Antioxidant and Inflammation-Attenuating Ability of Human Milk, Infant Formulas and Their Oligosaccharides †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Milk and Infant Formula Samples and Oligosaccharide Extraction
2.2. Antioxidant Assays
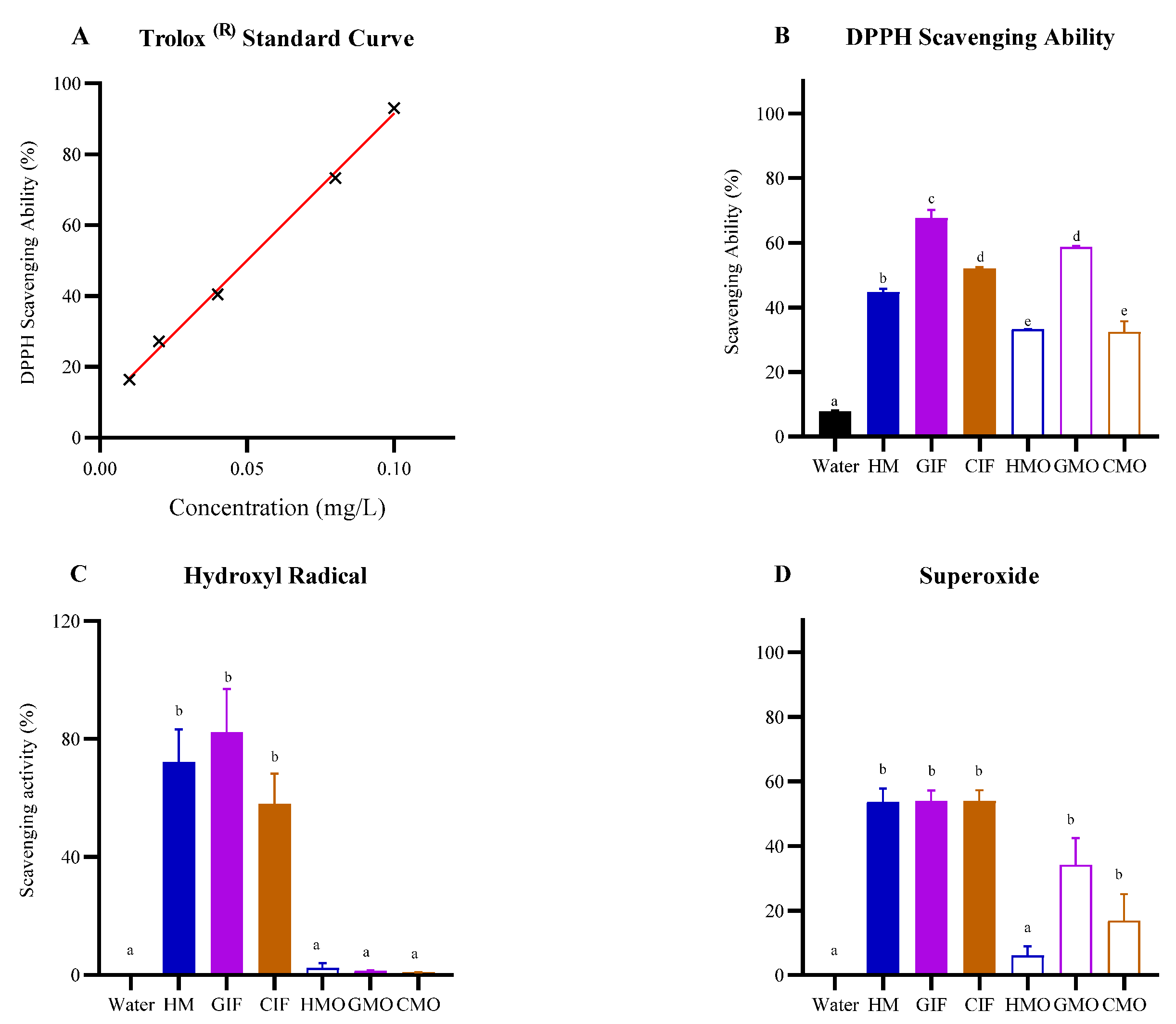
2.2.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
2.2.2. Hydroxyl Radical Scavenging Ability
2.2.3. Superoxide Radical Scavenging Ability
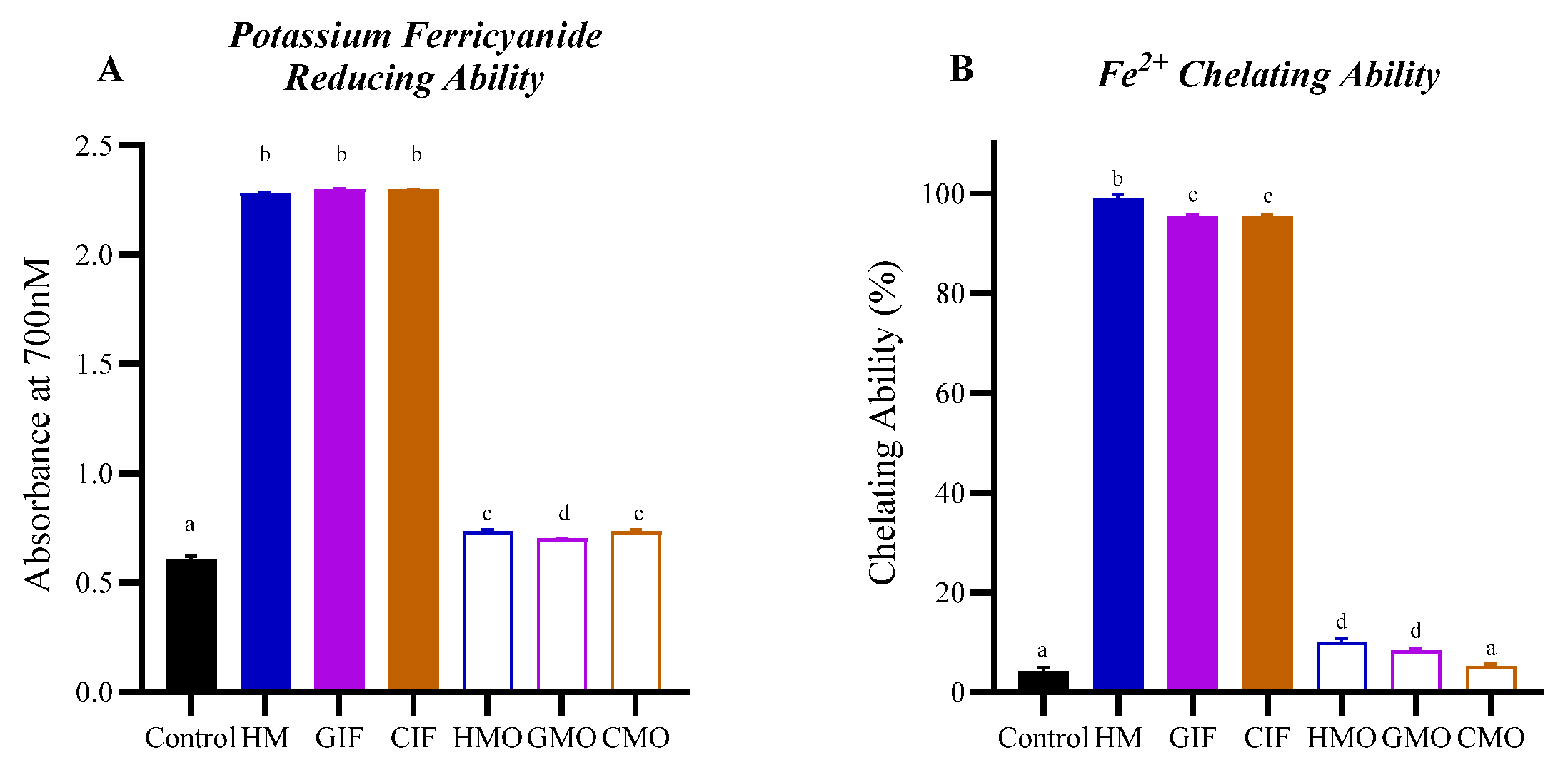
2.2.4. Potassium Ferricyanide Reducing Power (FER)
2.2.5. Fe2+-Chelating Ability (CA)
2.3. Cell Culture Conditions and Simulation of Inflammation Conditions
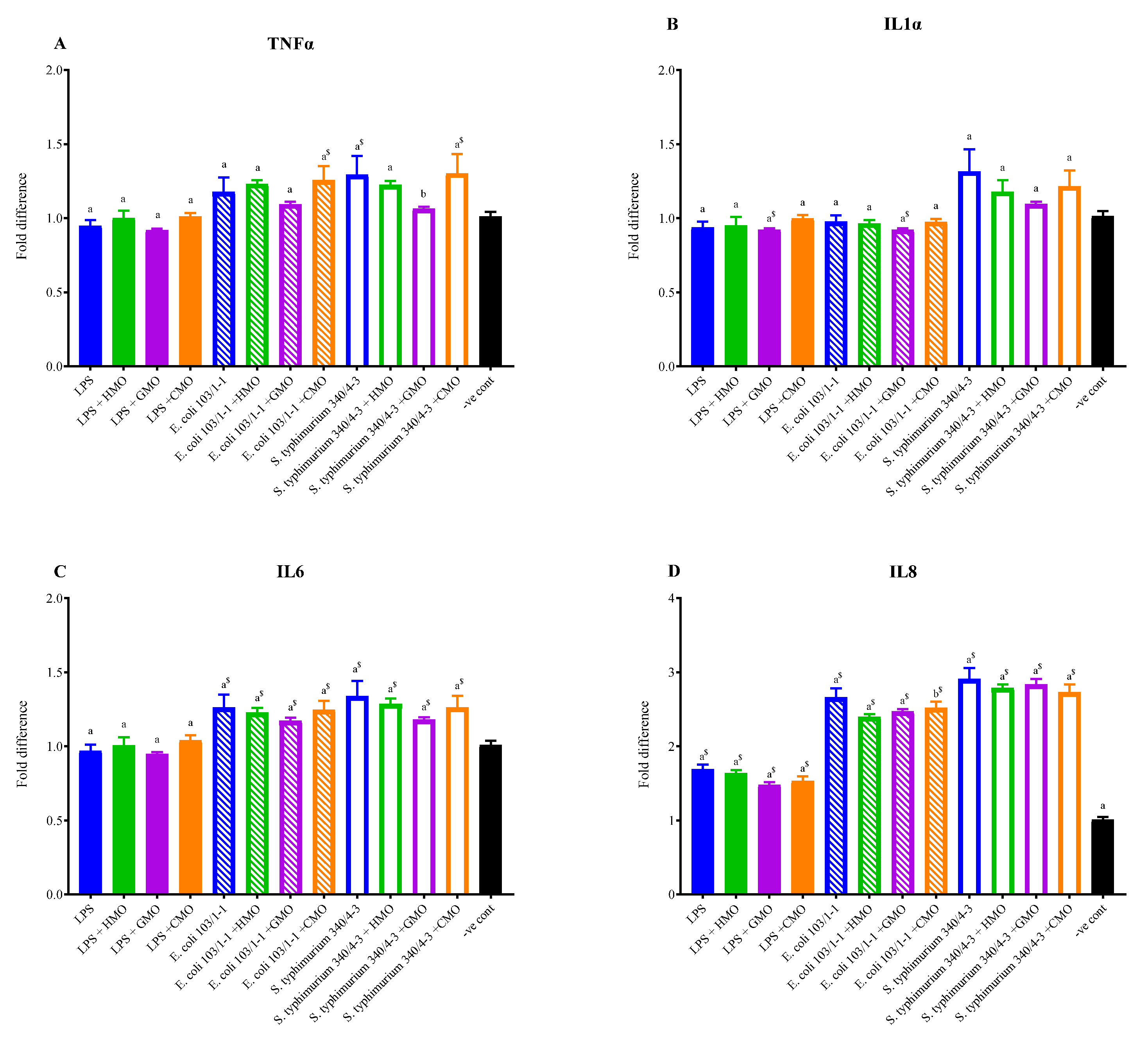
2.4. Cytokine Expression Levels
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Antioxidant Properties
3.1.1. Free Radical Scavenging Abilities
3.1.2. Iron-Reduction and Iron-Chelation Abilities
3.2. Effects of Milk Oligosaccharides on Reducing Inflammatory Cytokine Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Wiciński, M.; Sawícka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human milk oligosaccharides: Health benefits, potential applications in infant formulas, and pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Stewart, C.J. Untangling human milk oligosaccharides and infant gut microbiome. iScience 2022, 25, 103542. [Google Scholar] [CrossRef] [PubMed]
- Leong, A.; Mori, C.; Pillidge, C.; Gill, H. Do oligosaccharide-supplemented infant formulas improve infant gastrointestinal health? A systematic review of randomized clinical trials. Food Rev. Int. 2024, 41, 40–86. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef]
- Jacobs, J.P.; Lee, M.L.; Rechtman, D.J.; Sun, A.K.; Autran, C.; Niklas, V. Human milk oligosaccharides modulate the intestinal microbiome of healthy adults. Sci. Rep. 2023, 13, 14308. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef]
- Dinleyici, M.; Barbieur, J.; Dinleyici, C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef]
- Sánchez, C.; Fente, C.; Regal, P.; Lamas, A.; Lorenzo, M.P. Human milk oligosaccharides (HMOs) and infant microbiota: A scoping review. Foods 2021, 10, 1429. [Google Scholar] [CrossRef]
- Sodhi, C.P.; Ahmad, R.; Fulton, W.B.; Lopez, C.M.; Eke, B.O.; Scheese, D.; Duess, J.W.; Steinway, S.N.; Raouf, Z.; Moore, H.; et al. Human milk oligosaccharides reduce necrotizing enterocolitis-induced neuroinflammation and cognitive impairment in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 325, G23–G41. [Google Scholar] [CrossRef]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Tailored combinations of human milk oligosaccharides modulate the immune response in an in vitro model of intestinal inflammation. Biomolecules 2024, 14, 1481. [Google Scholar] [CrossRef] [PubMed]
- Živkovíć, J.; Sunaríć, S.; Trutíć, N.; Deníć, M.; Kocíć, G.; Jovanovíć, T. Antioxidants and antioxidant capacity of human milk. Acta Fac. Medicae Naissensis 2015, 32, 115–125. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, S.; Deng, X.; Luo, Z.; Chen, A.; Yu, R. Effects of antioxidants in human milk on bronchopulmonary dysplasia prevention and treatment: A review. Front. Nutr. 2022, 9, 924036. [Google Scholar] [CrossRef]
- Ribeiro, V.P.D.; Tinoco, R.B.; Chamon, A.L.B.; Pessoa, I.S.; Santos, T.C.D.; Silva, R.S.; Fronza, M. The influence of time and temperature on human milk storage antioxidant properties, oxidative stress, and total protein. J. Hum. Lact. 2023, 39, 308–314. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez, L.M.; Migacheva, N.; Mosselmans, J.-M.; Picaud, J.-C.; Possner, M.; et al. Human milk oligosaccharides: 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) in infant formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef]
- Nijman, R.M.; Liu, Y.; Bunyatratchata, A.; Smilowitz, J.T.; Stahl, B.; Barile, D. Characterization and quantification of oligosaccharides in human milk and infant formula. J. Agric. Food Chem. 2018, 66, 6851–6859. [Google Scholar] [CrossRef]
- Daddaoua, A.; Puerta, V.; Requena, P.; Martinez-Ferez, A.; Guadix, E.; de Medina, F.S.; Zarzuelo, A.; Suárez, M.D.; Boza, J.J.; Martinez-Augustin, O. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J. Nutr. 2006, 136, 672–676. Available online: http://jn.nutrition.org/content/136/3/672.full.pdf (accessed on 1 June 2023). [CrossRef]
- Lara-Villoslada, F.; Debras, E.; Nieto, A.; Concha, A.; Gálvez, J.; López-Huertas, E.; Boza, J.; Obled, C.; Xaus, J. Oligosaccharides isolated from goat milk reduce intestinal inflammation in a rat model of dextran sodium sulfate-induced colitis. Clin. Nutr. ESPEN 2006, 25, 477–488. [Google Scholar] [CrossRef]
- Wang, G.; Sun, W.; Pei, X.; Jin, Y.; Wang, H.; Tao, W.; Xiao, Z.; Liu, L.; Wang, M. Galactooligosaccharide pretreatment alleviates damage of the intestinal barrier and inflammatory responses in LPS-challenged mice. Food Funct. 2021, 12, 1569–1579. [Google Scholar] [CrossRef]
- Liu, Z.; Moate, P.; Cocks, B.; Rochfort, S. Simple liquid chromatography-mass spectrometry method for quantification of major free oligosaccharides in bovine milk. J. Agric. Food Chem. 2014, 62, 11568–11574. [Google Scholar] [CrossRef]
- Leong, A.; Liu, Z.; Almshawit, H.; Zisu, B.; Pillidge, C.; Rochfort, S.; Gill, H. Oligosaccharides in goats’ milk-based infant formula and their prebiotic and anti-infection properties. Br. J. Nutr. 2019, 122, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Alwasei, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- El-Fattah, A.A.; Azzam, M.; Elkashef, H.; Elhadydy, A. Antioxidant properties of milk: Effect of milk species, milk fractions and heat treatments. Int. J. Dairy Sci. 2020, 15, 1–9. [Google Scholar] [CrossRef]
- Zarban, A.; Taheri, F.; Chahkandi, T.; Sharifzadeh, G.; Khorashadizadeh, M. Antioxidant and radical scavenging activity of human colostrum, transitional and mature milk. J. Clin. Biochem. Nutr. 2009, 45, 150–154. [Google Scholar] [CrossRef]
- Zhu, K.; Yao, S.; Zhang, Y.; Liu, Q.; Xu, F.; Wu, G.; Dong, W.; Tan, L. Effects of in vitro saliva, gastric and intestinal digestion on the chemical properties, antioxidant activity of polysaccharide from Artocarpus heterophyllus Lam. (jackfruit) pulp. Food Hydrocoll. 2019, 87, 952–959. [Google Scholar] [CrossRef]
- Haber, F.; Weiss, J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1934, 147, 332–351. [Google Scholar] [CrossRef]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Imam, M.U.; Zhang, S.; Ma, J.; Wang, H.; Wang, F. Antioxidants mediate both iron homeostasis and oxidative stress. Nutrients 2017, 9, 671. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Methods for the assessment of antioxidant activity. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Oxford, UK, 2015; Chapter 12; pp. 287–333. [Google Scholar] [CrossRef]
- Liu, W.; Wang, H.; Pang, X.; Yao, W.; Gao, X. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. Int. J. Biol. Macromol. 2010, 46, 451–457. [Google Scholar] [CrossRef]
- Sundström, C.; Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef]
- Bahrami, B.; Macfarlane, S.; Macfarlane, G. Induction of cytokine formation by human intestinal bacteria in gut epithelial cell lines. J. Appl. Microbiol. 2011, 110, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.-H.; Shin, S.Y.; Song, J.H.; Kang, N.M. Effect of human breast milk on innate immune response: Up-regulation of bacterial pattern recognition receptors and innate cytokines in THP-1 monocytic cells. Eur. J. Inflamm. 2021, 19. [Google Scholar] [CrossRef]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-rich grape seed extract reduces inflammation and oxidative stress and restores tight junction barrier function in Caco-2 colon cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, M.; Xu, N.; Lv, Q.; Huang, N.; He, J.; Zhang, Y. miR-181a regulates inflammation responses in monocytes and macrophages. PLoS ONE 2013, 8, e58639. [Google Scholar] [CrossRef]
- Cho, H.; Choi, S.; Lee, S.-W.; Kim, Y.W.; Lee, C.K.; Lee, S.-W. Iopromide in combination with IFN-γ induces the activation of HMC-1 Cells via IL-4 and MCP-1 expression. Cell Immunol. 2015, 293, 95–103. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Åkesson, B. Antioxidative factors in milk. Br. J. Nutr. 2000, 84, 103–110. [Google Scholar] [CrossRef]
- Yao, L.; Friel, J.K.; Suh, M.; Diehl-Jones, W.L. Antioxidant properties of breast milk in a novel in vitro digestion/enterocyte model. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 670–676. [Google Scholar] [CrossRef]
- Hernández-Aguilar, M.T.; de la Torre, M.J.L.; Borja-Herrero, C.; Lasarte-Velillas, J.-J.; Martorell-Juan, L. Antioxidant properties of human milk. J. Ped. Biochem. 2013, 3, 161–167. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Ahmed, A.S.; El-Bassiony, T.; Elmalt, L.M.; Ibrahim, H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Res. Int. 2015, 74, 80–88. [Google Scholar] [CrossRef]
- Lorenzetti, S.; Plösch, T.; Teller, I.C. Antioxidative molecules in human milk and environmental contaminants. Antioxidants 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Lugonja, N.; Gorjanović, S.; Pastor, F.T.; Marinković, V.; Miličić, B.; Vrvić, M.; Spasić, S. Antioxidant capacity and quality of human milk and infant formula determined by direct current polarography. Food Anal. Meth. 2021, 14, 1987–1994. [Google Scholar] [CrossRef]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A comprehensive review on infant formula: Nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef]
- Soyyılmaz, B.; Mikš, M.H.; Röhrig, C.H.; Matwiejuk, M.; Meszaros-Matwiejuk, A.; Vigsnæs, L.K. The mean of milk: A review of human milk oligosaccharide concentrations throughout lactation. Nutrients 2021, 13, 2737. [Google Scholar] [CrossRef]
- Schalich, K.M.; Buendia, M.A.; Kaur, H.; Choksi, Y.A.; Washington, M.K.; Codreanu, G.S.; Sherrod, S.D.; McLean, J.A.; Peek, R.M., Jr.; Acra, S.A.; et al. A human milk oligosaccharide prevents intestinal inflammation in adulthood via modulating gut microbial metabolism. mBio 2024, 15, e00298-24. [Google Scholar] [CrossRef]
- Meyrand, M.; Dallas, D.; Caillat, H.; Bouvier, F.; Martin, P.; Barile, D. Comparison of milk oligosaccharides between goats with and without the genetic ability to synthesize αs1-casein. Small Rumin. Res. 2013, 113, 411–420. [Google Scholar] [CrossRef]
- van Leeuwen, S.S.; te Poele, E.M.; Chatziioannou, A.C.; Benjamins, E.; Haandrikmann, A.; Dijkhuizen, L. Goat milk oligosaccharides: Their diversity, quantity and functional properties in comparison to human milk oligosaccharides. J. Agric. Food. Chem. 2020, 68, 13469–13485. [Google Scholar] [CrossRef]
- van der Toorn, M.V.; Chatziioannou, C.; Pellis, L.; Haandrikmann, A.; van der Zee, L.; Dijkhuizen, L. Biological relevance of goat milk oligosaccharides to infant health. J. Agric. Food. Chem. 2023, 71, 13935–13949. [Google Scholar] [CrossRef]
- Sergius-Ronot, M.; Suwal, S.; Shama, S.; Chamberland, J.; Unger, S.; O’Connor, D.L.; Pouliot, Y.; Doyen, A. The ultrafiltration molecular weight cut-off has a limited effect on the concentration and protein profile during preparation of human milk protein concentrates. J. Dairy Sci. 2021, 104, 3820–3831. [Google Scholar] [CrossRef]
- Vieira, T.F.; Corrêa, R.C.G.; Peralta, R.A.; Peralta-Muniz-Moreira, R.F.; Bracht, A.; Peralta, R.M. An overview of structural aspects and health beneficial effects of antioxidant oligosaccharides. Curr. Pharm. Des. 2020, 26, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Triantis, V.; Bode, L.; Van Neerven, R. Immunological effects of human milk oligosaccharides. Front. Pediatr. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; van De Worp, W.R.; Stassen, R.; van Maastrigt, C.; Kettelarij, N.; Stahl, B.; Blijenberg, B.; Overbeek, S.A.; Folkerts, G.; Garssen, J.; et al. Human milk oligosaccharides promote immune tolerance via direct interactions with human dendritic cells. Eur. J. Immunol. 2019, 49, 1001–1014. [Google Scholar] [CrossRef] [PubMed]
- Zuurveld, M.; van Witzenburg, N.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L. Immunomodulation by human milk oligosaccharides: The potential role in prevention of allergic diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Li, A.; Li, Y.; Zhang, X.; Zhang, C.; Li, T.; Zhang, J.; Li, C. The human milk oligosaccharide 2′-fucosyllactose attenuates β-lactoglobulin–induced food allergy through the miR-146a–mediated toll-like receptor 4/nuclear factor-κB signaling pathway. J. Dairy Sci. 2021, 104, 10473–10484. [Google Scholar] [CrossRef]
- Del Fabbro, S.; Calder, P.C.; Childs, C.E. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc. Nutr. Soc. 2020, 79, 468–478. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leong, A.; Pillidge, C.; Gill, H. Antioxidant and Inflammation-Attenuating Ability of Human Milk, Infant Formulas and Their Oligosaccharides. Foods 2025, 14, 960. https://doi.org/10.3390/foods14060960
Leong A, Pillidge C, Gill H. Antioxidant and Inflammation-Attenuating Ability of Human Milk, Infant Formulas and Their Oligosaccharides. Foods. 2025; 14(6):960. https://doi.org/10.3390/foods14060960
Chicago/Turabian StyleLeong, Andrea, Christopher Pillidge, and Harsharn Gill. 2025. "Antioxidant and Inflammation-Attenuating Ability of Human Milk, Infant Formulas and Their Oligosaccharides" Foods 14, no. 6: 960. https://doi.org/10.3390/foods14060960
APA StyleLeong, A., Pillidge, C., & Gill, H. (2025). Antioxidant and Inflammation-Attenuating Ability of Human Milk, Infant Formulas and Their Oligosaccharides. Foods, 14(6), 960. https://doi.org/10.3390/foods14060960








