan, A.; Cooreman, M.P.; et al. Epitope-Specific Immunotherapy Targeting CD4-Positive T Cells in Celiac Disease: Safety, Pharmacokinetics, and Effects on Intestinal Histology and Plasma Cytokines with Escalating Dose Regimens of Nexvax2 in a Randomized, Double-Blind, Placebo-Controlled Phase 1 Study. EBioMedicine 2017, 26, 78–90. [Google Scholar] [CrossRef]
- Truitt, K.E.; Daveson, A.J.M.; Ee, H.C.; Goel, G.; MacDougall, J.; Neff, K.; Anderson, R.P. Randomised clinical trial: A placebo-controlled study of subcutaneous or intradermal NEXVAX2, an investigational immunomodulatory peptide therapy for coeliac disease. Aliment. Pharmacol. Ther. 2019, 50, 547–555. [Google Scholar] [CrossRef]
- Hardy, M.Y.; Goel, G.; Russell, A.K.; Chen Yi Mei, S.L.G.; Brown, G.J.E.; Wang, S.; Szymczak, E.; Zhang, R.; Goldstein, K.E.; Neff, K.M.; et al. A Sensitive Whole Blood Assay Detects Antigen-Stimulated Cytokine Release From CD4+ T Cells and Facilitates Immunomonitoring in a Phase 2 Clinical Trial of Nexvax2 in Coeliac Disease. Front. Immunol. 2021, 12, 661622. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Daveson, A.J.; Goel, G.; Goldstein, K.E.; Hand, H.L.; Neff, K.M.; Popp, A.; Taavela, J.; Maki, M.; Isola, J.; et al. Efficacy and safety of gluten peptide-based antigen-specific immunotherapy (Nexvax2) in adults with coeliac disease after bolus exposure to gluten (RESET CeD): An interim analysis of a terminated randomised, double-blind, placebo-controlled phase 2 study. Lancet Gastroenterol. Hepatol. 2023, 8, 446–457. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: Diagnostic and therapeutic implications. Clin. Gastroenterol. Hepatol. 2012, 10, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Leffler, D.A.; Kelly, C.P.; Abdallah, H.Z.; Colatrella, A.M.; Harris, L.A.; Leon, F.; Arterburn, L.A.; Paterson, B.M.; Lan, Z.H.; Murray, J.A. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am. J. Gastroenterol. 2012, 107, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.P.; Green, P.H.; Murray, J.A.; Dimarino, A.; Colatrella, A.; Leffler, D.A.; Alexander, T.; Arsenescu, R.; Leon, F.; Jiang, J.G.; et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: A randomised placebo-controlled study. Aliment. Pharmacol. Ther. 2013, 37, 252–262. [Google Scholar] [CrossRef]
- Leffler, D.A.; Kelly, C.P.; Green, P.H.; Fedorak, R.N.; DiMarino, A.; Perrow, W.; Rasmussen, H.; Wang, C.; Bercik, P.; Bachir, N.M.; et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: A randomized controlled trial. Gastroenterology 2015, 148, 1311–1319.e6. [Google Scholar] [CrossRef]
- Paterson, B.M.; Lammers, K.M.; Arrieta, M.C.; Fasano, A.; Meddings, J.B. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: A proof of concept study. Aliment. Pharmacol. Ther. 2007, 26, 757–766. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Durai, M.; Kitchens, K.; Tamiz, A.P.; Somerville, R.; Ginski, M.; Paterson, B.M.; Murray, J.A.; Verdu, E.F.; Alkan, S.S.; et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 2012, 35, 86–94. [Google Scholar] [CrossRef]
- Biopharma. 9 Meters Biopharma Announces Interim Analysis of Phase 3 Study of Larazotide for Celiac Disease Does Not Support Trial Continuation (Press Release 21 June 2022). Available online: https://www.biospace.com/poor-results-end-9-meters-phase-iii-trial-for-larazotide (accessed on 8 March 2025).
- Hoilat, G.J.; Altowairqi, A.K.; Ayas, M.F.; Alhaddab, N.T.; Alnujaidi, R.A.; Alharbi, H.A.; Alyahyawi, N.; Kamal, A.; Alhabeeb, H.; Albazee, E.; et al. Larazotide acetate for treatment of celiac disease: A systematic review and meta-analysis of randomized controlled trials. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101782. [Google Scholar] [CrossRef]
- Pardin, C.; Pelletier, J.N.; Lubell, W.D.; Keillor, J.W. Cinnamoyl inhibitors of tissue transglutaminase. J. Org. Chem. 2008, 73, 5766–5775. [Google Scholar] [CrossRef]
- Klock, C.; Jin, X.; Choi, K.; Khosla, C.; Madrid, P.B.; Spencer, A.; Raimundo, B.C.; Boardman, P.; Lanza, G.; Griffin, J.H. Acylideneoxoindoles: A new class of reversible inhibitors of human transglutaminase 2. Bioorg Med. Chem. Lett. 2011, 21, 2692–2696. [Google Scholar] [CrossRef]
- Hausch, F.; Halttunen, T.; Maki, M.; Khosla, C. Design, synthesis, and evaluation of gluten peptide analogs as selective inhibitors of human tissue transglutaminase. Chem. Biol. 2003, 10, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Garabuczi, E.; Sarang, Z.; Vereb, G.; Vamosi, G.; Aeschlimann, D.; Blasko, B.; Becsi, B.; Erdodi, F.; Lacy-Hulbert, A.; et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J. Immunol. 2009, 182, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Sarang, Z.; Molnar, P.; Nemeth, T.; Piacentini, M.; Mastroberardino, P.G.; Falasca, L.; Aeschlimann, D.; Kovacs, J.; Kiss, I.; et al. Transglutaminase 2-/- mice reveal a phagocytosis-associated crosstalk between macrophages and apoptotic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 7812–7817. [Google Scholar] [CrossRef]
- Rauhavirta, T.; Oittinen, M.; Kivisto, R.; Mannisto, P.T.; Garcia-Horsman, J.A.; Wang, Z.; Griffin, M.; Maki, M.; Kaukinen, K.; Lindfors, K. Are transglutaminase 2 inhibitors able to reduce gliadin-induced toxicity related to celiac disease? A proof-of-concept study. J. Clin. Immunol. 2013, 33, 134–142. [Google Scholar] [CrossRef]
- Sulic, A.M.; Kurppa, K.; Rauhavirta, T.; Kaukinen, K.; Lindfors, K. Transglutaminase as a therapeutic target for celiac disease. Expert Opin. Ther. Targets 2015, 19, 335–348. [Google Scholar] [CrossRef]
- Schuppan, D.; Mäki, M.; Lundin, K.E.A.; Isola, J.; Friesing-Sosnik, T.; Taavela, J.; Popp, A.; Koskenpato, J.; Langhorst, J.; Hovde, Ø.; et al. A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease. N. Engl. J. Med. 2021, 385, 35–45. [Google Scholar] [CrossRef]
- Dotsenko, V.; Tewes, B.; Hils, M.; Pasternack, R.; Isola, J.; Taavela, J.; Popp, A.; Sarin, J.; Huhtala, H.; Hiltunen, P.; et al. Transcriptomic analysis of intestine following administration of a transglutaminase 2 inhibitor to prevent gluten-induced intestinal damage in celiac disease. Nat. Immunol. 2024, 25, 1218–1230. [Google Scholar] [CrossRef]
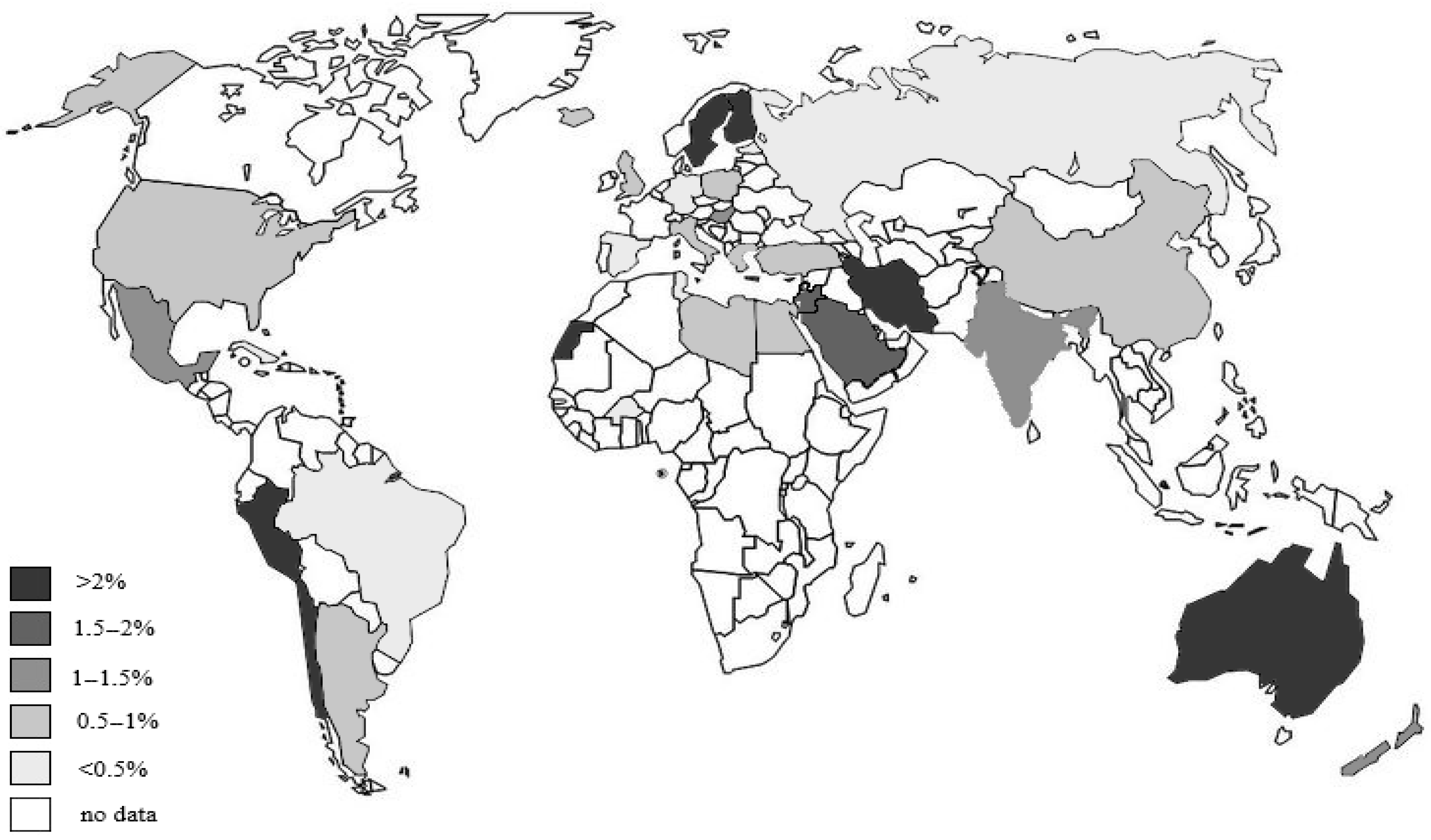

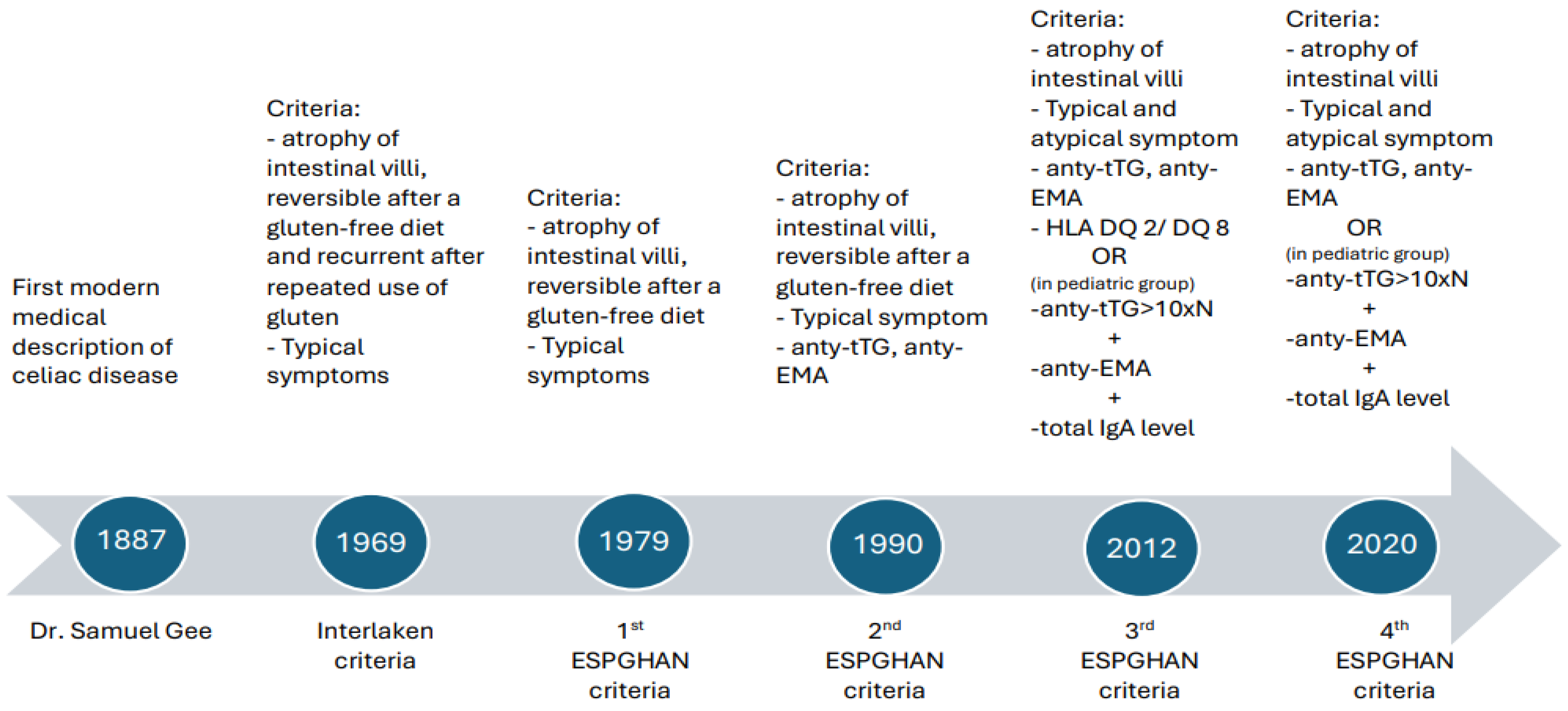
| Author | Years of Study | Country | Age | No. of Participation | Sex | Osteoporosis Frequency |
|---|---|---|---|---|---|---|
| Zanchetta et al. [23,25] | 2011–2015 | Argentina | Adults | 30 | Women | n/c |
| Bai et al. [21] | n/c | Argentina | Adults | 25 | Women | n/c |
| Galli et al. [29] | 2010–2021 | Itali | Adults | 291 | Women–men 3:1 | Osteopenia + osteoporosis 63.5% |
| Ganji et al. [30] | Review of 56 article tile 2018 | UK, Brazil, India, Hungary, Poland | Adults | Women–men 3:1 | 14.4% | |
| Ganji et al. [31] | 2014–2019 | Iran | Adults under 55 | 387 | Women–men 3:1 | 16.4% |
| Walker et al. [32] | USA | Adults | 721 | Women–men 3:1 | 19.6% | |
| Sayar et al. [33] | 2010–2019 | Turkey | Adults under 50 | 84 | Women and men | 15.2% |
| Soracka et al. [34] | Poland | Adults under 50 | 30 | Women | 13.3% |
| Country/Region | Years of Study | Studied Population | Test Used for Screening | Studied Population Number | Prevalence of CD |
|---|---|---|---|---|---|
| North America -USA [68] | Anty-EMA, anty-tTG, HLA DQ2/DQ8 | ||||
| 1996–2001 | At all ages | 13,145 | 0.75% | ||
| South America [79] | 2000–2013 | Metanalyses of 72 seroprevalence studies, at all ages | Anty-tTG, anty-EMA | 0.46–0.64% | |
| Europe [73] | 1991–2012 | Metanalyses of 49 seroprevalence studies, at all age | Anty-tTG | 1.3% | |
| -Finland [64,71] | 1994 | Schoolchildren | Anty-EMA, anty-tTG | 3645 | 1.5% |
| 2000–2001 | Adults (30–93 years old) | Anty-EMA, anty-tTG, biopsies | 6403 | 2.4% | |
| -German [71] | 1990, 2001 | Adults (25–74 years old) | Anty-EMA, anty-tTG, biopsies | 9201 | 0.3% |
| -Italy [71] | 2000–2002 | At all ages | Anty-EMA, anty-tTG, biopsies | 7427 | 0.7% |
| -Russian [80] | 1997–2001 | At all ages | Anty-EMA, anty-tTG, biopsies | 11,070 | 0.2–0.57% |
| Asia [66] | Metanalyses of 19 seroprevalence studies, at all ages | Anty-tTG, anty-EMA | 1.6% | ||
| -China [81] | Metanalyses of 18 seroprevalence studies, at all age | Anty-tTG, anty-DGP | 0.56% | ||
| -India [82] | 1994–1995 | Seroprevalence at all ages | Anty-tTG | 10,488 | 1.44% |
| -Australia [83] | 2007–2015 | Seroprevalence at all ages | Anty-tTG | 3011 | 1.56% |
| -Iran [84] | Seroprevalence, schoolchildren | Anty-tTG | 1500 | 2% | |
| -Saudi Arabia [85] | Healthy adults aged 20–60 years | Anty-EMA, anty-tTG, biopsies | 980 | 1.9% | |
| -Israel [86] | 2012–2013 | Seroprevalence at all ages | Anty-tTG | 403,283 | 1.56% |
| Africa: | |||||
| -Tunisia [87] | Adults (blood donors) | Anty-EMA | 2500 | 0.28% | |
| -Egypt [88] | Children | Anty-tTG | 1500 | 0.53% | |
| -Saharawi population [89] | At all ages | Anty-tTG, anty-EMA | 975 | 5.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, M.K.; Domżał-Magrowska, D.; Małecka-Wojciesko, E. Celiac Disease—Narrative Review on Progress in Celiac Disease. Foods 2025, 14, 959. https://doi.org/10.3390/foods14060959
Kowalski MK, Domżał-Magrowska D, Małecka-Wojciesko E. Celiac Disease—Narrative Review on Progress in Celiac Disease. Foods. 2025; 14(6):959. https://doi.org/10.3390/foods14060959
Chicago/Turabian StyleKowalski, Marek K., Danuta Domżał-Magrowska, and Ewa Małecka-Wojciesko. 2025. "Celiac Disease—Narrative Review on Progress in Celiac Disease" Foods 14, no. 6: 959. https://doi.org/10.3390/foods14060959
APA StyleKowalski, M. K., Domżał-Magrowska, D., & Małecka-Wojciesko, E. (2025). Celiac Disease—Narrative Review on Progress in Celiac Disease. Foods, 14(6), 959. https://doi.org/10.3390/foods14060959







